Abstract
HIV prevention method preferences were evaluated among 512 U.S. men who have sex with men (MSM; median age: 22 years). Approximately 90% consistently preferred one option across pairwise comparisons of condoms, daily oral pre-exposure prophylaxis (PrEP), and long-acting PrEP delivered via either an injectable or one of two types of PrEP implants differing in visibility. Condoms were most frequently preferred (33.8%), followed by non-visible implants (21.5%), and oral PrEP (17.0%); HIV risk was reported by more choosing implants. In a follow-up question comparing the four PrEP options only, daily oral pills and non-visible implants were most frequently preferred (35.5% and 34.3%, respectively), followed by injections (25.2%) and visible implants (4.3%). An inductive, open-coding approach determined that convenience, duration of protection, and privacy were the most commonly cited reasons for a PrEP method choice, and associated with self-report of HIV risk. Tailoring PrEP product development to privacy and other concerns important to those at highest HIV risk may improve HIV prevention.
Keywords: HIV, Homosexuality, Male, Pre-Exposure Prophylaxis, Prevention, Sexual Behavior
INTRODUCTION
In the U.S., an estimated 67% of all diagnosed HIV infections occurred in men who have sex with men (MSM) in 2014 (1). The number of new infections among adolescent and adult MSM increased 9% between 2010 and 2014 (2). This is the only population subgroup that experienced an increase in number of HIV infections. For populations at high risk of HIV infection such as MSM, the CDC recommends daily oral pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF or Truvada®) and a recent estimate reported that PrEP was indicated for one in four MSM (3, 4). Adherence to daily or almost daily pill-taking is key for optimal PrEP efficacy. For example, the IPrEx study demonstrated fewer infections among MSM with higher adherence (5). Across other trials, higher adherence to daily oral PrEP was associated with higher efficacy (i.e., HIV protection) (6–10), whereas no HIV protection was found in the FEM-PrEP (9) and VOICE (11) trials in which adherence to PrEP appeared to have been very low. Consequently, the CDC’s Clinical Practice Guideline advises medication adherence counseling as part of daily oral FTC/TDF PrEP to prevent HIV; in addition, consistent condom use is also recommended to prevent infections other than HIV (3).
There is a dearth of research on adherence to daily oral PrEP among MSM in real-world settings despite the growing literature on awareness of and willingness to use daily oral PrEP (12–21). As an exception, PrEP adherence among MSM has been reported in the US PrEP Demonstration Project (22). Alternatives to circumvent the need for frequent oral PrEP dosing are starting to be studied (23, 24). Other options include long-acting injectable antiretroviral drugs (25–27) and a subcutaneous implant with controlled, sustained release of tenofovir alafenamide (28). In addition to addressing problems with adherence to daily oral PrEP, there may be alternative reasons why these may appeal to some. Indeed, lessons from the longer history of development of contraceptive methods support that a diversity of options can enhance uptake to suit a variety of user needs and preferences. Few studies have yet addressed how such new potential PrEP delivery options (29) could be acceptable to users as alternatives to daily or frequent oral PrEP (25, 28, 30–32).
The aims of this mixed method study were to: (a) identify preferences for current and future HIV prevention options; and (b) explore reasons for preferences among HIV prevention options including potential new long-acting delivery options. An online sample of MSM completed paired preference tests, followed by open-ended questioning to explore reasons for preferences given the dearth of knowledge about this. Paired preference tests are commonly used in marketing research to determine consumer preferences by presenting consumers with two discriminable products and asking them to report which one they prefer or to indicate if they have no preference (33–35). Five current or potential HIV prevention methods were evaluated in pairwise comparisons: condoms and four PrEP choices - daily oral pill, a long-acting injectable, or two subcutaneous implants that differed in perceptibility (visible to others or able to be felt under the skin by others). The four different PrEP delivery options in this study varied in their administration, duration of protection, and perceptibility - whether or not the method was noticeable to others.
It is important to learn what might make a long-acting PrEP option more acceptable to potential users, particularly for those not now adhering to current recommendations for daily oral PrEP and consistent use of condoms. In this study, a choice between condoms plus current daily oral PrEP versus condoms plus a new long-acting form of PrEP (injectable or implantable delivery system) may lead those who do not use condoms consistently to randomly choose either option because neither may be appealing with condoms included in the decision-making. In addition, prevention of just HIV, arguably the most chronic and socially burdensome of sexually transmitted infections (STIs), could be critically important “harm reduction” even if all other STIs were not prevented. For these reasons, participants were presented with an either-or choice of an HIV prevention method without allowing an option of both condoms and a PrEP method.
METHODS
Study Design
The study consisted of a one-time Internet survey. After consent, interested individuals completed a demographic screener to determine study eligibility. Eligible participants then completed a 15-minute survey. Participation was voluntary and compensation was not provided. Northwestern University’s Institutional Review Board approved study procedures.
Sample
Eligible study candidates were male at birth and at least 18 years of age. The analytic sample only included men who identified their sexual orientation as gay or bisexual. Recruitment primarily occurred through the placement of advertisements on Facebook based on users’ self-reported characteristics (e.g., being “interested in men” or “interested in men and women”). Participants were also recruited through the placement of advertisements on Twitter feeds and through email via a research participant registry. Individuals who clicked through the advertisements or email links were directed to an information screen on the survey platform, which was hosted on a secure server. Those who consented to participate were administered the survey.
Procedures
Participants completed an anonymous online survey on demographics, relationships, sexual health, and PrEP awareness and use. Next, they were provided with information on each of the prevention methods: condoms, oral PrEP, injectable PrEP, and two proposed PrEP implant options. Each description highlighted key attributes relating to administration, duration, and perceptibility. Sources of information for the descriptions were based on manufacturer materials for existing products (e.g., Truvada pill by Gilead) and similar products currently on the market (e.g., Nexplanon implant by Merck), in addition to study team expertise in the fields of infectious disease, sexual and reproductive health, and engineering (supplementary materials are available from the corresponding author upon request). Participants were then presented with a series of 10 randomized, pairwise comparisons (i.e., two prevention methods at a time) across five HIV prevention options and asked to choose their preferred option in each pairwise comparison. The 10 comparisons represented all possible pairwise combinations of the five options (e.g., Pill vs. Condoms, Pill vs. Injection, etc.). Following this, participants were presented with a table describing the four PrEP delivery options in terms of administration, duration, and perceptibility (visible or able to be felt under the skin). They were asked to choose the one they preferred and to provide their reasons for this choice in an open-ended response format.
Measures
Demographic variables
The survey collected age, birth sex, sexual orientation, race and ethnicity, geographic residence, educational attainment, annual income, and presence of a primary health care provider.
Relationships and sexual health
An instrument collected information on relationship status, sexual behaviors (past 6 months), and HIV testing history (most recent test and test result).
PrEP awareness and use
Two items assessed PrEP awareness and use (17): “How familiar are you with PrEP (or Truvada)?” (0 = “I’ve never heard of it before today” to 4 = “I know a lot about it”) and “Have you taken PrEP (or Truvada) in the past three months?” (0 = “No” and 1 = “Yes”).
Prevention options
Participants were provided with information related to administration, duration, and perceptibility for each of the prevention methods (condoms, oral PrEP, injectable PrEP, and two proposed PrEP implant options). The descriptions for administration described how each method would be administered (e.g., minimal surgery with only local pain-numbing medicine in an outpatient doctor’s office for the implant options). For duration, the descriptions noted how long the HIV prevention method would last (e.g., “The injection would protect against HIV infection for 2 months”.) Perceptibility indicated the extent to which others could perceive the method through touch or vision. For the pairwise comparisons, the information about the PrEP options, and not condoms, was summarized in tabular form. To illustrate, administration for oral PrEP was presented as “Pill,” duration was presented as “24 hours and has to be taken every day,” and perceptibility was presented as “Do not feel or see on person.” Similar descriptions were presented for the other options: injectable PrEP (injection; repeated bi-monthly; not perceptible), PrEP Implant option 1 (two implants; 12-month duration; tactile, but no visible perception), and PrEP Implant option 2 (two implants; 12-month duration; tactile and visible perception).
Analytic Approach
Quantitative data
A preference was assigned to one particular prevention method if that one method was consistently selected over each of the other options in all pairwise comparisons that included it. If participants did not consistently choose one option over the other four in all pairwise comparisons, then “Undecided” was assigned. Distributions were compared by whether the respondent had a preferred method of HIV prevention or was Undecided, using Kruskal-Wallis Tests (age, education, and income) and Fisher’s Exact Tests (remaining variables). If there was at least one significant difference in the overall test, pairwise tests were performed, using the Dwass-Steel-Critchlow-Fligner (DSCF) multiple comparison analysis or Fisher’s Exact Test with a Bonferroni correction where appropriate. SAS 9.4 and a significance level of 0.05 were used for statistical analysis.
Qualitative data
Participants were asked to choose a preferred method among the four PrEP delivery options and then to describe in their own words the reasons for their choice. There were 409 participants who responded and thus included in this portion of the analysis. The first author and two Masters’-level research staff used open coding to identify and refine themes through constant comparison techniques (36–38). This resulted in the identification of 10 primary themes: (a) convenience; (b) protection duration; (c) privacy; (d) dislike/fear of other options; (e) pain/discomfort of other options; (f) protection evidence; (g) health risk; (h) effectiveness; (i) avoiding the need for condoms for HIV prevention (if not for preventing other sexually transmitted infections); and (j) cost. In order to identify which of the 10 themes emerged in each written response, the coding team developed a codebook with descriptions and excerpts for each theme. Two research staff members independently rated the open-ended responses. For each response, the coders indicated whether or not each of the 10 themes were applicable to the response (0 = “No” and 1 = “Yes”). As such, each participant’s response could receive between 1 and 10 codes depending on the number of themes the participant included in their response. Cohen’s Kappa was used to evaluate inter-rater reliability for each of the 10 themes in SPSS. The Kappa computed across the two coders was .81, suggesting strong agreement (39). The two coders together resolved the remaining discrepancies with a third coder brought in to make a final decision in situations where opinions could not be reconciled to create a final set of 10 binary variables.
Mixed Method Data
Fisher’s Exact Tests in SPSS 22.0 were used to detect differences in endorsed themes based on race, sexual orientation, relationship status, condom preference, and preferred method. A significance level of 0.05 was used for statistical analysis.
RESULTS
The primary recruitment source was Facebook. In less than 6 days, the Facebook ads generated 1,540 clicks on the study links for a click through rate of 2.76% and a total of 562 individuals completed the survey. In response to the Twitter feed campaign (less than 4 days), 512 individuals clicked on the link, and 6 completed the survey. For the research participant registry, study information was emailed to 195 individuals, 18 clicked on the link, and 6 completed the survey over a period of 3 days.
Demographics
Of the 624 participants who completed the survey, 112 were excluded from analyses due to having an HIV-positive status (n = 28), reporting a sexual orientation other than gay or bisexual (e.g., heterosexual, unsure, other; n = 29), and/or submitting incomplete paired-preference responses (n = 55). This resulted in an analytic sample of 512 (see Table 1). The participants resided in 45 states, in addition to Washington, D.C., and there was even distribution across the U.S. Census regions (Midwest = 28.5%, Northeast = 17.4%, South = 26.4%, West = 24.4%). They were predominantly White (n = 404, 78.9%) and had a median age of 22 years (range = 18 to 71). All participants were male and 88.9% (n = 455) identified their sexual orientation as gay, versus bisexual (n = 57, 11.1%). The majority of participants were single (n = 311, 60.7%) and 38.9% (n = 199) were involved in a romantic relationship (two participants did not answer this question). In terms of educational attainment, 24.2% (n = 124) reported a high school education or less, 48.6% (n = 249) attended trade school or some college, and 27.2% (n = 139) received an undergraduate degree or higher. Participants were distributed across the following annual household income categories: 37.1% (n = 190) reported an income of less than $20,000; 29.3% (n = 150) reported income from $20,000 to $39,000; 13.5% (n = 69) reported income from $40,000 to $59,000; 19.7% (n = 101) reported earning greater than $60,000. (Two participants did not answer this question.) When asked if they had a primary care provider, 69.3% (n = 355) responded affirmatively. Nearly two-thirds of participants (n = 328, 64.1%) reported ever testing for HIV and having an HIV-negative test result; the remaining participants did not respond if they ever had HIV testing (n = 2), responded that they were never tested (n = 181) or responded that they were tested but did not provide a result (n = 1). The majority (n = 293; 57.2%) of participants reported having condomless anal sex in the past 6 month. Of the 237 men who ever heard of PrEP (46.3%), only 6 (2.5%) used PrEP in the last 3 months.
Table 1.
Participant characteristics by paired preference of HIV prevention method (n=512)
| Characteristic | Condom n=173 (33.8%) |
Pill n=87 (17.0 %) |
Injection n=73 (14.3%) |
Implant-1 n=110 (21.5%) |
Implant-2 n=19 (3.7%) |
Undecided n=50 (9.8%) |
Over all p-value |
|---|---|---|---|---|---|---|---|
| Agea | 21 (19–24) | 22 (19–26) | 23 (20–27) | 24 (21–29) | 21 (20–26) | 22 (19–26) | 0.01 |
| Sexual Orientation | 0.73 | ||||||
| Gay | 153 (88.4) | 77 (88.5) | 64 (87.7) | 101 (91.8) | 18 (94.7) | 42 (84.0) | |
| Bisexual | 20 (11.6) | 10 (11.5) | 9 (12.3) | 9 (8.2) | 1 (5.3) | 8 (16.0) | |
| Race | 0.72 | ||||||
| Caucasian | 134 (77.5) | 72 (82.8) | 59 (80.8) | 84 (76.4) | 17 (89.5) | 38 (76.0) | |
| Other | 39 (22.5) | 15 (17.2) | 14 (19.2) | 26 (23.6) | 2 (10.5) | 12 (24.0) | |
| Education | 0.01 | ||||||
| ≤High school | 46 (26.6) | 29 (33.3) | 9 (12.3) | 22 (20.0) | 7 (36.8) | 11 (22.0) | |
| Some collegeb | 88 (50.9) | 40 (46.0) | 37 (50.7) | 56 (50.9) | 6 (31.6) | 22 (44.0) | |
| ≥Bachelor’s | 39 (22.5) | 18 (20.7) | 27 (37.0) | 32 (29.1) | 6 (31.6) | 17 (34.0) | |
| Annual Household Income (in thousands) | 0.10 | ||||||
| < 20 | 70 (40.5) | 38 (43.7) | 19 (26.0) | 36 (32.7) | 3 (15.8) | 24 (48.0) | |
| 20–39 | 45 (26.0) | 26 (29.9) | 23 (31.5) | 38 (34.5) | 10 (52.6) | 8 (16.0) | |
| 40–59 | 21 (12.1) | 12 (13.8) | 8 (11.0) | 18 (16.4) | 3 (15.8) | 7 (14.0) | |
| ≥60 | 36 (20.8) | 11 (12.6) | 22 (30.1) | 18 (16.4) | 3 (15.8) | 11 (22.0) | |
| Missing | 1 (0.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| In a Relationship | 0.11 | ||||||
| Yes | 63 (36.4) | 27 (31.0) | 37 (50.7) | 46 (41.8) | 9 (47.4) | 17 (34.0) | |
| No | 110 (63.6) | 60 (69.0) | 35 (47.9) | 63 (57.3) | 10 (52.6) | 33 (66.0) | |
| Missing | 0 (0.0) | 0 (0.0) | 1 (1.4) | 1 (0.9) | 0 (0.0) | 0 (0.0) | |
| Primary Care Provider | 0.08 | ||||||
| Yes | 122 (70.5) | 49 (56.3) | 55 (75.3) | 82 (74.5) | 12 (63.2) | 35 (70.0) | |
| No | 51 (29.5) | 38 (43.7) | 18 (24.7) | 28 (25.5) | 7 (36.8) | 15 (30.0) | |
| Familiar with PrEP | 0.16 | ||||||
| Yes | 68 (39.3) | 41 (47.1) | 37 (50.7) | 61 (55.5) | 8 (42.1) | 22 (44.0) | |
| No | 105 (60.7) | 46 (52.9) | 36 (49.3) | 49 (44.5) | 11 (57.9) | 28 (56.0) |
The median and 25th and 75th percentiles are listed for age. All the rest are frequency and percent.
Some college or trade school.
Paired Preferences among All Prevention Options
A preference for HIV prevention methods was assigned if one of the five options was always selected over the other four options in the paired preference test (see Table 1). Approximately 90% of participants consistently preferred one prevention option over all others; that is, only 9.8% (n = 50) did not consistently choose one option over the other four (coded as “Undecided”). Among the five options, the most frequently endorsed preference was Condoms (33.8%), however, 56.5% of participants preferred a non-condom option. Among the PrEP options, the most preferred delivery method was Implant-1 (21.5%; two implants; 12-month duration; tactile, but not visible perception by others), followed in order by daily oral PrEP (17.0%; “Pill”; taken daily; not perceptible), injectable PrEP (14.3%; “Injection”; repeated bi-monthly; not perceptible), and Implant-2 (3.7%; two implants; 12-month duration; tactile and visible perception).
Sociodemographic differences in preferences
No significant differences in sexual orientation, race, relationship status, having a primary care provider, or being familiar with PrEP were associated with a preference for any single prevention method (using Fisher’s Exact Tests, see Table 1). There were significant differences in the distribution of age (p = 0.01) and education (p = 0.01) across preferences (using Kruskal-Wallis Tests, see Table 1). Those who preferred Condoms had a significantly younger median age than those who preferred Implant-1, 21 and 24 respectively (p = 0.01, using DSCF multiple comparisons analyses). There was also more advanced educational attainment reported by those preferring Injection as compared to those preferring Condoms and Pill (p = 0.04 and p = 0.02, respectively, using DSCF multiple comparisons analyses).
Differences in reported condom use based on prevention preference
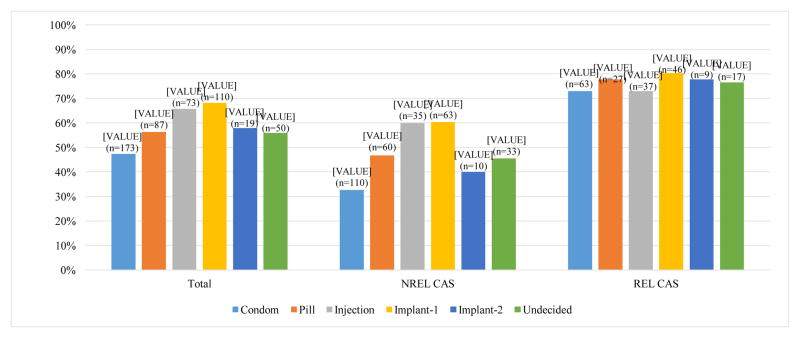
Condomless anal sex (CAS) was scored Yes (1) if participants reported any anal sex without condoms in the past 6 months, regardless of relationship status (Figure 1). Participants who preferred Implant-1 had a higher percentage of reported CAS than those who preferred Condoms (68.2% vs 47.4%; p<0.001, Fisher’s Exact Test with a Bonferroni correction). Figure 1 also presents rates of CAS by preferred HIV prevention method separately for men who reported being in relationships and being single. Results for men not in relationships (n = 311) were qualitatively similar to results for the overall sample: participants who preferred Condoms reported the lowest percentage of CAS compared to all other groups (32.7%), while those who preferred Implant-1 and Injection reported the highest percentage (60.3% and 60.0%, respectively). In contrast, men in relationships (n = 199), reported similar rates of CAS regardless of preferred prevention method (80.4%), all at higher frequency than reported by single men (73.0%). Small cell sizes limited statistical analyses by relationship status.
Figure 1.
Rates of condomless anal sex (CAS) by preferred HIV prevention method among the total sample, men reporting not being in a relationship (NREL, n = 311), and men reporting being in a relationship (REL, n = 199)a
aTwo participants did not report relationship status.
Forced-Choice among Only the Four PrEP Delivery Options
The most frequently endorsed options were Pill (35.4%) and Implant-1 (34.4%), followed by Injection (25.2%) and Implant-2 (4.3%). Four respondents did not answer this question (0.8%). Table 2 presents participants’ single forced choice of the four different PrEP delivery methods as compared to their paired preferences among the initial five options, including condoms. Of the participants who consistently preferred condoms in the paired preference test, the most frequent forced choice preference for PrEP delivery was Pill (52.6%), followed by Implant-1 (24.3%), and Injection (20.2%). Of the participants who were Undecided in the paired preference test, 40.0% chose Injection, 38.0% chose Implant-1, and 20.0% chose Pill. The remaining participants were generally consistent in their responses between the initial pairwise comparisons and subsequent forced choice preferences among only PrEP options. For example, 86.2% of those who chose Pill in the pairwise comparisons also chose Pill in the question about preference among only the four PrEP options. As an exception, those who chose Implant-2 in the initial pairwise comparisons were split between Implant-1 (42.1%) and Implant-2 (52.6%) in the PrEP-only question.
Table 2.
PrEP-only forced choice preferences of HIV prevention method by paired preferences (n=512)
| Paired Preference | Pill n (%) 181 (35.4%) |
Injection n (%) 129 (25.2%) |
Implant-1 n (%) 176 (34.3%) |
Implant-2 n (%) 22 (4.3%) |
Missing n (%) 4 (0.8%) |
|---|---|---|---|---|---|
| Condom | 91 (52.6) | 35 (20.2) | 42 (24.3) | 5 (2.9) | 0 (0.0) |
| Pill | 75 (86.2) | 9 (10.3) | 0 (0.0) | 0 (0.0) | 3 (3.4) |
| Injection | 5 (6.8) | 62 (84.9) | 5 (6.8) | 1 (1.4) | 0 (0.0) |
| Implant-1 | 0 (0.0) | 2 (1.8) | 102 (92.7) | 5 (4.5) | 1 (1.0) |
| Implant-2 | 0 (0.0) | 1 (5.3) | 8 (42.1) | 10 (52.6) | 0 (0.0) |
| Undecided | 10 (20.0) | 20 (40.0) | 19 (38.0) | 1 (2.0) | 0 (0.0) |
Qualitative Responses
Table 3 lists the themes and axial codes of reasons for preferred PrEP-only prevention method, along with the number of excerpts that were coded with each theme. On average, approximately three codes were applied to each open-ended response (M = 2.8, SD = 1.6; range = 1 to 7 codes).
Table 3.
Themes and axial codes of reasons for preferred PrEP-only prevention method
| Theme/Axial Code | Full Sample n=409a n (%) |
Pill n=145a n (%) |
Injection n=109a n (%) |
Implant-1 n=138a n (%) |
Implant-2 n=17a n (%) |
|---|---|---|---|---|---|
| Convenience | 263 (64.3) | 80 (55.2) | 84 (77.1) | 90 (65.2) | 9 (52.9) |
| Ease of useb | 174 (66.2) | 27 (33.8) | 70 (83.3) | 70 (77.8) | 7 (77.8) |
| Integrationb | 54 (20.5) | 25 (31.3) | 15 (17.9) | 14 (15.6) | 0 (0.0) |
| Administrationb | 23 (8.7) | 9 (11.3) | 4 (4.8) | 10 (11.1) | 0 (0.0) |
| Not specifiedb | 42 (16.0) | 24 (30.0) | 4 (4.8) | 12 (13.3) | 2 (22.2) |
| Protection Duration | 152 (37.2) | 6 (4.1) | 54 (49.5) | 86 (62.3) | 6 (35.3) |
| Privacy | 132 (32.3) | 14 (9.7) | 35 (32.1) | 83 (60.1) | 0 (0.0) |
| Dislike/Fear of Other Options | 130 (31.8) | 61 (42.1) | 54 (49.5) | 13 (9.4) | 2 (11.8) |
| Pain/Discomfort of Other Options | 51 (12.5) | 35 (24.1) | 14 (12.8) | 2 (1.4) | 0 (0.0) |
| Protection Evidence | 23 (5.6) | 1 (0.7) | 1 (0.9) | 13 (9.4) | 8 (47.1) |
| Health Risk | 23 (5.6) | 7 (4.8) | 12 (11.0) | 3 (2.2) | 1 (5.9) |
| Effectiveness | 24 (5.9) | 6 (4.1) | 9 (8.3) | 8 (5.8) | 1 (5.9) |
| Eliminates Need for Condoms | 21 (5.1) | 5 (3.4) | 6 (5.5) | 10 (7.2) | 0 (0.0) |
| Cost | 8 (2.0) | 3 (2.1) | 1 (0.9) | 4 (2.9) | 0 (0.0) |
Number of excerpts.
Percent calculated using the n for the Convenience theme of each column as the denominator.
Convenience
Convenience was the most frequently cited reason for selecting a particular prevention method across all participants (263 excerpts). Within the convenience theme, four axial codes emerged: “ease of use/remembering,” “integration into current routine,” “administration setting,” and “general/not specified”. “General/not specified” refers to participants who simply stated that their preferred prevention strategy is easy or convenient (e.g., “For me, pills are more convenient”). Ease of use/remembering (174 excerpts) and integration into current routines (54 excerpts) were the two most commonly referenced axial codes.
The following quotes are examples of responses that were coded with one or more of the convenience subcodes. With regard to ease of use, participant who selected the Injection prevention method stated: “I would forget to take a pill every day. An injection is quick and easy to get over with” (age: 19; ethnicity/race: White) and “I’m use to getting shots since I used to get allergy shots on a regular.” (age: 24; ethnicity/race: African American). The “integration into current routines” axial code was applied to excerpts in which participants referred to being able to add the new prevention method into their daily health routine or use the prevention method with little to no effect on their lifestyle. One example is from a participant (age: 22; ethnicity/race: Hispanic) who preferred the Pill: “It seems to be the easiest just like talking a vitamin.” The axial code “administration setting” refers to the location in which the prophylaxis is administered (e.g., at-home, a doctor’s office, or pharmacy). It was not as commonly referred to as other responses (23 excerpts). Of the participants who did mention the setting being important, most liked the ability to administer their preferred prevention method at home instead of visiting doctors’ offices. One participant (age: 33; ethnicity/race: White) who preferred the Pill wrote, “I do not think that having an implant is really an effective choice. It would mean going in and having a [doctor] cut you open a little. Where if it’s a pill, you can take it at home.”
Protection duration
Participants’ references to the length of time a prevention method lasts was coded as protection duration. This was the second most cited reason for choosing a particular prevention method (152 excerpts) and was discussed more frequently by individuals who preferred Implant-1 (86 excerpts) and Injections (54 excerpts). A participant (age: 22; ethnicity/race: White) mindful of the length of time his preferred prevention method lasts, as well as its convenience, wrote:
“The implant seems like a really easy in and out procedure that won’t require me to take a pill everyday or go get an injection every few months. It’s a longer term preventative and will give me just one less thing to worry about. It reminds me of the anti-pregnancy implant for women in a way. Also chose the one that’s not seen simply for cosmetic reasons.”
A participant (age: 23; ethnicity/race: White) who preferred the Injection noted, “Pills can be annoying and you can forgot to take them. Injection last 2 months and you can keep track of everything. Implants are [too] risky.” In addition to protection duration, this quote highlights the perceived difficulty in taking pills daily, as well as the perceived potential for health risks associated with implant surgery.
Privacy
Privacy was the third most cited reason for participants’ choice in prevention method (132 excerpts). References to the ease of keeping a method discrete or unnoticed were coded as privacy: “I can take the pill and not worry about anyone seeing it” (age: 22; ethnicity/race: African American). Participants who preferred Implant-1 were the group that most frequently cited privacy as a concern (83 excerpts). They often referenced the visible implant in explaining their prevention method choice as is illustrated in the following excerpt: “Implants have the longest span of protection and [this option] is the least noticeable of the two implant options” (age: 19; ethnicity/race: White).
There were also participants who cited the potential stigma of other methods, such as attributing a visible implant to someone being HIV-positive, as a reason for wanting a discrete prevention method. An example comes from a participant (age: 24; ethnicity/race: White) who wrote:
“I think that people are afraid of stigmatization. Having an implant that can be felt will imply the presence of it but not immediately let everyone know that you are somebody who is taking steps to protect themselves against HIV. I think that the assumption that people will make when they see that you have an implant will be that you have HIV or are extremely promiscuous.”
Another participant (age: 20; ethnicity/race: White) who chose Implant-1 echoed these sentiments: “I want my partner to realize I have it but I don’t want it to be regularly seen by others for fear of judgment.”
Attitudes toward other prevention methods
Dislike/fear of other prevention methods and pain/discomfort of other prevention methods rounded out the top reasons that participants chose a particular method (130 and 51 excerpts, respectively). These two themes are similar in that they express participants’ negative attitudes toward other prevention methods. The excerpt, “I have a phobia of needles so a pill would be my best option” (age: 26; ethnicity/race: White) is an example of an excerpt coded as dislike or fear of a prevention method. Participants for whom pain or discomfort were a concern frequently referred to the pain of needles or discomfort of having an implant as being a deterrent. For example, one participant (age: 20; ethnicity/race: Hispanic) who preferred that injection noted his belief that, “Implants are sometimes not effective and hurt if working out.” Some participants cited both dislike/fear and pain/discomfort in their explanations for their preferred prevention method: “I’d prefer taking something that didn’t involve anything breaking my skin like a needle or something. I’m also afraid of needles so that doesn’t help either” (age: 19; ethnicity/race: White).
Other themes
The remaining themes were infrequently endorsed by participants. Each was referenced by less than six percent of the total sample. Protection evidence was mentioned more often by participants who chose either of the implant prevention methods (21of 23 excerpts). This included being able to show the implant to a sexual partner as proof of protection: “The implant lasts for a year and you can prove that you have it. It doesn’t matter to me if you could see it or not but as long as you can feel it then you can show potential partners that you have it” (age: 20; ethnicity/race: White). Health risk concerns pertaining to prevention options were often described as complications from implant surgery. One participant (age: 24; ethnicity/race: White) who chose the Pill said, “Injections or implants seem more invasive and potentially more harmful. I like being able to control when and how I take the medicine with the immediate choice of stopping taking the medicine if I want.” Another participant (age: 20; ethnicity/race: multiracial) who chose the Injection explained his choice by saying, “Implants have higher risk of complications.” Effectiveness referred to a participant’s preferred method being more likely to be effective or the other methods being less likely to work at preventing HIV. Many participants simply described a method as effective without going into greater explanation into why they believed this to be true as shown in the following example: “Injections would be the most effective while being the most convenient” (age: 21; ethnicity/race: Asian). Eliminates the need for condoms referred to instances in which participants indicated using their preferred prevention method in place of condoms. An example of this can be seen in the following excerpt from a participant (age: 21; ethnicity/race: White) who preferred the Pill: “I don’t mind taking pills and my boyfriend prefers no condom.” A participant (age: 21; ethnicity/race: White) who liked Injections best as a prevention method offered more insight into his dislike of condoms:
“As for condoms, well, I’ll start by saying I’d never engage in casual sex. Therefore when it comes to sex with someone I trust I’d never use a condom. Either giving or receiving, I do not like the feel, and I believe it detracts from the overall pleasure.”
Additionally, a participant (age: 18; ethnicity/race: multiracial) who selected Implant-1 noted, “I would like that long-term protection and still have sexual freedom and not have to worry about condoms or pills all the time.” Finally, the cost of prevention methods was mentioned in eight excerpts. One participant (age: 20; ethnicity/race: White) who preferred Implant-1 reported: “It would be easy and simple and you could prove [you’re] protected and it would most likely be the most economical solution.”
Taken together, participants’ most frequently referred to convenience as a reason for selecting a particular option. For those who preferred the Pill (n=145), other common responses included dislike/fear (42.1%) and pain/discomfort (24.1%) associated with the other options. For those who preferred the Injection (n=109), other frequently cited reasons were protection duration (49.5%), dislike/fear of other options (49.5%), and privacy (32.1%). Among participants that preferred Implant-1 (n=138), protection duration (62.3%) and privacy (60.1%) were frequently cited also, while protection evidence (47.1%) and protection duration (35.3%) were commonly endorsed for Implant-2 (n=17).
Mixed Methods Analyses
With regard to demographic differences in theme endorsement, White participants were more likely to endorse a dislike/fear of other methods as a reason for their prevention choice compared to participants in all other race/ethnicity categories (35.0% vs. 22.7%, p = .03). There were no differences found for the other nine themes. Participants who consistently preferred condoms in the initial pairwise comparisons were compared to those who endorsed a PrEP delivery method over condoms in at least one of the pairwise comparisons (percentages of theme endorsement and Fisher’s Exact Tests for differences across endorsed themes in Table 4).
Table 4.
Fisher’s Exact Test differences in theme endorsement between condom-preferred and other method-preferred from paired preferences (n=409)
| Other Method Preferred (n=275) | Condom-Preferred (n=134) | p- value | |
|---|---|---|---|
|
| |||
| Count (%) | |||
| Convenience | 189 (68.7) | 74 (55.2) | < .01 |
| Protection Duration | 112 (40.7) | 40 (29.9) | < .05 |
| Protection Evidence | 17 (6.2) | 6 (4.5) | 0.65 |
| Health Risk | 14 (5.1) | 9 (6.7) | 0.50 |
| Dislike/Fear | 75 (27.3) | 55 (41.0) | < .01 |
| Pain/Discomfort | 28 (10.2) | 23 (17.2) | < .05 |
| Privacy | 100 (36.4) | 32 (23.9) | < .05 |
| Effectiveness | 15 (5.5) | 9 (6.7) | 0.66 |
| Cost | 8 (2.9) | 0 (0.0) | 0.06 |
| Eliminates Need for Condoms | 20 (7.3) | 1 (0.7) | < .01 |
Participants who preferred condoms were more likely to endorse dislike/fear of other methods (41.0% vs. 27.3%, p < .01) and pain/discomfort of other methods (17.2% vs. 10.2%, p < .05) as a reason for their HIV prevention preference, compared to participants who preferred other options. In contrast, participants who preferred other methods were more likely to endorse convenience (68.7% vs. 55.2%, p < .01), privacy (36.4% vs. 23.9%, p < .05), and avoiding the need to use condoms (7.3% vs. 0.7%, p < .01).
Comparisons were also made of how often the different qualitative themes explaining choice were made across those who preferred different PrEP delivery options in the forced-choice question (percentages of theme endorsement and Fisher’s Exact Tests for differences in endorsed themes across methods in Table 5). The convenience theme was most frequently endorsed by participants who preferred the Injection (77.1% of excerpts) compared to those who preferred other methods. Protection duration was most frequently coded for responses from participants who preferred the Injection or Implant-1 (49.5% and 62.3% of excerpts, respectively). Protection evidence was endorsed most often by participants who preferred Implant-2 (47.1% of excerpts). The health risk theme was most frequently endorsed by the Injection-preferring participants (11.0% of excerpts). Dislike/fear of other methods was endorsed most often by Pill-preferring (42.1% of excerpts) and Injection-preferring (49.5% of excerpts) participants. The theme of pain/discomfort of other methods was chosen most frequently by Pill-preferring participants (24.1% of excerpts). The privacy theme was most commonly endorsed by participants who preferred Implant-1 (60.1% of excerpts). There were no differences by preferred method across the effectiveness, cost, and preventing the need for condoms themes.
Table 5.
Fisher’s Exact Test differences in theme endorsement based on preferred method (n=409)
| Pill | Injection | Implant-1 | Implant-2 | p- value | |
|---|---|---|---|---|---|
|
| |||||
| Count (%) | |||||
| Convenience | 80 (55.2) | 84 (77.1) | 90 (65.2) | 9 (52.9) | < .01a |
| Protection Duration | 6 (4.1) | 54 (49.5) | 86 (62.3) | 6 (35.3) | <.001a |
| Protection Evidence | 1 (0.7) | 1 (0.9) | 13 (9.4) | 8 (47.1) | < .001a |
| Health Risk | 7 (4.8) | 12 (11.0) | 3 (2.2) | 1 (5.9) | < .05 |
| Dislike/Fear | 61 (42.1) | 54 (49.5) | 13 (9.4) | 2 (11.8) | < .001 |
| Pain/Discomfort | 35 (24.1) | 14 (12.8) | 2 (1.4) | 0 (0.0) | < .001a |
| Privacy | 14 (9.7) | 35 (32.1) | 83 (60.1) | 0 (0.0) | < .001a |
| Effectiveness | 6 (4.1) | 9 (8.3) | 8 (5.8) | 1 (5.9) | 0.53 |
| Cost Eliminates | 3 (2.1) | 1 (0.9) | 4 (2.9) | 0 (0.0) | 0.68 |
| Need for Condoms | 5 (3.4) | 6 (5.5) | 10 (7.2) | 0 (0.0) | 0.48 |
Result also significant when analysis was limited to condom-preferring participants.
DISCUSSION
This paper aimed to understand preferences for HIV prevention options that included existing as well as potential future strategies among an online sample of MSM in the U.S. This analysis began with evaluation of paired preferences for HIV prevention using either condoms, daily oral PrEP, or systemic, long-acting PrEP delivered via sustained release from either injections or implants. Approximately 90% of participants consistently preferred one prevention option above all others. Condoms were the most frequently preferred method in the paired preference test. This is surprising given the well-documented rates of incorrect and inconsistent condom use among MSM (40, 41), but may be related to extensive familiarity with condoms and low awareness of PrEP among these study participants, as also reported in other research (42, 43).
Long-acting systemic delivery represents a promising alternative to oral PrEP because the need for adherence would be less demanding than either daily or “on-demand” sex act-dependent oral regimens (25, 28, 30, 31). Recent preliminary reports suggest efficacy of injectable ARVs (25–27) and a vaginal ring matrix (44–47) for the sustained delivery of PrEP; other systems are in development, including a subdermal implant (28). However, there has been limited assessment of potential user preferences across existing and potential future methods of PrEP administration including sustained delivery systems. An earlier study of various HIV risk groups in seven countries other than the U.S. found that bimonthly PrEP injections were preferred, followed by a monthly injection in the arm, while a daily pill and a pill before and after sex were the least preferred options (17).
In the present study, although condoms were selected by one-third of the participants (33.8%) as their preferred HIV prevention option, over half (56.5%) preferred a non-condom, PrEP option in the paired preference tests. After condoms, the yearly non-visible implants were the next most frequently preferred modality, followed by the daily oral daily pill. Among the PrEP-only options, daily oral pills and the yearly non-visible implants were the most frequently preferred options. Of note, a larger proportion of younger participants and those with less educational attainment (high school or less) preferred condoms when compared to those who preferred the implant that was not visibly perceptible and the injection, respectively. There were no other demographic differences across participants’ preferences for prevention options. These findings may be understood in the context of prior research that found associations between willingness to use PrEP with younger age (29, 48, 49) and with lower levels of education (50). While the current results contrast with such research, our findings are consistent with one study reporting that PrEP interest was associated with higher levels of education (43). This suggests the hypothesis that better education about current and future PrEP options may increase awareness and interest in use among younger MSM.
Differences in sexual risk were also observed across HIV prevention options. Participants who preferred the implants that were not visible reported the highest percentage of CAS, while those who preferred condoms reported the lowest percentage of CAS. This pattern of results was the same for single men and for those in relationships. Similarly, other studies have found positive associations between more sexual risk behaviors (49, 51, 52) and willingness to use oral PrEP. Taken together, the research suggests that some MSM who may benefit from PrEP due to more frequent CAS may be receptive to using it as a protective intervention (51). And while emerging research may alleviate concerns about the efficacy of daily oral PrEP in real-world settings (22, 53, 54), concerns persist about risk compensation and sexually transmitted infection acquisition, as suggested in the PROUD (54), IPrEx (7), and US PrEP Demonstration Project (22) studies.
Qualitative analyses revealed that privacy, convenience, and protection duration were commonly cited reasons explaining a choice of a long-acting systemic PrEP delivery method. Participants who preferred the pill often described issues pertaining to perceived pain/discomfort associated with of other prevention methods, as well as dislike/fear of the non-pill options. In contrast, participants who preferred injections most commonly described issues relating to convenience and protection duration. Surprisingly, less than 10% of all coded responses referred to cost, effectiveness, health risks, or eliminating the need for condoms as important drivers in HIV prevention option decision-making. This stands in contrast to previous research underscoring the associations between willingness to use oral PrEP and medication cost and effectiveness (50, 55, 56). In this study, it may be that the primary attributes presented for each of the prevention options (i.e., administration, duration, and perceptibility) primed open-ended responses describing reasons for their preferences.
With regard to the implant options for PrEP delivery, participants who preferred the implants that were not visible most commonly described issues relating to protection duration and privacy. Given that these implants were chosen more frequently than the visible implants, it appears that having visible evidence of protection against HIV may be a less common rationale for an implant than a desire for privacy. Such discretion may be prompted by concern with the stigma of taking an HIV medication. For participants who preferred the visible implant, having evidence of protection was a key rationale for that choice. This finding is consistent with research indicating that it is not uncommon for MSM to seek potential sex partners who disclose using biomedical HIV prevention strategies (e.g., HIV-negative MSM on PrEP use and HIV-positive MSM on antiretroviral treatment) (57). Such disclosure, or “proof” of HIV protection as offered by a visible PrEP implant, may become an emerging prevention strategy because it does not solely rely on a partner’s self-reported HIV status.
Limitations
The results of this study should be considered in light of several limitations. One limitation is potential sampling bias. Some individuals were recruited from a research participant and it is not clear to what extent their exposure to prior research, if any, may have influenced their participation in this study, or generalizability of their responses to the survey items. In addition, the Internet-based sample may not be representative of the broader MSM population in the U.S. While the sample included participants from 45 states, online samples may vary in demographic and behavioral and characteristics compared to samples recruited from other venue types (58). Also, the majority of participants were White (78.9%), so there was less representation of some of the highest HIV risk groups, such as Black and Hispanic MSM (59, 60). However, the median age of the sample was 22 years and, thus, the study findings are likely to be relevant for young U.S. MSM. This population subgroup has experienced the sharpest increases in new HIV infections in recent years (59). Another limitation is that the research was cross-sectional and does not allow inferences about causality. Additionally, a potential limitation is the range of product attributes that were presented to participants for making choices among existing and future HIV prevention options in this brief survey. For example, the current study did not evaluate different frequencies of PrEP pill-taking or “on-demand” PrEP before and after sexual activity, despite a recent trial adding support for “on-demand” PrEP (61). In addition to examining dosing as an important attribute for HIV prevention options in future research, other important characteristics to consider are effectiveness, side effects, and cost, particularly given the limited discussion of these attributes in participants’ reasoning for their method of choice in this study. The survey also did not compare options of condoms plus oral PrEP (the current recommendation) versus condoms plus a future long-acting PrEP delivery method (a likely future recommendation). Recommendations to use both condoms and a long-acting PrEP option may lead to a decrease in acceptability of the long-acting PrEP option compared to what was surveyed here. However, if an individual has access to PrEP in any form as an HIV prevention option, condoms will likely also be readily available from the same source so that it will not be necessary to choose only one or the other. Despite these several different limitations, the current study is one of the first of potential user preferences across existing and anticipated methods of PrEP administration among a U.S. population of MSM, and is consistent with an earlier report from other countries that found acceptability for long-acting systemic PrEP (17).
Conclusions
Limited awareness and low uptake of PrEP among MSM in the U.S. was again seen in this online sample, despite findings from recent studies indicating 86% effectiveness rates in reducing the risk of new HIV infections (54, 61) and the fact that Medicaid and private companies are currently covering the cost of PrEP. The demographic characteristics that are associated with higher risk for HIV infection among MSM, including younger age and limited education, are also characteristics that render them less likely to be adequately informed about PrEP, despite being the best candidates for its use. However, in this study MSM who reported being receptive to using PrEP for protection reported more often engaging in sexual HIV risk behaviors (e.g., CAS), suggesting that those with the greatest need may be receptive to using PrEP. Future research should continue to evaluate efforts to improve PrEP awareness and use patterns over time, as new research, different delivery methods, and clinical guidelines continue to influence the course of PrEP implementation in the U.S. Future uptake of long-acting PrEP with less-frequent adherence requirements now in development that will be acceptable to those who would benefit the most from an HIV prevention other than condoms could be enhanced by aligning development with user concerns and preferences. The finding that majority of study participants preferred a non-condom HIV prevention option with convenience, protection duration, and privacy as the most important attributes suggests that incorporation of such features into new PrEP options may enhance future uptake of long-acting PrEP with less-frequent adherence requirements.
Acknowledgments
Funding: Northwestern University Feinberg School of Medicine and Northwestern Medicine supported this research. The National Institute of Allergy and Infectious Diseases of the National Institutes of Health funded preparation of this manuscript under award number UM1AI120184. The authors declare that they have no conflicts of interest to disclose.
Northwestern University Feinberg School of Medicine and Northwestern Medicine supported this research. The National Institute of Allergy and Infectious Diseases of the National Institutes of Health funded preparation of this manuscript under award number UM1 AI120184. The authors appreciate the Northwestern University infrastructure support provided by the IMPACT LGBT Health and Development Program and the Third Coast Center for AIDS Research, an NIH-funded center (P30 AI 117943). This research could not have been conducted without research staff support from Antonia Clifford, Krystal Madkins, and Dan Ryan. We also appreciate expert marketing consulting advice from Robert Schieffer and critical manuscript review by Rebecca Giguere.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Northwestern University Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.CDC. Diagnoses of HIV Infection in the United States and Dependent Areas, 2014. HIV Surveillance Report. 2015;26:1–123. [Google Scholar]
- 2.CDC. HIV Surveillance - Men who have sex with men (MSM) 2015 Dec 15; Available from: http://www.cdc.gov/hiv/library/slideSets/index.html.
- 3.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DK, Van Handel M, Wolitski RJ, Stryker JE, Hall HI, Prejean J, et al. Vital Signs: Estimated Percentages and Numbers of Adults with Indications for Preexposure Prophylaxis to Prevent HIV Acquisition--United States, 2015. 2015;64(46):1291–5. doi: 10.15585/mmwr.mm6446a4. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Science translational medicine. 2012;4(151):151ra25. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 7.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 9.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. New England Journal of Medicine. 2012;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grohskopf LA, Chillag KL, Gvetadze R, Liu AY, Thompson M, Mayer KH, et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013;64(1):79–86. doi: 10.1097/QAI.0b013e31828ece33. [DOI] [PubMed] [Google Scholar]
- 11.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellerman SE, Hutchinson AB, Begley EB, Boyett BC, Clark HA, Sullivan P. Knowledge and use of HIV pre-exposure prophylaxis among attendees of minority gay pride events, 2004. J Acquir Immune Defic Syndr. 2006;43(3):376–7. doi: 10.1097/01.qai.0000234085.18914.d5. [DOI] [PubMed] [Google Scholar]
- 13.Voetsch AC, Heffelfinger JD, Begley EB, Jafa-Bhushan K, Sullivan PS. Knowledge and use of preexposure and postexposure prophylaxis among attendees of Minority Gay Pride events, 2005 through 2006. J Acquir Immune Defic Syndr. 2007;46(3):378–80. doi: 10.1097/QAI.0b013e3181576874. [DOI] [PubMed] [Google Scholar]
- 14.Liu AY, Kittredge PV, Vittinghoff E, Raymond HF, Ahrens K, Matheson T, et al. Limited knowledge and use of HIV post- and pre-exposure prophylaxis among gay and bisexual men. J Acquir Immune Defic Syndr. 2008;47(2):241–7. [PubMed] [Google Scholar]
- 15.Barash EA, Golden M. Awareness and use of HIV pre-exposure prophylaxis among attendees of a seattle gay pride event and sexually transmitted disease clinic. AIDS Patient Care STDS. 2010;24(11):689–91. doi: 10.1089/apc.2010.0173. [DOI] [PubMed] [Google Scholar]
- 16.Rucinski KB, Mensah NP, Sepkowitz KA, Cutler BH, Sweeney MM, Myers JE. Knowledge and use of pre-exposure prophylaxis among an online sample of young men who have sex with men in New York City. AIDS Behav. 2013;17(6):2180–4. doi: 10.1007/s10461-013-0443-y. [DOI] [PubMed] [Google Scholar]
- 17.Golub SA, Kowalczyk W, Weinberger CL, Parsons JT. Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. J Acquir Immune Defic Syndr. 2010;54(5):548–55. doi: 10.1097/QAI.0b013e3181e19a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauermeister JA, Meanley S, Pingel E, Soler JH, Harper GW. PrEP awareness and perceived barriers among single young men who have sex with men. Curr HIV Res. 2013;11(7):520–7. doi: 10.2174/1570162x12666140129100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eaton LA, Driffin DD, Smith H, Conway-Washington C, White D, Cherry C. Psychosocial factors related to willingness to use pre-exposure prophylaxis for HIV prevention among Black men who have sex with men attending a community event. Sex Health. 2014;11(3):244–51. doi: 10.1071/SH14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grov C, Whitfield TH, Rendina HJ, Ventuneac A, Parsons JT. Willingness to take PrEP and potential for risk compensation among highly sexually active gay and bisexual men. AIDS Behav. 2015 doi: 10.1007/s10461-015-1030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strauss BB, Greene GJ, Phillips G, 2nd, Bhatia R, Madkins K, Parsons JT, et al. Exploring Patterns of Awareness and Use of HIV Pre-Exposure Prophylaxis Among Young Men Who Have Sex with Men. AIDS Behav. 2016 doi: 10.1007/s10461-016-1480-0. [DOI] [PMC free article] [PubMed]
- 22.Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med. 2016;176(1):75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amico KR, Stirratt MJ. Adherence to preexposure prophylaxis: current, emerging, and anticipated bases of evidence. Clin Infect Dis. 2014;59(Suppl 1):S55–60. doi: 10.1093/cid/ciu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansergh G, Koblin BA, Sullivan PS. Challenges for HIV pre-exposure prophylaxis among men who have sex with men in the United States. PLoS Med. 2012;9(8):e1001286. doi: 10.1371/journal.pmed.1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Current opinion in HIV and AIDS. 2013;8(6):565–71. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolis DA, Boffito M. Long-acting antiviral agents for HIV treatment. Current opinion in HIV and AIDS. 2015;10(4):246–52. doi: 10.1097/COH.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson A, McGowan I. Long-acting rilpivirine for HIV prevention. Current opinion in HIV and AIDS. 2015;10(4):253–7. doi: 10.1097/COH.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 28.Gunawardana M, Remedios-Chan M, Miller CS, Fanter R, Yang F, Marzinke MA, et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrobial agents and chemotherapy. 2015;59(7):3913–9. doi: 10.1128/AAC.00656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisingerich AB, Wheelock A, Gomez GB, Garnett GP, Dybul MR, Piot PK. Attitudes and acceptance of oral and parenteral HIV preexposure prophylaxis among potential user groups: a multinational study. PLoS One. 2012;7(1):e28238. doi: 10.1371/journal.pone.0028238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boffito M, Jackson A, Owen A, Becker S. New approaches to antiretroviral drug delivery: challenges and opportunities associated with the use of long-acting injectable agents. Drugs. 2014;74(1):7–13. doi: 10.1007/s40265-013-0163-7. [DOI] [PubMed] [Google Scholar]
- 31.Dolgin E. Long-acting HIV drugs advanced to overcome adherence challenge. 2014;20(4):323–4. doi: 10.1038/nm0414-323. [DOI] [PubMed] [Google Scholar]
- 32.Parsons JT, Rendina HJ, Whitfield TH, Grov C. Familiarity with and Preferences for Oral and Long-Acting Injectable HIV Pre-exposure Prophylaxis (PrEP) in a National Sample of Gay and Bisexual Men in the U.S. AIDS Behav. 2016;20(7):1390–9. doi: 10.1007/s10461-016-1370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone H, Bleibaum R, Thomas HA. Sensory evaluation practices. 4. London: Elsevier/AP; 2012. [Google Scholar]
- 34.Courcoux P, Semenou M. Preference data analysis using a paired comparison model. Food Qual Prefer. 1997;8(5–6):6. [Google Scholar]
- 35.Day RL. Systematic Paired Comparisons in Preference Analysis. Journal of Marketing Research. 1965;2(4):406–12. [Google Scholar]
- 36.Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine Publishing Company; 1967. p. x.p. 271. [Google Scholar]
- 37.Carey MA, Smith MW Capturing the Group. Qualitative Health Research. 1994. Effect in Focus Groups: A Special Concern in Analysis; p. 4. [Google Scholar]
- 38.Ryan GW, Bernard HR. Techniques to identify themes. Field Methods. 2003;15(1):85. [Google Scholar]
- 39.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 40.Smith DK, Herbst JH, Zhang X, Rose CE. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2015;68(3):337–44. doi: 10.1097/QAI.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 41.Mustanski B, Ryan DT, Garofalo R. Associations of sexually transmitted infections with condom problems among young men who have sex with men. Sex Transm Dis. 2014;41(7):427–32. doi: 10.1097/OLQ.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolezal C, Frasca T, Giguere R, Ibitoye M, Cranston RD, Febo I, et al. Awareness of Post-Exposure Prophylaxis (PEP) and Pre-Exposure Prophylaxis (PrEP) Is Low but Interest Is High Among Men Engaging in Condomless Anal Sex With Men in Boston, Pittsburgh, and San Juan. AIDS Educ Prev. 2015;27(4):289–97. doi: 10.1521/aeap.2015.27.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mustanski B, Johnson AK, Garofalo R, Ryan D, Birkett M. Perceived likelihood of using HIV pre-exposure prophylaxis medications among young men who have sex with men. AIDS Behav. 2013;17(6):2173–9. doi: 10.1007/s10461-012-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palanee-Phillips T, Schwartz K, Brown ER, Govender V, Mgodi N, Kiweewa FM, et al. Characteristics of Women Enrolled into a Randomized Clinical Trial of Dapivirine Vaginal Ring for HIV-1 Prevention. PLoS One. 2015;10(6):e0128857. doi: 10.1371/journal.pone.0128857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016 doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Nel A, et al. A Phase III Trial of the Dapivirine Vaginal Ring for HIV-1 Prevention in Women. Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, WA. 2016. [Google Scholar]
- 47.Nel A, Kapiga S, Bekker LG, Devlin B, Borremans M, Rosenberg Z. Safety and Efficacy of Dapivirine Vaginal Ring for HIV-1 Prevention in African Women. Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, WA. 2016. [Google Scholar]
- 48.Amico KR, McMahan V, Goicochea P, Vargas L, Marcus JL, Grant RM, et al. Supporting study product use and accuracy in self-report in the iPrEx study: next step counseling and neutral assessment. AIDS Behav. 2012;16(5):1243–59. doi: 10.1007/s10461-012-0182-5. [DOI] [PubMed] [Google Scholar]
- 49.Aghaizu A, Mercey D, Copas A, Johnson AM, Hart G, Nardone A. Who would use PrEP? Factors associated with intention to use among MSM in London: a community survey. Sex Transm Infect. 2013;89(3):207–11. doi: 10.1136/sextrans-2012-050648. [DOI] [PubMed] [Google Scholar]
- 50.Mimiaga MJ, Case P, Johnson CV, Safren SA, Mayer KH. Preexposure antiretroviral prophylaxis attitudes in high-risk Boston area men who report having sex with men: limited knowledge and experience but potential for increased utilization after education. J Acquir Immune Defic Syndr. 2009;50(1):77–83. doi: 10.1097/QAI.0b013e31818d5a27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krakower DS, Mimiaga MJ, Rosenberger JG, Novak DS, Mitty JA, White JM, et al. Limited awareness and low immediate uptake of pre-exposure prophylaxis among men who have sex with men using an Internet social networking site. PLoS One. 2012;7(3):e33119. doi: 10.1371/journal.pone.0033119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saberi P, Gamarel KE, Neilands TB, Comfort M, Sheon N, Darbes LA, et al. Ambiguity, ambivalence, and apprehensions of taking HIV-1 pre-exposure prophylaxis among male couples in San Francisco: a mixed methods study. PLoS One. 2012;7(11):e50061. doi: 10.1371/journal.pone.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, et al. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin Infect Dis. 2015 doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galea JT, Kinsler JJ, Salazar X, Lee SJ, Giron M, Sayles JN, et al. Acceptability of pre-exposure prophylaxis as an HIV prevention strategy: barriers and facilitators to pre-exposure prophylaxis uptake among at-risk Peruvian populations. Int J STD AIDS. 2011;22(5):256–62. doi: 10.1258/ijsa.2009.009255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King HL, Keller SB, Giancola MA, Rodriguez DA, Chau JJ, Young JA, et al. Pre-exposure prophylaxis accessibility research and evaluation (PrEPARE Study) AIDS Behav. 2014;18(9):1722–5. doi: 10.1007/s10461-014-0845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newcomb ME, Mongrella MC, Weis B, McMillen SJ, Mustanski B. Partner Disclosure of PrEP Use and Undetectable Viral Load on Geosocial Networking Apps: Frequency of Disclosure and Decisions About Condomless Sex. J Acquir Immune Defic Syndr. 2016;71(2):200–6. doi: 10.1097/QAI.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grov C. HIV Risk and Substance Use in Men Who Have Sex with Men Surveyed in Bathhouses, Bars/Clubs, and on Craigslist org: Venue of Recruitment Matters. AIDS and Behavior. 2012;16(4):807–17. doi: 10.1007/s10461-011-9999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.CDC. HIV among gay and bisexual men. CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of HIV/AIDS Prevention; 2015. [Google Scholar]
- 60.Purcell DW, Johnson CH, Lansky A, Prejean J, Stein R, Denning P, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J. 2012;6:98–107. doi: 10.2174/1874613601206010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015;373(23):2237–46. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]