Abstract
Advanced imaging techniques including CT angiography, CT perfusion, MR angiography, MR with diffusion- and perfusion-weighted imaging, and, more recently, resting-state BOLD (Blood Oxygen Level Dependent) functional MRI (rs-fMRI) are increasingly used to evaluate patients with acute ischemic stroke. Advanced imaging allows for identification of patients with ischemic stroke and determination of the size of infarcted and potentially salvageable tissue, all of which yield crucial information for proper stroke management. The addition of rs-fMRI for ischemia adds information at the microvascular level, thereby improving the understanding of pathophysiologic mechanisms of impaired cerebral perfusion and tissue oxygenation beyond the known concepts at the macrovascular level. As such, it may further delineate functional and dysfunctional neuronal networks, guide stroke interventions, and improve prognosis and monitoring of patient outcomes.
Keywords: resting-state fMRI, BOLD, perfusion, ischemia, stroke, connectivity
INTRODUCTION
Stroke is one of the leading causes of mortality and disability in the United States, and it accounts for 130,000 deaths annually, which will be augmented by shifting demographics in the U.S population [1]. The impact of stroke on the U.S. health care system is immense and is estimated to cost about $34 billion per year [2].
Recent randomized clinical trials have shown a strong benefit for endovascular mechanical thrombectomy of acute ischemic stroke caused by large vessel occlusion (LVO) [3–8]. The SWIFT PRIME and EXTEND-IA trials demonstrated that endovascular treatment guided by vascular and perfusion-weighted imaging leads to a three-fold increase in functional independence. These trials underline the crucial role of advanced stroke imaging in the risk stratification and triage of patients to endovascular recanalization, despite the potential negative effect of adding delay to treatment of these patients. With advanced imaging, the time window for endovascular therapy may be extended so that more patients may benefit from early endovascular recanalization [9].
Many neuroimaging protocols exist for acute stroke patients, and these protocols vary between different centers. Advanced modalities used in acute stroke imaging include CT angiography (CTA), CT perfusion (CTP), MRI with diffusion-weighted imaging (DWI), MR angiography (MRA), gadolinium-based MR perfusion (MRP), and other non-contrast measures of cerebral perfusion such as arterial spin labeling (ASL) and resting-state functional MRI (rs-fMRI). Infarcted cerebral tissue (“core” infarction) is best detected on DWI and manifests as restricted diffusion. Blood flow to the brain may be measured with CTP, MRP, or ASL, and these techniques can identify underperfused but viable tissue (“penumbra”). The optimal endovascular stroke candidate has a relatively small core infarct with a large surrounding penumbra caused by an LVO; tissue at risk in these patients may be salvaged if the blocked artery is reopened [10]. Hence, advanced imaging is important because it can reliably identify patients who may significantly benefit from rapid endovascular treatment, specifically those with (1) a small core infarction, (2) tissue at risk for infarction, and (3) a LVO.
Despite the benefits of current advanced imaging techniques, there are specific limitations. For example, CT, CTA, and CTP all use ionizing radiation. CTP also uses cerebral blood flow (CBF) or cerebral blood volume to estimate the core infarction size; as such, the true extent of infarcted tissue may be under- or over- estimated when compared to diffusion-weighted MRI. However, while MRI is most accurate in determining infarct burden, it is not available 24 hours a day at many centers, and it requires robust setup and troubleshooting for optimal diagnostic quality. In addition, both CTP and MRP use intravenous contrast agents (iodinated-based contrast for CT and gadolinium-based contrast for MRI), which may be contraindicated in patients with renal impairment or contrast allergy. Of note, the recent demonstration of gadolinium deposition in the brain raises concern for the long-term safety of MRI contrast agents [11–15] although the clinical significance of gadolinium deposition remains to be elucidated. Furthermore, all current perfusion techniques use indirect measures such as CBF to estimate neuronal viability rather than direct measures such as oxygen consumption or neuronal metabolism. Additional neuroimaging techniques are, therefore, needed to assess cerebral tissue viability and impaired blood flow.
While infarct size on MRI predicts patient outcome in some series [9], the variable functional recovery of patients after ischemic stroke suggest that other factors aside from the location, time, and size of injury may contribute to patient recovery. A study by Hakimelahi et al. found poor correlation between infarct volume and time after stroke onset, suggesting that there are factors more powerful than time in determining infarct size [16, 17]. Recently, more advanced imaging techniques, which may aid in the understanding of stroke pathology and guide therapeutic interventions as well as disease and therapy monitoring, have become feasible. In this article, we will review one of these advanced imaging techniques, resting-state fMRI, which is based on blood oxygen level dependent (BOLD) contrast, and highlight both the technical concepts and current and potential clinical applications for this technique, specifically in the arena of perfusion and ischemia.
THE BOLD PRINCIPLE
Neurons have poor metabolic reserve, and their metabolism depends on blood oxygen for aerobic glycolysis [18]. During neuronal activity, an increase in oxygen consumption and local blood flow can be observed. This mechanism is termed “neurovascular coupling.” The increase in blood flow exceeds the increase in oxygen consumption, leading to a relative increase in oxyhemoglobin concentration relative to deoxyhemoglobin concentration in regions of higher neuronal activity. The relatively decreased deoxyhemoglobin concentration in regions of high neuronal activity can be detected by MRI as a transient increase in T2*-weighted signal [19], because deoxyhemoglobin is paramagnetic. This principle is called the BOLD principle. BOLD imaging is feasible with clinical 1.5 or 3 Tesla MR scanners. Requirements for BOLD sequences are high temporal resolution and sensitivity for T2* effects. Therefore, ultrafast echo-planar imaging (EPI) sequences are frequently applied. To accommodate for high temporal resolution, EPI sequences are usually obtained with small matrices, yielding a lower spatial resolution. The acquired BOLD contrast is relatively poor, as it accounts for only a low percentage of the signal variation. Parallel imaging techniques can be used to increase temporal resolution and reduce the artifacts in echo planar sequences by reducing the readout time. Limitations of BOLD technique are the distance between activated neurons and vascular variation in the oxyhemoglobin to deoxyhemoglobin ratio, which can cause imprecisions when locating the true zone of activation. Other limitations are motion artifacts caused by head motion, vessel pulsation, and breathing as well as magnetic susceptibility (e,g, signal loss at brain-bone interfaces and interfaces with intracranial hemorrhage, signal changes in post-operative patients).
CURRENT APPLICATIONS FOR BOLD MRI
Oxygenation Mapping
One application for BOLD MRI in stroke imaging is referred to as oxygen mapping. The concept is based on the theory that it is possible to estimate cerebral oxygen extraction fraction (OEF) with BOLD MRI. OEF is a critical marker for tissue viability and is, therefore, of particular interest in stroke imaging. In normal subjects, OEF is about 40%, but this can increase if CBF decreases in order to maintain oxygen supply to brain tissue. It is thought to be elevated in the penumbra, due to its decreased CBF [20]. The OEF is related to the deoxyhemoglobin concentration in venules and capillaries of the brain [21]. OEF can therefore be assessed with BOLD MRI based on the T2* signal changes resulting from change in the deoxyhemoglobin concentration. Different approaches of BOLD MRI have been used to extract data related to cerebral oxygenation; these include analysis of T2 data [22, 23], T2* data without [24, 25] and with oxygen breathing challenge [26, 27], and T2’ data [28, 29].
T2 imaging is relatively insensitive for changes in signal dropout related to the deoxyhemoglobin concentration and is markedly affected by vasogenic edema, which commonly occurs in evolving ischemic stroke. T2* imaging has better sensitivity for deoxyhemoglobin detection but is also affected by vasogenic edema. Studies using T2* with oxygen breathing challenge demonstrated promising results [26, 27], but were limited because they did not account for potential changes in cerebral blood volume in ischemic stroke, which will affect correct oxygen map calculations. T2’ imaging eliminates spin-spin interactions and thus helps to reduce measurement errors from T2 signal prolongation such as vasogenic edema, but it is limited by image noise and processing artifacts as shown in two studies [28, 29].
An additional method based on BOLD imaging is to measure the cerebral metabolic rate of oxygen consumption (CMRO2). With this technique, a multi-echo sequence is performed, which allows for the derivation of OEF, based on R2’ and the venous blood volume fraction. After multiplying OEF with CBF from a separate MR perfusion sequence, it is possible to calculate CMRO2. The concept has shown to be feasible in both animal and clinical studies [30, 31], but clinical application is limited due to poor signal-to-noise ratios on applied MR sequences and due to the inaccuracies introduced by quantitative measurements from dynamic susceptibility contrast perfusion MRI.
Oxygenation mapping techniques yield promising results for penumbra imaging in experimental studies but have significant limitations, which prevent routine clinical use at this time. As mentioned before, these include T2 signal prolongation in edematous parenchyma, which may mask OEF-related changes, local changes in the cerebral blood volume occurring in ischemic stroke affecting the interpretation of the BOLD signal [32], and a low hematocrit in hypoperfused brain regions leading to overestimation of OEF [33].
Task-based fMRI
Currently, BOLD MRI techniques are often used for fMRI applications. The most common application of fMRI is so-called “task-based” fMRI, which involves using different tasks (“paradigms”) presented to the patient during imaging acquisition to extract neuronal activity in respective speech, language, or motor areas. The temporal coherence of the BOLD signal is then compared to the timing of the task to extract the location of voxels that are active during the performance of the task. fMRI has been used extensively to study motor, sensory, and language activation and is clinically used as a complementary imaging technique in neuropsychiatric and neurodegenerative diseases such as schizophrenia [34], attention deficit and hyperactivity syndrome [35], and depression [36]. It may also be used to determine hemispheric language dominance in the pre-operative setting (e.g., before temporal lobectomy or hemispherectomy in patients with medically refractory epilepsy) or for surgical planning in the setting of brain masses [37]. This non-invasive method to determine language dominance is often preferred to more invasive methods such WADA testing.
Resting-state BOLD MRI
In contrast to task-based fMRI, resting-state fMRI is acquired during a physiologic “resting state.” During resting state fMRI, the patient does not perform a task during imaging and is usually asked to keep their eyes open and think of nothing in particular. The goal is to simulate a functional state without specific or targeted neuronal activity. Such a paradigm is advantageous in stroke patients as there is no particular demand for patient compliance. Resting-state fMRI uses the same physical concept of the BOLD principle as task-based fMRI.
Resting-state fMRI can be used as a measure of temporal coherence between brain regions. In the absence of a task, very small, low frequency (<0.1 Hz) amplitude modulations of the T2*-weighted signal intensity are observed (Fig. 1). These fluctuations were previously thought to represent noise and were removed on task-based fMRI [38]. However, it was eventually discovered that these fluctuations were not noise, but instead represented regions that show temporal correlation for regions that are anatomically connected [39]. For example, there is overlap of temporal curves from motor-related areas including the supplemental motor area and primary motor cortex, secondary somatosensory area, premotor cortex, putamen, thalamus, and contralateral cerebellum, all of which are interconnected to perform appropriate motor tasks.
FIGURE 1.
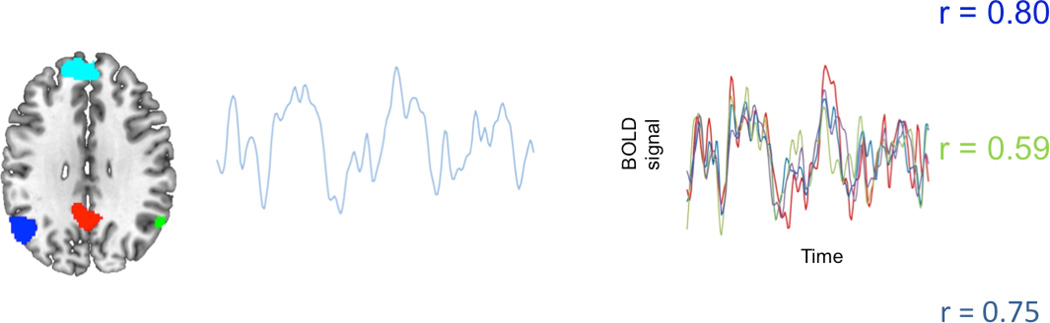
Resting-state fMRI connectivity map of the default network nodes are marked in blue, green, red and teal. The curves on the right show temporal coherence between the four regions during resting state, demonstrating the ability to functionally image connected neuronal networks at rest (Courtesy of Dr. William Shirer).
It is currently hypothesized that even more of these anatomical correlations exist, but the detection of temporal correlation is limited by the relatively slow temporal resolution of fMRI, which leads to loss of high frequency signals. Studies with animal models and human subjects have proven a link between rs-fMRI connectivity and anatomical connections [39–42]. Support for clinical value of this data is the observation that distributed spatiotemporal network organization is highly reproducible across subjects [43].
Connectivity studies with rs-fMRI in stroke patients have identified disruptions in the functional neuronal architecture, both in animal and human models [44, 45]. In a human study, it was shown that impaired sensorimotor function correlated with a loss of interhemispheric connectivity between sensorimotor regions, whereas recovery of function weeks after an ischemic insult correlated with normalization of interhemispheric connectivity [44].
Motor recovery imaging studies with rs-fMRI have been performed to study the effect of stroke on disrupted cerebral neuronal networks. In one study [46], investigators were able to explain why some stroke patients have better recovery after specific brain injury to the same region than others by analyzing the affected neuronal networks through rs-fMRI. They found that in stroke patients, changes in neuronal activity are closely associated with functional recovery. Increases in the rs-fMRI motor activity of the supplementary motor cortex, lateral premotor cortex, and superior parietal cortex in the first 14 days after infarct correlated with greater improvement of hand motor function during this period.
These findings of resting state neuronal activity question the traditional concepts of stroke pathology. The traditional theories assume a direct connection between a particular spatial lesion in the brain and an associated neurological deficit. For example, a patient who suffers ischemic injury to the inferior frontal lobe in the dominant hemisphere (e.g., Broca’s area) may lead to aphasia. These concepts are based on autopsy studies of ischemic brains and respective correlation with patients’ symptoms [47].
Resting-state BOLD MRI, however, is able to structurally and functionally test a new pathophysiological concept; the hypothesis is that in addition to injury to particular loci, injury to the connecting white matter tracts also play a crucial role in the symptom–location correlation equation [48]. One example for this theory would be that the injury of the white matter tracts connecting the speech centers (e.g., medial longitudinal fasciculus) may lead to more pronounced, global aphasia than isolated injury to the speech centers themselves. There is evidence to believe that disconnectivity disorders may not be a complicating factor of ischemic injury but a major contributor to associated symptom complexes [49].
Resting-state BOLD Imaging for Perfusion
BOLD imaging is used to derive neuronal activity in functional MRI. As detailed in the previous paragraph, rs-fMRI assesses synchronous neuronal activity that occurs in the absence of dedicated paradigms [50, 51]. In addition to using temporal coherence to identify different neuronal networks, rs-fMRI can be used to extract data that allow derivation of temporal dynamics of low frequency fluctuations, which can indicate the effects of perfusion changes in the brain [52, 53]. One example is that a higher magnitude of BOLD fluctuations, which can be quantified as the normalized standard deviation of the low frequency fluctuations, is proportional to cerebral blood volume and venous oxygenation [54]. An easy way to understand this is that if a region of the brain has no blood in it, the BOLD fluctuations will disappear. Another surprising discovery is that by time-shifting temporal fluctuations in each voxel and comparing the correlation of the signal with a reference region (either whole brain or the superior sagittal sinus), it is possible to map arterial arrival delays that have traditionally been measured using time-based metrics such as the time-to-peak of the residue function (Tmax) or mean transit time (Fig. 1) [54, 55]. In a sense, every fluctuation in blood flow (through every cardiac or breathing cycle) represents a bolus of oxygenated blood that can be measured with BOLD techniques. These measured fluctuations contain information about cerebral perfusion, oxygenation, and vascular reactivity [54, 56].
Fluctuations in the resting-state BOLD signal were used by Lv et al. to demonstrate a correlation between brain tissue exhibiting significant delay in BOLD signal and areas of hypoperfusion identified by dynamic susceptibility contrast-based perfusion MRI in patients with acute ischemic stroke [57]. In this study the maximum of correlation between the global signal averaged over the whole brain and the signal from tissues was delayed up to nine seconds in regions with concurrent prolonged mean transit time (MTT). Another similar study compared the perfusion characteristics derived from rs-fMRI to contrast bolus perfusion MRI data and showed excellent agreement between the modalities [58]. Christen et al. applied similar concepts to map arterial delays in patients with moyamoya disease, a chronic cerebrovascular disease characterized by proximal arterial stenoses and collateral formation. Imaging times of around 5 minutes or even less make rs-fMRI perfusion applicable for acute stroke cases [54, 57]. We present two clinical cases of patients presenting with acute ischemic infarcts, who underwent advanced imaging with rs-fMRI. In both cases, the rs-fMRI perfusion maps correlated well with the contrast DSC bolus perfusion imaging, demonstrating the feasibility of non-contrast based perfusion with rs-fMRI in the clinical setting. In case 1 (Fig. 2), rs-fMRI correctly identified a completed late acute to early subacute ischemic infarction with no significant penumbra. Based on the imaging findings, the patient was treated with conservative medical therapy rather than with endovascular intervention. Case 2 is an example of treatment monitoring. In this case, successful intravenous tPA treatment for a LVO with right hemispheric penumbra was performed, and subsequent MRI showed complete recanalization of the occluded vessel and reperfusion of tissue at risk (Fig. 3).
FIGURE 2.
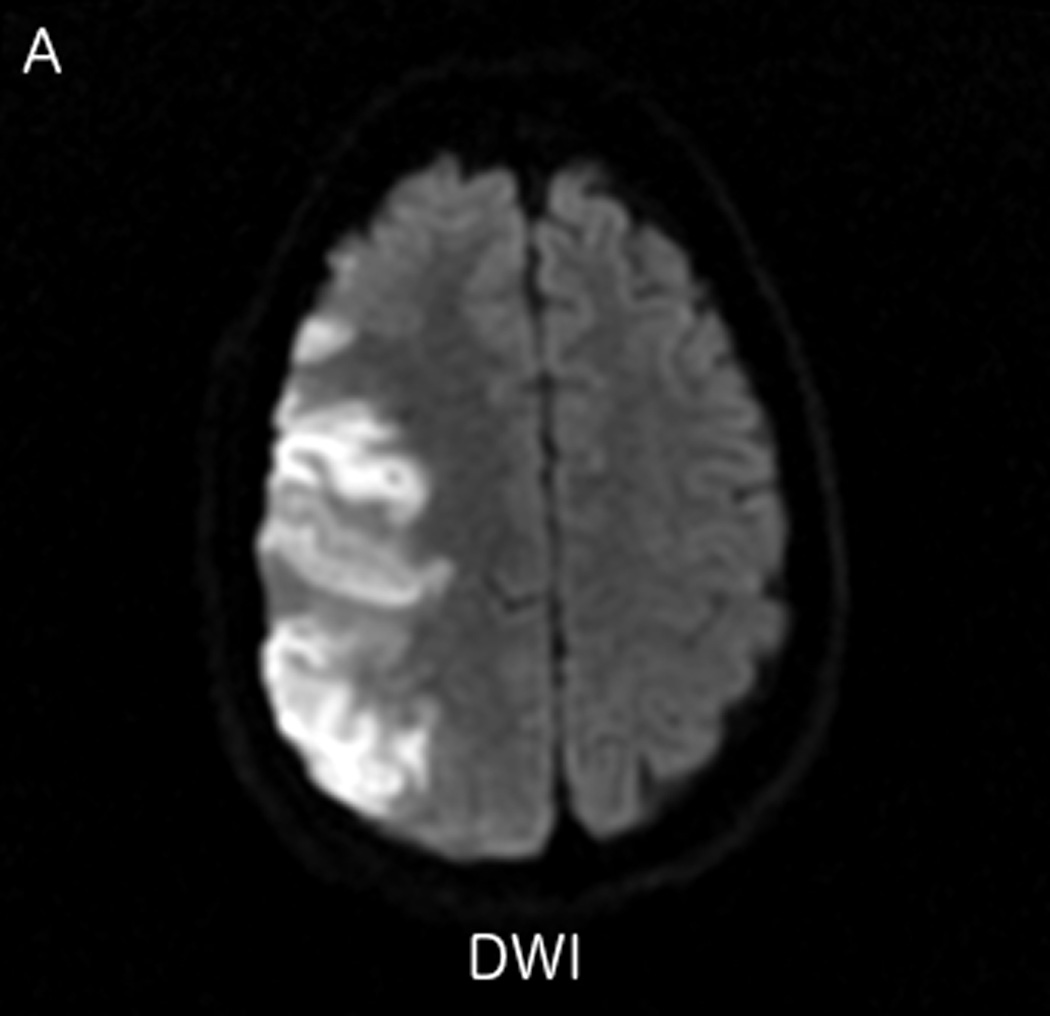
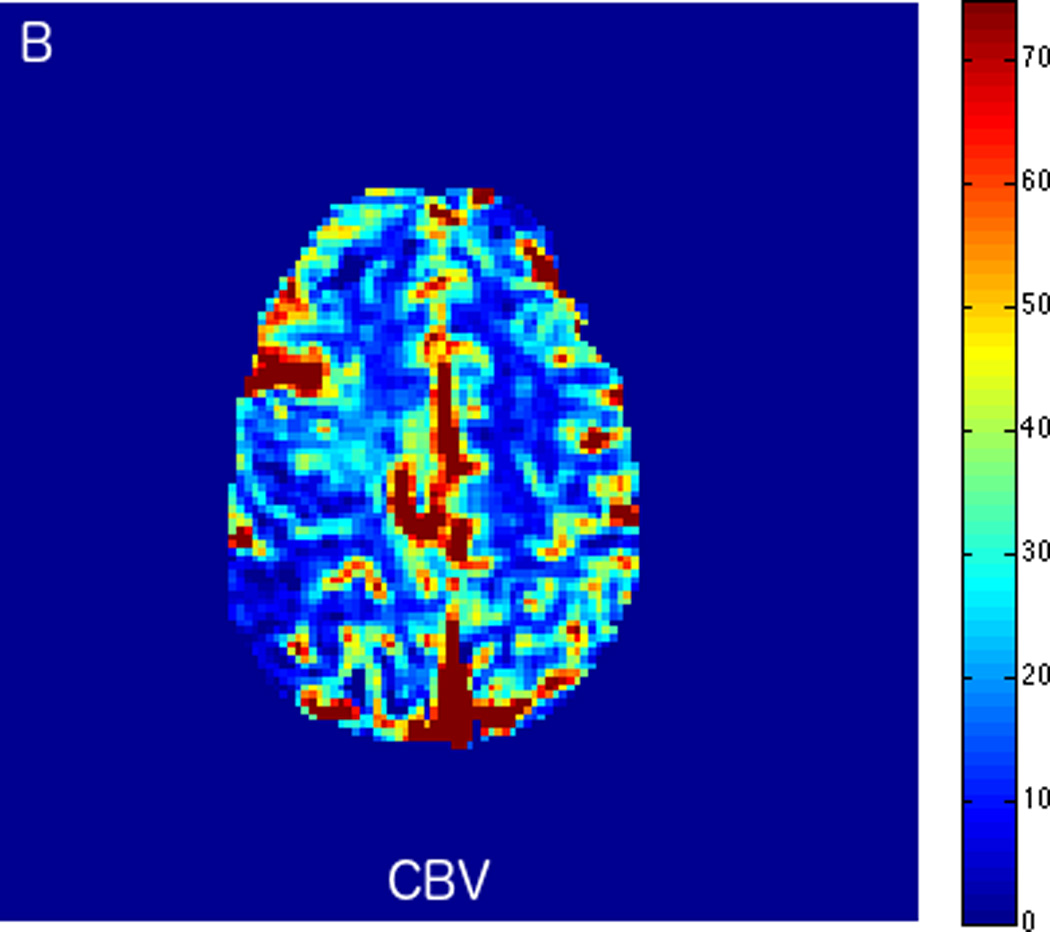
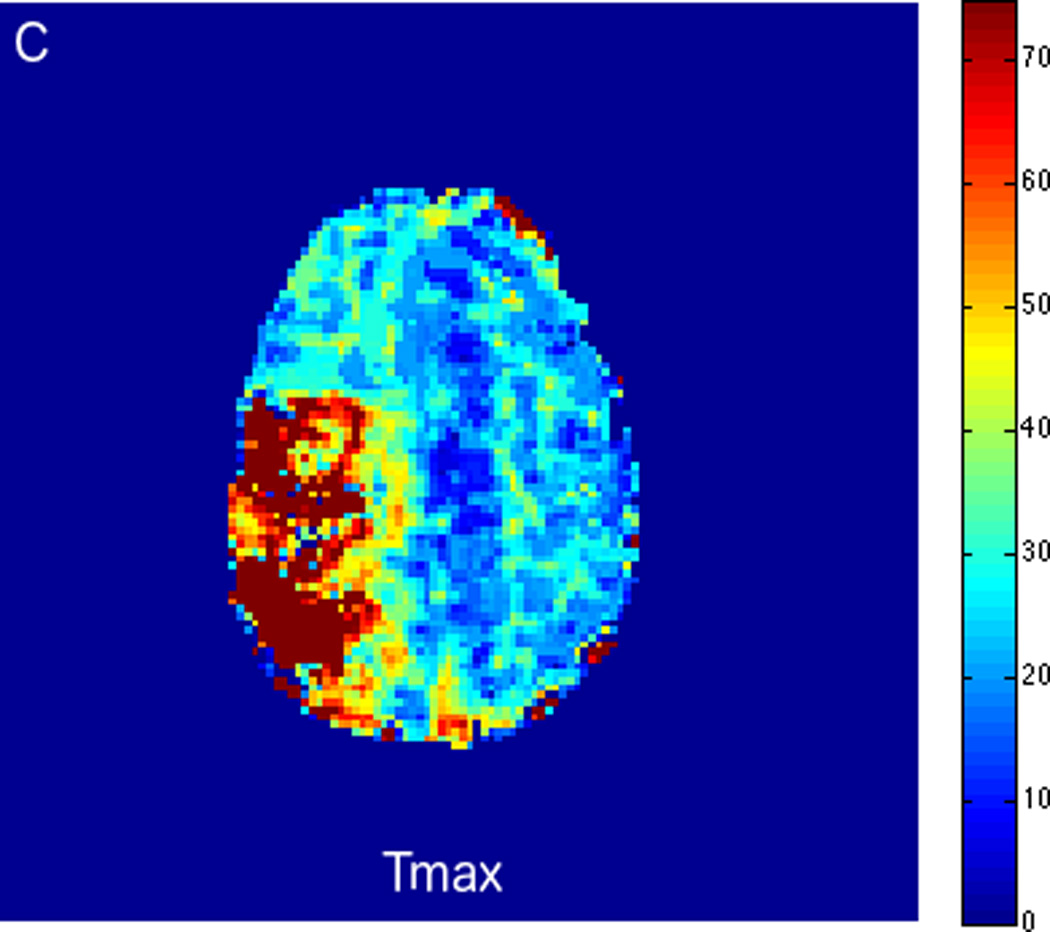
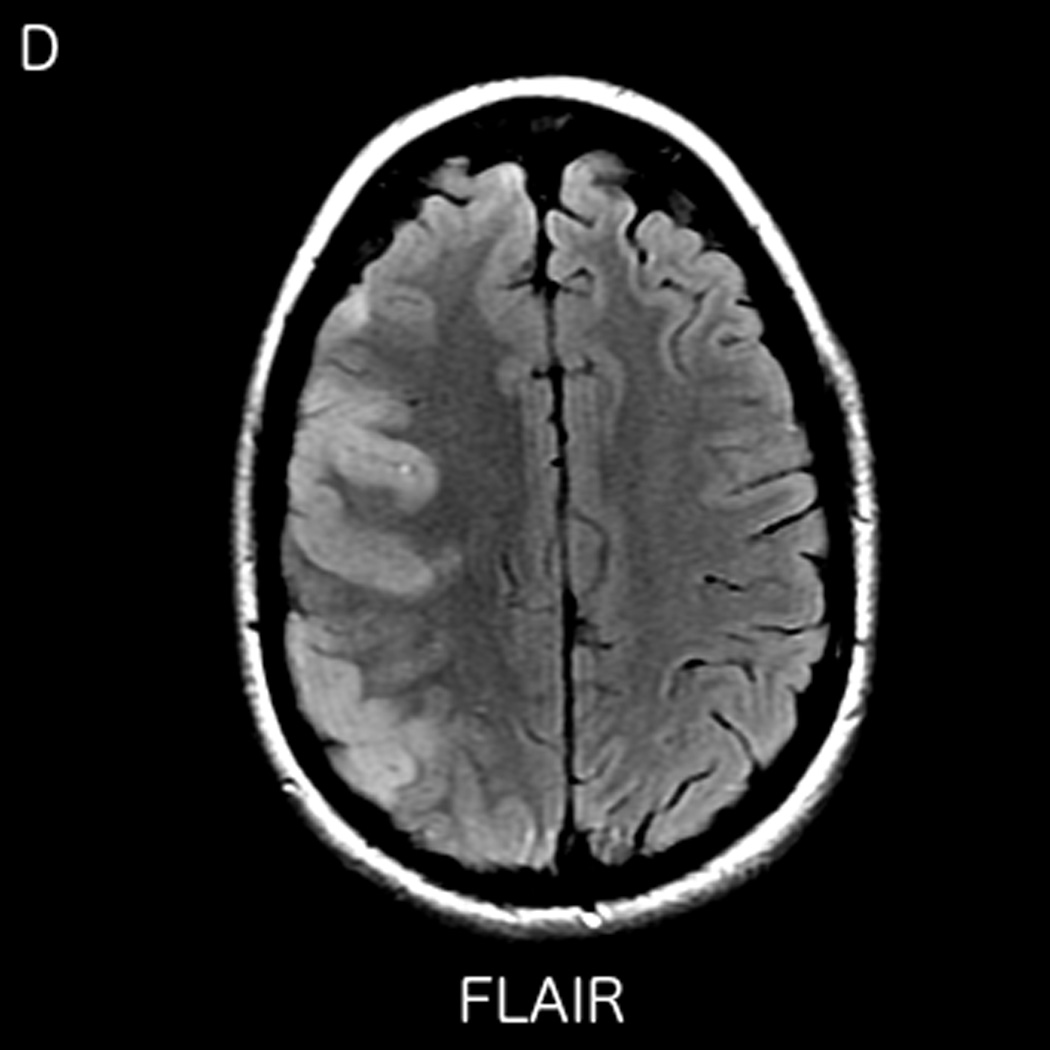
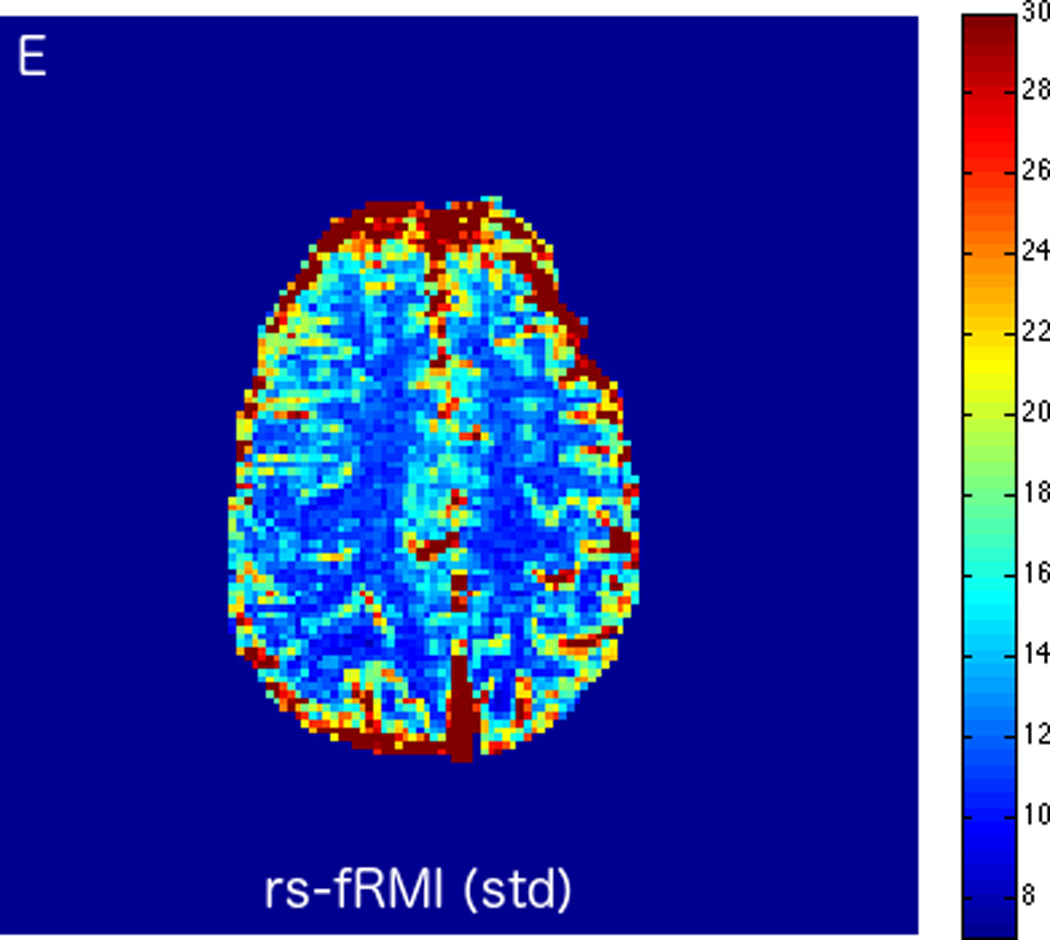
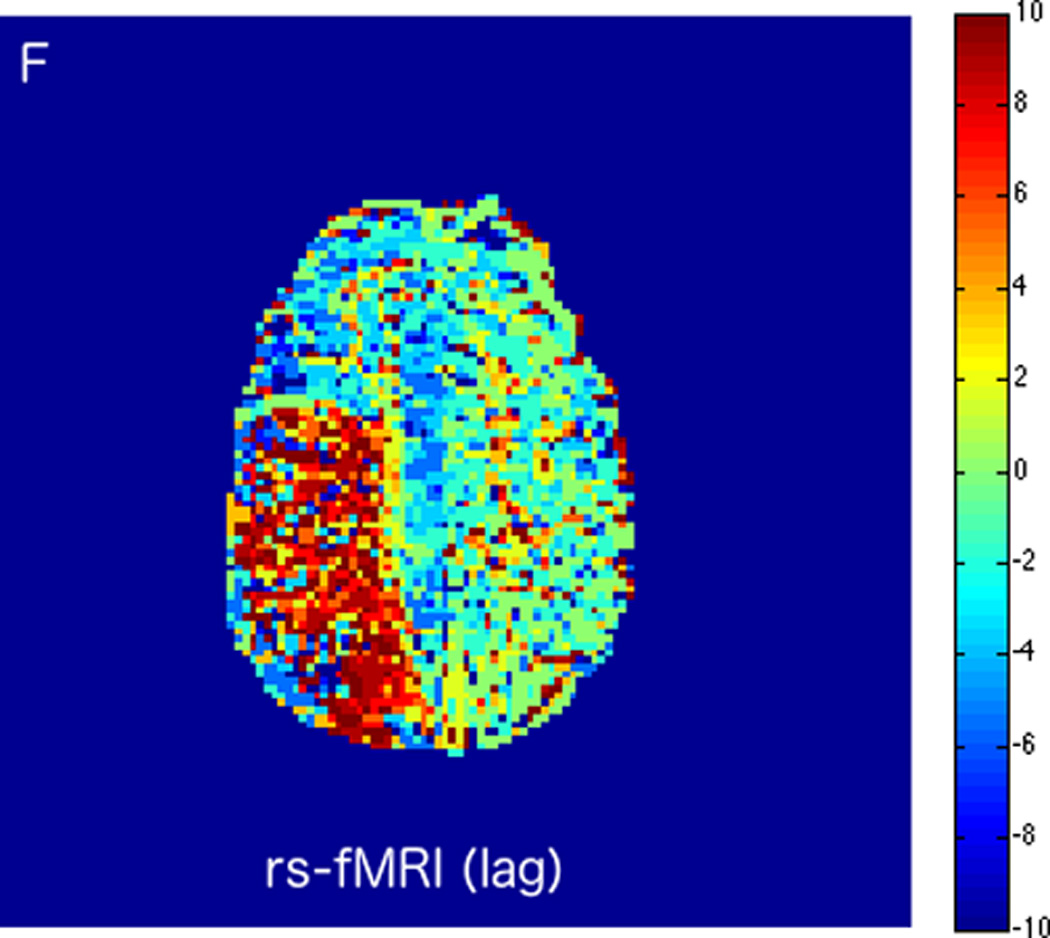
MRI including diffusion, perfusion with dynamic susceptibility contrast (DSC), and resting-state BOLD fMRI in a 37-year-old female patient who presented with acute right-sided headache as well as left-sided facial droop and weakness (NIH stroke scale 15). DWI (A) and FLAIR (D) MR images show abnormal signal in the right middle cerebral artery (MCA) territory, compatible with a late acute to early subacute infarction. DSC perfusion images demonstrate mildly decreased cerebral blood volume (CBV) (B) and prolonged Tmax (C) in the right MCA territory. The standard deviation of low frequency fluctuations (E) and delay correlation analysis (F) from resting-state fMRI show excellent correlation with the respective DSC bolus perfusion maps. The perfusion defect is matched with the diffusion abnormality and represents completed infarction without penumbra.
FIGURE 3.

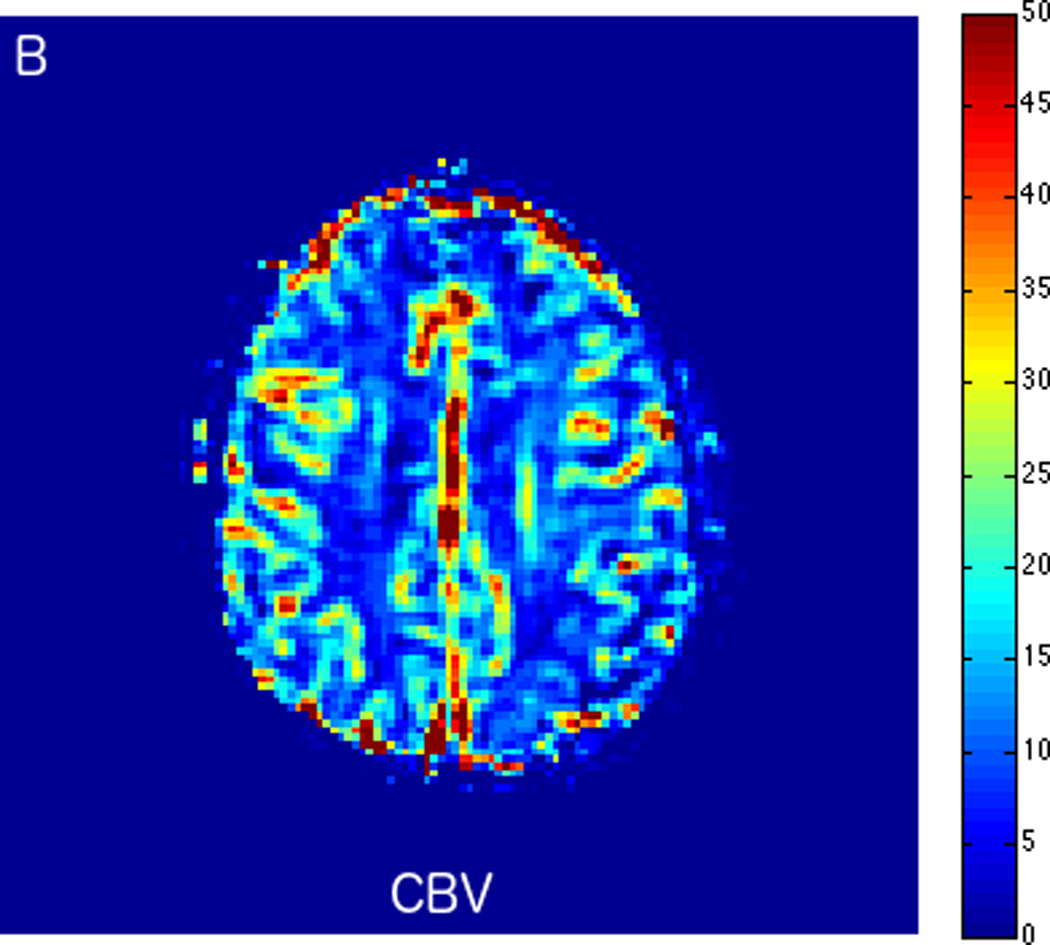
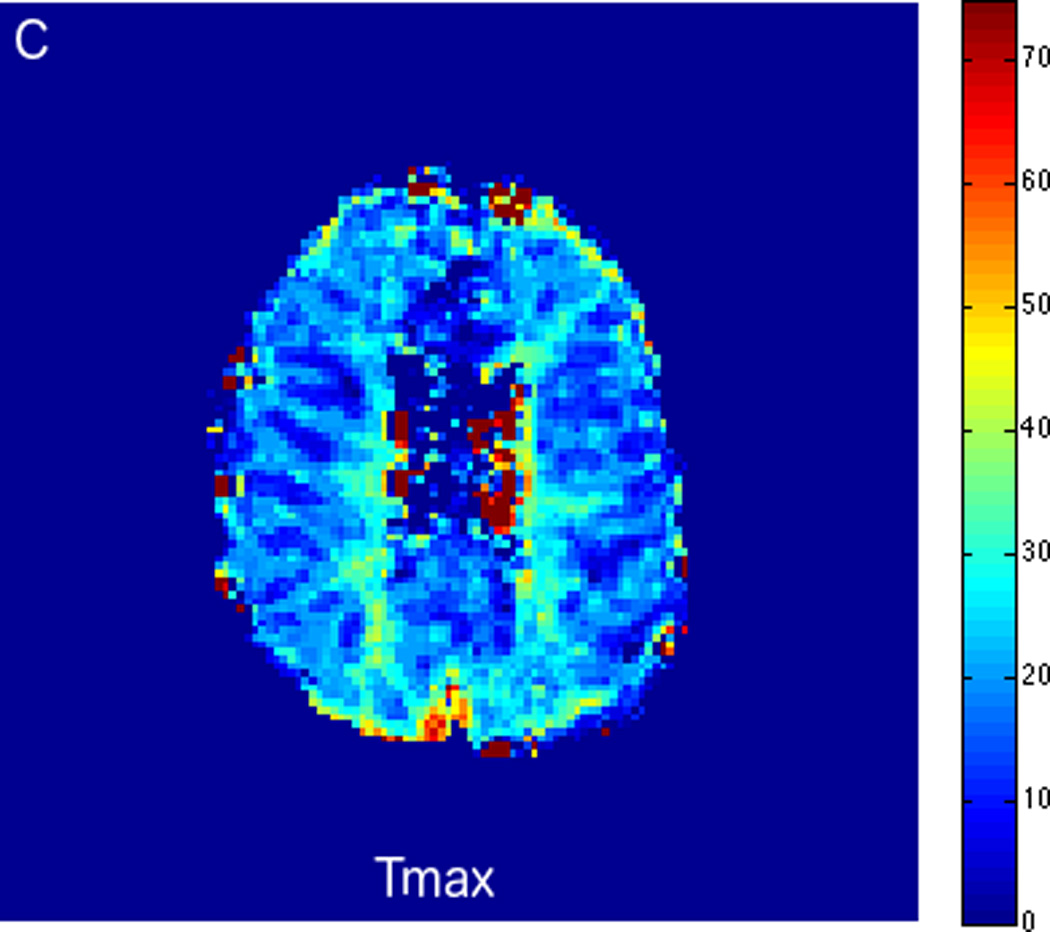
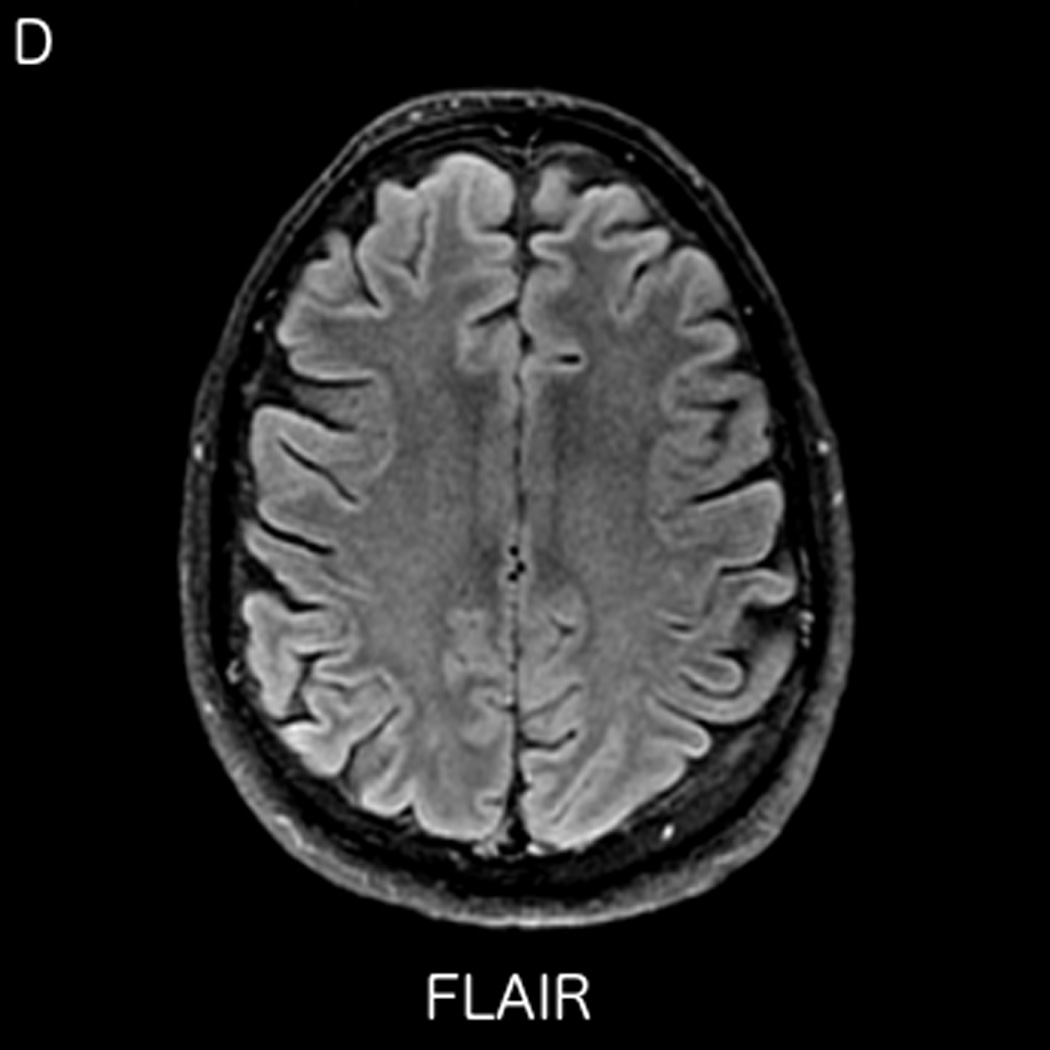
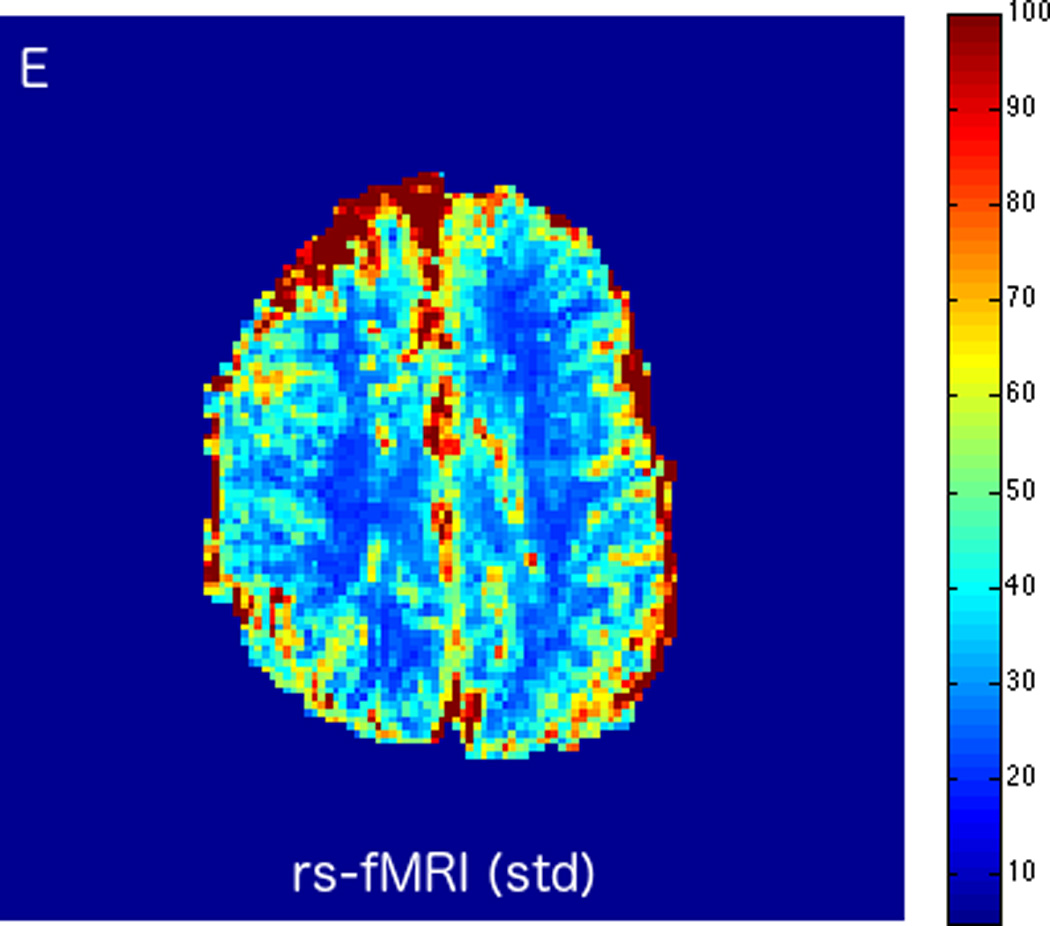
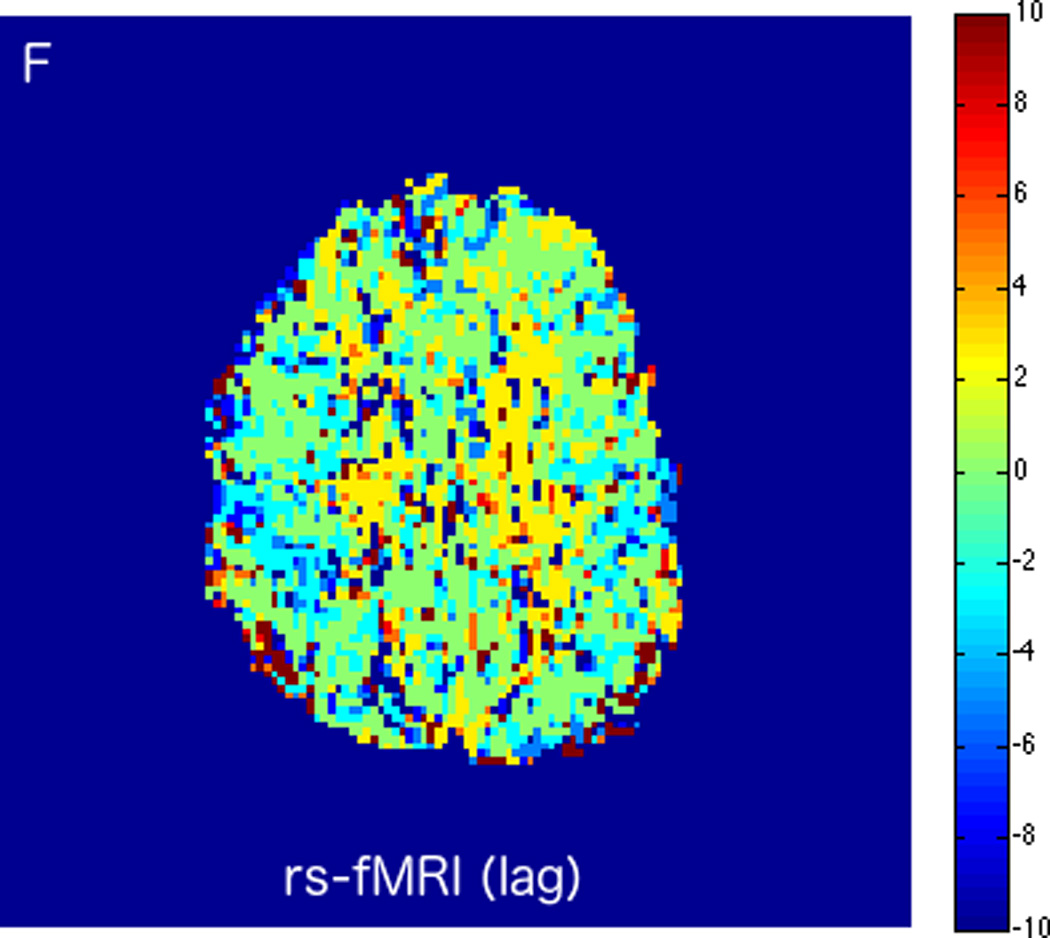
MRI including diffusion, perfusion with dynamic susceptibility contrast (DSC), and resting-state BOLD fMRI in a 66-year-old male patient with history of hypertension, hyperlipidemia, and diabetes, who presented with acute left hand tingling, left mouth numbness, and left visual field cut (NIH stroke scale 3). CT angiography, which was performed prior to the MRI, showed occlusion of the right posterior cerebral artery (not shown), after which intravenous tPA was administered. DWI (A) and FLAIR (D) MR images demonstrate no cortical signal abnormality to suggest the presence of acute infarction. DSC perfusion images demonstrate normal and symmetric cerebral blood volume (CBV) (B) and Tmax (C). The standard deviation of low frequency fluctuations (E) and delay correlation analysis (F) from resting-state fMRI demonstrate excellent correlation with the corresponding DSC perfusion maps. These findings indicate complete recanalization of the occluded vessel without evidence of ischemia. Follow-up MR angiography confirmed resolution of the previously identified right posterior cerebral artery occlusion (not shown).
Resting-state fMRI has multiple advantages. It is feasible to assess brain perfusion without using contrast media, a concept that is helpful in patients with contraindications to contrast administration such a renal impairment or in complicated cases requiring multiple repeated imaging attempts. In addition, rs-fMRI may add information to the microvascular perfusion and oxygenation of brain tissue, whereas traditional contrast bolus perfusion techniques typically assess macrovascular perfusion. Finally, rs-fMRI perfusion data may be combined with connectivity analyses, which may enable the study of neuronal networks and their hemodynamic behavior simultaneously.
To date, there are several limitations of rs-fMRI in stroke imaging. While multiple experimental studies prove the validity of the concept in feasibility studies, there are no current studies assessing robustness in clinical routine. This is important because stroke patients may move more than most clinical patients, and rs-fMRI is known to be sensitive to patient motion. Repeatability of the tests also has to be validated under clinical conditions with larger trials.
CONCLUSIONS
Resting-state fMRI in stroke imaging is a promising advanced imaging technique that can help to further explain the underlying pathophysiology of ischemic stroke through delineation of functional or dysfunctional neuronal networks with connectivity studies. It may also serve as a non-contrast material dependent technique for brain perfusion imaging with the possibility of extracting additional valuable information such as cerebral blood volume and tissue oxygenation. The combination of connectivity and perfusion rs-fMRI data may open new options for ischemic stroke treatment guidance and monitoring and, potentially, help to predict the functional recovery and outcome of stroke patients.
Acknowledgments
The authors acknowledge support from the National Institute of Health/National Institute of Neurological Disorders and Stroke, grant number R01NS066506. In addition, JH is supported by the Radiological Society of North America Research Scholar Award RSCH1610.
Footnotes
Disclosure: All authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
REFERENCES
- 1.Underlying Cause of Death 1999–2015 [Centers for Disease Control and Prevention Website] Data from the Multiple Cause of Death Files, 1999–2013, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. [Accessed December 2, 2016]; Available at: https://wonder.cdc.gov/ucd-icd10.html.
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 6.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 7.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 9.Marks MP, Lansberg MG, Mlynash M, et al. Angiographic outcome of endovascular stroke therapy correlated with MR findings, infarct growth, and clinical outcome in the DEFUSE 2 trial. Int J Stroke. 2014;9(7):860–865. doi: 10.1111/ijs.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heit JJ, Wintermark M. Imaging selection for reperfusion therapy in acute ischemic stroke. Curr Treat Options Neurol. 2015;17(2):332. doi: 10.1007/s11940-014-0332-3. [DOI] [PubMed] [Google Scholar]
- 11.Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834–841. doi: 10.1148/radiol.13131669. [DOI] [PubMed] [Google Scholar]
- 12.Radbruch A, Weberling LD, Kieslich PJ, et al. High-Signal Intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Invest Radiol. 2015;50(12):805–810. doi: 10.1097/RLI.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 13.Ramalho J, Castillo M, AlObaidy M, et al. High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology. 2015;276(3):836–844. doi: 10.1148/radiol.2015150872. [DOI] [PubMed] [Google Scholar]
- 14.Roberts DR, Holden KR. Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev. 2016;38(3):331–336. doi: 10.1016/j.braindev.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Stojanov DA, Aracki-Trenkic A, Vojinovic S, et al. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol. 2016;26(3):807–815. doi: 10.1007/s00330-015-3879-9. [DOI] [PubMed] [Google Scholar]
- 16.Hakimelahi R, Vachha BA, Copen WA, et al. Time and diffusion lesion size in major anterior circulation ischemic strokes. Stroke. 2014;45(10):2936–2941. doi: 10.1161/STROKEAHA.114.005644. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler HM, Mlynash M, Inoue M, et al. The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. Int J Stroke. 2015;10(5):723–729. doi: 10.1111/ijs.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gore JC. Principles and practice of functional MRI of the human brain. J Clin Invest. 2003;112(1):4–9. doi: 10.1172/JCI19010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furlan M, Marchal G, Viader F, et al. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40(2):216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- 21.Baron JC, Bousser MG, Rey A, et al. Reversal of focal "misery-perfusion syndrome" by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke. 1981;12(4):454–459. doi: 10.1161/01.str.12.4.454. [DOI] [PubMed] [Google Scholar]
- 22.Grohn OH, Kettunen MI, Penttonen M, et al. Graded reduction of cerebral blood flow in rat as detected by the nuclear magnetic resonance relaxation time T2: a theoretical and experimental approach. J Cereb Blood Flow Metab. 2000;20(2):316–326. doi: 10.1097/00004647-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Ida M, Mizunuma K, Hata Y, et al. Subcortical low intensity in early cortical ischemia. AJNR Am J Neuroradiol. 1994;15(7):1387–1393. [PMC free article] [PubMed] [Google Scholar]
- 24.Donswijk ML, Jones PS, Guadagno JV, et al. T2*-weighted MRI versus oxygen extraction fraction PET in acute stroke. Cerebrovasc Dis. 2009;28(3):306–313. doi: 10.1159/000229017. [DOI] [PubMed] [Google Scholar]
- 25.Tamura H, Hatazawa J, Toyoshima H, et al. Detection of deoxygenation-related signal change in acute ischemic stroke patients by T2*-weighted magnetic resonance imaging. Stroke. 2002;33(4):967–971. doi: 10.1161/01.str.0000013672.70986.e2. [DOI] [PubMed] [Google Scholar]
- 26.Robertson CA, McCabe C, Gallagher L, et al. Stroke penumbra defined by an MRI-based oxygen challenge technique: 1. Validation using [14C]2-deoxyglucose autoradiography. J Cereb Blood Flow Metab. 2011;31(8):1778–1787. doi: 10.1038/jcbfm.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santosh C, Brennan D, McCabe C, et al. Potential use of oxygen as a metabolic biosensor in combination with T2*-weighted MRI to define the ischemic penumbra. J Cereb Blood Flow Metab. 2008;28(10):1742–1753. doi: 10.1038/jcbfm.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geisler BS, Brandhoff F, Fiehler J, et al. Blood-oxygen-level-dependent MRI allows metabolic description of tissue at risk in acute stroke patients. Stroke. 2006;37(7):1778–1784. doi: 10.1161/01.STR.0000226738.97426.6f. [DOI] [PubMed] [Google Scholar]
- 29.Siemonsen S, Fitting T, Thomalla G, et al. T2' imaging predicts infarct growth beyond the acute diffusion-weighted imaging lesion in acute stroke. Radiology. 2008;248(3):979–986. doi: 10.1148/radiol.2483071602. [DOI] [PubMed] [Google Scholar]
- 30.An H, Liu Q, Chen Y, et al. Evaluation of MR-derived cerebral oxygen metabolic index in experimental hyperoxic hypercapnia, hypoxia, and ischemia. Stroke. 2009;40(6):2165–2172. doi: 10.1161/STROKEAHA.108.540864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JM, Vo KD, An H, et al. Magnetic resonance cerebral metabolic rate of oxygen utilization in hyperacute stroke patients. Ann Neurol. 2003;53(2):227–232. doi: 10.1002/ana.10433. [DOI] [PubMed] [Google Scholar]
- 32.Yablonskiy DA, Haacke EM. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med. 1994;32(6):749–763. doi: 10.1002/mrm.1910320610. [DOI] [PubMed] [Google Scholar]
- 33.Ulatowski JA, Bucci E, Nishikawa T, et al. Cerebral O2 transport with hematocrit reduced by cross-linked hemoglobin transfusion. Am J Physiol. 1996;270(2 Pt 2):H466–H475. doi: 10.1152/ajpheart.1996.270.2.H466. [DOI] [PubMed] [Google Scholar]
- 34.Keshavan MS, Eack SM, Prasad KM, et al. Longitudinal functional brain imaging study in early course schizophrenia before and after cognitive enhancement therapy. Neuroimage. doi: 10.1016/j.neuroimage.2016.11.060. http://dx.doi.org/10.1016/j.neuroimage.2016.11.060 [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Emond V, Joyal C, Poissant H. [Structural and functional neuroanatomy of attention-deficit hyperactivity disorder (ADHD)] Encephale. 2009;35(2):107–114. doi: 10.1016/j.encep.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Fiorelli M, Aceti F, Marini I, et al. Magnetic Resonance Imaging Studies of Postpartum Depression: An Overview. Behav Neurol. 2015;2015:913843. doi: 10.1155/2015/913843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer PR, Reitsma JB, Houweling BM, et al. Can fMRI safely replace the Wada test for preoperative assessment of language lateralisation? A meta-analysis and systematic review. J Neurol Neurosurg Psychiatry. 2014;85(5):581–588. doi: 10.1136/jnnp-2013-305659. [DOI] [PubMed] [Google Scholar]
- 38.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44(1):162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 39.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 40.De Luca M, Smith S, De Stefano N, et al. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp Brain Res. 2005;167(4):587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- 41.Vincent JL, Patel GH, Fox MD, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 42.Fox MD, Corbetta M, Snyder AZ, et al. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter AR, Astafiev SV, Lang CE, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67(3):365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Meer MP, van der Marel K, Wang K, et al. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30(11):3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rehme AK, Fink GR, von Cramon DY, et al. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex. 2011;21(4):756–768. doi: 10.1093/cercor/bhq140. [DOI] [PubMed] [Google Scholar]
- 47.Broca P. Sur le siege de la faculte du langage articule. Bulletin de la Societe d’anthropologie. 1865;6:337–393. [Google Scholar]
- 48.Corbetta M. Functional connectivity and neurological recovery. Dev Psychobiol. 2012;54(3):239–253. doi: 10.1002/dev.20507. [DOI] [PubMed] [Google Scholar]
- 49.Corbetta M, Ramsey L, Callejas A, et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85(5):927–941. doi: 10.1016/j.neuron.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purdon PL, Weisskoff RM. Effect of temporal autocorrelation due to physiological noise and stimulus paradigm on voxel-level false-positive rates in fMRI. Hum Brain Mapp. 1998;6(4):239–249. doi: 10.1002/(SICI)1097-0193(1998)6:4<239::AID-HBM4>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong Y, Frederick BD. Concurrent fNIRS and fMRI processing allows independent visualization of the propagation of pressure waves and bulk blood flow in the cerebral vasculature. Neuroimage. 2012;61(4):1419–1427. doi: 10.1016/j.neuroimage.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tong Y, Frederick BD. Time lag dependent multimodal processing of concurrent fMRI and near-infrared spectroscopy (NIRS) data suggests a global circulatory origin for low-frequency oscillation signals in human brain. Neuroimage. 2010;53(2):553–564. doi: 10.1016/j.neuroimage.2010.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christen T, Jahanian H, Ni WW, et al. Noncontrast mapping of arterial delay and functional connectivity using resting-state functional MRI: a study in Moyamoya patients. J Magn Reson Imaging. 2015;41(2):424–430. doi: 10.1002/jmri.24558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erdogan SB, Tong Y, Hocke LM, et al. Correcting for blood arrival time in global mean regression enhances functional connectivity analysis of resting state fMRI-BOLD signals. Front Hum Neurosci. 2016;10:311. doi: 10.3389/fnhum.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jahanian H, Christen T, Moseley ME, et al. Measuring vascular reactivity with resting-state blood oxygenation level-dependent (BOLD) signal fluctuations: A potential alternative to the breath-holding challenge? J Cereb Blood Flow Metab. 2016 Sep 28; doi: 10.1177/0271678X16670921. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lv Y, Margulies DS, Cameron Craddock R, et al. Identifying the perfusion deficit in acute stroke with resting-state functional magnetic resonance imaging. Ann Neurol. 2013;73(1):136–140. doi: 10.1002/ana.23763. [DOI] [PubMed] [Google Scholar]
- 58.Amemiya S, Kunimatsu A, Saito N, et al. Cerebral hemodynamic impairment: assessment with resting-state functional MR imaging. Radiology. 2014;270(2):548–555. doi: 10.1148/radiol.13130982. [DOI] [PubMed] [Google Scholar]


