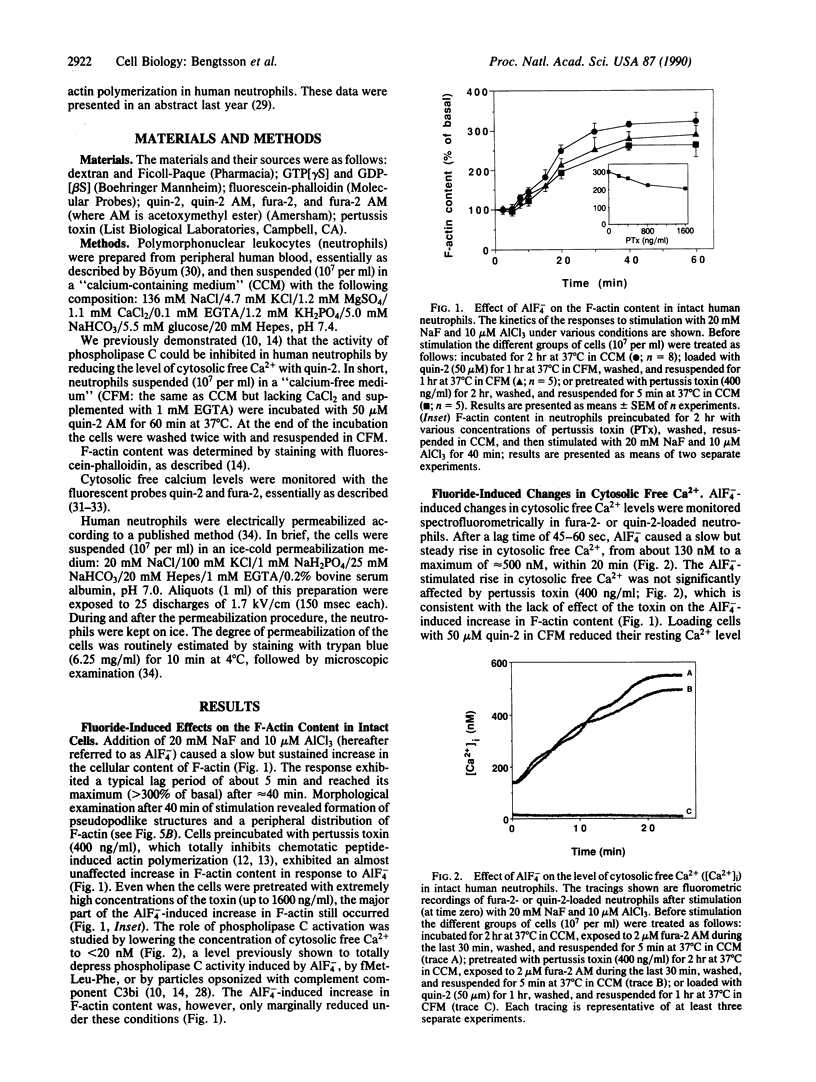
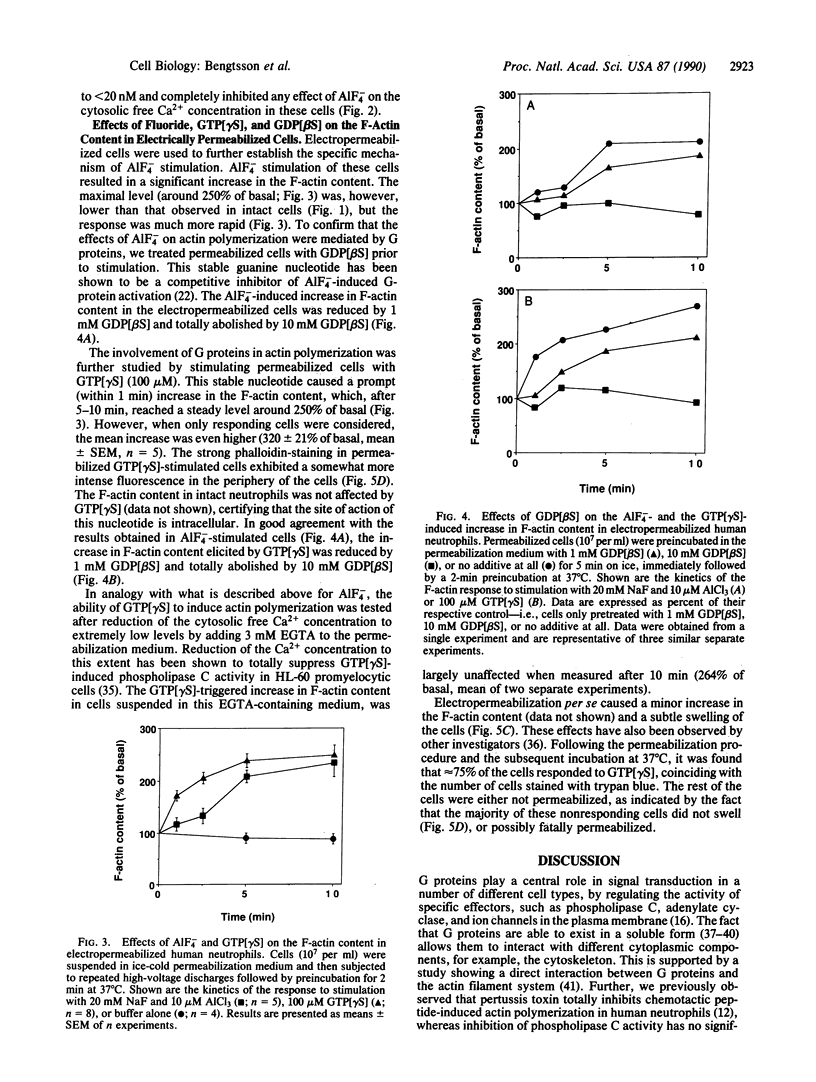
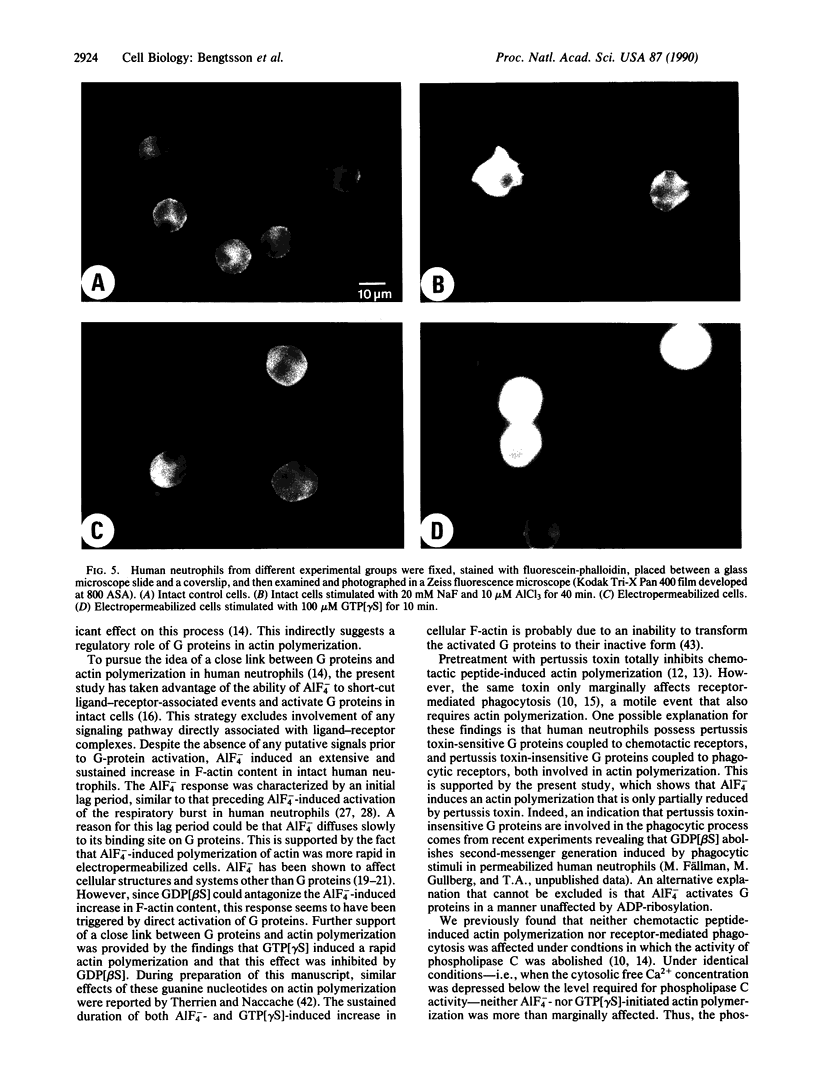
Abstract
The motility of human neutrophils, which is of vital importance for the role of these cells in host defense, is based on rapid and dynamic changes of the filamentous actin F-actin) network. Consequently, to understand how neutrophils move and ingest particles, we need to know how polymerization and depolymerization of actin are regulated. Previous studies by several investigators have, based on indirect evidence obtained with pertussis toxin, suggested a role for GTP-binding protein(s) (G protein) in chemotaxis-induced, but not phagocytosis-induced, reorganization of the F-actin network. The aim of the present investigation was to study the effects of directly activated G proteins (i.e., without prior ligand-receptor complex formation) on the F-actin content in human neutrophils. AlF4- induced a pronounced and sustained increase in F-actin in intact neutrophils. This effect coincided with an increase in cytosolic free Ca2+, indicating that phospholipase C and the subsequent transduction mechanism were also activated. Inhibition of phospholipase C activity by extensive depression of the cytosolic free Ca2+ level (less than 20 nM) only marginally affected the AlF4(-)-induced rise in F-actin content. The major part of the AlF4(-)-induced rise in F-actin content was also resistant to pertussis toxin, suggesting that pertussis toxin-insensitive G proteins in neutrophils are also able to trigger actin polymerization. The specificity of AlF4- in activating G proteins was also tested in permeabilized cells. In this case the effect was more rapid and could be totally abolished by guanosine 5'-[beta-thio]diphosphate. In analogy, in permeabilized cells guanosine 5'-[gamma-thio]triphosphate mimicked the effect of AlF4- on actin polymerization, and the effect induced by this nonhydrolyzable GTP analogue could also be totally abolished by guanosine 5'-[beta-thio]diphosphate. In summary, the present data support our previous hypothesis that G proteins are intimately linked to actin polymerization in human neutrophils.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Dahlgren C., Pozzan T., Stendahl O., Lew P. D. Characterization of fMet-Leu-Phe receptor-mediated Ca2+ influx across the plasma membrane of human neutrophils. Mol Pharmacol. 1986 Nov;30(5):437–443. [PubMed] [Google Scholar]
- Andersson T., Schlegel W., Monod A., Krause K. H., Stendahl O., Lew D. P. Leukotriene B4 stimulation of phagocytes results in the formation of inositol 1,4,5-trisphosphate. A second messenger for Ca2+ mobilization. Biochem J. 1986 Dec 1;240(2):333–340. doi: 10.1042/bj2400333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Kanaho Y., Kermode J. C. Nature and functioning of the pertussis toxin-sensitive G protein of neutrophils. Biomed Pharmacother. 1987;41(6):289–297. [PubMed] [Google Scholar]
- Bengtsson T., Rundquist I., Stendahl O., Wymann M. P., Andersson T. Increased breakdown of phosphatidylinositol 4,5-bisphosphate is not an initiating factor for actin assembly in human neutrophils. J Biol Chem. 1988 Nov 25;263(33):17385–17389. [PubMed] [Google Scholar]
- Bengtsson T., Stendahl O., Andersson T. The role of the cytosolic free Ca2+ transient for fMet-Leu-Phe induced actin polymerization in human neutrophils. Eur J Cell Biol. 1986 Dec;42(2):338–343. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Bickford K., Bohl B. P. Subcellular localization and quantitation of the major neutrophil pertussis toxin substrate, Gn. J Cell Biol. 1988 Jun;106(6):1927–1936. doi: 10.1083/jcb.106.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Brandt S. J., Dougherty R. W., Lapetina E. G., Niedel J. E. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham D. N., Tyagi S. R., Uhlinger D. J., Lambeth J. D. Diacylglycerol generation and phosphoinositide turnover in human neutrophils: effects of particulate versus soluble stimuli. Arch Biochem Biophys. 1989 Feb 15;269(1):345–353. doi: 10.1016/0003-9861(89)90116-1. [DOI] [PubMed] [Google Scholar]
- Carlson K. E., Woolkalis M. J., Newhouse M. G., Manning D. R. Fractionation of the beta subunit common to guanine nucleotide-binding regulatory proteins with the cytoskeleton. Mol Pharmacol. 1986 Nov;30(5):463–468. [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. G-proteins, the inositol lipid signalling pathway, and secretion. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):247–265. doi: 10.1098/rstb.1988.0075. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Taylor J. A. Fluoroaluminates mimic guanosine 5'-[gamma-thio]triphosphate in activating the polyphosphoinositide phosphodiesterase of hepatocyte membranes. Role for the guanine nucleotide regulatory protein Gp in signal transduction. Biochem J. 1987 Jan 15;241(2):409–414. doi: 10.1042/bj2410409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combeau C., Carlier M. F. Probing the mechanism of ATP hydrolysis on F-actin using vanadate and the structural analogs of phosphate BeF-3 and A1F-4. J Biol Chem. 1988 Nov 25;263(33):17429–17436. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M., Karnovsky M. L. Fluoride-mediated activation of the respiratory burst in human neutrophils. A reversible process. J Clin Invest. 1979 Apr;63(4):637–647. doi: 10.1172/JCI109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Bianca V., Grzeskowiak M., Dusi S., Rossi F. Fluoride can activate the respiratory burst independently of Ca2+, stimulation of phosphoinositide turnover and protein kinase C translocation in primed human neutrophils. Biochem Biophys Res Commun. 1988 Feb 15;150(3):955–964. doi: 10.1016/0006-291x(88)90722-x. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Grinstein S. Receptor-mediated actin assembly in electropermeabilized neutrophils: role of intracellular pH. Biochem Biophys Res Commun. 1989 Apr 14;160(1):18–24. doi: 10.1016/0006-291x(89)91614-8. [DOI] [PubMed] [Google Scholar]
- Eide B., Gierschik P., Milligan G., Mullaney I., Unson C., Goldsmith P., Spiegel A. GTP-binding proteins in brain and neutrophil are tethered to the plasma membrane via their amino termini. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1398–1405. doi: 10.1016/s0006-291x(87)80287-5. [DOI] [PubMed] [Google Scholar]
- English D., Debono D. J., Gabig T. G. Relationship of phosphatidylinositol bisphosphate hydrolysis to calcium mobilization and functional activation in fluoride-treated neutrophils. J Clin Invest. 1987 Jul;80(1):145–153. doi: 10.1172/JCI113040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fällman M., Lew D. P., Stendahl O., Andersson T. Receptor-mediated phagocytosis in human neutrophils is associated with increased formation of inositol phosphates and diacylglycerol. Elevation in cytosolic free calcium and formation of inositol phosphates can be dissociated from accumulation of diacylglycerol. J Clin Invest. 1989 Sep;84(3):886–891. doi: 10.1172/JCI114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Honeycutt P. J., Niedel J. E. Cytochalasin B enhancement of the diacylglycerol response in formyl peptide-stimulated neutrophils. J Biol Chem. 1986 Dec 5;261(34):15900–15905. [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- Krause K. H., Schlegel W., Wollheim C. B., Andersson T., Waldvogel F. A., Lew P. D. Chemotactic peptide activation of human neutrophils and HL-60 cells. Pertussis toxin reveals correlation between inositol trisphosphate generation, calcium ion transients, and cellular activation. J Clin Invest. 1985 Oct;76(4):1348–1354. doi: 10.1172/JCI112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Olson C. V., Grewal I. S. A step sensitive to pertussis toxin and phorbol ester in human neutrophils regulates chemotaxis and capping but not phagocytosis. FEBS Lett. 1986 May 5;200(1):91–96. doi: 10.1016/0014-5793(86)80517-8. [DOI] [PubMed] [Google Scholar]
- Lange A. J., Arion W. J., Burchell A., Burchell B. Aluminum ions are required for stabilization and inhibition of hepatic microsomal glucose-6-phosphatase by sodium fluoride. J Biol Chem. 1986 Jan 5;261(1):101–107. [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Evidence that the phosphatidylinositol cycle is linked to cell motility. Exp Cell Res. 1988 Jan;174(1):1–15. doi: 10.1016/0014-4827(88)90136-x. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Robinson J. D., Davis R. L., Steinberg M. Fluoride and beryllium interact with the (Na + K)-dependent ATPase as analogs of phosphate. J Bioenerg Biomembr. 1986 Dec;18(6):521–531. doi: 10.1007/BF00743148. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Koesling D., Hinsch K. D., Seifert R., Bigalke M., Schultz G., Rosenthal W. G-protein alpha-subunits in cytosolic and membranous fractions of human neutrophils. Mol Cell Endocrinol. 1989 May;63(1-2):143–153. doi: 10.1016/0303-7207(89)90090-7. [DOI] [PubMed] [Google Scholar]
- Shefcyk J., Yassin R., Volpi M., Molski T. F., Naccache P. H., Munoz J. J., Becker E. L., Feinstein M. B., Sha'afi R. I. Pertussis but not cholera toxin inhibits the stimulated increase in actin association with the cytoskeleton in rabbit neutrophils: role of the "G proteins" in stimulus-response coupling. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1174–1181. doi: 10.1016/0006-291x(85)90309-2. [DOI] [PubMed] [Google Scholar]
- Sheterline P., Rickard J. E., Richards R. C. Fc receptor-directed phagocytic stimuli induce transient actin assembly at an early stage of phagocytosis in neutrophil leukocytes. Eur J Cell Biol. 1984 May;34(1):80–87. [PubMed] [Google Scholar]
- Smith C. D., Chang K. J. Regulation of brain phosphatidylinositol-4-phosphate kinase by GTP analogues. A potential role for guanine nucleotide regulatory proteins. J Biol Chem. 1989 Feb 25;264(6):3206–3210. [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad C. F., Parente J. E., Wong K. Use of fluoride ion as a probe for the guanine nucleotide-binding protein involved in the phosphoinositide-dependent neutrophil transduction pathway. FEBS Lett. 1986 Sep 29;206(1):20–24. doi: 10.1016/0014-5793(86)81332-1. [DOI] [PubMed] [Google Scholar]
- Strnad C. F., Wong K. Calcium mobilization in fluoride activated human neutrophils. Biochem Biophys Res Commun. 1985 Nov 27;133(1):161–167. doi: 10.1016/0006-291x(85)91855-8. [DOI] [PubMed] [Google Scholar]
- Särndahl E., Lindroth M., Bengtsson T., Fällman M., Gustavsson J., Stendahl O., Andersson T. Association of ligand-receptor complexes with actin filaments in human neutrophils: a possible regulatory role for a G-protein. J Cell Biol. 1989 Dec;109(6 Pt 1):2791–2799. doi: 10.1083/jcb.109.6.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrien S., Naccache P. H. Guanine nucleotide-induced polymerization of actin in electropermeabilized human neutrophils. J Cell Biol. 1989 Sep;109(3):1125–1132. doi: 10.1083/jcb.109.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urumow T., Wieland O. H. Stimulation of phosphatidylinositol 4-phosphate phosphorylation in human placenta membranes by GTP gamma S. FEBS Lett. 1986 Oct 27;207(2):253–257. doi: 10.1016/0014-5793(86)81499-5. [DOI] [PubMed] [Google Scholar]
- Wallace P. J., Wersto R. P., Packman C. H., Lichtman M. A. Chemotactic peptide-induced changes in neutrophil actin conformation. J Cell Biol. 1984 Sep;99(3):1060–1065. doi: 10.1083/jcb.99.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]