Abstract
Less invasive approaches continue to be explored and refined for diseases of the colon and rectum. The current gold standard for the surgical treatment of rectal cancer, total mesorectal excision (TME), is a technically precise yet demanding procedure with outcomes measured by both oncologic and functional outcomes (including bowel, urinary, and sexual). To date, the minimally invasive approach to rectal cancer has not yet been perfected, leaving ample opportunity for rectal surgeons to innovate. Transanal TME has recently emerged as a safe and effective technique for both benign and malignant diseases of the rectum. While widespread acceptance of this surgical approach remains tempered at this time due to lack of long-term oncologic outcome data, short-term outcomes are promising and there is great excitement surrounding the promise of this technique.
Keywords: total mesorectal excision, transanal, rectal cancer
Since the description and popularization of rectal dissection in the “Holy Plane” by Professor R.J. Heald, total mesorectal excision (TME) has become the standard of care for rectal cancer surgery, dramatically impacting local recurrence and functional outcomes.1 2 3 4 TME, however, is a technically challenging procedure, particularly in an obese patient with a narrow pelvis and/or bulky tumor, and can result in significant morbidity including anastomotic complications and genitourinary and bowel dysfunction.5 6
More than 20 years ago, laparoscopy was introduced as an alternative to the traditional open surgical approach with the hope of facilitating recovery and decreasing morbidity following colon and rectal surgery. The CLASICC trial demonstrated better short-term outcomes, including shorter hospital stay and recovery following laparoscopic surgery when compared with open resections for colorectal cancer, although this study simultaneously raised concerns about positive circumferential resection margin (CRM) following rectal resection.7 Subsequently, the COLOR II trial revealed noninferiority of a laparoscopic approach to an open approach for rectal cancer.8 Most recently, two large randomized trials have demonstrated that the laparoscopic approach does not meet criteria for noninferiority compared with an open approach when evaluating a composite of outcomes including CRM positivity and completeness of the TME.9 10 The data gathered from these studies will eventually produce long-term oncologic results, but these findings also highlight the opportunity for alternative minimally invasive surgical approaches for rectal cancer. As a result of these studies, coupled with the technical complexity of the surgery, the laparoscopic approach for rectal cancer has not been routinely adopted in the same way it has for colon cancer.11
Laparoscopic and robotic proctectomies are technically challenging procedures for several reasons, including challenging exposure, tissue retraction, maneuverability, smoke accumulation, and lack of tactile sensation.12 13 Furthermore, endoscopic staplers are not designed to be optimally positioned in the narrow pelvis, often requiring multiple stapler applications which can result in angulated, crossing staple lines and an increased risk of anastomotic leak.14 Ultimately, these technical issues can lead to high conversion to open surgery, lower sphincter preservation rates, and incomplete specimens with positive CRM and distal margins.7 12 15 16 17
In response to these challenges, alternative techniques are being explored as potential improvements to traditional open and laparoscopic techniques. Laparoscopic transanal TME (taTME), also referred to as “bottom-to-up” TME, is emerging as a novel approach that allows for a caudal to cephalad minimally invasive rectal dissection.18 The primary advantage of this technique is that the surgeon can directly visualize and define the distal resection margin of the tumor and enter the mesorectal dissection plane at its most caudal aspect. Direct visualization allows safe dissection around the critical structures that envelop the narrow pelvis including the vagina, prostate, and pelvic neurovascular structures. Pneumoinflation of the TME plane provides a significant amount of tissue retraction, further facilitating the rectal dissection and mobilization.12 13 19 20 Consequently, this technique has been found to have improved histologic outcomes with fewer positive circumferential and distal margins compared with other minimally invasive surgical options.13 20 21 22 While early studies may validate the safety and efficacy of this technique, no long-term oncologic or functional data have been published.
Indications
Indications for taTME include both benign and malignant diseases of the rectum. A consensus was recently published by the Second International Transanal Total Mesorectal Excision Conference23 held in July 2014. Consensus members suggest that the taTME approach is optimally designed for men, patients with narrow and/or deep pelvis, visceral obesity and/or body mass index (BMI) >30, prostatic hypertrophy, tumor diameter > 4 cm, distorted tissue planes such as irradiated fields, difficult to palpate tumors, and failure to progress from a traditional open or laparoscopic operative approach.
Operative Technique
The patient is placed in a lithotomy position with the right arm tucked and patient secured to the bed. Both the abdominal and perineal fields are prepped. The abdominal portion commences laparoscopically with the objectives of mobilizing the left colon and upper rectum, dividing the superior hemorrhoidal vessels, assisting in the anastomosis, and creating the protective loop ileostomy. The perineal dissection begins with the placement of the GelPOINT Path access sleeve (Applied Medical, Inc., Rancho Santa Margarita, CA) into the anal canal with the proximal/cranial ridge positioned above the levators. The distal/caudal ridge is secured to the perianal skin with sutures (Fig. 1). A LoneStar Rectractor System (Cooper Surgical, Inc, Stafford, TX) may be used to efface the anus when the semi-rigid access channel cannot be placed atraumatically (Fig. 2). Alternatively, a narrow anal canal or strictured sphincter complex may require the use of the more malleable Covidien SILS port (Covidien, Minneapolis, MN) for access. Finally, rigid platforms from Storz or Wolf can be used based on surgeon expertise and preference. Very low rectal tumors with invasion of the sphincter complex may require an initial intersphincteric dissection, which can be performed transanally prior to port placement.
Fig. 1.
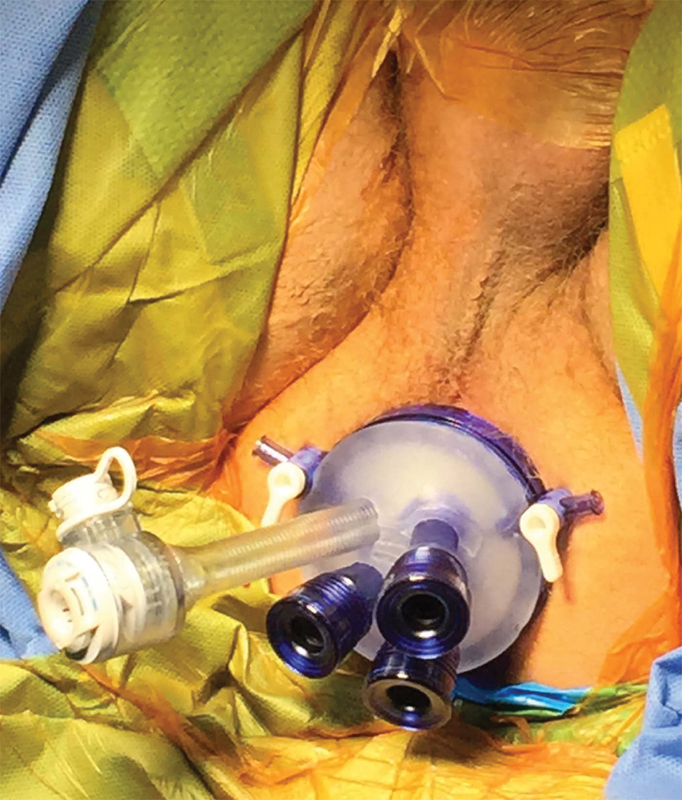
Placement of GelPOINT Path access sleeve into anal canal.
Fig. 2.
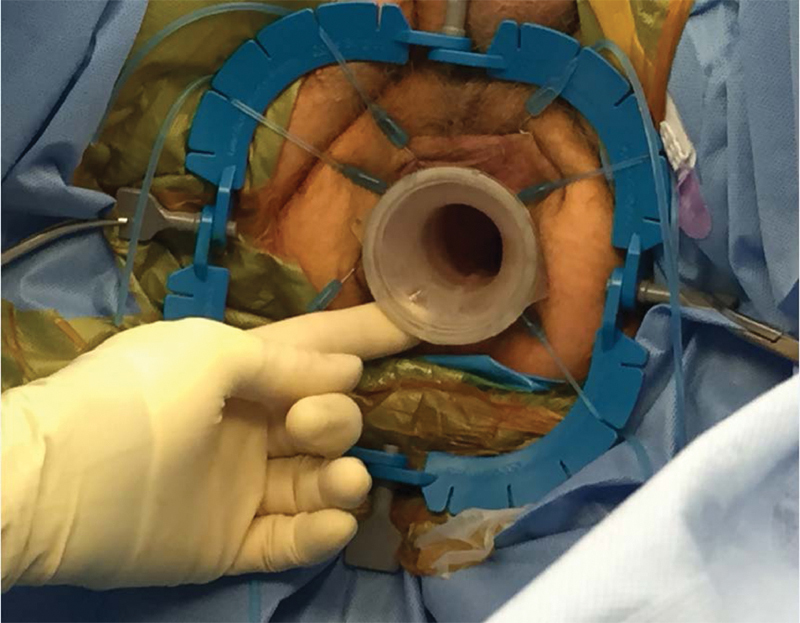
Effacement of anus with LoneStar Retractor.
Most commonly, pneumorectum is obtained using an AirSeal system (SurgiQuest, Milford, CT), and the tumor is visualized. Cautery marks are placed circumferentially 1 cm distal to the lowest extent of the tumor to mark the exact location for purse-string placement (Fig. 3). A 2–0 Prolene (Ethicon, Inc., Somerville, NJ) purse string is used to close the rectal lumen either via an open method with a standard needle driver through the access channel or laparoscopically, utilizing luminal insufflation (Fig. 4). Tight rectal closure prevents stool spillage, isolates the tumor from the dissection plane, and allows insufflation of the TME plane (Fig. 5).
Fig. 3.
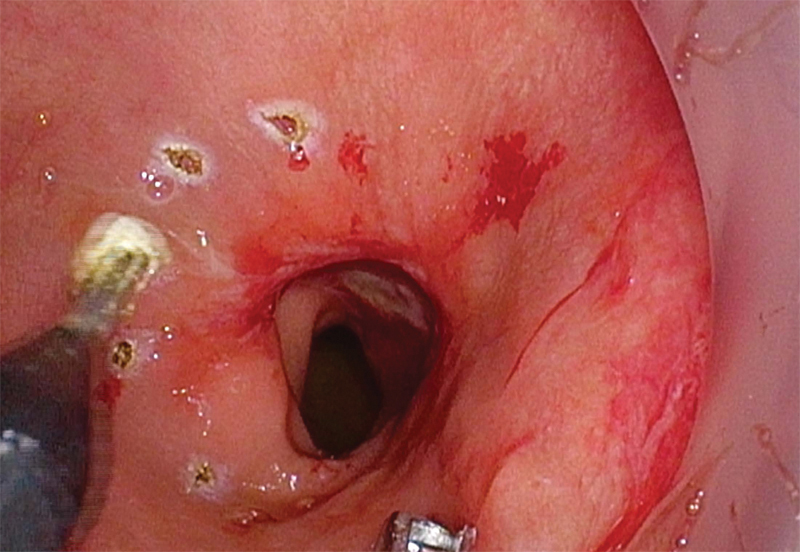
Circumferential cautery marks placed 1 cm distal to lowest extent of tumor (placement of purse-string suture).
Fig. 4.
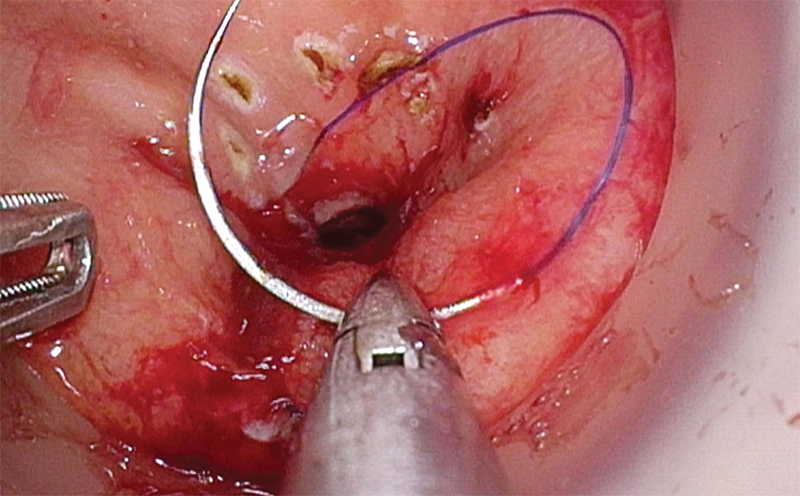
Purse-string placement.
Fig. 5.
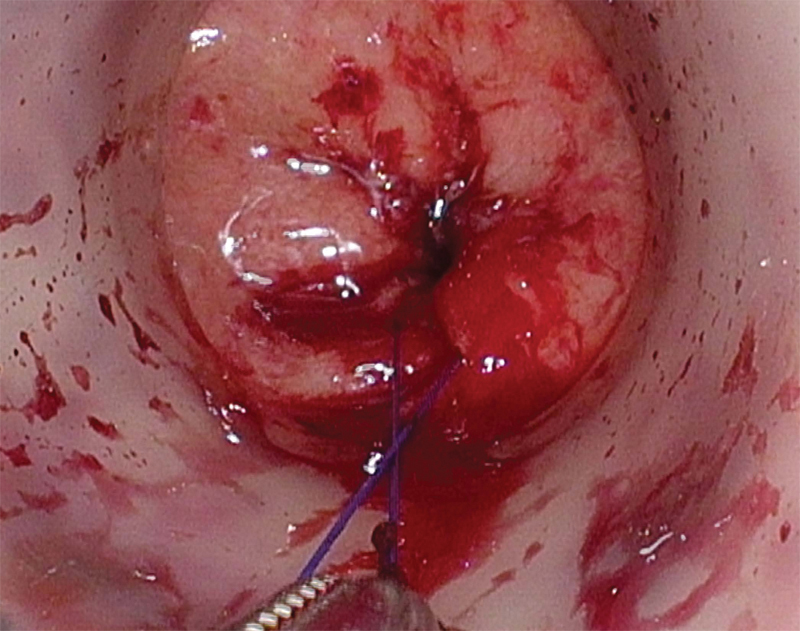
Completed purse-string.
Full-thickness, circumferential division of the rectum is then performed using electrocautery 1 cm distal to the closed purse string stitch. The TME plane is best entered either posteriorly between the rectum and the presacral plane or anteriorly between the rectum and vagina or prostate (Fig. 6). In the posterior position, it is critical to bring the dissection plane directly downward, staying outside the fascia propria of the mesorectum. A common mistake is to bring the dissection into the intramesorectal plane along the rectal wall. As the TME dissection is followed laterally, the lateral autonomic nerve fibers are encountered, marking the lateral border of the dissection plane. A second common mistake is to follow the plane out laterally and then anteriorly, mobilizing the prostate en bloc with the rectum, potentially resulting in urethral injury. When properly identified and entered, the circumferential TME dissection plane is followed proximally/cranially until the abdominal cavity is entered, either anteriorly or posteriorly. This communication of the two dissection fields is done in a coordinated fashion with the abdominal and pelvic teams providing tissue retraction and exposure for each other.
Fig. 6.
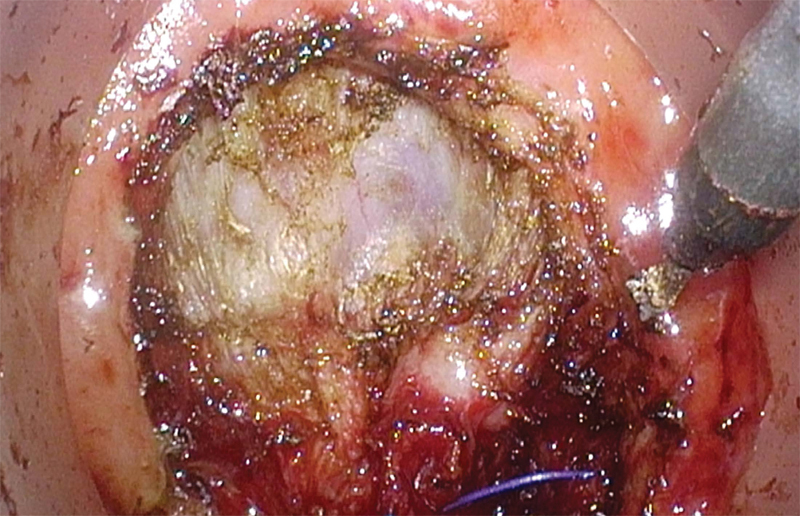
Entry into TME plane anteriorly.
Extraction can be performed through an abdominal incision or transperineally, depending on patient's anatomy and tumor/specimen bulk (Figs. 7 and 8). The anastomosis is created by placing the EEA anvil in the proximal colon, and a 2–0 Prolene purse string transanally at the top of the open distal rectal stump. A 19F round Blake drain is passed through the rectal stump opening and into the pelvis. The purse string is then tied down snugly around the drain, which acts as a guide for the EEA stapler post. It is important to ensure that the distal rectal stump is completely free of the levators and vagina or prostate prior to placing and tying down the purse string suture. The open EEA stapler post is then inserted into the open end of the drain, and under direct visualization from the abdominal laparoscope, guided through the mid aspect of the rectum. The abdominal surgeon detaches the drain and mates the previously placed EEA anvil to the trocar. The perineal surgeon closes and fires the stapler, inspects the staple line, and an air-leak test is then performed. A diverting loop ileostomy is routinely created to protect the anastomosis (Fig. 9).
Fig. 7.
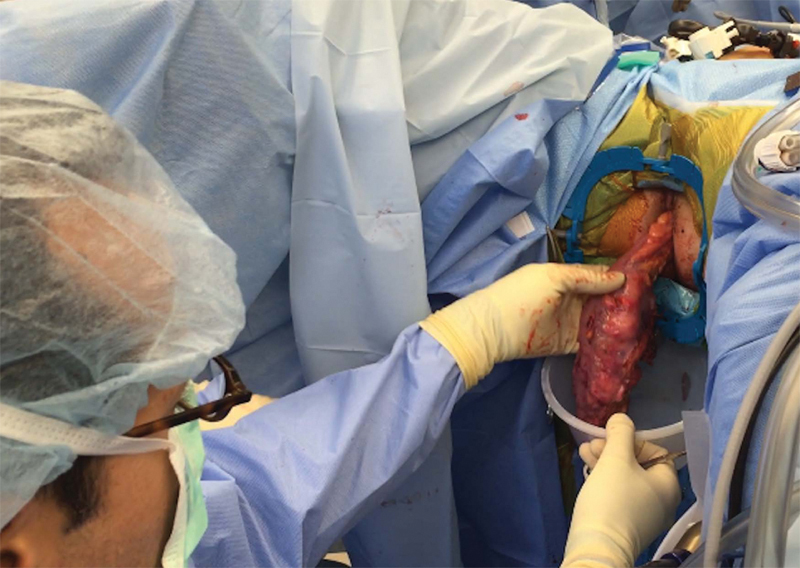
Transperineal extraction of specimen.
Fig. 8.
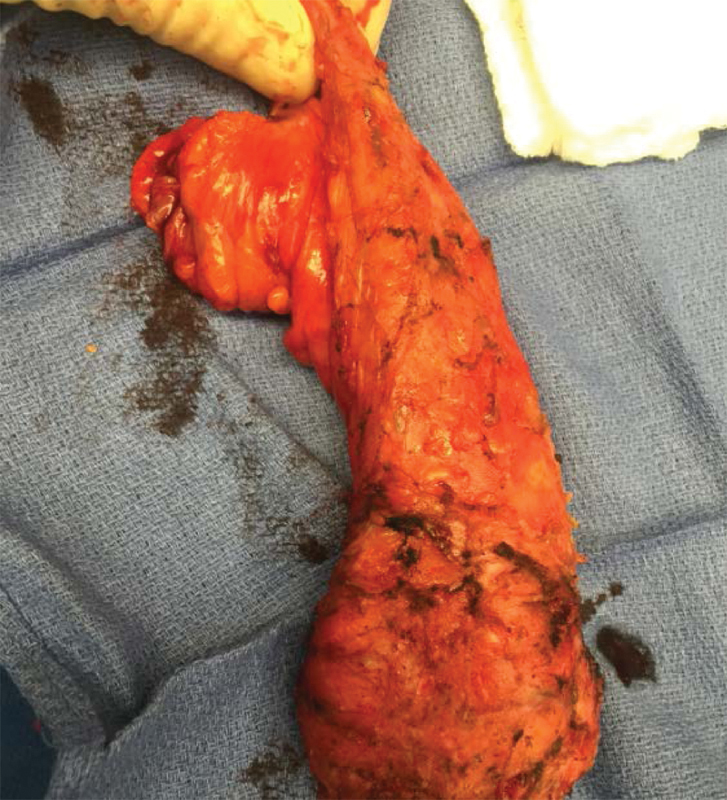
Specimen with optimal TME quality.
Fig. 9.
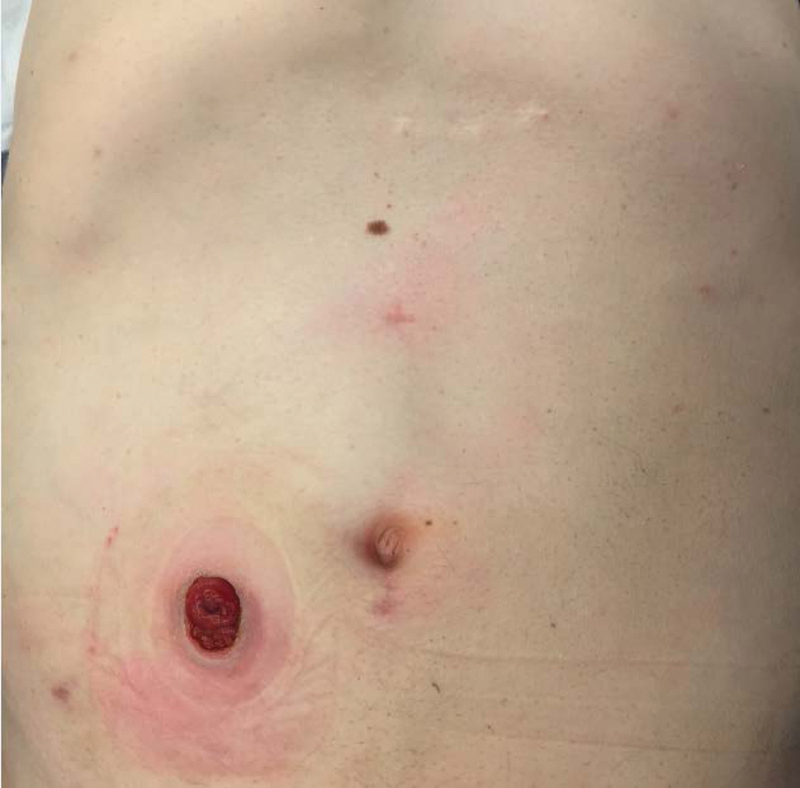
Diverting loop ileostomy and incision sites.
Reported Experience
The largest study to date was published in the Journal of the American College of Surgeons in 2015. Lacy et al13 performed a single-arm prospective study evaluating the outcomes from 140 taTME procedures performed between 2011 and 2014. Patients with adenocarcinoma within 15 cm from the anal verge were included. T4 tumors restaged after neoadjuvant therapy and those requiring an abdominoperineal resection were excluded. The authors utilized a simultaneous two-surgeon approach with a meeting point for both teams at the peritoneal reflection. The perianal device used was the GelPOINT Path Transanal Platform with specimen extraction via a Pfannenstiel incision or transanally. Very distal rectal tumors required hand-sewn coloanal anastomosis, whereas most anastomoses were created using a stapled anastomosis (71%). The majority of patients received a protective diverting ileostomy (84%).
The authors reported a mean operating time (OR) time of 166 ± 57 minutes (range 60–360 minutes) with no conversions to open and no intraoperative complications. Median length of hospital stay was 6 days (range 3–39 days). Minor complications (Clavien-Dindo I and II) occurred in 24.2% of patients, and 10% experienced major complications (Clavien-Dindo III and IV). Twelve patients (8.6%) developed anastomotic leak with nine of those patients requiring reoperation. According to Quirke classification, 136 (97.1%) patients had complete specimens. CRM positivity (<1 mm) was reported in nine (6.4%) patients, all of whom were predicted preoperatively by MRI. Lymph node harvest mean was 14.7 ± 6.8. Overall, the authors reported a lower overall conversion rate, comparable complication rates, and superior pathologic outcomes when compared with published series using a laparoscopic approach for rectal cancer.13
Published Indications
The literature to date includes both men and women with ages ranging from 22 to 80 years. Patient BMI range was wide, with Atallah et al reporting a range of 18 to 41 kg/m2 and Tuech et al reporting a range of 20 to 42 kg/m2, although most patients reported had BMIs less than 30 kg/m2.24 25 The most common indication for performing taTME is rectal adenocarcinoma located 0 to 15 cm from the anal verge. The most common site of tumor was at the mid to low rectum, and a large proportion of these patients underwent neoadjuvant chemoradiation therapy. Few studies have reported performing taTME for benign disease such as supralevator abscess, malignant polyps, Crohn proctitis, or ulcerative colitis (Table 1).26 27 28 29 30
Table 1. Details of TaTME studies.
| Reference | Study period | Patient characteristics |
Operative characteristics |
Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n, age, sex (% male), BMIa | Pathology | Distance from anal verge | Neoadjuvant therapy (n, %) |
Transanal platform, sequence of procedures | OR time | Protective stoma (n, %) |
Complications | LN retrieved, quality of specimen, CRM | ||
| Funahashi et al 200944 | 6, 62.8 y, 67% male, BMI 29.8 | Adenocarcinoma | Not reported | 0 (0%) | Lap disc mini, transanal/abdominal | 64 min (perineal only) | 6 (100%) | 0 (0%) | Surgical margin histologically free, mean distance from rectal stump 24 mm (17–30) | |
| Marks et al 201021 | 1998–2008 | 79, 59.2 y (22–85), 68% male, BMI 26.2 ± 5.2 | Adenocarcinoma | 1.2 cm (-0.5–3) from anorectal ring | 79 (100%) radiation, 77 (97%) chemotherapy | None, transanal/abdominal | Not reported | 102 (100%) | 4 (5%) rectal prolapse, 3 (3.7%) wound infection, 6 (7.6%) anastomotic stenosis, 2 (2.5%) each pelvic abscess, SBO, stoma prolapse, 3 (3.7%) urinary retention | Mean distal margin 1.9 cm (0–7.5), 1 (1.3%) positive distal margin, 5 (6.3%) positive CRM, mean LN 11.4 (1–93) |
| Sylla et al 201018 | 1, 76 y, 0% male, BMI 20 | Adenocarcinoma | 8 cm | 1 (100%) | TEM proctoscope, simultaneous abdominal and perineal | 270 min | 1 (100%) | 0 (0%) | Negative distal and CRM, 23 LN | |
| Gaujoux et al 201145 | 2, 59 and 61 y, 0% male, nonobese | Adenocarcinoma | Not reported | 2 (100%) | None, transanal/abdominal | 195, 210 min | 2 (100%) | 0 (0%) | R0 resection with negative distal and CRM, 12 and 16 LN | |
| Tuech et al 201146 | 1, 45 y, 0% male, BMI 20 | Adenocarcinoma | 3 cm from dentate | Not reported | Endorec Trocar, transanal/abdominal | 300 min | 1 (100%) | None reported | Complete TME, 15 LN | |
| Zorron et al 201232 | 2, 54 and 74 y, 50% male, BMI not reported | Adenocarcinoma | 8, 6 cm | 0 (0%) | Perirectal NOTES access, transanal/abdominal | 350, 360 min | 2 (100%) | 2 (100%) transient foot paresthesia | Adequate TME, negative distal and CRM | |
| Wolthuis et al 201227 | 1, 51 y, 0% male, BMI not reported | Recurrent supralevator abscess | Not reported | 0 (0%) | SSL port, transanal/abdominal | 122 min | 1 (100%) | None reported | Not reported | |
| Dumont et al 201215 | Feb 2011–Feb 2012 | 4, 66.8 y, 100% male, BMI 23.5 | Adenocarcinoma | 5.3 cm | 4 (100%) | GelPOINT, transanal/abdominal | 360 min | 4 (100%) | 1 (25%) anastomotic fistula | Median distal margin 22.5 mm (10–40), median CRM 7.4 mm (1.5–15), median LN 16 (8–22) |
| Han et al 201347 | May 2010–Oct 2011 | 34, 56.5 y (41–78), 55.8% male, BMI 24.6 (19.8–30.3) | Adenocarcinoma | 11.5 cm (4–30) | Not reported | TEM proctoscope, abdominal/transanal | 151.6 ± 25.93 min | 0 (0%) | 6 (17.6%) anastomotic leak with 1 (3%) requiring diverting ileostomy | LN harvest 12.92 ± 2.2, distal margin 2.43 ± 1.34 cm, 100% R0 resection |
| Lacy et al 201348 | Mar 2012–Apr 2012 | 3, 73 y, 33% male, BMI 21.7 | Adenocarcinoma | 9.67 cm | 2 (66%) | GelPOINT, simultaneous abdominal and perineal | 143 min | 3 (100%) | 1 (33%) readmission for dehydration | Satisfactory mesorectal resection, negative distal and CRM |
| Zhang et al 201331 | 1, 48 y, 0% male, BMI 20 | Adenocarcinoma | 8 cm | 0 (0%) | PPH anoscope, transanal only | 300 min | 0 (0%) | Transient anal incontinence | Negative distal and CRM, 12 LN | |
| Rouanet et al 201335 | Jan 2009–June 2011 | 30, 65 y, 100% male, BMI 26 | Adenocarcinoma (advanced or recurrent) | 20 (66%) 0–5 cm, 10 (33%) 5–10 cm | 29 (97%) | TEO, transanal/abdominal | Not reported | 30 (100%) | 6% conversion, 2 (6.7%) urethral injury, 2 (6.7%) sepsis, 1 (3%) bowel obstruction, 1 (3%) air embolism | Mesorectal dissection good, R0 in 26 (86.7%), median CRM 7 (0–17) mm, OS 12 mon 96.6%, OS 21 mon 80.5%, relapse-free survival 12 mon 93.3%, relapse-free survival 24 mon 88.9% |
| Velthuis et al 201349 | June 2012–Aug 2012 | 5, 69.4 y, 60% male, BMI not reported | Adenocarcinoma | 6 cm | 5 (100%) | SILS, transanal/abdominal | 175 min | 5 (100%) | 1 (20%) presacral abscess | Clear surgical margins, intact mesorectal fascia, median LN 12 (11–17) |
| Choi et al 201350 | June 2009–Apr 2011 | 22, 65.8 y, 45% male, BMI 21.2 ± 0.6 | Adenocarcinoma | 6.1 ± 0.4 cm | 3 (13.6%) | None, abdominal/perianal | 260 min | 12 (55%) | 1 (5%) pancreatitis, 2 (10%) urinary retention, 1 (5%) SBO, 1 (5%) anastomotic leak | Median distal margin 2 (0.3–4) cm, median LN 22 (9–42) |
| de Lacy et al 201351 | Aug 2011–July 2012 | 20, 65 ±10.2 y, 55% male, BMI 25.3 ± 3.8 | Adenocarcinoma or high-grade dysplasia | 6.5 ± 3.3 cm | 14 (70%) | GelPOINT, simultaneous abdominal and perineal |
234.7 ± 56 min | 16 (80%) | 4 (20%) minor complications, 0 (0%) major complications | Distal margin mean 2.6 ± 1.6 cm, CRM 1.8 ± 0.7 cm, mean LN 15.9 ± 4.3 |
| Sylla et al 201352 | Nov 2011–May 2012 | 5, 48.6 y, 60% male, BMI 48.6 ± 2.3 | Adenocarcinoma | 5.7 ± 2.4 cm | 2 (40%) | TEO, abdominal/transanal | 274.6 ± 85.4 min | 5 (100%) | 2 (40%) transient urinary dysfunction, 1 (20%) ileus | Complete mesorectal excision in all, negative distal and CRM |
| Leroy et al 201328 | Jan 2012–Jan 2013 | 1, 56 y, 0% male, BMI not reported | Tubulovillous adenoma with low grade dysplasia | Midrectum | 0 (0%) | TEO, transanal only | 190 min | 0 (0%) | None reported | No invasive nature demonstrated, 16 LN |
| Denost et al 201420 | June 2008–Feb 2012 | 50, 64 y (39–82), 74% male, BMI 25.1 (17.3–33.2) | Adenocarcinoma | 4 cm (2–6) | 40 (80%) | Handheld straight retractors, Transanal/abdominal | 240 min (170–380) | 50 (100%) | Overall morbidity 16 (32%), Clavien-Dindo III–V 6 (12%), 1 (2%) anastomotic leak, 6 (12%) bowel obstruction, 2 (4%) reoperation, 3 (6%) urologic morbidity | Distal margin 10 mm (0–30), positive distal margin 1 (2%), CRM 7 mm (0–20), positive CRM 2 (4%), LN harvest 17 (2–30) |
| Wolthuis et al 201426 | 14, 65 y, 36% male, BMI 25 (17–32) | 9 (64%) malignant, 5 (36%) benign | Not reported | 0 (0%) | GelPOINT, transanal/abdominal | 55 min (35–95) transanal portion only | 3 (21%) temporary, 1 (7%) permanent, 6 (43%) end colostomy | 2 (14%) conversion, 1 (7%) rectal perforation, 1 (7%) pelvic hematoma, 3 (21%) UTI | Not reported | |
| Meng and Lau 201453 | 3, 80 y, 66% male, BMI not reported | 2 (66%) adenocarcinoma, 1 (33%) villous adenoma | 4–10 cm | 2 (66%) | Perineal set of KOL, transanal/abdominal | 400 min, not reported, 330 min | 0 (0%) | 0 (0%) | Clear margins in all | |
| Chouillard et al 201429 | Feb 2011–May 2013 | 16, 57.7 y, 38% male, BMI 27.9 (21–38) | Adenocarcinoma or severe dysplasia | 0–12 cm | Not reported | GelPOINT, SILS port, transanal/abdominal or transanal only | 265 min (155–440) | 4 (25%) | 2 (13%) SBO, 1 (6.5%) pelvic abscess | Resection margins negative in all, median LN 17 (12–81) |
| Atallah et al 201424 | Nov 2010–Aug 2013 | 20, 57 y, 70% male, BMI 24 (18–41) | Adenocarcinoma | 5 cm (1–9) | 12 (60%) | GelPOINT path, abdominal/transanal | 243 min (140–495) | 23 (100%) | 2 (9%) wound infection, 4 (18%) pelvic abscess, 4 (18%) prolonged ileus | 90% negative margins, 85% complete or near-complete intact mesorectal envelope, no locoregional recurrence at 6 months, 1 (5%) distant met at 6 months |
| Velthuis et al 201439 | June 2012–July 2013 | 25, age not reported, 72% male, BMI 25 (20–36) | Adenocarcinoma | 8 cm (0–16) | 13 (52%) | SILS port, transanal/abdominal | Not reported | 25 (100%) | Not reported | 96% complete mesorectum, 94% negative CRM, mean LN 14 (7–24) |
| Atallah et al 201437 | Mar 2013–Feb 2014 | 3, 45 y, 66% male, BMI 32 (21–38.5) | Adenocarcinoma | Distal 5 cm of rectum | 2 (66%) | GelPOINT, abdominal/transanal (Robot) | 376 min | 3 (100%) | 1 (33%) PE, 1 (33%) dehydration | Surgical margins negative in all, LN 31 (18–39) |
| Zorron et al 201454 | Nov 2009–June 2010 | 9, 62.6 y, 55% male, BMI not reported | Adenocarcinoma | Not reported | 4 (44%) | Triport or flexible endoscope, perineal only | 311 min | 9 (100%) | 1 (11%) anastomotic leak, 2 (22%) conversion, 1 (11%) transient paresthesia of feet, 1 (11%) tumor rupture | TME adequate 6 (66%), mean LN 13 |
| Verheijen et al 201433 | 1, 48 y, 0% male, BMI 23.6 | Adenocarcinoma | 8 cm | 1 (100%) | GelPOINT, transanal only (Robot) | 205 min | 1 (100%) | 0 (0%) | Complete mesorectal excision with free distal and CRM | |
| Gómez Ruiz et al 201530 | Aug 2013–Jan 2014 | 5, 57 y, 80% male, BMI 25.8 ± 2.7 | Adenocarcinoma | 5 ± 1 cm | 4 (80%) | Transanal Access Port proctoscope, transanal only (Robot) | 398 ± 88 min | 5 (100%) | 1 (20%) anastomotic leak | Complete mesorectal excision and negative proximal, distal and CRM in all |
| Huscher et al 201555 | Jan 2014–Apr 2014 | 7, 63.2 y, 43% male, BMI 29.9 ± 6.1 | Adenocarcinoma | 2 cm (1–6.5) | 0 (0%) | GelPOINT, abdominal/transanal (Robot) | 165.7 ± 54.4 min | 7 (100%) | 1 (14%) rectal bleeding | 86% complete mesorectal excision, 14% nearly complete mesorectal excision, mean LN 14 ± 3, 100% R0 resection, CRM 3.2 ± 1.8 mm |
| Muratore et al 201534 | Jan 2012–Dec 2013 | 26, 65.8 y, 61.5% male, BMI 26.2 (16.9–38.2) | Adenocarcinoma | 4.4 cm (3–6) | 19 (73%) | SILS port, transanal/abdominal | 241 min (150–360) | 26 (100%) | 2 (7.7%) anastomotic leak, 2 (7.7%) SBO, 1 (4%) urinary retention | 88.5% complete TME quality, distal resection margin 1.9 (0.2–5) cm, CRM 1.1 (0.3–1.6) cm |
| Knol et al 201556 | Dec 2012–Oct 2013 | 10, 60.5 y, 80% male, BMI 26.5 (22–34) | Adenocarcinoma | 28.9 ± 12.2 mm from anorectal junction | 10 (100%) | GelPOINT, abdominal/transanal | 235 min (150–290) | 10 (100%) | 1 (10%) gastroparesis and high ileostomy output | 90% complete mesorectal specimen, mean distal margin 1.9 ± 1 cm, mean CRM 1.4 ± 0.5 cm, median LN 10.5 (5–15) |
| Fernández-Hevia et al 201536 | Nov 2011–Mar 2013 | 37, 64.5 y, 65% male, BMI 23.7 ± 3.6 | Adenocarcinoma | Mid 1 ± 1.7 cm, low 3.5 ± 1.2 cm | 27 (72.9%) | GelPOINT, simultaneous abdominal and transanal | 215 ± 60 min | 32 (86%) | 2 (5.4%) anastomotic leak, 4 (11%) ileus, 3 (8%) second look surgery | 92% complete mesorectal specimen, LN 14.3 ± 6 |
| Tuech et al 201525 | Feb 2010–June 2012 | 56, 65 (39–83) y, 73% male, BMI 27 (20–42) | Adenocarcinoma | Median tumor height 40 mm (0–50) | 47 (84%) | Endorec Trocar, GelPOINT, SILS port, transanal/abdominal | 270 min (150–495) | 50 (89%) | Overall 14 (26%), 3 anastomotic leak, 3 pelvic sepsis, 5 transient urinary disorders, 2 blood transfusion, 1 CVA | 47 (84%) intact mesorectum, 9 (16%) nearly complete, median CRM 8 (0–20) mm, median distal margin 10 (3–40) mm, median LN 12 (7–29) |
| de'Angelis et al 201522 | Jan 2011–Dec 2014 | 32, 64.9 y, 66% male, BMI 25.19 ± 3.52 | Adenocarcinoma | 4 cm (2.5–5) | 27 (84.4%) | GelPOINT, transanal/abdominal | 195 ± 43.6 min | 32 (100%) | 30-days morbidity 8 (25%), Clavien-Dindo I 3 (9.4%), II 3 (9.4%), III 1 (3.1%), IV 1 (3.1%) | CRM 9.68 ± 4.57 mm, positive CRM 1 (3.1%), positive distal margin 2 (6.2%), LN harvest 17.06 ± 7.14 |
| Lacy et al 201513 | Oct 2011–Nov 2014 | 140, 65.5 ± 12.7 y, 63.6% male, BMI 25.2 ± 3.9 | Adenocarcinoma | Up to 15 cm | 94 (67%) | GelPOINT path, simultaneous abdominal and perineal |
166 ± 57 min | 117 (83.6%) | 34 (24.2%) minor complications 14 (10%) major complications |
136 (97.1%) complete 9 (6.4%) positive CRM 108 (77.1%) less than 12 LN, mean LN 14.7 ± 6.8 |
Abbreviations: BMI, body mass index; CRM, circumferential resection margin; CVA, cerebrovascular accident; LM, lymph node; SBO, small bowel obstruction; SILS, single-incision laparoscopic surgery; SSL, single-site laparoscopic; TEO, transanal endoscopic operation device; TME, total mesorectal excision.
BMI in kg/m2.
Procedure Variability
The majority of studies published perform taTME in a hybrid fashion with the abdominal portion performed either laparoscopically or robotically. Six studies reported a purely transanal TME with Verheijen et al and Gómez Ruiz et al reporting the use of the robot to perform the transanal portion.28 29 30 31 32 33 The most commonly used transanal platform is the GelPOINT Path over the single-incision laparoscopic surgery (SILS) port or rigid platforms. Hybrid procedures most often report performing the transanal portion before the abdominal portion of the procedure. The majority of studies reported placing a protective stoma if an anastomosis was created. Operative times ranged from as little as 35 minutes for the perianal portion alone to 495 minutes for the entirety of the procedure (Table 1).24 27
Postoperative Outcomes
In general, taTME demonstrates similar complication rates when compared with an open or laparoscopic approach, including conversion to open for the laparoscopic approach, anastomotic leak, small bowel obstruction and ileus, and transient urinary retention. Only a single postoperative death has been reported from all the series published due to acute myocardial infarction.34 Rouanet et al were the only authors to report urethral injury in 2 (6.7%) of their cohort of 30 patients.35 The authors indicated that this occurred early in their experience or in a patient with a large T4 lesion extending into the prostate. Both injuries were repaired intraoperatively, and no long-term effects were noted.
A retrospective study that compared 37 patients undergoing taTME and 37 matched patients undergoing laparoscopic TME demonstrated shorter OR time (252 vs. 215 minutes), lower distal margin (1.8 vs. 2.7 cm), and fewer early readmissions (22 vs. 6%) in favor of the taTME approach.36 No difference was noted in 30-day postoperative complications.
Histological Outcomes
Of those who reported histologic outcomes, lymph node harvest was satisfactory and negative distal and CRMs were superior to previous reports of open and laparoscopic TME. Mean CRM was reported to be 7 to 18 mm with positive CRM in four studies with a rate of 3.1 to 6.4%.13 20 21 22 Positive distal margins were reported in three series with rates of 2 to 6.3% (Table 1).13 20 21
Early Lessons Learned
The taTME technique is safe and feasible, providing potential solutions to the numerous technical challenges plaguing laparoscopic rectal surgery, including tissue retraction, visualization, oncologic margin determination, distal rectal division, and creation of a low pelvic anastomosis. This new surgical approach has been shown to facilitate anastomosis creation in lower tumors and is associated with lower conversion rates.2 13 37 38 Additionally, there may be an oncologic benefit with improved distal and CRM and equivalent lymph node harvest in several series.13 20 21 22 Theoretical advantages also include less pain due to fewer laparoscopic ports, lower risk of wound infection and hernia formation, and better visualization and preservation of the pelvic autonomic nerves.5 39 The simultaneous, or two-team, approach may result in shorter operative times due to abdominal transanal collaboration.13 40 Furthermore, with direct access to the anal canal and distal rectal dissection and division, this approach may ultimately improve sphincter preservation rates, although long-term studies have yet to validate this potential advantage.19 20
Potential risks to taTME include damage to the urethra at the level of the prostatic urethra, particularly in a previously irradiated pelvis, in patients with prostatic hypertrophy, or following prior prostate surgery. Moreover, it is possible to dissect outside of the TME plane, laterally causing injury to the pelvic sidewall autonomic nerves or posteriorly beneath the endopelvic fascia, exposing the sacral venous plexus.41 Intramesorectal dissection resulting in a compromised mesorectal excision may result in higher rates of local recurrence, although no data have demonstrated this at this time.42
This technique is specifically suited for patients with mid to low rectal tumors less than 10 to 12 cm from the anal verge. Upper rectal cancers are better approached with a standard open or laparoscopic tumor-specific mesorectal excision. Most authors suggest the greatest opportunity is realized in the most challenging cases with limited pelvic exposure, such as obese males with narrow pelvises. Conversely, the more easily identified anterior dissection plane between the rectum and vagina (compared with the plane separating the low rectum from the prostate) makes this operation more straightforward in women. The choice of operating platform (flexible vs. rigid) is based on surgeon's comfort and patient's anatomy. A two-team approach is strongly recommended to help facilitate the progress of this technically challenging operation.
One of the greatest challenges to the adoption of this technique is related to the steep learning curve and development of a structured team. Regardless of your experience in rectal cancer surgery, this operation turns things “upside down,” and requires expert understanding of pelvic anatomy and tissue plane identification, advanced laparoscopic skills, and mastery of low anastomotic techniques. Because of this, early experience should commence with benign conditions such as inflammatory bowel disease.
Successful implementation of a taTME program requires a structured approach. The team of surgeons should enroll in a cadaver-based course to learn from experienced colleagues. A team including OR staff should be assembled at the home institution. A commitment to the program will be solidified with capital purchases of an advanced insufflation system and access platforms. A surgical mentor should be available and present for early cases until mastery has been achieved. There is no reason why multiple surgeons at multiple institutions should have to progress though a challenging learning curve independently; instead, the nuances of the operation should be transferred in real-time with the goal of optimizing patient outcomes.
Scholarly Study
Before this approach can be recommended widely, as with all surgical innovations, the short- and long-term outcomes of the taTME procedure must be recorded and reported. The COLOR III trial is an international, multicenter, randomized superiority trial evaluating transanal TME compared with laparoscopic TME for mid to low rectal cancers.43 Primary endpoint is involvement of CRM with secondary endpoints of mesorectum completeness, morbidity and mortality, local recurrence, and survival. Accrual of 1,098 patients over a 4-year period is expected. As of October 1, 2015, the OSTRiCh Consortium (Optimizing the Surgical Treatment of Rectal Cancer) taTME Registry opened for recording of all taTME cases being performed in the United States. A multicenter, prospective trial supported by the American Society of Colon and Rectal Surgeons will soon be launched to study outcomes of patients at the 10 centers offering this approach in the United States.
Conclusion
Transanal TME is a novel minimally invasive approach that has emerged in response to the challenges associated with traditional open and laparoscopic surgical approaches to rectal cancer. Although taTME is technically challenging, at least initially, its technical advantages as well as the potential oncologic benefit are exciting. Preliminary studies are promising with ongoing studies that will determine long-term oncologic and functional outcomes. The initiation of a taTME program is feasible but must be done systematically using a team approach to ensure safe practices. The potential procedure-specific operative risks including autonomic nerve injury, sphincter injury, and ureteral injury must be recognized and prevented. Moving forward, partnership with industry and the development of new devices that make the operation less technically challenging may facilitate widespread adoption.
References
- 1.Heald R J. A new approach to rectal cancer. Br J Hosp Med. 1979;22(3):277–281. [PubMed] [Google Scholar]
- 2.Heald R J. A new solution to some old problems: transanal TME. Tech Coloproctol. 2013;17(3):257–258. doi: 10.1007/s10151-013-0984-0. [DOI] [PubMed] [Google Scholar]
- 3.Weaver K L, Grimm L M Jr, Fleshman J W. Changing the way we manage rectal cancer-standardizing TME from open to robotic (including laparoscopic) Clin Colon Rectal Surg. 2015;28(1):28–37. doi: 10.1055/s-0035-1545067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wibe A, Møller B, Norstein J. et al. A national strategic change in treatment policy for rectal cancer--implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum. 2002;45(7):857–866. doi: 10.1007/s10350-004-6317-7. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa S, Takahashi R, Hida K, Kawada K, Sakai Y. Transanal total mesorectal excision for rectal cancer. Surg Today. 2016;46(6):641–653. doi: 10.1007/s00595-015-1195-2. [DOI] [PubMed] [Google Scholar]
- 6.Havenga K, Enker W E, McDermott K, Cohen A M, Minsky B D, Guillem J. Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg. 1996;182(6):495–502. [PubMed] [Google Scholar]
- 7.Guillou P J, Quirke P, Thorpe H. et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 8.van der Pas M H, Haglind E, Cuesta M A. et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(3):210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson A R, Solomon M J, Lumley J W. et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363. doi: 10.1001/jama.2015.12009. [DOI] [PubMed] [Google Scholar]
- 10.Fleshman J, Branda M, Sargent D J. et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Z, Kim J, Adam M A. et al. Minimally invasive versus open low anterior resection: equivalent survival in a national analysis of 14,033 patients with rectal cancer. Ann Surg. 2016;263(6):1152–1158. doi: 10.1097/SLA.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 12.Maykel J A. Laparoscopic transanal total mesorectal excision (taTME) for rectal cancer. J Gastrointest Surg. 2015;19(10):1880–1888. doi: 10.1007/s11605-015-2876-2. [DOI] [PubMed] [Google Scholar]
- 13.Lacy A M, Tasende M M, Delgado S. et al. Transanal total mesorectal excision for rectal cancer: outcomes after 140 patients. J Am Coll Surg. 2015;221(2):415–423. doi: 10.1016/j.jamcollsurg.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 14.Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N. Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis. 2008;23(7):703–707. doi: 10.1007/s00384-008-0470-8. [DOI] [PubMed] [Google Scholar]
- 15.Dumont F, Goéré D, Honoré C, Elias D. Transanal endoscopic total mesorectal excision combined with single-port laparoscopy. Dis Colon Rectum. 2012;55(9):996–1001. doi: 10.1097/DCR.0b013e318260d3a0. [DOI] [PubMed] [Google Scholar]
- 16.Maslekar S, Pillinger S H, Monson J R. Transanal endoscopic microsurgery for carcinoma of the rectum. Surg Endosc. 2007;21(1):97–102. doi: 10.1007/s00464-005-0832-z. [DOI] [PubMed] [Google Scholar]
- 17.Nagtegaal I D van de Velde C J van der Worp E Kapiteijn E Quirke P van Krieken J H; Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control J Clin Oncol 20022071729–1734. [DOI] [PubMed] [Google Scholar]
- 18.Sylla P, Rattner D W, Delgado S, Lacy A M. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24(5):1205–1210. doi: 10.1007/s00464-010-0965-6. [DOI] [PubMed] [Google Scholar]
- 19.Marks J, Nassif G, Schoonyoung H. et al. Sphincter-sparing surgery for adenocarcinoma of the distal 3 cm of the true rectum: results after neoadjuvant therapy and minimally invasive radical surgery or local excision. Surg Endosc. 2013;27(12):4469–4477. doi: 10.1007/s00464-013-3092-3. [DOI] [PubMed] [Google Scholar]
- 20.Denost Q, Adam J P, Rullier A, Buscail E, Laurent C, Rullier E. Perineal transanal approach: a new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg. 2014;260(6):993–999. doi: 10.1097/SLA.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 21.Marks J, Mizrahi B, Dalane S, Nweze I, Marks G. Laparoscopic transanal abdominal transanal resection with sphincter preservation for rectal cancer in the distal 3 cm of the rectum after neoadjuvant therapy. Surg Endosc. 2010;24(11):2700–2707. doi: 10.1007/s00464-010-1028-8. [DOI] [PubMed] [Google Scholar]
- 22.de'Angelis N, Portigliotti L, Azoulay D, Brunetti F. Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbecks Arch Surg. 2015;400(8):945–959. doi: 10.1007/s00423-015-1350-7. [DOI] [PubMed] [Google Scholar]
- 23.Motson R W, Whiteford M H, Hompes R. et al. Current status of trans-anal total mesorectal excision (TaTME) following the 2nd International consensus conference. Colorectal Dis. 2016;18(1):13–18. doi: 10.1111/codi.13131. [DOI] [PubMed] [Google Scholar]
- 24.Atallah S, Martin-Perez B, Albert M. et al. Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol. 2014;18(5):473–480. doi: 10.1007/s10151-013-1095-7. [DOI] [PubMed] [Google Scholar]
- 25.Tuech J J, Karoui M, Lelong B. et al. A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg. 2015;261(2):228–233. doi: 10.1097/SLA.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 26.Wolthuis A M, de Buck van Overstraeten A, D'Hoore A. Dynamic article: transanal rectal excision: a pilot study. Dis Colon Rectum. 2014;57(1):105–109. doi: 10.1097/DCR.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 27.Wolthuis A M, Cini C, Penninckx F, D'Hoore A. Transanal single port access to facilitate distal rectal mobilization in laparoscopic rectal sleeve resection with hand-sewn coloanal anastomosis. Tech Coloproctol. 2012;16(2):161–165. doi: 10.1007/s10151-011-0795-0. [DOI] [PubMed] [Google Scholar]
- 28.Leroy J Barry B D Melani A Mutter D Marescaux J No-scar transanal total mesorectal excision: the last step to pure NOTES for colorectal surgery JAMA Surg 20131483226–230., discussion 231 [DOI] [PubMed] [Google Scholar]
- 29.Chouillard E, Chahine E, Khoury G. et al. NOTES total mesorectal excision (TME) for patients with rectal neoplasia: a preliminary experience. Surg Endosc. 2014;28(11):3150–3157. doi: 10.1007/s00464-014-3573-z. [DOI] [PubMed] [Google Scholar]
- 30.Gómez Ruiz M, Parra I M, Palazuelos C M. et al. Robotic-assisted laparoscopic transanal total mesorectal excision for rectal cancer: a prospective pilot study. Dis Colon Rectum. 2015;58(1):145–153. doi: 10.1097/DCR.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Zhang Y S, Jin X W, Li M Z, Fan J S, Yang Z H. Transanal single-port laparoscopic total mesorectal excision in the treatment of rectal cancer. Tech Coloproctol. 2013;17(1):117–123. doi: 10.1007/s10151-012-0882-x. [DOI] [PubMed] [Google Scholar]
- 32.Zorron R, Phillips H N, Coelho D, Flach L, Lemos F B, Vassallo R C. Perirectal NOTES access: “down-to-up” total mesorectal excision for rectal cancer. Surg Innov. 2012;19(1):11–19. doi: 10.1177/1553350611409956. [DOI] [PubMed] [Google Scholar]
- 33.Verheijen P M, Consten E C, Broeders I A. Robotic transanal total mesorectal excision for rectal cancer: experience with a first case. Int J Med Robot. 2014;10(4):423–426. doi: 10.1002/rcs.1594. [DOI] [PubMed] [Google Scholar]
- 34.Muratore A, Mellano A, Marsanic P, De Simone M. Transanal total mesorectal excision (taTME) for cancer located in the lower rectum: short- and mid-term results. Eur J Surg Oncol. 2015;41(4):478–483. doi: 10.1016/j.ejso.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Rouanet P, Mourregot A, Azar C C. et al. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013;56(4):408–415. doi: 10.1097/DCR.0b013e3182756fa0. [DOI] [PubMed] [Google Scholar]
- 36.Fernández-Hevia M, Delgado S, Castells A. et al. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261(2):221–227. doi: 10.1097/SLA.0000000000000865. [DOI] [PubMed] [Google Scholar]
- 37.Atallah S, Martin-Perez B, Pinan J. et al. Robotic transanal total mesorectal excision: a pilot study. Tech Coloproctol. 2014;18(11):1047–1053. doi: 10.1007/s10151-014-1181-5. [DOI] [PubMed] [Google Scholar]
- 38.Cahill R A. Single port surgery for rectal cancer-going up or down? Dis Colon Rectum. 2013;56(11):1199–1200. doi: 10.1097/DCR.0b013e3182a5660d. [DOI] [PubMed] [Google Scholar]
- 39.Velthuis S, Nieuwenhuis D H, Ruijter T E, Cuesta M A, Bonjer H J, Sietses C. Transanal versus traditional laparoscopic total mesorectal excision for rectal carcinoma. Surg Endosc. 2014;28(12):3494–3499. doi: 10.1007/s00464-014-3636-1. [DOI] [PubMed] [Google Scholar]
- 40.Atallah S, Albert M, DeBeche-Adams T, Nassif G, Polavarapu H, Larach S. Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): a stepwise description of the surgical technique with video demonstration. Tech Coloproctol. 2013;17(3):321–325. doi: 10.1007/s10151-012-0971-x. [DOI] [PubMed] [Google Scholar]
- 41.Atallah S. Transanal total mesorectal excision: full steam ahead. Tech Coloproctol. 2015;19(2):57–61. doi: 10.1007/s10151-014-1254-5. [DOI] [PubMed] [Google Scholar]
- 42.Bülow S Christensen I J Iversen L H Harling H; Danish Colorectal Cancer Group. Intra-operative perforation is an important predictor of local recurrence and impaired survival after abdominoperineal resection for rectal cancer Colorectal Dis 201113111256–1264. [DOI] [PubMed] [Google Scholar]
- 43.Deijen C L, Velthuis S, Tsai A. et al. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016;30(8):3210–3215. doi: 10.1007/s00464-015-4615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funahashi K, Koike J, Teramoto T. et al. Transanal rectal dissection: a procedure to assist achievement of laparoscopic total mesorectal excision for bulky tumor in the narrow pelvis. Am J Surg. 2009;197(4):e46–e50. doi: 10.1016/j.amjsurg.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 45.Gaujoux S, Bretagnol F, Au J, Ferron M, Panis Y. Single port access proctectomy with total mesorectal excision and intersphincteric resection with a primary transanal approach. Colorectal Dis. 2011;13(9):e305–e307. doi: 10.1111/j.1463-1318.2011.02676.x. [DOI] [PubMed] [Google Scholar]
- 46.Tuech J J, Bridoux V, Kianifard B. et al. Natural orifice total mesorectal excision using transanal port and laparoscopic assistance. Eur J Surg Oncol. 2011;37(4):334–335. doi: 10.1016/j.ejso.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Han Y, He Y G, Zhang H B. et al. Total laparoscopic sigmoid and rectal surgery in combination with transanal endoscopic microsurgery: a preliminary evaluation in China. Surg Endosc. 2013;27(2):518–524. doi: 10.1007/s00464-012-2471-5. [DOI] [PubMed] [Google Scholar]
- 48.Lacy A M, Adelsdorfer C, Delgado S, Sylla P, Rattner D W. Minilaparoscopy-assisted transrectal low anterior resection (LAR): a preliminary study. Surg Endosc. 2013;27(1):339–346. doi: 10.1007/s00464-012-2443-9. [DOI] [PubMed] [Google Scholar]
- 49.Velthuis S van den Boezem P B van der Peet D L Cuesta M A Sietses C Feasibility study of transanal total mesorectal excision Br J Surg 20131006828–831., discussion 831 [DOI] [PubMed] [Google Scholar]
- 50.Choi B J, Lee S C, Kang W K. Single-port laparoscopic total mesorectal excision with transanal resection (transabdominal transanal resection) for low rectal cancer: initial experience with 22 cases. Int J Surg. 2013;11(9):858–863. doi: 10.1016/j.ijsu.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 51.de Lacy A M, Rattner D W, Adelsdorfer C. et al. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: “down-to-up” total mesorectal excision (TME)--short-term outcomes in the first 20 cases. Surg Endosc. 2013;27(9):3165–3172. doi: 10.1007/s00464-013-2872-0. [DOI] [PubMed] [Google Scholar]
- 52.Sylla P, Bordeianou L G, Berger D. et al. A pilot study of natural orifice transanal endoscopic total mesorectal excision with laparoscopic assistance for rectal cancer. Surg Endosc. 2013;27(9):3396–3405. doi: 10.1007/s00464-013-2922-7. [DOI] [PubMed] [Google Scholar]
- 53.Meng W, Lau K. Synchronous laparoscopic low anterior and transanal endoscopic microsurgery total mesorectal resection. Minim Invasive Ther Allied Technol. 2014;23(2):70–73. doi: 10.3109/13645706.2014.887022. [DOI] [PubMed] [Google Scholar]
- 54.Zorron R, Phillips H N, Wynn G, Neto M P, Coelho D, Vassallo R C. “Down-to-Up” transanal NOTES Total mesorectal excision for rectal cancer: preliminary series of 9 patients. J Minim Access Surg. 2014;10(3):144–150. doi: 10.4103/0972-9941.134878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huscher C G, Bretagnol F, Ponzano C. Robotic-assisted transanal total mesorectal excision: the key against the Achilles' heel of rectal cancer? Ann Surg. 2015;261(5):e120–e121. doi: 10.1097/SLA.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 56.Knol J J, D'Hondt M, Souverijns G, Heald B, Vangertruyden G. Transanal endoscopic total mesorectal excision: technical aspects of approaching the mesorectal plane from below--a preliminary report. Tech Coloproctol. 2015;19(4):221–229. doi: 10.1007/s10151-015-1275-8. [DOI] [PubMed] [Google Scholar]


