Abstract
Background
While fibrosis seems to be prognostic for adverse outcomes in adults with idiopathic dilated cardiomyopathy (IDC), little is known about the prevalence and development of fibrosis in pediatric IDC hearts. We hypothesize there is less activation of fibrosis at a molecular level in pediatric IDC hearts than in the failing adult heart.
Methods and Results
Pediatric hearts were analyzed histologically to determine the prevalence of fibrosis. Left ventricular tissue from adult and pediatric IDC hearts and adult and pediatric non-failing (NF) hearts were subjected to qRT-PCR to study the expression of important mRNAs that affect fibrosis. We found age-specific differences between IDC and NF hearts in regulation of non-coding galectin 3, Corin, MMP-2, MMP-9, TIMP-2, and TIMP-3. We also found markers that were similarly altered in both adult and pediatric IDC (ST2L, TIMP-1, and TIMP-4). Finally, microRNAs 29a-c were significantly decreased in the pediatric IDC patients.
Conclusion
Pediatric IDC patients demonstrate age-specific differences in the molecular pathways implicated in fibrosis in the adult heart. At the ultrastructural level the unique gene expression pattern appears to limit fibrosis in the failing pediatric heart.
Keywords: Pediatric idiopathic cardiomyopathy, fibrosis, gene expression
Introduction
The most common cause of heart failure (HF) in pediatric patients is idiopathic dilated cardiomyopathy (IDC) (1, 2). Although the myocellular mechanisms involved in pediatric IDC are mostly unexplored, children are treated with the same medications as adult HF patients. Therapies for adult HF patients have lowered mortality; however, the same therapies have failed to improve outcomes for pediatric patients (3). It is becoming increasingly clear that HF in pediatric patients is a separate disease entity from that of adult HF (4–7).
Fibrosis is an important pathologic response that is found in the majority of adults with IDC and the extent of fibrosis has been associated with worse outcomes (8–12). In a healthy heart, cardiomyocytes are supported by a fibrillar-collagen matrix composed of type I and III collagen. However under pathological stress, chronic activation of the renin-angiotensin-aldosterone system (RAAS) leads to an imbalance of synthesis and degradation of extracellular matrix components by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) (12–17). Structurally, increased deposition of the extracellular matrix increases cardiac stiffness and decreases cardiac output. There are multiple medications targeting fibrosis in HF patients through modulation of RAAS (eg aldosterone antagonists and angiotensin II type 1 receptor antagonists) but none have been systematically studied or demonstrated benefit in children. Little is known about fibrosis in pediatric hearts but the few imaging studies using cardiac MRI in pediatric HF patients show less fibrosis than in the failing adult heart (18–20). A recently convened NHLBI working group recommended a better understanding of fibrosis in pediatric HF patients (21).
Although the fibrotic process is incompletely understood, extensive investigation in adult HF has elucidated several important signaling pathways involved in fibrosis. In the adult, 3 promising biomarkers have been identified: Galectin-3 (Gal-3), Corin and ST2L (22–27). Circulating levels of these molecules in adult HF patients have been related to myocardial fibrosis and dysfunction. Since little is known about the expression of these genes in pediatric HF, it is important to determine if the gene expression of these molecules is different between adult and pediatric HF patients. Another well documented signaling pathway involved in fibrosis in adult HF patients involves MMPs and TIMPs which regulate extracellular matrix composition (12–17). In addition, the expression of the microRNA 29 (miRNA-29) family has been shown to attenuate fibrosis through regulation of several downstream targets (28, 29). However, it is not known if there are age-specific differences in the expression of this miRNA family.
The overall purpose of this study was to investigate age-related differences in pathologic fibrosis and selected fibrosis gene expression in children and adults with IDC. Little is known about the prevalence and development of fibrosis in pediatric IDC hearts, but based on prior studies (18–20), we hypothesize there is less activation of fibrosis at a molecular level in pediatric IDC hearts than in the failing adult heart. Indeed, the current study demonstrates an age difference in regulation of fibrotic genes in the HF population. The results of this study could help determine whether fibrosis should be a therapeutic target for children.
Materials and Methods
Subjects
All human tissue was from pediatric (n = 42; age < 18 years) and adult (n = 10; age 20–60; median: 51 years) patients who underwent transplant due to end-stage IDC. Adults with ischemic heart disease and children with congenital heart disease were excluded. Samples were donated to the IRB approved Pediatric or Adult Cardiac Transplant Tissue Bank at the University of Colorado Denver (Table 1). Non-failing control samples (pediatric: n = 22; adult: n = 10) were from donor hearts with normal function that could not be placed for technical reasons (eg size or blood type mismatch). At the time of heart explant in the operating room, the left ventricle (LV) was rapidly dissected, flash frozen, and stored at −80°C until further use.
Table 1. Patient demographics.
Neither pathology reports nor slides were available for 3 patients; therefore, they were excluded from classification of fibrosis. Inotropes include dopamine, dobutamine, vasopressin, epinephrine, norepinephrine, and milrinone. NA = not available. EF = Ejection Fraction, ACEI = Angiotensin Converting Enzyme Inhibitor.
| Adult | Pediatric | |||
|---|---|---|---|---|
| NF | IDC | NF | IDC | |
| Sample size | 10 | 10 | 22 | 42 |
| Sex | 50% M | 50% M | 54% M | 48% M |
|
Median Age At Tissue Collection (Years) |
42.5 (19–61) | 51 (20–60) | 12.7 (1.3–17) | 4.0 (0.05–17.7) |
| Mean EF ± SD (%) | 56.4 ± 10.8 | 14.0 ± 5.2 | 48.3 ± 15.2 | 25.4 ± 6.0 |
| Inotropes | 7 (70%) | 2 (20%) | - | 32 (76%) |
| Digoxin | - | 2 (20%) | - | 15 (35%) |
| ACEI | - | 2 (20%) | - | 31 (74%) |
| Beta-Blocker | - | 3 (30%) | - | 8 (19%) |
| Presence of Fibrosis | NA | NA | NA | 21 (50%) |
| Type of Fibrosis | NA | NA | NA | 17 (81%) Endocardial; 2 (9.5%) Interstitial; 2 (9.5%) Both |
Histologic evaluation
Sections of myocardium were isolated from children with IDC as a routine part of clinical care at the time of heart explant and transplantation. These slides were evaluated by H&E and/or trichrome staining to assess for fibrosis. Interpretation was performed by a pediatric pathologist and a formal report entered into the medical record for each patient. For this study, all available slides were reviewed in a blinded fashion by a single investigator (A.K.S) and the presence and location of fibrosis was determined. If there was a discrepancy between the investigator’s evaluation and the clinical pathology report, a pediatric cardiac pathologist (C.G.) reviewed the slides and made the final determination. For those patients for whom slides were not available the presence of fibrosis was based on the pathology report only.
Determination of percent area of fibrosis
There were H&E stained slides available for 30 patients (71%), but only 5 patients (17%) had slides also stained with trichrome. First, Image J was used to analyze fibrotic area of the slides stained with trichrome to establish a standard (30). The analysis was then performed on H&E sections from the same patient for comparison. The analysis method yielded similar results of percent area of fibrosis for trichrome and H&E slides (% area fibrosis with trichrome = 7.6% ± 0.6; % area fibrosis with H&E = 6.9% ± 1.5; p = 0.87). Therefore, for each patient, the percent area of fibrosis was determined by Image J analysis in 6 random H&E images and averaged to obtain the final percent area of fibrosis for each patient.
mRNA expression
Total RNA from LV tissue was extracted using MirVana™ RNA Isolation Kit (Ambion #AM1561) as previously published by our group (5). miRNA reverse transcription (RT) was performed using the miScript Reverse Transcription Kit (Qiagen) as described previously (31). Each sample was run in triplicate and dissociation curves were determined to assure a single amplified sequence. Primer sequences are listed (Supplemental Table 1). The internal control for mRNA was 18S whereas U6 was the internal control for miRNA. Due to the number of samples and 96 well format of the system all primers were run on 2 plates, one containing the adult samples and one containing the pediatric samples. The expression within the diseased samples was normalized to age-match NF control expression.
Data analysis
All qRT-PCR data were normalized to age-matched NF control expression. All statistical procedures were performed with GraphPad Prism software (GraphPad Software, Inc). Shapiro-Wilks normality test was performed on all data. Normally distributed data was compared using an unpaired Student’s t-test with Welch’s correction (2 groups) or ANOVA (3 groups). In cases where the data was not normally distributed, a Mann Whitney test (2 groups) or a Kruskal-Wallis test (3 groups) was conducted. To determine the relationship between age at HF onset or duration and percent area fibrosis, a linear regression analysis was conducted. Statistical significance was set a priori at p < 0.05 and all data are presented as mean ± SEM in the figures.
Results
LVs from pediatric IDC patients have significantly less fibrosis than LVs from adult IDC patients
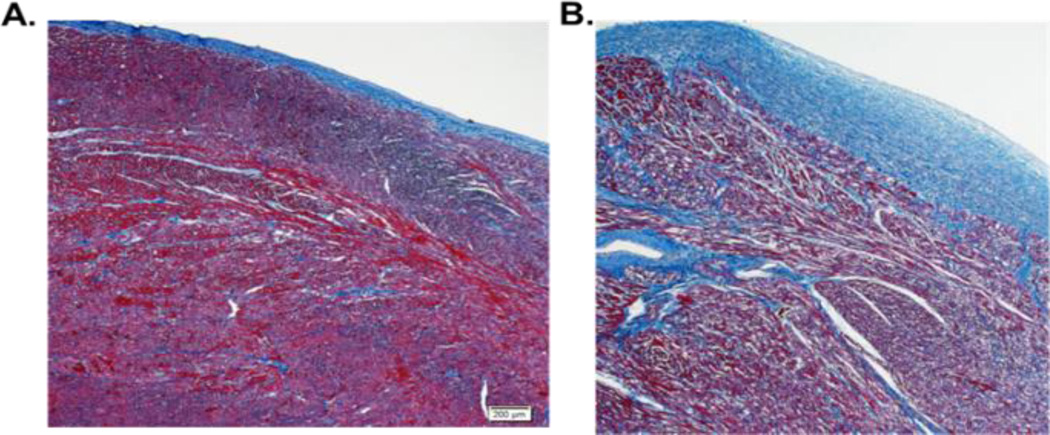
In our cohort of pediatric patients, LV tissue was available for gene expression analysis on all 42 patients, the presence of pathologic fibrosis was determined in 39 of the 42 patients (no pathology reports or slides were available for 3 patients) and percent area of fibrosis was defined for the 30 patients with pathology slides available for re-review. Only about half (21 of 39 or 54%) of pediatric IDC hearts showed histologic signs of fibrosis. The majority of fibrosis noted on pathologic analysis of these pediatric hearts was endocardial (90% of patients) while only 19% had interstitial fibrosis (Figure 1 and Table 1). Interestingly, the distribution of fibrosis reported in adults tends to be interstitial (16).
Figure 1. Representative trichrome-stained sections of pediatric IDC LV.
A. Pediatric IDC patient with no fibrosis (duration of disease 3.76 years). Magnification = 5x. B. Pediatric IDC patient with endocardial fibrosis (duration of disease 0.32 years). Magnification = 5x.
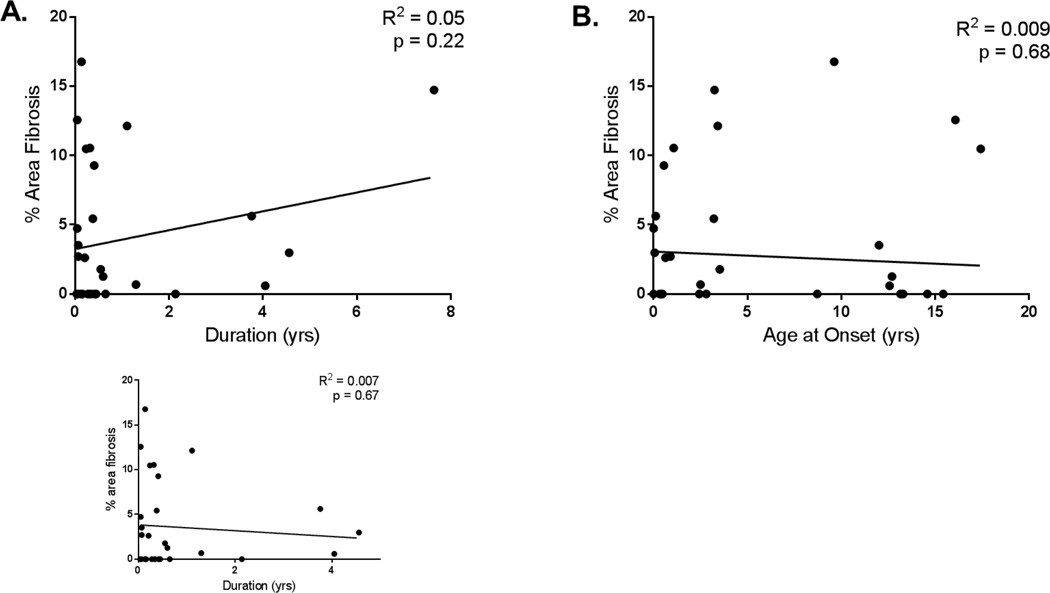
Further, in the pediatric population we studied, the time from HF onset to transplant averaged 1 year (Range = 0.01 to 7.64 years; mean = 0.9 years; median = 0.33 years). There was no correlation between duration of HF nor age at onset of HF and percent area of fibrosis noted on histology in the pediatric patients (Figure 2A and B). There was also no correlation between age at onset or duration of HF and gene expression for any of the genes studied (data not shown).
Figure 2. Percent area of fibrosis related to duration of HF and age at HF onset in pediatric IDC.
A. There is no correlation between percent area fibrosis and duration of HF in pediatric IDC patients. R2 = 0.05, n = 30, p = 0.22. Subset graph demonstrates no correlation with patient with 8 years of HF removed. R2 = 0.007, n = 29, p = 0.67. B. There is no correlation between percent area fibrosis and age at HF onset in pediatric IDC patients. R2 = 0.006, n = 30, p = 0.68. Linear regression analysis was done to determine the relationship between % area fibrosis and each parameter was analyzed for linearity, homoscedasticity, and normality of errors.
Different than adults, non-coding Gal-3 or Corin gene expression is unchanged in pediatric IDC patients
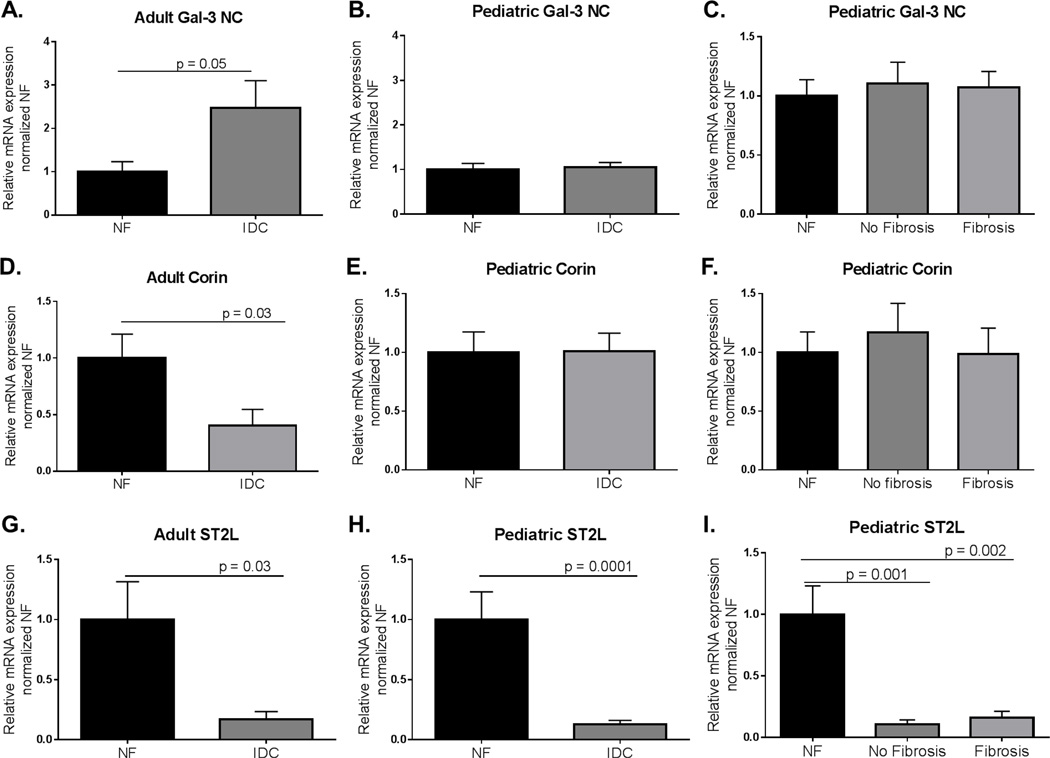
Using qRT-PCR, we analyzed expression of non-coding Gal-3, Corin, and ST2L in LV tissue from adult and pediatric NF and IDC hearts (Figure 3). The expression of non-coding Gal-3 was elevated in adult IDC (NF = 1.00±0.23; IDC = 2.47±0.63, p = 0.05), but not in pediatric IDC LV compared to age-matched NF control LV (NF = 1.00±0.14; IDC = 1.06±0.10) (Figure 3A and B). Corin expression was lower in adult IDC, but not pediatric IDC LV compared to NF (Adult NF = 1.00±0.21; Adult IDC = 0.40±0.14, p = 0.03; Ped NF = 1.00±0.17; Ped IDC = 1.01±0.15) (Figure 3D and E). We also found neither non-coding Gal-3 nor Corin expression changed with the pathologic presence of fibrosis in the pediatric IDC patients (Figure 3C and 3F). Further, we analyzed ST2L expression in the LV of pediatric and adults with IDC. We found ST2L was significantly lower in both adult and pediatric IDC LV (Adult NF = 1.00±0.32; Adult IDC = 0.169±0.07, p = 0.03; Ped NF = 1.00±0.23; Ped IDC = 0.130±0.20, p = 0.0001) (Figure 3G and H). Interestingly, this decreased expression of the membrane-receptor was seen in pediatric hearts with and without fibrosis (Figure 3I).
Figure 3. Expression of Gal-3NC, Corin, and ST2L in adult and pediatric IDC LV normalized to age-matched NF controls.
A. Expression of Gal-3NC was significantly higher in adult IDC LVs compared to NF controls. Significance was determined using unpaired Student’s t-test with Welch’s correction: p = 0.05. B. Expression of Gal-3NC in pediatric IDC LV normalized to age-matched NF controls. Unpaired Student’s t-test with Welch’s correction was used to determine significance. C. Expression of Gal-3NC in pediatric failing IDC hearts with and without fibrosis. Significance was determined by one way ANOVA with Sidak’s multiple comparison test. D. Expression of Corin in adult IDC LV normalized to NF controls. Significance was determined by Mann-Whitney analysis: p = 0.03. E. Corin expression in pediatric IDC LV normalized to age-matched controls. Unpaired Student’s t-test with Welch’s correction determined there is no difference in relative expression. F. Comparison of Corin expression in pediatric IDC LV in the presence of fibrosis. There was not a significant difference in Corin expression as determined by Kruskal-Wallis analysis with Sidak’s multiple comparison test. G. Expression of ST2L in adult IDC LVs normalized to NF controls. Significance was determined by an unpaired Student’s t-test with Welch’s correction: p = 0.03. H. ST2L expression in pediatric IDC LV normalized to NF controls. Significance was determined by unpaired Student’s t-test with Welch’s correction: p = 0.0001. I. Comparison of ST2L expression in pediatric IDC LV in the presence of fibrosis. Significance was determined by one way ANOVA with Sidak’s multiple comparison test: NF v no fibrosis p = 0.001; NF v Fibrosis p = 0.002.
MMPs and TIMPs are differentially dysregulated in pediatric IDC LVs
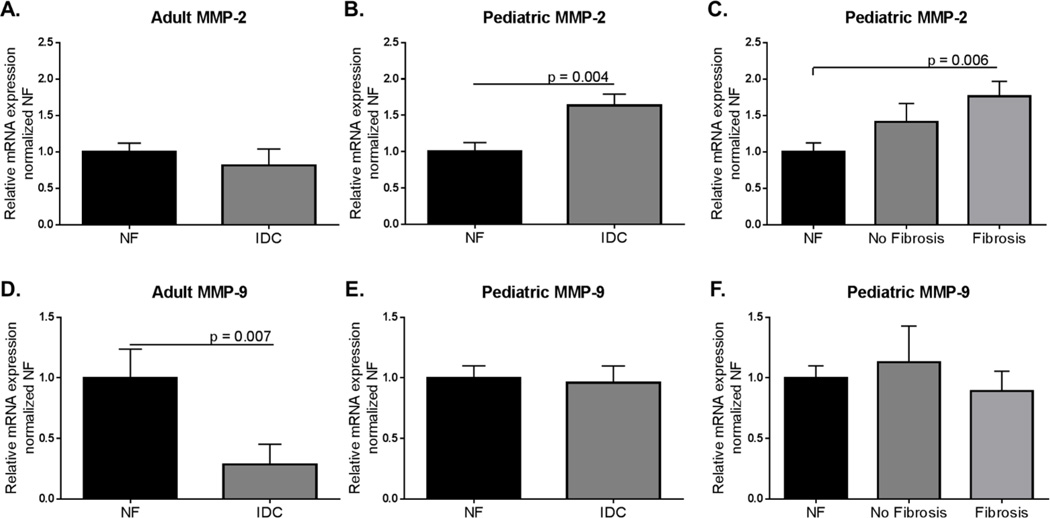
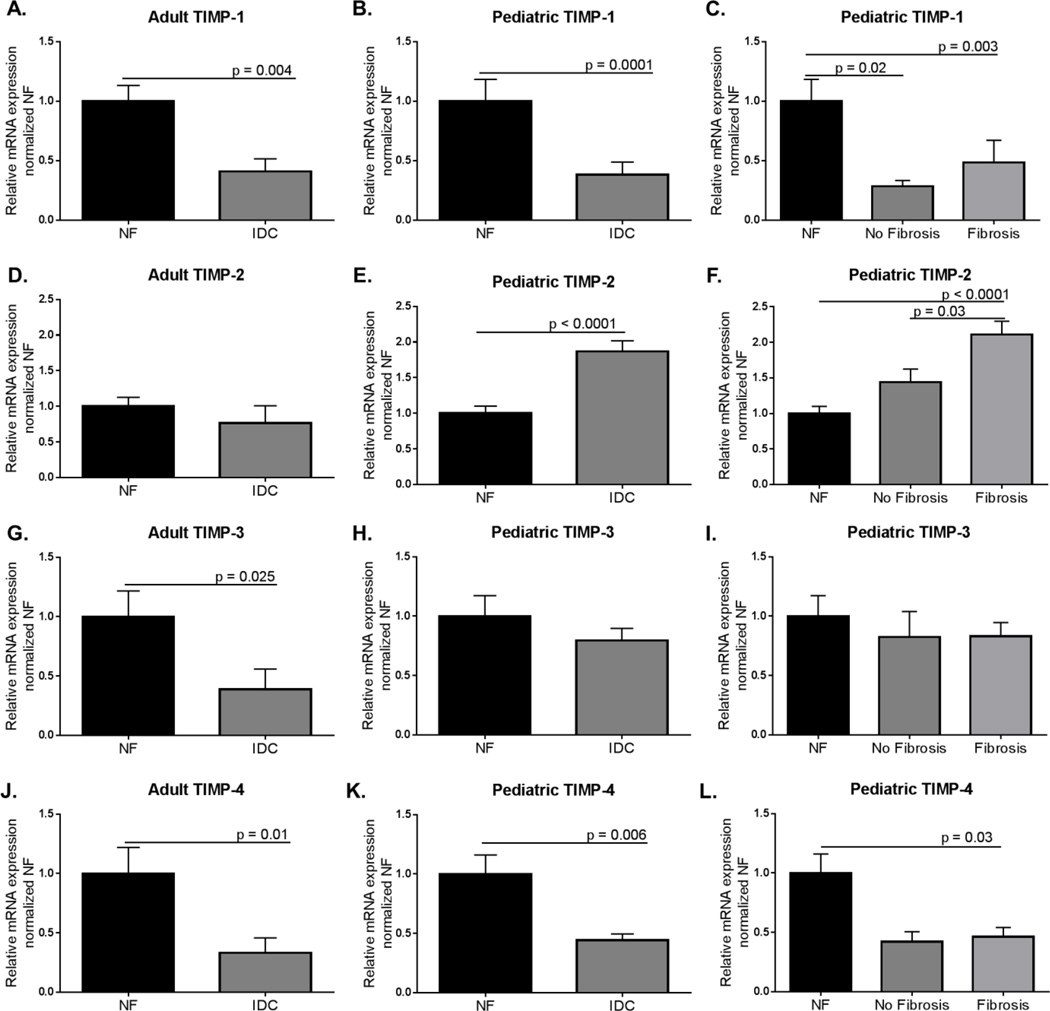
We measured the expression of MMP-2 and MMP-9 in the LVs from pediatric and adult NF and IDC hearts and found differential results (Figure 4). In the adult IDC hearts there was no difference in MMP-2 expression (NF = 1.00±0.12; IDC = 0.82±0.22), whereas it is upregulated in pediatric IDC LVs (NF = 1.00±0.12; IDC 1.64±0.16, p = 0.004) (Figure 4A and B). MMP-2 was higher in the pediatric hearts with fibrosis compared to NF controls (Ped IDC: No fibrosis = 1.11±0.25; Ped IDC: Fibrosis = 1.76± 0.21, NF v Fibrosis, p = 0.006) (Figure 4C) but was unchanged in pediatric hearts without fibrosis. In contrast, MMP-9 was significantly lower in adult IDC LV, but unchanged in pediatric IDC LV compared to NF controls (Adult NF = 1.00±0.24; Adult IDC = 0.29±0.17, p = 0.007; Pediatric NF = 1.00±0.10; Pediatric IDC = 0.96±0.14) (Figure 4D and E). Since TIMPs inhibit MMPs, we also explored the expression of TIMPs 1–4 (Figure 5). Interestingly, TIMP-1, 3 and 4 were downregulated in the LVs from adult IDC hearts (TIMP-1: NF = 1.00±0.13; IDC = 0.41±0.11, p = 0.003; TIMP-3: NF = 1.00±0.22; IDC = 0.39±0.17, p = 0.025; TIMP-4: NF = 1.00±0.22; IDC = 0.33±0.13, p = 0.01) (Figures 5A, G, and J). TIMP-1 and 4 were also significantly down-regulated in pediatric IDC LVs (TIMP-1: NF = 1.00±0.18; IDC = 0.38±0.11, p = 0.0001; TIMP-4: NF = 1.00±0.16; IDC = 0.44±0.05, p = 0.006) (Figures 5B and K). TIMP-1 was down-regulated in presence and absence of fibrosis (Figure 5C), while TIMP-4 was down-regulated in pediatric LV with fibrosis (Figure 5L). Importantly, TIMP-2 was upregulated in pediatric but not adult IDC LVs (Adult NF = 1.00±0.13; Adult IDC = 0.77±0.24; Ped NF = 1.00±0.10; Ped IDC = 1.87±0.15, p < 0.0001) (Figure 5D and E), and is associated with the presence of fibrosis (Figure 5F).
Figure 4. Expression of MMP-2 and MMP-9 in adult and pediatric IDC LV normalized to age-matched NF controls.
A. Expression of MMP-2 in adult IDC LV normalized to age-matched NF controls. Significance was determined using unpaired Student’s t-test with Welch’s correction. B. MMP-2 expression was significantly higher in pediatric IDC LV than in age-matched NF controls as determined by an unpaired Student t-test with Welch’s correction: p = 0.004. C. Expression of MMP-2 in pediatric IDC LVs separated by presence of fibrosis. This data was analyzed using one way ANOVA with Sidak’s multiple comparison test: NF v fibrosis p = 0.006. D. Expression of MMP-9 in adult IDC LV normalized to NF controls. Significance was determined by unpaired Student’s t-test with Welch’s correction: p = 0.007. E. Expression of MMP-9 in pediatric IDC LV normalized to age-matched NF controls. This data was analyzed using an unpaired Student’s t-test with Welch’s correction. F. Expression of MMP-9 in pediatric IDC LVs separated by presence of fibrosis. This data was analyzed using one way ANOVA with Sidak’s multiple comparisons test.
Figure 5.
Expression of TIMP-1, TIMP-2, TIMP-3, and TIMP-4 in adult and pediatric IDC LV normalized to age-matched NF controls. A. Expression of TIMP-1 in adult IDC LV normalized to NF controls. Significance was determined using unpaired Student’s t-test with Welch’s correction: p = 0.003. B. Expression of TIMP-1 in pediatric IDC LV normalized to age-matched NF controls. Mann-Whitney analysis was used to determine significance: p = 0.0001. C. Expression of TIMP-1 in pediatric failing IDC LV separated by presence of fibrosis. Significance was determined by one way ANOVA with Sidak’s multiple comparison test: NF v no fibrosis p = 0.02; NF v fibrosis p = 0.003. D. Expression of TIMP-2 in adult IDC LV normalized to NF controls. Significance was determined by unpaired Student’s t-test with Welch’s correction. E. TIMP-2 expression in pediatric IDC LV normalized to age-matched controls. Unpaired Student’s t-test with Welch’s correction determined there is a significant increase in TIMP-2 expression in pediatric IDC LV compared with age-matched NF controls: p <0.0001. F. Comparison of TIMP-2 expression in pediatric fibrotic and not fibrotic LV. One-way ANOVA with Sidak’s multiple comparison test demonstrated significant differences between the groups: NF v no fibrosis p = 0.03; NF v fibrosis p < 0.0001. G. Expression of TIMP-3 in adult IDC LVs normalized to NF controls. Significance was determined by an unpaired Student’s t-test with Welch’s correction: p = 0.025. H. TIMP-3 expression in pediatric IDC LV normalized to NF controls. Significance was determined by unpaired Student’s t-test with Welch’s correction. I. Comparison of TIMP-3 expression in pediatric fibrotic and non-fibrotic IDC hearts. There was no significant difference between the groups as assessed by one way ANOVA with Sidak’s multiple comparison test. J. Expression of TIMP-4 in adult IDC LVs normalized to NF controls. Significance was determined by an unpaired Student’s t-test with Welch’s correction: p = 0.01. H. TIMP-4 expression in pediatric IDC LV normalized to NF controls. Significance was determined by unpaired Student’s t-test with Welch’s correction: p = 0.006. I. Comparison of TIMP-4 expression in pediatric fibrotic and non-fibrotic IDC hearts. Significance was determined by one way ANOVA with Sidak’s multiple comparison test: NF v fibrosis p = 0.03.
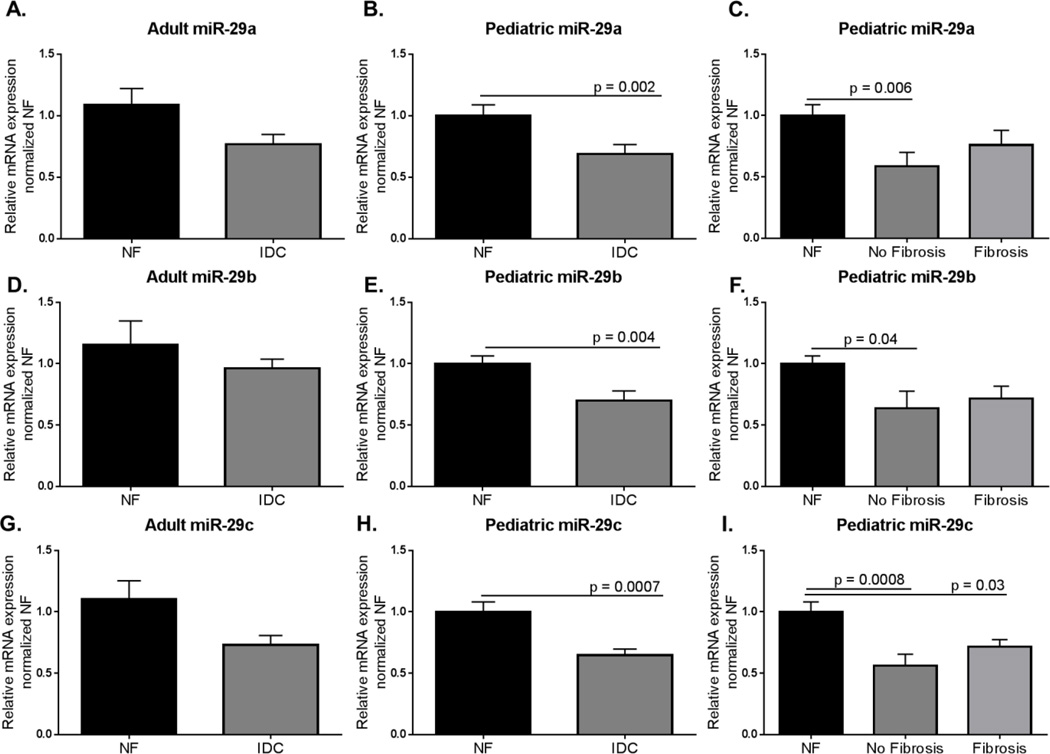
miRNA-29 a, b, and c are downregulated in pediatric IDC LVs
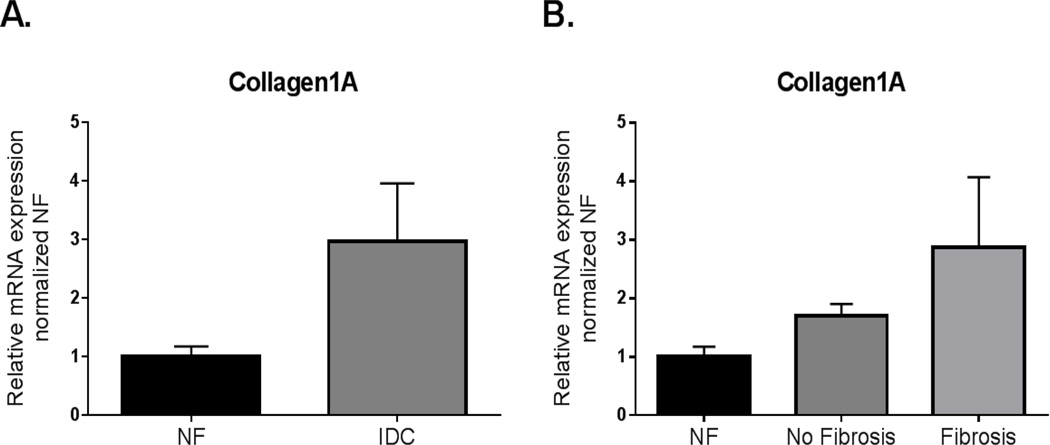
MicroRNAs (miRNA, miR) target the 3’UTR of mRNAs and promote RNA degradation and translation suppression. The miRNA-29 family of microRNAs target fibrotic processes including the components of the extracellular matrix, and increased expression of miRNA-29a, b, or c have been found to inhibit fibrosis (28, 29). While miRNA-29a, b, and c expression trended to decrease in adult IDC LV, there was significant downregulation of each of the 3 family members of the miRNA-29 family in pediatric IDC LV compared to NF control (miRNA-29a: Adult NF = 1.09±0.14; Adult IDC =0.77±0.08, p = 0.06; Pediatric NF = 1.00± 0.09; Pediatric IDC = 0.69± 0.08, p = 0.002; miR-29b: Adult NF = 1.15±0.20; Adult IDC = 0.96±0.07, p = 0.3; Pediatric NF = 1.00±0.06; Pediatric IDC = 0.70±0.08, p = 0.004; miR-29c: Adult NF = 1.10±0.15; Adult IDC = 0.73±0.08, p = 0.1; Pediatric NF = 1.00±0.08; Pediatric IDC = 0.65±0.05, p = 0.0007) (Figure 6A,B,D,E,G, and H). Interestingly, there was a significant downregulation in miRNA-29a, b, and c expression in pediatric HF patients without fibrosis (miRNA-29a: Pediatric NF = 1.00±0.09; Pediatric IDC with no fibrosis = 0.59±0.11, p = 0.006; Pediatric IDC with fibrosis = 0.76±0.12; miRNA29b: Pediatric NF = 1.00±0.06; Pediatric IDC with no fibrosis = 0.64±0.14, p = 0.004; Pediatric IDC with fibrosis = 0.72±0.10; miRNA-29c: Pediatric NF = 1.00±0.08; Pediatric IDC with no fibrosis = 0.56±0.09, p = 0.0008, Pediatric IDC with fibrosis = 0.72±0.06, p = 0.03) (Figure 6C, F, and I). Importantly, a target of miR-29, collagen 1A, as expected showed a trend to higher expression in the LV from pediatric IDC hearts (NF = 1.00± 0.17; IDC = 2.97± 0.99, p = 0.06) (Figure 7). This is only one of the many targets of the miR-29 family, but its upregulation supports miR-29’s influence on collagen gene expression.
Figure 6. Expression of miR29a, miR29b, and miR29c in adult and pediatric IDC LV normalized to age-matched NF controls.
A. Expression of miR-29a in adult IDC LVs compared to NF controls. Significance was determined using unpaired Student’s t-test with Welch’s correction. B. Expression of miR-29a was lower in pediatric IDC LV compared to age-matched NF controls. Significance was determined by Mann-Whitney analysis: p = 0.002. C. Expression of miR-29a in pediatric failing IDC hearts with and without fibrosis. Significance was determined by Kruskal-Wallis analysis with Sidak’s multiple comparison test: NF v No fibrosis p = 0.006. D. Expression of miR-29b in adult IDC LV normalized to NF controls. Significance was determined by unpaired Student’s t-test with Welch’s correction. E. miR-29b expression in pediatric IDC LV normalized to age-matched controls. Unpaired Student’s t-test with Welch’s correction determined significant decreased in miR-29b expression: p = 0.004. F. Comparison of miR-29b expression in pediatric IDC LV hearts in the presence of fibrosis. miR-29b was significantly lower in pediatric IDC LVs with no fibrosis as determined by one way ANOVA with Sidak’s multiple comparison test: NF v No fibrosis p = 0.04. G. Expression of miR-29c in adult IDC LVs normalized to NF controls. Significance was determined by an unpaired Student’s t-test with Welch’s correction. H. miR-29c expression in pediatric IDC LV normalized to NF controls. Significance was determined by unpaired Student’s t-test with Welch’s correction: p = 0.0007. I. Comparison of miR-29c expression in pediatric IDC LV in presence of fibrosis. Significance was determined by one way ANOVA with Sidak’s multiple comparison test: NF v no fibrosis p = 0.0008; NF v Fibrosis p = 0.003.
Figure 7. Expression of collagen 1A in pediatric IDC.
LV normalized to age-matched NF controls. A. Expression of collagen 1A in pediatric IDC LV. Significance was analyzed using an unpaired Student’s t-test with Welch’s correction. B. Expression in the pediatric hearts with IDC LVs separated by the presence of fibrosis. Data was analyzed by one way ANOVA with Sidak’s multiple comparison test.
Discussion
Fibrosis is an important component of myocardial remodeling in the adult heart. Based on histologic evaluation, we found that only 54% of pediatric IDC hearts in this cohort had any fibrosis, with interstitial fibrosis being very uncommon (13% of the entire cohort and 19% of those with any fibrosis). This finding is different from prior adult studies which describe 50–60% of patients having significant interstitial fibrosis on explant (16). We hypothesized that there would be differences in expression of key fibrotic markers in the pediatric and adult failing hearts. The current study demonstrates that failing pediatric hearts have different expression of important mRNAs that affect fibrosis.
In order to elucidate the differences between fibrosis in pediatric and adult IDC patients, we studied expression of several known fibrotic markers. Galectin-3 (Gal-3), Corin and ST2L, are all considered promising biomarkers that correlate with fibrosis in adult hearts (22–24, 26). Gal-3 is a β-galactoside binding lectin that activates fibroblasts which leads to increased deposition of collagen I (32–34). Corin is a cardiac serine protease that converts BNP to its active form (35). Corin is decreased in adults with HF which leads to impaired BNP processing (24, 25). ST2 is a member of the IL-1 receptor family which has 2 forms: ST2L, a transmembrane receptor and sST2 which is a soluble decoy receptor (26). When ST2L binds IL-33 it attenuates cardiac fibrosis and hypertrophy (27). Interestingly, 2 of the promising biomarkers that have been related to the amount of fibrosis and LV dysfunction in adult patients, Corin and Gal-3, did not show dysregulated expression in pediatric IDC LV. There was also no difference in either marker when comparing expression in pediatric hearts with or without fibrosis; this indicates Corin and Gal-3 may not be predictive of fibrosis in pediatric IDC patients. Meanwhile, ST2L, a counterpart in the ST2 signaling pathway which also has promising biomarker potential, was distinctly downregulated in both pediatric and adult IDC LVs. Despite the fact that the ST2 signaling pathway is altered in both pediatric and adult IDC patients, our study and others demonstrate a lower prevalence of fibrosis in the pediatric heart (8, 18). It is possible other components of the ST2 regulatory system (such as sST2, the circulating decoy receptor) have differential expression in children suggesting further study of this circulating biomarker in the pediatric population is relevant.
We also determined the expression of metalloproteinases and their inhibitors. An imbalance of these important regulators of extracellular matrix synthesis and degradation leads to fibrosis. Many studies have found both dysregulation of MMPs (breakdown of ECM) and TIMPs (breakdown of MMPs) lead to fibrosis in IDC in both adults and animal models (36–40). Importantly we found a significant upregulation of MMP-2 and TIMP-2 in pediatric IDC hearts in contrast to no significant changes in adult LV. MMP-9 and TIMP-3 were significantly downregulated in adult hearts but not in pediatric LV, and TIMP-1 and TIMP-4 were downregulated in both adult and pediatric hearts. The increased expression of MMP-2 in pediatric hearts could be a compensatory reaction to increased pro-fibrotic stimuli and could influence the reduced fibrotic phenotype in pediatric patients. TIMP-2 expression was increased specifically in those children with fibrosis and could represent an attempt to maintain the balance of MMPs. Interestingly, the ratio between MMP-2 and TIMP-2 in pediatric IDC patients did not significantly differ (NF = 1.05±0.30; IDC no fibrosis = 0.98±0.27; IDC with fibrosis = 0.90±0.32). Importantly, MMPs and TIMPs are known to impact intracellular targets as well as extracellular matrix (41–43). These intracellular targets cover many areas of cellular function including metabolism, cytoskeleton, transcription, translation, signal transduction, and apoptosis (41). MMP-2 has been reported to be upregulated in ischemia/reperfusion models in the heart and contributes to cardiac dysfunction by degrading cytoskeletal components such as Troponin I (42). Inhibition of MMP-2 in cardiomyocytes in vitro demonstrated an increase in contractility and improved metabolism (44). Given the fact these molecules can affect so many different intracellular mechanisms, it is possible that the upregulation of MMP-2 could contribute to cardiomyocyte dysfunction in the failing pediatric heart. So in addition to balancing fibrotic deposition, these molecules could potentially contribute to other aspects of the state of the disease. Further, it has been reported that current commonly used HF therapies inhibit MMPs and TIMPs (45). Since all analyzed patients were treated with a variety of these medications, the expression of the proteinases and TIMPs may be affected by medical intervention.
Finally, we determined the expression of miR-29a, b, and c, a family of microRNAs deeply involved in the fibrotic process (29). In the literature, miR-29 downregulation has been associated with the infarct zone in a mouse model of myocardial infarction (MI) (29). Additionally, we showed that miR-29 expression post-MI is time dependent; expression of miR-29c was down-regulated two weeks post-MI, but expression of miR-29b and -29c was upregulated two months post-MI. Similarly, there has been some disagreement about whether these microRNAs are up or downregulated in adult heart failure. Lai et al (2015) (46) reported miR-29 is upregulated in heart failure whereas Dawson et al (2013) (47) demonstrated its downregulation in adults with heart failure. These differences could be related to disease duration. Interestingly, we found all 3 miR-29s are downregulated in pediatric IDC patient hearts, and no changes in the adult IDC heart. It is possible that these differences are related to disease duration in the two populations. Importantly, we found a trend toward upregulation of collagen1A in the pediatric IDC hearts, a known target of miR-29 (28, 29). However, expression of miR-29a-c and collagen gene expression was not differentially regulated based on the presence or absence of pathological fibrosis in children with IDC, suggesting that there are additional regulators of the fibrotic process that are in play.
Taken together these data demonstrate a difference between adult and pediatric IDC LV fibrosis and fibrotic signaling. It has been hypothesized that the reason adult HF patients develop fibrosis is due to the length of disease duration which averages 5 years (48, 49). The average duration of disease (age of onset to time of transplant) in the patient population we studied was 1 year, with a range of 0.01 to 7.6 years. Another interesting consideration given these variable outcomes is the idea that fibrosis is a protective mechanism to preserve cardiac function during injury. In a pressure-overload mouse model, lack of collagen VIII led to decreased fibrosis but premature mortality (50). It is possible the decreased presence of fibrosis in pediatric IDC patients represents an inappropriate response to injury, leading to progression to failure at a more rapid pace than adult hearts. This remarkable variability in prevalence of fibrosis and duration of disease reinforces the concept that there are different disease mechanisms and responses to existing therapeutics between adult and pediatric IDC patients.
Overall, given the dysregulation of mRNAs associated with fibrosis, it seems that the pediatric patients are in a pre-fibrotic state. Whether they are better able to compensate for the increased pro-fibrotic signaling (by upregulating MMP-2 and TIMP-2) or if there is a separate disease process preventing fibrotic deposition that could be detrimental is unclear at this point. However, these data indicate that larger, prospective studies are needed to better understand the utility of anti-fibrotic medical therapy in children with HF.
Limitations
There are important limitations to this study that warrant consideration. First, the selection of genes does not encompass all possible pro-fibrotic pathways. Instead, we chose, a priori, to investigate genes based primarily on clinical relevance as biomarkers (Gal-3, Corin, ST2L), those impacted by the RAAS (TIMPs and MMPs), collagen as a global marker of fibrosis and miR29 due to its potential role as a regulator of varied pro-fibrotic genes. Second, while we did not find an association between duration of HF or age at onset of HF and percent area of fibrosis on histology or gene expression we may not have had adequate power to answer this question with finality. While we are unable to determine at which age a pediatric heart transitions into an adult heart with respect to myocardial response to HF, our data emphasizes that age remains an important consideration in the study of the failing heart. Finally, we do not know if there is a differential cellular profile or fibroblast content in the pediatric hearts based on age or in comparison to the adult hearts. Studies in the developing murine heart have demonstrated an increase in fibroblast production over the first week of life as the ventricular extracellular matrix is remodeled to adapt to increased postnatal afterload (51, 52). By one month of age the mouse heart cellular make-up resembles the adult phenotype (53). While we did not find a correlation with age and percent area fibrosis it is possible that variable fibroblast number or inflammatory cell components play a role in the gene expression findings and fibrotic response in HF.
Conclusion
Children with IDC demonstrate age-specific differences in the molecular pathways implicated in fibrosis in the adult heart. At the molecular level the unique gene expression pattern appears to limit fibrosis in the failing pediatric heart. Furthermore, the degree of fibrosis in this cohort of patients was not related to duration of disease as is suggested by the adult experience. Taken together these data provide the framework to support additional studies regarding the efficacy of RAAS inhibitors in children with HF. Expanding our understanding of the mechanisms that limit fibrotic deposition in the pediatric heart could lead to new therapeutic targets.
Acknowledgments
This work was supported by gifts from the Boedecker Foundation, Boulder, CO, and the Nair family, and by funding from National Institutes of Health (NIH) grant R01 HL07715 (BLS), R21 HL097123 (BLS, CCS, SDM), T32HL007171 (KCW), and American Heart Association (AHA) grant 16POST29970010 (KCW). Further this work was supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The authors would like to thank Anis Karimpour-fard for her overview and expert advice regarding the statistical analysis of the data. The authors would also like to thank and acknowledge the Children’s Hospital Colorado Heart Transplant Team for their contributions to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kathleen C. Woulfe, Department of Cardiology, University of Colorado, 12700 E 19th Avenue, B139, Aurora, CO 80045.
Austine K. Siomos, Children’s Hospital Colorado, 13123 E. 16th Avenue, B100, Aurora, CO 80045.
Hieu Nguyen, Children’s Hospital Colorado, 13123 E. 16th Avenue, B100, Aurora, CO 80045.
Megan SooHoo, Children’s Hospital Colorado, 13123 E. 16th Avenue, B100, Aurora, CO 80045.
Csaba Galambo, Children’s Hospital Colorado, 13123 E. 16th Avenue, B100, Aurora, CO 80045.
Brian L. Stauffer, Division of Cardiology, Denver Health and Hospital Authority, Denver, CO.
Carmen Sucharov, University of Colorado, 12700 E 19th Avenue, B139, Aurora, CO 80045.
Shelley Miyamoto, Children’s Hospital Colorado, 13123 E. 16th Avenue, B100, Aurora, CO 80045; Phone #: 303-724-6146.
References
- 1.Kirk R, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: thirteenth official pediatric heart transplantation report--2010. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29(10):1119–1128. doi: 10.1016/j.healun.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. Jama. 2006;296(15):1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 3.Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. Journal of the American College of Cardiology. 2010;55(13):1377–1384. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 4.Rossano JW, Shaddy RE. Heart failure in children: etiology and treatment. J Pediatr. 2014;165(2):228–233. doi: 10.1016/j.jpeds.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto SD, Stauffer BL, Nakano S, Sobus R, Nunley K, Nelson P, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. European heart journal. 2014;35(1):33–41. doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto SD, Karimpour-Fard A, Peterson V, Auerbach SR, Stenmark KR, Stauffer BL, et al. Circulating microRNA as a biomarker for recovery in pediatric dilated cardiomyopathy. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34(5):724–733. doi: 10.1016/j.healun.2015.01.979. [DOI] [PubMed] [Google Scholar]
- 7.Nakano SJ, Miyamoto SD, Movsesian M, Nelson P, Stauffer BL, Sucharov CC. Age-Related Differences in Phosphodiesterase Activity and Effects of Chronic Phosphodiesterase Inhibition in Idiopathic Dilated Cardiomyopathy. Circulation Heart failure. 2014 doi: 10.1161/CIRCHEARTFAILURE.114.001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. Jama. 2013;309(9):896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 9.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. Journal of the American College of Cardiology. 2008;51(25):2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. Journal of the American College of Cardiology. 2006;48(10):1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 11.Lehrke S, Lossnitzer D, Schob M, Steen H, Merten C, Kemmling H, et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart. 2011;97(9):727–732. doi: 10.1136/hrt.2010.205542. [DOI] [PubMed] [Google Scholar]
- 12.Unverferth DV, Baker PB, Swift SE, Chaffee R, Fetters JK, Uretsky BF, et al. Extent of myocardial fibrosis and cellular hypertrophy in dilated cardiomyopathy. The American journal of cardiology. 1986;57(10):816–820. doi: 10.1016/0002-9149(86)90620-x. [DOI] [PubMed] [Google Scholar]
- 13.Brilla CG, Maisch B, Weber KT. Renin-angiotensin system and myocardial collagen matrix remodeling in hypertensive heart disease: in vivo and in vitro studies on collagen matrix regulation. Clin Investig. 1993;71(5 Suppl):S35–S41. doi: 10.1007/BF00180074. [DOI] [PubMed] [Google Scholar]
- 14.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. Journal of the American College of Cardiology. 1989;13(7):1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 15.Ferrans VJ. Pathologic anatomy of the dilated cardiomyopathies. The American journal of cardiology. 1989;64(6):9C–11C. doi: 10.1016/0002-9149(89)90677-2. [DOI] [PubMed] [Google Scholar]
- 16.de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM. Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int. 2001;14(5):299–306. doi: 10.1007/s001470100339. [DOI] [PubMed] [Google Scholar]
- 17.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. The Journal of clinical investigation. 2007;117(3):568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latus H, Gummel K, Klingel K, Moysich A, Khalil M, Mazhari N, et al. Focal myocardial fibrosis assessed by late gadolinium enhancement cardiovascular magnetic resonance in children and adolescents with dilated cardiomyopathy. J Cardiovasc Magn Reson. 2015;17:34. doi: 10.1186/s12968-015-0142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, He L, Cai J, Lv T, Yi Q, Xu Y, et al. Measurements in Pediatric Patients with Cardiomyopathies: Comparison of Cardiac Magnetic Resonance Imaging and Echocardiography. Cardiology. 2015;131(4):245–250. doi: 10.1159/000381418. [DOI] [PubMed] [Google Scholar]
- 20.Etesami M, Gilkeson RC, Rajiah P. Utility of late gadolinium enhancement in pediatric cardiac MRI. Pediatr Radiol. 2015 doi: 10.1007/s00247-015-3526-2. [DOI] [PubMed] [Google Scholar]
- 21.Burns KM, Byrne BJ, Gelb BD, Kuhn B, Leinwand LA, Mital S, et al. New mechanistic and therapeutic targets for pediatric heart failure: report from a National Heart, Lung, and Blood Institute working group. Circulation. 2014;130(1):79–86. doi: 10.1161/CIRCULATIONAHA.113.007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frunza O, Russo I, Saxena A, Shinde AV, Humeres C, Hanif W, et al. Myocardial Galectin-3 Expression Is Associated with Remodeling of the Pressure-Overloaded Heart and May Delay the Hypertrophic Response without Affecting Survival, Dysfunction, and Cardiac Fibrosis. Am J Pathol. 2016;186(5):1114–1127. doi: 10.1016/j.ajpath.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French B, Wang L, Ky B, Brandimarto J, Basuray A, Fang JC, et al. Prognostic Value of Galectin-3 for Adverse Outcomes in Chronic Heart Failure. Journal of cardiac failure. 2016;22(4):256–262. doi: 10.1016/j.cardfail.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong N, Chen S, Wang W, Zhou Y, Wu Q. Corin in clinical laboratory diagnostics. Clin Chim Acta. 2012;413(3–4):378–383. doi: 10.1016/j.cca.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee R, Xu B, Rame JE, Felkin LE, Barton P, Dries DL. Regulated inositol-requiring protein 1-dependent decay as a mechanism of corin RNA and protein deficiency in advanced human systolic heart failure. J Am Heart Assoc. 2014;3(6):e001104. doi: 10.1161/JAHA.114.001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Januzzi JL., Jr ST2 as a cardiovascular risk biomarker: from the bench to the bedside. J Cardiovasc Transl Res. 2013;6(4):493–500. doi: 10.1007/s12265-013-9459-y. [DOI] [PubMed] [Google Scholar]
- 27.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. The Journal of clinical investigation. 2007;117(6):1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, et al. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45(2):287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sucharov CC, Sucharov J, Karimpour-Fard A, Nunley K, Stauffer BL, Miyamoto SD. Micro-RNA expression in hypoplastic left heart syndrome. Journal of cardiac failure. 2015;21(1):83–88. doi: 10.1016/j.cardfail.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19(7–9):527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 33.Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33(1):67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 34.Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circulation Heart failure. 2013;6(1):107–117. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 35.Ichiki T, Huntley BK, Burnett JC., Jr BNP molecular forms and processing by the cardiac serine protease corin. Adv Clin Chem. 2013;61:1–31. doi: 10.1016/b978-0-12-407680-8.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polyakova V, Hein S, Kostin S, Ziegelhoeffer T, Schaper J. Matrix metalloproteinases and their tissue inhibitors in pressure-overloaded human myocardium during heart failure progression. Journal of the American College of Cardiology. 2004;44(8):1609–1618. doi: 10.1016/j.jacc.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Polyakova V, Loeffler I, Hein S, Miyagawa S, Piotrowska I, Dammer S, et al. Fibrosis in endstage human heart failure: severe changes in collagen metabolism and MMP/TIMP profiles. Int J Cardiol. 2011;151(1):18–33. doi: 10.1016/j.ijcard.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 38.Herpel E, Pritsch M, Koch A, Dengler TJ, Schirmacher P, Schnabel PA. Interstitial fibrosis in the heart: differences in extracellular matrix proteins and matrix metalloproteinases in end-stage dilated, ischaemic and valvular cardiomyopathy. Histopathology. 2006;48(6):736–747. doi: 10.1111/j.1365-2559.2006.02398.x. [DOI] [PubMed] [Google Scholar]
- 39.Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovascular research. 2000;46(2):307–315. doi: 10.1016/s0008-6363(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 40.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97(17):1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 41.Cauwe B, Opdenakker G. Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2010;45(5):351–423. doi: 10.3109/10409238.2010.501783. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106(12):1543–1549. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- 43.DeCoux A, Lindsey ML, Villarreal F, Garcia RA, Schulz R. Myocardial matrix metalloproteinase-2: inside out and upside down. Journal of molecular and cellular cardiology. 2014;77:64–72. doi: 10.1016/j.yjmcc.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin HB, Sharma K, Bialy D, Wawrzynska M, Purves R, Cayabyab FS, et al. Inhibition of MMP-2 expression affects metabolic enzyme expression levels: proteomic analysis of rat cardiomyocytes. J Proteomics. 2014;106:74–85. doi: 10.1016/j.jprot.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 45.Hopps E, Caimi G. Matrix metalloproteases as a pharmacological target in cardiovascular diseases. Eur Rev Med Pharmacol Sci. 2015;19(14):2583–2589. [PubMed] [Google Scholar]
- 46.Lai KB, Sanderson JE, Izzat MB, Yu CM. Micro-RNA and mRNA myocardial tissue expression in biopsy specimen from patients with heart failure. Int J Cardiol. 2015;199:79–83. doi: 10.1016/j.ijcard.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 47.Dawson K, Wakili R, Ordog B, Clauss S, Chen Y, Iwasaki Y, et al. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation. 2013;127(14):1466–1475. 75e1-28. doi: 10.1161/CIRCULATIONAHA.112.001207. [DOI] [PubMed] [Google Scholar]
- 48.Blechman I, Arad M, Nussbaum T, Goldenberg I, Freimark D. Predictors and outcome of sustained improvement in left ventricular function in dilated cardiomyopathy. Clinical cardiology. 2014;37(11):687–692. doi: 10.1002/clc.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada Y, Saito S, Nishinaka T, Yamazaki K. Myocardial size and fibrosis changes during left ventricular assist device support. ASAIO J. 2012;58(4):402–406. doi: 10.1097/MAT.0b013e31825b9826. [DOI] [PubMed] [Google Scholar]
- 50.Skrbic B, Engebretsen KV, Strand ME, Lunde IG, Herum KM, Marstein HS, et al. Lack of collagen VIII reduces fibrosis and promotes early mortality and cardiac dilatation in pressure overload in mice. Cardiovascular research. 2015;106(1):32–42. doi: 10.1093/cvr/cvv041. [DOI] [PubMed] [Google Scholar]
- 51.Borg TK, Rubin K, Lundgren E, Borg K, Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol. 1984;104(1):86–96. doi: 10.1016/0012-1606(84)90038-1. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz-Villalba A, Ziogas A, Ehrbar M, Perez-Pomares JM. Characterization of epicardial-derived cardiac interstitial cells: differentiation and mobilization of heart fibroblast progenitors. PloS one. 2013;8(1):e53694. doi: 10.1371/journal.pone.0053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR. Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci. 2008;1123:30–40. doi: 10.1196/annals.1420.005. [DOI] [PubMed] [Google Scholar]