Abstract
Metastasis is the consequence of a cancer cell that disperses from the primary tumor, travels throughout the body, and invades and colonizes a distant site. Based on Paget’s 1889 hypothesis, the majority of modern metastasis research focuses on the properties of the metastatic “seed and soil,” but the implications of the primary tumor “soil” have been largely neglected. The rare lethal metastatic “seed” arises as a result of the selective pressures in the primary tumor. Optimal foraging theory describes how cancer cells adopt a mobile foraging strategy to balance predation risk and resource reward. Further selection in the dispersal corridors leading out of the primary tumor enhances the adaptive profile of the potentially metastatic cell. This review focuses on the selective pressures of the primary tumor “soil” that generate lethal metastatic “seeds” which is essential to understanding this critical component of prostate cancer metastasis.
Implications
Elucidating the selective pressures of the primary tumor “soil” that generate lethal metastatic “seeds” is essential to understand how and why metastasis occurs in prostate cancer.
Keywords: optimal foraging theory, prostate cancer metastasis, dispersal corridor, seed and soil hypothesis, primary tumor selective pressures
INTRODUCTION
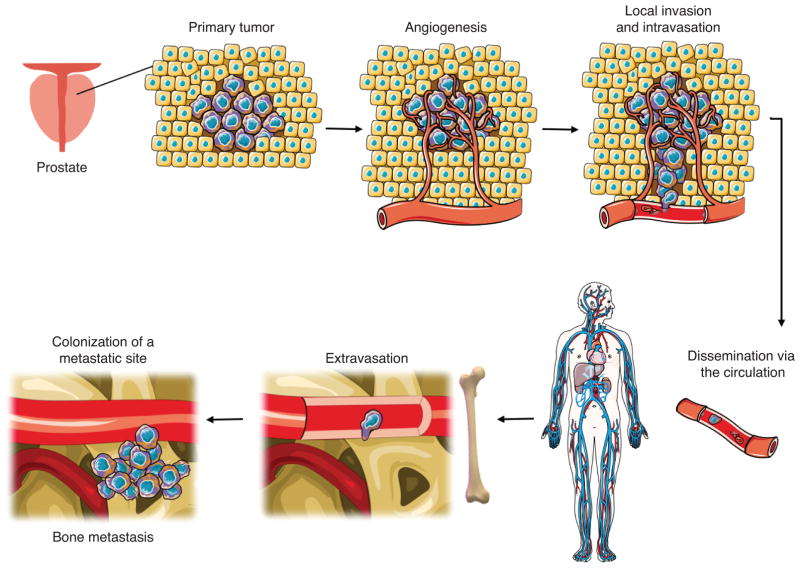
Metastatic prostate cancer is responsible for approximately 26,000 deaths per year in the United States. Despite clinical advances improving survival of men with localized prostate cancer, metastatic disease remains incurable (1,2). Prostate cancer non-randomly metastasizes to the bone, resulting in high patient morbidity and mortality (3). Over the last several decades, models have emerged to describe the general sequential steps of the metastatic process: detachment from the basement membrane, local invasion, intravasation into the vasculature, systemic dissemination, cellular extravasation, and metastatic colonization (Fig. 1) (4,5). The research investigating the selective colonization of the bone is largely based on Paget’s “seed and soil” hypothesis that suggests that metastatic cancer cell “seeds” must fall on congenial target organ “soil” (6–9). While the compatibility between a metastatic prostate cancer cell and the bone metastatic site has been extensively described, little work has investigated the relationship of the pre-metastatic primary prostate cancer cell “seed” and the site of origin “soil.”
Figure 1. Steps of the metastatic process.
Metastasis is characterized by a series of sequential steps: primary tumor formation, recruitment of blood vessels through angiogenesis, cancer cell invasion of local tissue, and entry into dispersal corridors such as blood vessels. Disseminated cells travel through the circulation and upon reaching a suitable secondary site such as the bone, extravasate from the blood vessels and colonize to form bone metastases. (Modified from Servier Medical Art by Servier licensed under CC BY 3.0.)
Applying evolutionary ecology principles to the study of cancer has provided a deeper understanding of the selective pressures that give rise to the eventually successful metastatic seed (10–19). Previous work has demonstrated that cancer cells move within the primary tumor and that there is variability in movement patterns among individual cells (14,20–22). The cells that readily move in the primary tumor may be predisposed to disseminate, underscoring the importance of uncovering the origins of cell motility within a tumor. Despite the advancements in understanding mechanisms of cell movement (20–22), the determinants of cell movement remain unclear. Optimal foraging theory (OFT), a subdiscipline of evolutionary ecology, can be used to describe and predict movement in a heterogeneous environment and may be applied to prostate cancer to elucidate the influences of cell movement within a tumor.
Studying cancer as an invasive species provides insight into the necessary phenotypic characteristics of the metastatic “seed” and how those traits are selected for. In order to disseminate to a distant secondary habitat, invasive species utilize established dispersal corridors, regions of uninhabitable geography linking two distant favorable habitats. Metastatic prostate cancer cells emigrate from the primary tumor via distinct corridors: blood vessels, lymphatics, and nerves (Fig 4) (23–29). Understanding the selective pressures that promote or inhibit a cell’s entry into these corridors will provide a better understanding of the requirements for a successful metastatic “seed.” These ecological principles shed light on how the primary tumor “soil” and metastatic routes select for successful metastatic cells.
Figure 4. Path of dispersing prostate cancer cells from the primary tumor.
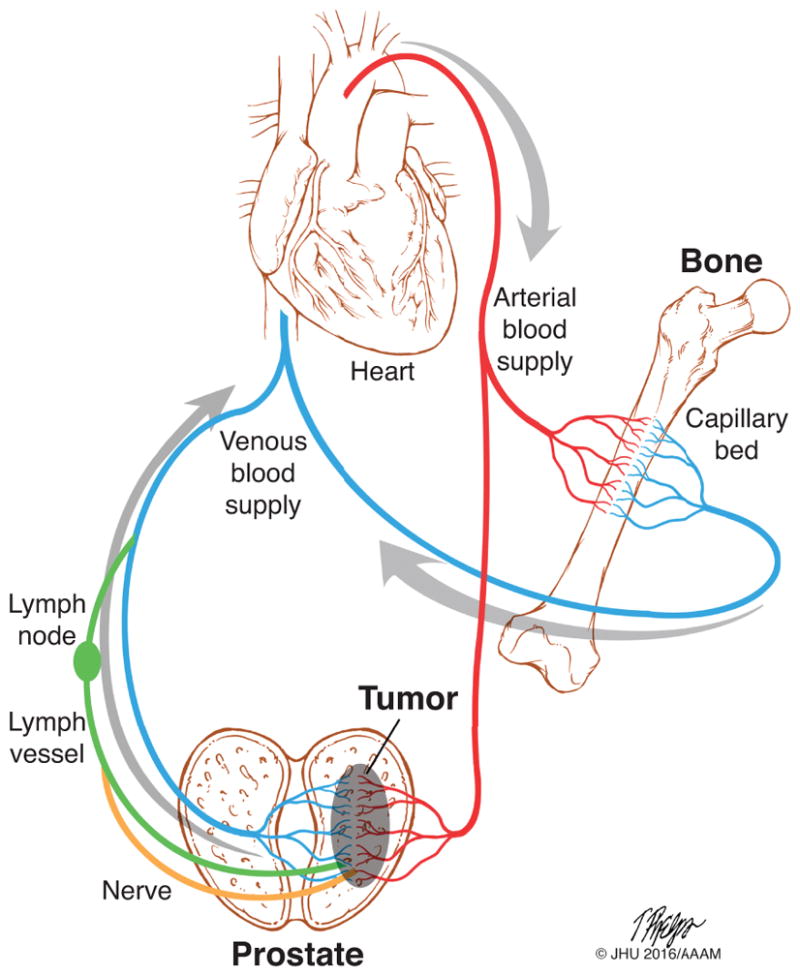
Prostate cancer cells disseminate from the primary tumor via venous blood vessels (blue), lymph vessels (green), or nerves (orange). Nerve-disseminated cells enter the lymph and all cancer cells in the lymph pass through at least one lymph node before entering the venous blood supply. CTCs are then carried through the body via the blood circulation. CTCs pass through the through the heart and lungs to enter the arterial blood supply. CTCs are carried with the blood through the arterial system, entering distant organ capillary beds at random. Upon reaching a suitable secondary site, such as the bone, cells must extravasate from the blood vessel to colonize the metastatic site. Image printed here with permission from the source: Tim Phelps (C)JHU/AAAM 2016, Department of Art as Applied to Medicine, The Johns Hopkins University School of Medicine.
Optimal foraging of prostate cancer
One of the universal properties of life, as an animal, a plant, or a cell, is the need for acquiring resources to fulfill the basic metabolic needs to support life. Resources are the consumable and depletable factors essential for the survival, proliferation, and movement of an individual (Table 1). In order to find and consume these resources, an organism must forage by employing different strategies dependent on the balance of risk, reward, and ability (Fig. 2). OFT states that the optimal foraging strategy for a particular organism is one that provides maximal resources at minimal cost to the individual (30,31). Optimal foraging strategies vary among members of a species and depend on the state of the individual as well as the properties of its environment (31). Foraging strategies are described broadly as a combination of stationary versus mobile techniques. Stationary foragers remain in one place and wait for local resources to replenish. In contrast, mobile foragers optimize foraging potential by moving among resource patches as they become depleted over time. Organisms will adapt their foraging strategies based on the immediate characteristics of their habitat, and the fittest individuals will have adopted the most successful or optimal foraging strategies. In this way, OFT describes when and how an organism should move through its environment so as to balance the benefits and costs of foraging.
Table 1.
Definitions of prostate cancer optimal foraging theory and dispersal
| Term | Definition | Examples | |
|---|---|---|---|
| Ecology | Prostate Cancer Biology | ||
| Resource | a depletable factor essential for survival, movement, or proliferation | nuts, water, oxygen | sugars, lipids, oxygen, etc. |
| Patch | a depletable area of localized resource | oak tree | region adjacent to a blood vessel |
| Habitat | the physical abiotic region in which an individual resides, segmented into resource patches | forest | prostate tumor |
| Species | a group of individuals with a shared lineage and similar functional traits | squirrels, hawks | T-cells, prostate cells, fibroblasts |
| Individual | a species member that functions independently and consumes resources | squirrel | cell |
| Foraging | the search for and consumption of resources in a habitat (mobile or stationary strategy) | squirrel forages for nuts | cancer cell forages for oxygen |
| Stationary forager | an individual that forages without moving to another patch regardless of patch depletion | sponge | epithelial cell |
| Mobile forager | an individual that forages by moving among patches | squirrel, hawk | mesenchymal cell |
| Predator | an individual that attacks and kills another individual | hawk | T-cell, M1 macrophage |
| Dispersal corridor | an inhabitable path through an inhospitable region linking two or more favorable habitats | railroad tracks, river | blood vessel, lymph vessel, nerve |
| Adaptation | a fitness-enhancing phenotypic trait that is selected for by an external selective pressure | white fur color of a snowshoe hare | resistance to anoikis by a circulating tumor cell |
Figure 2. Optimal foraging of prostate cancer cells.
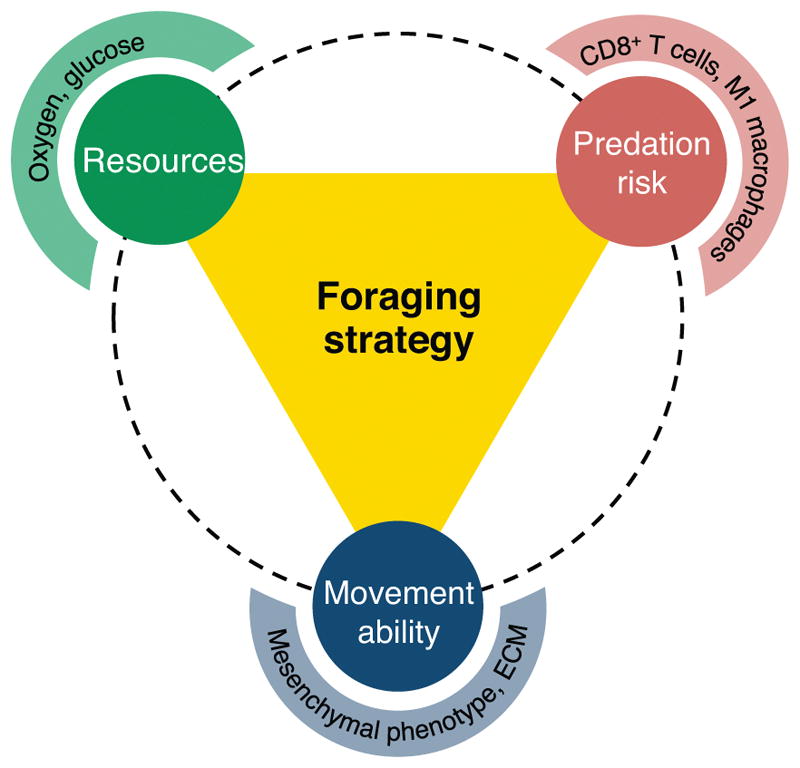
The optimal foraging strategy employed by a cancer cell is influenced by three interacting factors: available resources (e.g. oxygen, glucose), predation risk (e.g. cytotoxic T cells, M1 macrophages), and movement ability (e.g. mesenchymal phenotype, permissible ECM).
Cancer cells are generally stationary foragers that focus their energetic efforts on proliferation rather than movement (32). A select few, likely those that undergo an epithelial-mesenchymal transition (EMT), will have the option to employ a mobile foraging strategy. Generally these cells have higher energy requirements and consume more resources than their stationary epithelial-like counterparts (33). Since very few cells in the primary tumor undergo EMT, the number of stationary foraging cells is likely to vastly outnumber mobile foraging cells.
Applying OFT to cancer biology introduces the novel concept of cancer cell foraging in which a cancer cell’s potential to disseminate from the primary tumor is influenced by its foraging strategy. The optimal foraging strategy of an individual is influenced by movement ability, resource distribution, and predation risk (Fig. 2). These same influences dictate cancer cell foraging behavior. While the primary tumor habitat promotes both foraging strategies, the few cells that adopt mobile foraging are more likely to possess the traits necessary for successful metastasis.
Movement ability is essential for mobile foraging of pre-metastatic prostate cancer cells
The ability of an organism to move through space is determined both by the organism’s intrinsic capacity to move as well as the environmental constraints on movement. For example, a sea sponge is obligatorily stationary because it possesses little capacity to move. In contrast, a squirrel in a forest has the capability to act as a mobile forager by transporting itself from tree to tree in search of resources. However, if confined by a cage, the squirrel can no longer act as a mobile forager because its environment does not permit movement, forcing it to adopt a stationary foraging strategy. Thus, characteristics of both the individual and the environment limit whether an individual can incorporate movement into its foraging strategy.
These same physical limits constrain cancer cells in the primary tumor: the cell must have the capacity to move and the tumor environment must permit movement. The genetic and molecular bases for cell movement behaviors and metastatic propensity in prostate cancer have been extensively studied (21,33–35). Cell tracking experiments reveal that mesenchymal prostate cancer cells exhibit increased general non-directed movement compared to epithelial cells derived from the same parent prostate cancer cell line in vitro (33). This increased movement phenotype is a direct product of the cell’s intrinsic properties, predisposing it to a certain behavior.
In addition to a cell’s inherent capacity to move, the tumor environment must permit cell movement. Many physical properties of the tumor including extracellular matrix (ECM) organization, pH, and interstitial fluid pressure influence tumor cell dissemination (21,22,36,37). Variance in these physical conditions may determine whether the environment is conducive for cell movement. For example, the ECM may facilitate cell motility by providing a stiff substrate for cellular focal adhesion necessary for cell movement (38). Conversely, the ECM structure and organization can inhibit movement depending on characteristics such as fiber composition and alignment (39). As a classical example, the basement lamina confines benign cells to the gland lumen (40).
In addition to the physical properties of the tumor, other environmental factors, such as other cell species within the tumor, make the environment more or less permissible to movement. For example, cancer-associated fibroblasts and M2-like tumor associated macrophages secrete enzymes that remodel the ECM thereby increasing cancer cell movement opportunities by altering the physical scaffolding of the environment (41).
In OFT, the environment characteristics coupled with cellular movement phenotype determines the cell’s ability to incorporate movement into its foraging strategy. While an individual’s capacity for movement determines its ability to adopt a mobile foraging strategy, it does not mean that the cells will employ the option of mobile foraging. Movement through a heterogeneous environment is associated with certain risks (i.e. predation) and rewards (i.e. resources). The optimal foraging behavior of a cell will not only depend on its ability to move but also the predation risks and resource rewards associated with mobile foraging behaviors (Fig. 2).
Resources are distributed heterogeneously into patches
A major determinate of an individual’s foraging strategy is the availability of resources and their distribution throughout the habitat. Resources include all of the depletable factors consumed for survival, proliferation, and movement (Table 1). Resources are often distributed heterogeneously throughout a habitat. For example, acorns are necessarily concentrated on their parental tree or on the ground nearby, but are scarce in the adjacent space. Therefore, a foraging squirrel’s encounter rate with acorns increases as it approaches the tree. OFT defines these discrete areas of localized resource as “patches” (Table 1, Fig. 3B). Because patches are distributed non-homogenously both in geographical space and in time as resources are consumed by all members of the community, patchy habitats promote movement throughout a region as an optimal foraging strategy.
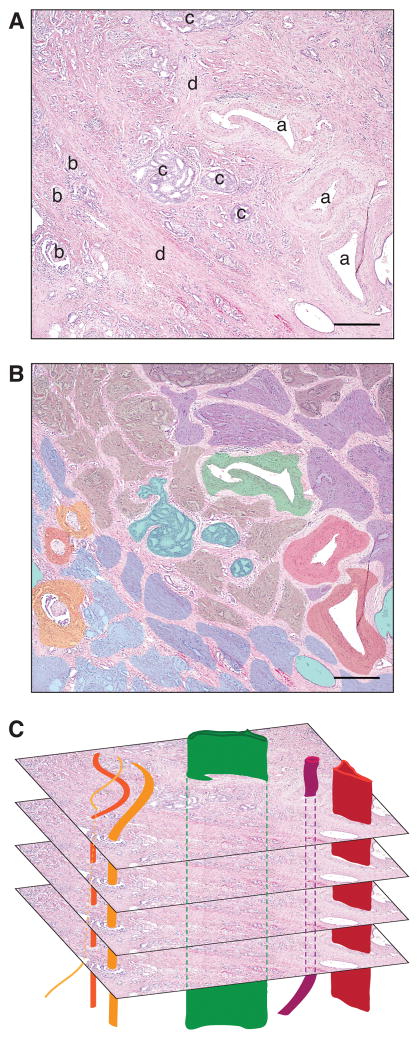
Figure 3. Prostate cancer resource patches and dispersal corridors.
(A) Primary prostate tumor from prostate cancer patient radical prostatectomy. (a: lymphovascular vessel, b: nerve, c: intraductal carcinoma, d: stromal infiltration) (B) Prostate cancer resource patches: Colored regions represent patches within the primary tumor and depict spatial heterogeneity at single moment in time. Variations in color represent variations in patch characteristics (i.e. resource and predation risk). Importantly, though not depicted, patch geography and characteristics change over time. (C) Dispersal corridors including blood vessels (red and maroon), lymph vessels (green), and nerves (orange) intersect primary tumor patches and provide a route for long-distance dissemination out of the primary tumor habitat. (H&E; scale bar = 300 μm; image courtesy of Dr. Tamara Lotan, Johns Hopkins University)
A similar pattern of patchy resource distribution has been observed in tumor habitats (Fig. 3A) (42–46). In the case of cancer cells, while the complete repertoire of resources has not been defined, resources likely include oxygen (47), carbon and nitrogen sources (sugars, amino acids, and lipids) (48), and metal ions (49). These resources are supplied to the tumor by the local ECM environment, cell debris, and tributary-like influxes from nerves and the blood and lymph vasculatures. These resource suppliers are distributed heterogeneously throughout the tumor (39,43,50) providing different areas of the tumor habitat with different resource levels and types (Fig. 3B).
Patches with higher levels of resource will take longer to deplete, allowing the organism increased resource consumption. Once a patch is depleted, stationary foragers will reduce their metabolic needs to stay in place and wait for resources in the patch to replenish. Under the same pressure of resource decline, mobile foragers will desert the depleted patch and physically move in search of for a more favorable resource patch elsewhere (assuming no confounding factors such as increased risk of predation).
In addition to resource abundance, however, the variety of resource types in a given patch also influences foraging strategy. Since each organism requires a variety of resources to fulfill a variety of energy requirements, an organism’s time in a patch also depends on the types of resources the patch contains. For example, a squirrel requires both a source of carbohydrates such as nuts and a source of water. A patch that offers nuts but no available water will be less valuable and abandoned earlier than a patch that offers nuts and readily available water (51). Thus, an organism’s response to the amount, type, and distribution of resource contributes to its foraging strategy and movement behavior throughout a habitat.
This OFT concept of patches has been applied to cancer to explain and model the distribution and abundance of varying resource types throughout the tumor (42). As with ecology, the resources in a patch diminish and the foraging individual must search for more. Overcrowding, such as when a large number of cancer cells deplete the oxygen supply and create regions of hypoxia, may accelerate resource depletion (10,46). To search for resource, the majority of cells remain in the same location to wait for local resource to replenish, but a select few invade through or out of the local tumor space in pursuit of more advantageous resource patches. The latter technique has been observed in cell lines with oxygen gradients in three dimensional matrix models in vitro (46). As with ecology, available resource abundance and variety affect whether an individual adopts a mobile foraging strategy: a patch with high levels of sugars will likely take longer to diminish and require less movement to optimally forage. However, if the patch lacks an essential resource such as oxygen, then an optimal foraging strategy would include movement out of that patch after a shorter amount of time. Thus, cell movement throughout the primary tumor in the context of OFT is in large part a response to resource distribution in the primary tumor.
Predation risk alters foraging strategy
Foraging strategies of virtually all organisms are strongly impacted by the predation risk associated exploiting or moving between resource patches (52). Predation risk describes the likelihood of being attacked by a predator as determined by the number of predators nearby, the predator’s lethality if encountered, and the prey’s ability to escape or evade detection. An organism’s ability to camouflage can reduce predation risk even in areas with large numbers of predators by reducing the likelihood of detection and attack. However, when the organism is eventually detected, it must evade the predator’s attack in order to survive. Evasion can take the form of moving away from areas of high predation risk (31). An ecological example of managing predation risk is observed in rabbit phenotypes and behavior. In the winter, snowshoe hares molt from brown to white fur. The color change serves them well in the snow that inevitably occurs in the boreal forests of Canada. In addition to camouflage, hares also have their rapid hopping gait as a means for evading attack by a predator such as a lynx.
In the prostate cancer setting, predators include the anti-tumor immune cells such as CD8+ cytotoxic T cells and M1 macrophages which seek out, destroy, and consume their prey: the cancer cell (Table 1) (53,54). As such, a risky patch for the tumor cell will have higher CD8+ T cell and M1 macrophage infiltrate. These immune cell predators are heterogeneously dispersed throughout the tumor (55), presumably with higher concentrations near the blood and lymph vessels where they enter the tumor habitat. Therefore, predation risk likely increases with proximity to the resource-high lymphovasculature with additional risk as they enter lymph or blood vessels. Cancer cells decrease their predation risk by expressing programmed death ligand 1 (PD-L1) which camouflages them from these predators by inhibiting CD8+ T cells proliferation and cytokine secretion (56). PD-L1-expressing cells may be able to forage successfully and more thoroughly in the high resource patches located closer to the vasculature regardless of the increased immune cell presence. In addition to camouflage, the optimal foraging strategy of cancer cells in response to predation risk will include increased movement in order to evade attack and move away from high concentrations of immune cells. Therefore, a cancer cell’s tactic for immune evasion is part of its foraging strategy and helps explain its movement behavior within the tumor.
Adaptive phenotypic traits selected for in the primary tumor: stationary versus mobile foragers
Movement ability, resources, and predation risk each contribute to determining an individual’s foraging strategy thereby explaining the primary tumor influences on cancer cell behavior (Fig. 2). Applying OFT to prostate cancer reveals that a variety of foraging strategies are adopted by cancer cells which creates phenotypic heterogeneity within the tumor. The most successful of these strategies are selected within the primary tumor. While a stationary foraging is a common successful strategy among primary tumor cells, some cells adopt mobile strategies with behavior similar to invasive species. The adaptations of mobile foragers, such as the ability to move or evade predation, likely increase their ability for metastatic behaviors such as intravasation or survival in the high-predation circulation. Because of these adaptations, mobile foragers are more suited for successful dissemination from the primary tumor and potential metastasis. Clinical evidence for the selection of mobile foragers by primary tumor conditions includes the positive correlation between hypoxic primary prostate tumors and biochemical recurrence (47). This study exemplifies how cell adaptations resulting from low-resource primary tumor conditions may promote metastatic capability.
Tributary-proximal patches positively select for potential disseminating cells
The richest resource patches in the primary tumor are located in close proximity or adjacent to the blood vessel, lymph vessel, and nerve tributaries that provide a constant supply of high levels of resources (Fig. 3C). Delivered resources include oxygen and sugars supplied by the blood and nerve growth factor and other neurotrophins supplied by nerves (57). In addition to providing high-resource patches, as source of entry for host immune cells these tributary-proximal patches are also characterized by high inherent predation risk. Cancer cells are attracted to high resource areas and therefore attracted to these patches. The cells that move into and remain in these patches must also have the ability to evade predation, either through camouflage (e.g. PD-L1 expression) and/or high movement ability. The same phenotypic traits selected for in the tributary-proximal mobile foragers are also favorable for successful metastasizing cells: preference for high cell movement, ability to invade the local tumor space, and ability to sense and evade predation by immune cells. These traits not only increase fitness within tributary-proximal patches but also confer metastatic potential. In this way, tributary-proximal patches positively select for cells predisposed to metastatic behavior.
Each of the three resource tributary types also acts as a metastatic route (23–25). As a cell moves into patches near these routes, a cell’s likelihood of entering the route and dispersing from the primary tumor increases. By exhibiting traits suited for metastasis and by increasing their encounters with metastatic routes, mobile foragers increase their potential for dissemination and metastasis. Evolution within the tumor unwittingly selects for cancer cells capable of metastasizing and selects for movement and patch use behaviors that place a mobile foraging cancer cell type near blood, lymph and nerve routes of dispersal.
Dispersal corridors are barriers to dissemination
Prostate cancer acts as an invasive species as cancer cells leave their native primary tumor to establish colonies in distant secondary sites, most commonly in bone (58). In order for an invasive individual to colonize a distant site, it must first escape its native habitat. In ecology, invasive species often escape via dispersal corridors, pathways that connect two or more distant regions (Table 1). In general, dispersal corridors themselves cannot sustain the population either because of limited resource availability or high predation risk. They do, however, provide a low level of resources and relative safety from the surrounding hostile environment. A common ecological corridor is a railroad track that is used by animals to travel through inhospitable urban habitats. While the tracks do not provide the essential requirements of a coyote habitat (ample resources, shelter, etc.), the coyote may move between suitable habitats without encountering the extreme and unfamiliar predation risk of a city (59).
In addition to providing a relatively safe and unhindered passage across hostile landscape between favorable habitats, a dispersal corridor also acts as a selection barrier. The dispersal corridor environment may include unfamiliar physical conditions, varied predation risk, and different resource levels than the primary habitat (60). Organisms utilizing the corridor must possess the necessary characteristics for entry into and survival within this unfamiliar environment. Therefore, while these selective corridors allow for rapid and long-distance expansion of a subset of potential invaders, they simultaneously prevent the spread of the organisms lacking the characteristics for corridor entry and survival. Thus, dispersal is limited to individuals with particular traits.
Invasive prostate cancer cells escape the primary tumor and metastasize via three dispersal corridors: hematogeneous spread via blood vessels, lymphatic spread via lymph vessels, and perineural invasion via nerves (Fig. 3C) (23–29). Similar to ecological dispersal corridors, these routes of dissemination function as filters between two distinct habitats, only permitting cells with particular phenotypes to utilize the corridors and potentially establish clinical metastases while confining others cells to the primary tumor (11,61). The selective properties of dispersal corridors are two-fold: barriers to entry into the corridor and barriers to long-distance dispersal once in the corridor.
Selection pressures of entry into dispersal corridors
The entry barriers faced when entering the dispersal corridor are determined by the corridor’s characteristics. For example, a river with thick underbrush and ground cover on its banks will have a high barrier to entry. Only animals that physically break through the underbrush will be able to enter the dispersal corridor. Thus, the corridor exerts selective pressure for certain characteristics and only organisms with the appropriate characteristics will have the opportunity to attempt dispersal along that corridor. Importantly, however, barriers to entry are context-dependent: different organisms with varying adaptations will be able to invade the depending on the selective properties of the barrier.
In a primary prostate cancer tumor, there are different barriers to entry depending on the characteristics of the dispersal corridor. Blood and lymph vessels are corridors with constant one-dimensional fluid currents analogous to a river. When entering a blood or lymph vessel, a primary tumor cell faces physical barriers such as the endothelial cell lining and surrounding basal lamina. Cells enter these vessels by two mechanisms: active intravasation or passive sloughing. Passive sloughing is most likely when entry barriers are low as typically observed in vessels with permeable basal lamina and endothelial cell layers (62). Vessels that allow entry by passive sloughing select for a wide range of cells that are capable of detachment by an external force such as a current. This cell population includes both mobile (mesenchymal) and stationary (epithelial) foragers.
Vessels that require entry by active intravasation have less permeable basal lamina and endothelial cell layers (62) and select for mobile foragers with high capacity for locomotion and invasion (32). Invasive prostate cancer cells overcome the ECM barrier surrounding blood vessels by secreting matrix metalloproteases (MMPs) to cleave key elements of the basal lamina (63) and undergo cytoskeleton remodeling to transmigrate between the endothelial cells as they move into the vessel (64). These characteristics required for active intravasation are also necessary for successful mobile foraging. A cell’s ability to manipulate its environment and itself in order to move in search for resources predisposes it to overcoming intravasation barriers. In this way, the entry barriers for blood and lymph vessels select for mobile foragers while keeping cells that lack the required characteristics out of the dispersal corridor thus preventing them from dispersing.
The third dispersal corridor, the nerve, is analogous to a railroad track with clear physical delineations, but lack of directionality. Prostate cancer cell entry into this corridor is called perineural invasion (PNI), which is invasion of prostate cancer cells in, around, and through the layers of the nerve (29). Nerves lack a surrounding matrix or cell layer and thus do not have high barriers to entry, but do require cells to move as mobile foragers in order to enter and traverse the nerve corridor. Once again a high movement foraging strategy proves to be a prerequisite for entry and long-distance dispersal.
Selection pressures of long-distance dispersal within corridors
Though all cells can enter corridors via passive sloughing, not all can survive the corridor barriers to dispersal. Corridors, such as rivers and railroads, are primarily suitable for dispersal as opposed to colonization as their conditions may provide just enough resources to allow the dispersing organism to stay in the corridor but are not suitable habitats for colonization. Likewise, blood vessels, lymph vessels, and nerves permit survival but not colonization. Only individuals with certain characteristics can disperse via these corridors and these individuals are selected for by the conditions of each corridor.
Long-distance dispersal along a corridor characterized by a unidirectional current, such as a river, selects for individuals with the ability to survive the drastically unfamiliar current conditions. In a prostate cancer primary tumor, cells that have entered the lymphovasculature must withstand increased predation risk and current forces during dispersal. Dispersing cells therefore are selected for a number of survival characteristics: immune evasion, ability to withstand sheering forces (64,65), and resistance to anoikis (induction of apoptosis due to a cell’s detachment from the extracellular matrix) (56,66). These barriers to dispersal act as selective pressures to limit successful dispersal to cells with the required characteristics.
In the case of unidirectional current corridors, the ability of the cell to move independently is not required because the current acts as an extrinsic displacement force. In contrast, in immobile delimited corridors, such as the railroad tracks used by coyotes (67), the organism must actively move in order to utilize the corridor. The nerves in the primary tumor act as stationary corridors: they provide a path along which the cell can travel but do not provide an external force to facilitate movement. In order to successfully disperse along this type of corridor, an individual must be able to transport itself along the corridor path. Thus, nerve dispersal selects for mobile cells that can survive the nerve fiber environment and locomote along the corridor. Thus, the adaptive mobile foraging phenotype is selected for in an ideal metastatic “seed” and is evidenced by the positive association of perineural invasion and development of bone metastasis (28).
While some tumor cells use the nerve and lymph as corridors for dispersal from the primary tumor, eventually all dispersing cancer cells enter the blood circulation (Fig. 4). Cancer cells that leave the primary tumor via nerve corridors likely use the nerve-surrounding lymphatic space in the prostate to enter the lymph circulation, joining the cells that originally left the primary tumor through the lymph corridor (26,68). Transferring to the lymph corridor induces lymph-associated barriers that were not present in nerve dispersal. Thus, in order to successfully disseminate, even cells that escape the tumor via nerve must also possess the characteristics required for entry and survival in a one-dimensional corridor (i.e. immune evasion, resistance to anoikis, etc.). Cells dispersing in the lymph pass through lymph nodes before draining into the blood. Some dispersing prostate cancer cells in the lymph will be trapped in and colonize the lymph node (28,69), resulting in regional lymph node metastasis.
Jump dispersal of prostate cancer cells through the vasculature
Eventually, all dispersing prostate cancer cells enter the venous blood circulation (Fig. 4). Once in circulation, circulating tumor cells (CTCs) are outwardly indistinguishable from each other regardless of initial dispersal corridor or mode of entry into the corridor (passive or active) (69,70). After entering the blood circulation, cells undergo a high velocity dispersal resembling the ecological phenomenon of jump dispersal, the rapid, long-distance dispersal of an organism across inhospitable habitat (71). In ecology, successful jump dispersal events are rare but are required for dispersal to distant habitats. For example, a jump dispersal event was required for the spread of monkeys from Africa across the Atlantic Ocean to South America (72). Similarly, jump dispersal through the blood is a critical event for the establishment of distant prostate cancer metastases corresponding to CTCs and bone metastases in patients (73).
Jump dispersal provides additional selective pressure to corridor dissemination. As cells enter the larger blood circulation, current velocity increases and as a result shearing forces increase (74,75). Thus, successful jump dispersers must withstand much greater current velocities and shearing forces. Only cells with the characteristics required for overcoming these jump dispersal barriers will be able to successfully disseminate to distant organs such as the bone. Thus, jump dispersal events in prostate cancer metastasis select for cells that can enter the blood circulation and survive its inhabitable conditions.
Dissemination destination is stochastic and requires permissible “soil” for metastasis
To understand the inter-connectivity of the blood, lymph, and nerves, and the long-distance dispersal of CTCs, it is important to view the circulation as a one-way circuit with all the corridors merging into the systemic circulation (Fig. 4). In order for a clinical bone metastasis to arise, a dispersing cancer cell from the primary tumor in the prostate must travel through one of the dispersal corridors and eventually undergo a jump dispersal event into the venous circulation. Venous blood carrying the CTC travels through the heart and lungs and exits the heart as part of the arterial blood supply. The CTC is carried by the blood flow to distant regions of the body: potential secondary sites of metastasis. Importantly, the dispersing CTC does not have explicit control over its direction or final destination and “homing” to a specific site while in the circulation as a CTC is impossible (32). Therefore, landing in a favorable habitat is a stochastic event. A CTC is just as likely to disseminate to a bone capillary as a liver or muscle capillary.
Wherever the CTC lands, in order to survive, the cell must extravasate from the circulatory vessel and survive in the secondary site environment as a disseminated tumor cell (DTC). For a clinical metastasis to arise, the DTC must proliferate and colonize the secondary site, often observed years or decades after the primary tumor was removed. The success of this metastatic event, detailed by Stephen Paget’s “seed and soil hypothesis” is largely dependent on the characteristics of the secondary site “soil” which must permit or promote colonization. As prostate cancer preferentially develops clinical metastasis to bone (58), it is likely that the bone “soil” is highly favorable for prostate cancer DTC colonization or for DTC re-awakening to establish a clinical metastasis.
Prostate cancer metastasis is an inefficient process
The journey from foraging in a local patch in the primary tumor to colonization of a secondary site is laden with selection pressures making the metastatic process incredibly inefficient (Fig. 5). A successful metastatic event requires a cancer cell to survive in the primary tumor habitat, encounter a dispersal corridor, enter the corridor, disperse along the corridor, jump disperse through the circulation, land in a permissive secondary site, extravasate, and colonize (Fig. 1).
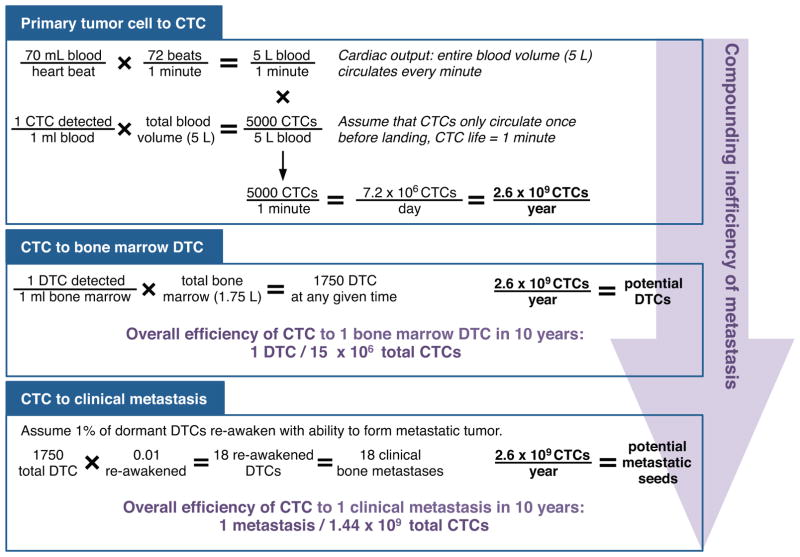
Figure 5. Inefficiency of prostate cancer metastasis.
Of the cells that disseminate from the primary tumor (detected as CTCs from the venous circulation), very few become bone DTCs and even fewer eventually form clinical metastases. The inefficiency of each of step compounds with progression along the metastatic process.
Based on the current detection techniques, men with metastatic prostate cancer have as many as 5000 cells in circulation at any given time (76). Assuming that a CTC will only survive a single pass through the circulation (as evidenced by the relatively low CTC count per total tumor burden) a prostate cancer tumor produces approximately 7 million CTCs per day. Despite the high numbers of CTCs introduced into the dispersal corridors, only a rare number of those CTCs are successful bone marrow DTCs, and even fewer are the seed for a clinical metastasis. For a single DTC to arise 10 years after primary tumor formation, more than 15 million CTCs would have been released from the primary tumor. Even more striking, the likelihood of a CTC seeding a metastasis over 10 years is 1 in 1.44 billion (Fig. 5).
The incredible inefficiency of metastasis underscores the necessity of a specialized subset of adaptations in order for a primary prostate cancer cell to successfully metastasize. Such adaptations arise from the selective pressures faced with each step in the metastatic cascade, including within the primary tumor and during dispersal. This highlights the necessity of understanding the unique environmental pressures of the selective “soil” to give rise to metastatic “seeds.”
Metastatic “seeds” are likely mobile foragers
The success of a metastatic “seed” is dependent on the cell’s ability to escape the primary tumor and colonize an unfamiliar secondary site. While some prostate cancer cells, including stationary foragers, exit the primary tumor through passive sloughing, it is unlikely that these cells will exhibit the necessary adaptive phenotype to equip them to survive dispersal or to thrive within a metastatic site. In contrast, mobile foragers, such as those with the capability to disseminate along the nerve, possess the adaptations required for overcoming selection pressures encountered along the metastatic cascade and therefore are selected for as metastatic “seeds.” The adaptations accumulated by mobile foraging prostate cancer cells in response to the selective pressure of the primary tumor primes the cancer cells to (a) encounter a greater number of dispersal corridors, (b) survive the severe dispersal event, and (c) have the capacity to invade a secondary site.
Acknowledgments
This work was supported by the Prostate Cancer Foundation and NIH grant nos. P01CA093900 and U54CA163124 to K.J.P.; NIH/NCI grant no. U54CA143970-05 and NSF grant no. CNS-1248080 to J.S.B.; and an American Cancer Society Postdoctoral Fellowship PF-16-025-01-CSM to S.R.A. The authors thank Dr. Tamara Lotan for the use of images. The authors thank the members of the Brady Urological Institute, especially Dr. Donald S. Coffey and the members of the Pienta laboratory, for thoughtful discussion.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Brower V. Researchers tackle metastasis, cancer’s last frontier. J Natl Cancer Inst. 2007;99(2):109–11. doi: 10.1093/jnci/djk047. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369(9574):1742–57. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast cancer research : BCR. 2008;10(Suppl 1):S2. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature reviews Cancer. 2003;3(6):453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 7.Langley RR, Fidler IJ. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. International journal of cancer. 2011;128(11):2527–35. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathot L, Stenninger J. Behavior of seeds and soil in the mechanism of metastasis: a deeper understanding. Cancer science. 2012;103(4):626–31. doi: 10.1111/j.1349-7006.2011.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paget S. The distribution of secondary growths in cancer of the breast. 1889 Cancer metastasis reviews. 1889;8(2):98–101. [PubMed] [Google Scholar]
- 10.Amend SR, Pienta KJ. Ecology meets cancer biology: the cancer swamp promotes the lethal cancer phenotype. Oncotarget. 2015;6(12):9669–78. doi: 10.18632/oncotarget.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang KR, Mooney SM, Zarif JC, Coffey DS, Taichman RS, Pienta KJ. Niche inheritance: a cooperative pathway to enhance cancer cell fitness through ecosystem engineering. Journal of cellular biochemistry. 2014;115(9):1478–85. doi: 10.1002/jcb.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greaves M. Evolutionary Determinants of Cancer. Cancer discovery. 2015;5(8):806–20. doi: 10.1158/2159-8290.CD-15-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–13. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Sprouffske K, Huang Q, Maley CC. Solving the puzzle of metastasis: the evolution of cell migration in neoplasms. PloS one. 2011;6(4):e17933. doi: 10.1371/journal.pone.0017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepper JW, Scott Findlay C, Kassen R, Spencer SL, Maley CC. Cancer research meets evolutionary biology. Evolutionary applications. 2009;2(1):62–70. doi: 10.1111/j.1752-4571.2008.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aktipis CA, Boddy AM, Jansen G, Hibner U, Hochberg ME, Maley CC, et al. Cancer across the tree of life: cooperation and cheating in multicellularity. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2015;370:1673. doi: 10.1098/rstb.2014.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnal A, Ujvari B, Crespi B, Gatenby RA, Tissot T, Vittecoq M, et al. Evolutionary perspective of cancer: myth, metaphors, and reality. Evolutionary applications. 2015;8(6):541–4. doi: 10.1111/eva.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatenby RA, Gillies RJ, Brown JS. Evolutionary dynamics of cancer prevention. Nature reviews Cancer. 2010;10(8):526–7. doi: 10.1038/nrc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, Polyak K. Non-cell autonomous tumor-growth driving supports sub-clonal heterogeneity. Nature. 2014;514(7520):54–8. doi: 10.1038/nature13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nature reviews Cancer. 2003;3(12):921–30. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 21.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nature reviews Cancer. 2003;3(5):362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 22.Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Current Opinion in Cell Biology. 2015;36:13–22. doi: 10.1016/j.ceb.2015.06.004. http://dx.doi.org/10.1016/j.ceb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Wong SY, Hynes RO. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle. 2006;5(8):812–7. doi: 10.4161/cc.5.8.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res. 2011;71(4):1214–8. doi: 10.1158/0008-5472.CAN-10-3277. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Sun Y, Gao D. Role of the nervous system in cancer metastasis. Oncol Lett. 2013;5(4):1101–11. doi: 10.3892/ol.2013.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21(1):77–82. doi: 10.1016/j.cytogfr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, et al. In vitro dorsal root ganglia and human prostate cell line interaction: Redefining perineural invasion in prostate cancer. The Prostate. 2001;49(3):213–23. doi: 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- 28.Ciftci S, Yilmaz H, Ciftci E, Simsek E, Ustuner M, Yavuz U, et al. Perineural invasion in prostate biopsy specimens is associated with increased bone metastasis in prostate cancer. The Prostate. 2015;75(15):1783–9. doi: 10.1002/pros.23067. [DOI] [PubMed] [Google Scholar]
- 29.Amit M, Na’ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nature reviews Cancer. 2016;16(6):399–408. doi: 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- 30.Odum EP, Barrett Gary W. Fundamentals of Ecology. Belmont, CA: Brooks Cole; 2004. [Google Scholar]
- 31.Brown JS. Vigilance, patch use and habitat selection: Foraging under predation risk. Evolutionary Ecology Research. 1999;1(1):49–71. [Google Scholar]
- 32.Amend SR, Roy S, Brown JS, Pienta KJ. Ecological paradigms to understand the dynamics of metastasis. Cancer letters. 2016;380(1):237–42. doi: 10.1016/j.canlet.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiraishi T, Verdone JE, Huang J, Kahlert UD, Hernandez JR, Torga G, et al. Glycolysis is the primary bioenergetic pathway for cell motility and cytoskeletal remodeling in human prostate and breast cancer cells. Oncotarget. 2015;6(1):130–43. doi: 10.18632/oncotarget.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banyard J, Chung I, Migliozzi M, Phan DT, Wilson AM, Zetter BR, et al. Identification of genes regulating migration and invasion using a new model of metastatic prostate cancer. BMC Cancer. 2014;14(1):1–15. doi: 10.1186/1471-2407-14-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roca H, Hernandez J, Weidner S, McEachin RC, Fuller D, Sud S, et al. Transcription Factors OVOL1 and OVOL2 Induce the Mesenchymal to Epithelial Transition in Human Cancer. PloS one. 2013;8(10):e76773. doi: 10.1371/journal.pone.0076773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, et al. Acidic extracellular microenvironment and cancer. Cancer cell international. 2013;13(1):89. doi: 10.1186/1475-2867-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rofstad EK, Galappathi K, Mathiesen BS. Tumor interstitial fluid pressure-a link between tumor hypoxia, microvascular density, and lymph node metastasis. Neoplasia. 2014;16(7):586–94. doi: 10.1016/j.neo.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang M-F, Siedlik MJ, Han S, Stallings-Mann M, Radisky DC, Nelson CM. Tissue stiffness and hypoxia modulate the integrin-linked kinase ILK to control breast cancer stem-like cells. Cancer Research. 2016 doi: 10.1158/0008-5472.CAN-16-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Kapoor A, Desai S, Inamdar MM, Sen S. Proteolytic and non-proteolytic regulation of collective cell invasion: tuning by ECM density and organization. Scientific reports. 2016;6:19905. doi: 10.1038/srep19905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelley LC, Lohmer LL, Hagedorn EJ, Sherwood DR. Traversing the basement membrane in vivo: A diversity of strategies. The Journal of cell biology. 2014;204(3):291–302. doi: 10.1083/jcb.201311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. Journal of cell science. 2012;125(Pt 23):5591–6. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 42.Lloyd MC, Rejniak KA, Brown JS, Gatenby RA, Minor ES, Bui MM. Pathology to enhance precision medicine in oncology: lessons from landscape ecology. Advances in anatomic pathology. 2015;22(4):267–72. doi: 10.1097/PAP.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heindl A, Nawaz S, Yuan Y. Mapping spatial heterogeneity in the tumor microenvironment: a new era for digital pathology. Laboratory investigation; a journal of technical methods and pathology. 2015;95(4):377–84. doi: 10.1038/labinvest.2014.155. [DOI] [PubMed] [Google Scholar]
- 44.Zellmer VR, Zhang S. Evolving concepts of tumor heterogeneity. Cell & bioscience. 2014;4:69. doi: 10.1186/2045-3701-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panteli JT, Forbes NS. Engineered bacteria detect spatial profiles in glucose concentration within solid tumor cell masses. Biotechnology and bioengineering. 2016 doi: 10.1002/bit.26006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis DM, Park KM, Tang V, Xu Y, Pak K, Eisinger-Mathason TSK, et al. Intratumoral oxygen gradients mediate sarcoma cell invasion. Proceedings of the National Academy of Sciences. 2016;113(33):9292–7. doi: 10.1073/pnas.1605317113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalonde E, Ishkanian AS, Sykes J, Fraser M, Ross-Adams H, Erho N, et al. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5-year biochemical recurrence of prostate cancer: a retrospective cohort study. The Lancet Oncology. 2014;15(13):1521–32. doi: 10.1016/S1470-2045(14)71021-6. [DOI] [PubMed] [Google Scholar]
- 48.Suburu J, Chen YQ. Lipids and Prostate Cancer. Prostaglandins & other lipid mediators. 2012;98(0):1–10. doi: 10.1016/j.prostaglandins.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Y, Selvaraj S, Varma A, Derry S, Sahmoun AE, Singh BB. Increase in Serum Ca(2+/)Mg(2+) Ratio Promotes Proliferation of Prostate Cancer Cells by Activating TRPM7 Channels. The Journal of Biological Chemistry. 2013;288(1):255–63. doi: 10.1074/jbc.M112.393918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balsat C, Blacher S, Signolle N, Beliard A, Munaut C, Goffin F, et al. Whole slide quantification of stromal lymphatic vessel distribution and peritumoral lymphatic vessel density in early invasive cervical cancer: a method description. ISRN obstetrics and gynecology. 2011;2011:354861. doi: 10.5402/2011/354861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotler BP, Dickman CR, Brown JS. The effects of water on patch use by two Simpson Desert granivores (Corvus coronoides and Pseudomys hermannsburgensis) Australian Journal of Ecology. 1998;23:574–8. [Google Scholar]
- 52.Brown JS, Kotler BP. Hazardous duty pay and the foraging cost of predation. Ecology Letters. 2004;7:999–1014. [Google Scholar]
- 53.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan Y. Modelling the spatial heterogeneity and molecular correlates of lymphocytic infiltration in triple-negative breast cancer. Journal of the Royal Society, Interface / the Royal Society. 2015;12(103) doi: 10.1098/rsif.2014.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. Journal of molecular medicine. 2003;81(5):281–7. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 57.Dolle L, El Yazidi-Belkoura I, Adriaenssens E, Nurcombe V, Hondermarck H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene. 2003;22(36):5592–601. doi: 10.1038/sj.onc.1206805. [DOI] [PubMed] [Google Scholar]
- 58.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–7. [PubMed] [Google Scholar]
- 59.Coffin AW. From roadkill to road ecology: A review of the ecological effects of roads. Journal of Transport Geography. 2007;15(5):396–406. http://dx.doi.org/10.1016/j.jtrangeo.2006.11.006. [Google Scholar]
- 60.Legrand D, Guillaume O, Baguette M, Cote J, Trochet A, Calvez O, et al. The Metatron: an experimental system to study dispersal and metaecosystems for terrestrial organisms. Nat Methods. 2012;9(8):828–33. doi: 10.1038/nmeth.2104. [DOI] [PubMed] [Google Scholar]
- 61.Boulangeat I, Gravel D, Thuiller W. Accounting for dispersal and biotic interactions to disentangle the drivers of species distributions and their abundances. Ecology Letters. 2012;15(6):584–93. doi: 10.1111/j.1461-0248.2012.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1(3):219–27. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 63.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature reviews Cancer. 2002;2(3):161–74. doi: 10.1038/nrc745. http://www.nature.com/nrc/journal/v2/n3/suppinfo/nrc745_S1.html. [DOI] [PubMed] [Google Scholar]
- 64.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nature reviews Cancer. 2011;11(7):512–22. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lipowsky HH. Shear Stress in the Circulation. In: Bevan JA, Kaley G, Rubanyi GM, editors. Flow-Dependent Regulation of Vascular Function. New York, NY: Springer New York; 1995. pp. 28–45. [Google Scholar]
- 66.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 67.Ehrlich PR. Attributes of invaders and the invading processes: vertebrates. Biological invasions: a global perspective. 1989:315–28. [Google Scholar]
- 68.Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas. 2007;35(3):218–23. doi: 10.1097/mpa.0b013e3180619677. [DOI] [PubMed] [Google Scholar]
- 69.Sleeman JP, Cady B, Pantel K. The connectivity of lymphogenous and hematogenous tumor cell dissemination: biological insights and clinical implications. Clin Exp Metastasis. 2012;29(7):737–46. doi: 10.1007/s10585-012-9489-x. [DOI] [PubMed] [Google Scholar]
- 70.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. The Journal of cell biology. 2011;192(3):373–82. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilkinson DM. eLS. Chichester: John Wiley & Sons Ltd; 2011. Dispersal: Biogeography. [Google Scholar]
- 72.Bond M, Tejedor MF, Campbell KE, Jr, Chornogubsky L, Novo N, Goin F. Eocene primates of South America and the African origins of New World monkeys. Nature. 2015;520(7548):538–41. doi: 10.1038/nature14120. http://www.nature.com/nature/journal/v520/n7548/abs/nature14120.html#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 73.Moreno JG, Miller MC, Gross S, Allard WJ, Gomella LG, Terstappen LW. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology. 2005;65(4):713–8. doi: 10.1016/j.urology.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Papaioannou TG, Stefanadis C. Vascular Wall Shear Stress: Basic Principles and Methods. Hellenic Journal of Cardiology. 2005;46(1):9–15. [PubMed] [Google Scholar]
- 75.Marieb ENH, Katja . Human anatomy & physiology. 9. Pearson Education; 2013. The Cardiovascular System:Blood Vessels; p. 712. [Google Scholar]
- 76.van der Toom EE, Verdone JE, Gorin MA, Pienta KJ. Technical challenges in the isolation and analysis of circulating tumor cells. Oncotarget. 2016 doi: 10.18632/oncotarget.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]