Abstract
Background/Objectives
Excessive infant weight gain in the first 6-months of life is a powerful predictor of childhood obesity and related health risks. In mice, omega-6 fatty acids (FA) serve as potent ligands driving adipogenesis during early development. The ratio of omega-6 relative to omega-3 (n-6/n-3) FA in human milk (HM) has increased 3-fold over the last 30 years, but the impact of this shift on infant adipose development remains undetermined. This study investigated how maternal obesity and maternal dietary FA (as reflected in maternal red blood cells (RBC) composition) influenced HM n-6 and n-3 FAs, and whether the HM n-6/n-3 ratio was associated with changes in infant adipose deposition between 2-weeks and 4-months postpartum.
Subjects/Methods
Forty-eight infants from normal-weight (NW), overweight (OW) and obese (OB) mothers were exclusively or predominantly breastfed over the first 4 months of lactation. Mid-feed HM and maternal RBC were collected at either transitional (2-weeks) or established (4-months) lactation, along with infant body composition assessed using air-displacement plethysmography. The FA composition of HM and maternal RBC was measured quantitatively by lipid mass spectrometry.
Results
In transitional and established HM, DHA was lower (P=0.008; 0.005) and the AA/DHA+EPA ratio was higher (P=0.05; 0.02) in the OB relative to the NW group. Maternal prepregnancy BMI and AA/ DHA+EPA ratios in transitional and established HM were moderately correlated (P=0.018; 0.001). Total infant fat mass was increased in the upper AA/DHA+EPA tertile of established HM relative to the lower tertile (P=0.019). The amount of changes in infant fat mass and % body fat were predicted by AA/EPA+DHA ratios in established HM (P=0.038; 0.010).
Conclusions
Perinatal infant exposures to a high AA/EPA+DHA ratio during the first 4-months of life, which is primarily reflective of maternal dietary FA, may significantly contribute to the way infants accumulate adipose.
Keywords: Human Milk, Fatty Acid Composition, Infant Adipose Deposition
Introduction
Childhood obesity rates in the United States have doubled in the last 30 years1. Maternal obesity increases offspring risk for childhood obesity2, 3, and up to 60% of U.S. women of childbearing age are overweight or obese4. Rapid and excessive infant weight gain over the first 6-months of life is a strong predictor of later obesity5–8, when infants typically double their percentage of body fat (% BF)5. Exclusive breastfeeding protects against excessive infant weight gain and lowers risk of childhood obesity9–12, but the mechanisms driving infant adipose deposition remain unclear and the degree of protection is influenced by maternal BMI13–15. Maternal pre-pregnancy BMI consistently associates with childhood overweight16, maternal diet strongly influences breast milk composition17, and both affect the fatty acid (FA) composition of human milk (HM)18. Accordingly, the variation in FA composition of HM may partially explain inconsistent protective effects of breastfeeding against excessive infant adipose deposition.
It is well established that adipose tissue expansion occurs through lipid filling of existing adipocytes (hypertrophy) and by increasing the population of resident adipocyte precursor cells (hypertrophy)19. Polyunsaturated FA (PUFA) are bioactive HM components contributing to the regulation of adipogenesis20, and the relative amount of n-6 to n-3 PUFA (n-6/n-3) present in HM has substantially increased in the United States over the last three decades17, 20. Functionally, excess n-6 FA activates adipogenesis in adipocyte culture and drives adipose tissue expansion in rodents21–24. In mice, maternal n-6 FA consumption in the perinatal window increases n-6 FA transmission to offspring, raises adipose tissue arachidonic acid (AA), and stimulates pathways implicated in adipose expansion in offspring and across generations22, 23, 25, 26. Lowering the n-6/n-3 FA ratio in mice, by maternal expression of the n-3 FA desaturase transgene (fat-1) or through perinatal dietary manipulation, protects the offspring from adult obesity22, 27, 28. Thus, in vivo evidence supports a direct role for n-6 FAs to drive offspring adipose tissue expansion during postnatal development21, 23, 24.
The impact of a high n-6/n-3 HM FA ratio on infant adipose deposition during gestation and lactation is less clear. An increase in HM n-6/n-3 FA ratio has potential to contribute to childhood obesity risk, similar to prenatal exposures for which a higher cord plasma n-6/n-3 was associated with risk of childhood adiposity at 3 years of age29. The present study utilized air-displacement plethysmography to assess infant adiposity. Quantitative lipid mass spectrometry was used to identify HM FA composition differences present in transitional milk (as secretory activation commences) and in established milk (following sustained breastfeeding) of normal-weight (NW), overweight (OW), and obese (OB) mothers. We hypothesized that perinatal exposures to elevated n-6/n-3 FA, whether driven by maternal obesity or diet, would associate with infant adipose deposition over the first 4-months of life.
METHODS
Participants
All participants were recruited and consented during pregnancy. The Colorado Multiple Institutional Review Board (COMIRB) approved all aspects of this study. Clinical trial registry number is NCT01693406. To be eligible, women needed to have a prepregnancy BMI ≤ 40 kg/m2, be carrying a singleton fetus, have the intention to exclusively breastfeed for at least 4 months, and be otherwise healthy. Women were excluded if they developed gestational diabetes or delivered their infant <37 weeks of gestation. Women were recruited into this study via two separate protocols. One protocol did not include maternal blood collection, and thus blood-based measurements were not performed on a subset of participants (n = 16). Maternal prepregnancy BMI was based on self-report of prepregnancy weight and height using standard BMI categories [normal weight (NW n = 26) 18.5–25, overweight (OW n = 12) = 25.1–30, and obese (OB n = 10) = 30.1–40]. Infant sex, birth weight, mode of delivery, and maternal gestational weight gain (GWG) were obtained from medical records. Women were classified as either inadequate, adequate, or excessive according to the IOM recommended GWG categories that are specific to each BMI category30.
Sample Collection
Mother/infant dyads were assessed at 2-weeks and 4-months postpartum. Visits occurred in the AM hours. Fasted (≥8 h) maternal venous blood and a mid-feed HM sample were collected. For HM, approximately halfway through a nursing session (estimated by the mother based on normal feeding time, sensation of milk removal, and breast softening), the infant was removed, nipple and areola wiped clean, and ~20 mL of HM was expressed by hand pump (One-Hand Manual Pump, Ameda, Buffalo Grove, IL). Milk was immediately placed on ice, transported back to the laboratory, and aliquots of whole milk stored at −80 °C until analysis.
During visits, mothers were administered a modified version of the Infant Feeding Practices II questionnaire that queries infant feeding practices and exclusivity over the preceding 7 days31. From this questionnaire, breastfeeding exclusivity calculated at each visit was expressed as a decimal (1.0 = exclusive breastfeeding, 0.0 = exclusive formula feeding). Mothers self-reported use of fish oil and any other dietary supplements. Infant weight and percentage body fat (%BF) were measured in duplicate by air-displacement plethysmography (PEA POD™; COSMED, Chicago, IL) at 2-weeks and 4-months postpartum.
HM total lipid extraction
Milk was briefly thawed at 37 °C, mixed well by inversion, and 50 µL was added to 200 µL of potassium phosphate buffer pH 7.0, then 400 µL of methanol was added and samples were vortexed vigorously. Total lipid extraction was performed as previously described32 with the following modifications. A 3:1 (vol/vol) isooctane/ethyl acetate solution was used to extract the milk fat and the samples were resuspended in 500 µL of isooctane. Percentage of fat was measured by creamatocrit, and fat g/L was estimated using the following approximation: g/L = (creamatocrit [%] – 0.59) / (0.146)33.
Quantification of milk triglyceride
Twenty-five µL of isooctane-suspended total milk lipid was evaporated to dryness, samples were resuspended in 200 µL dichloromethane containing 15 µL of a 10% nonaethylene glycol monododecyl ether (Sigma-Aldrich, St. Louis, MO) dissolved in dichloromethane (wt/vol). Samples were incubated for 5 min at 25 °C and taken to dryness at 40 °C for 25 min ensuring organic solvent was completely evaporated. Pellets contained triglyceride (TAG)/nonionic surfactant complexes, to which 200 µL of ultrapure water was carefully added without mixing and incubated at 40 °C for 10 min, followed by a gentle vortex. A standard regression curve 20, 10, 5, 2.5, 1.25, 0.625, and 0.3125 nmol was prepared from 80 nmol tripalmitin (Sigma-Aldrich) incubated with 25 µL of 10% nonaethylene glycol monododecyl ether, incubated, dried and resuspended in 100 µL of ultrapure water as above. Total TAG from the organic fraction was quantified using a modified colorimetric assay34 and expressed as mM concentrations. TAG Reagent and Free Glycerol Reagent (Sigma-Aldrich) were diluted according to the manufacturer’s instructions.
Human RBC lipid extraction
Maternal blood samples were collected into EDTA tubes and spun at 10,000 × g to separate plasma from RBCs. RBC were washed three times using 5 mL of sterile saline solution, collected by centrifugation, and saline was decanted before 500 µL of RBCs was transferred into cryovials and stored at −80 °C. RBCs were thawed on ice, mixed thoroughly by inversion, and 30 µL was added to 400 µL of ultrapure water containing stable isotope internal standards. One mL of HPLC grade isopropyl alcohol was added, samples were vortexed vigorously, and incubated at 27 °C for 10 min. Five hundred µL of dichloromethane was added, samples were vortexed vigorously, and incubated at 25 °C for 5 min. An additional 500 µL of dichloromethane was added and samples were vortexed. To complete phase separation, 500 µL of ultrapure water was added, samples were vortexed and incubated at 25 °C for 5 min before centrifugation at 300 × g for 10 min. FA results were normalized per mg of protein measured by Bradford assay.
HM and RBC FA quantification by GC/MS
The volume of organic extract representing 5 nmol of TAG in HM (equal TAG loading), or the 100 uL of total extracted maternal RBC lipid (above), containing 500 ng of internal stable isotope standards was saponified, extracted, and derivatized as previously described35. Pentafluorobenzyl FA esters were resuspended in 200 µL of hexane and diluted 1:10 into hexane for injection. Sample analysis was performed according to Rudolph and coworkers35, 36. The total n-6/n-3 FA ratio was the quantitative sum of all n-6 divided by the sum of all n-3 FAs, and the AA/eicosapentaenoic acid (EPA)+DHA ratio was the 20:4 n-6 divided by the sum of 20:5 n-3 plus 22:6 n-3.
Statistical analyses
Differences in participant characteristics by maternal BMI group were identified using one-way ANOVA with Tukey multiple testing correction. Differences in HM and RBC FA compositions between maternal BMI groups (NW vs. OW vs. OB) within transitional or established HM were identified using one-way ANOVA with Tukey multiple testing correction and plotted as the mean and s.e.m. Pearson correlation analysis was used to test correlations between the maternal prepregnancy BMI and FA ratios in HM or RBCs at 2-weeks and 4-months.
Multivariable linear regression evaluated two primary outcomes of infant adipose deposition between 2 weeks to 4 months: 1) the change in absolute fat mass (Δ-fat mass in g) and 2) the change in body fat percentage (Δ-%BF). The 2-week and 4-month FA ratio in HM considered as a predictor was the AA/DHA+EPA ratio. Models tested whether the AA/DHA+EPA ratio in HM predicted each infant outcome. GWG category, maternal prepregnancy BMI, infant sex, maternal use of fish oil supplements, birth weight, and breastfeeding exclusivity were retained as covariates due to their biological association with the outcome of infant adiposity. Statistical analyses were conducted using SPSS statistical software (IBM SPSS Statistics for Windows, Version 22.0 released 2013, Armonk, NY). GraphPad Prism was used for graphical presentation of data (version 6.0h for Mac, GraphPad Software, La Jolla California USA).
Results
Participant characteristics
Forty-eight mothers participated and ranged from 20 to 36 years old (Table 1). Fifty-four percent of the cohort were NW, 25% were OW and 21% were OB. No group differences in maternal age, mode of delivery, parity, or gestational age were observed. NW group mothers had a gestational weight gain (GWG) of 14.3 ± 0.9 kg, while the OW group tended to be higher (16.7 ± 1.8 kg) and the OB group tended to be lower (12.5 ± 2.4 kg), but neither group was significantly different from the NW group. At 2-weeks postpartum, infants in the OW group were significantly heavier those in the NW group (P = 0.022), and by 4-months, infants in the OW group were significantly heavier than those in the NW and OB groups (P < 0.001 and 0.03, respectively). There were no group differences in percent body fat (%BF) at 2-weeks and 4-months controlling for infant sex. Infants from OW mothers demonstrated greater overall weight gain from 2-weeks to 4-months, controlling for infant sex, relative to NW and OB groups (P = 0.036). The Infant Feeding Practices II scores for breastfeeding exclusivity were not different among groups (>98% exclusivity, Table 1). By 4-months there was a non-significant trend for lower breastfeeding exclusivity in the OB group relative to the NW group (P = 0.16).
Table 1.
Characteristics of mothers, infants, and breastfeeding by maternal prepregnancy BMI group
| All (n = 48) |
Normal weight (n = 26) |
Overweight (n = 12) |
Obese (n = 10) |
|
|---|---|---|---|---|
| Mothers | ||||
| Age, y | 30.5 ± 0.46 | 30.6 ± 0.5 | 30.6 ± 0.97 | 29.9 ± 1.45 |
| Prepregnancy BMI, kg/m2 | 25.3 ± 0.81 | 21.1 ± 0.4a | 27.1 ± 0.37b | 34.2 ± 0.91c |
| Vaginal delivery, % | 75 | 80 | 58 | 80 |
| Parity | 2.45 ± 0.18 | 2.46 ± 0.3 | 2.4 ± 0.33 | 2.5 ± 0.37 |
| Gestational age, wk | 39.91 ± 0.13 | 39.92 ± 0.14 | 39.89 ± 0.39 | 39.92 ± 0.29 |
| Gestational weight gain, kg | 14.5 ± 1.8 | 14.3 ± 0.9 | 16.7 ± 1.8 | 12.5 ± 2.4 |
| Fish oil supplementation, % | 26 | 35 | 17 | 10 |
| Infants | ||||
| Birth weight, g | 3362 ± 67 | 3147 ± 73a | 3576 ± 129b | 3665 ± 144b |
| Infant sex, % M | 60 | 46a | 83b | 70b |
| 2-week weight, g1 | 3665 ± 68 | 3509 ± 85a | 3938 ± 128b | 3744 ± 145a, b |
| 2-week fat, %1 | 11.1 ± 0.61 | 10.8 ± 0.86 | 12.2 ± 1.14 | 10.6 ± 1.22 |
| 4-month weight, g1 | 6482 ± 114 | 6174 ± 181a | 7195 ± 227b | 6426 ± 218a |
| 4-month fat, %1 | 22.9 ± 0.7 | 23.2 ± 0.8 | 24.1 ± 1.1 | 21.1 ± 2.0 |
| 2-wk to 4-mth Δ Weight (g)1 | 2817.1 | 2665.7a | 3257.7b | 2682.4a |
| Breastfeeding | ||||
| 2-week exclusivity2 | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.98 ± 0.02 | 0.99 ± 0.01 |
| 4-month exclusivity2 | 0.83 ± 0.05 | 0.92 ± 0.04 | 0.75 ± 0.13 | 0.69 ± 0.15 |
Results from one-way ANOVA with Tukey multiple testing correction presented as means ± SEMs unless otherwise noted.
Different superscript letters within a row indicate means are significantly different at P ≤ 0.05.
Controlling for infant sex
Breastfeeding exclusivity score of 1.0 = 100% exclusive
Maternal Obesity increases AA/DHA+EPA ratio but decreases DHA levels in HM
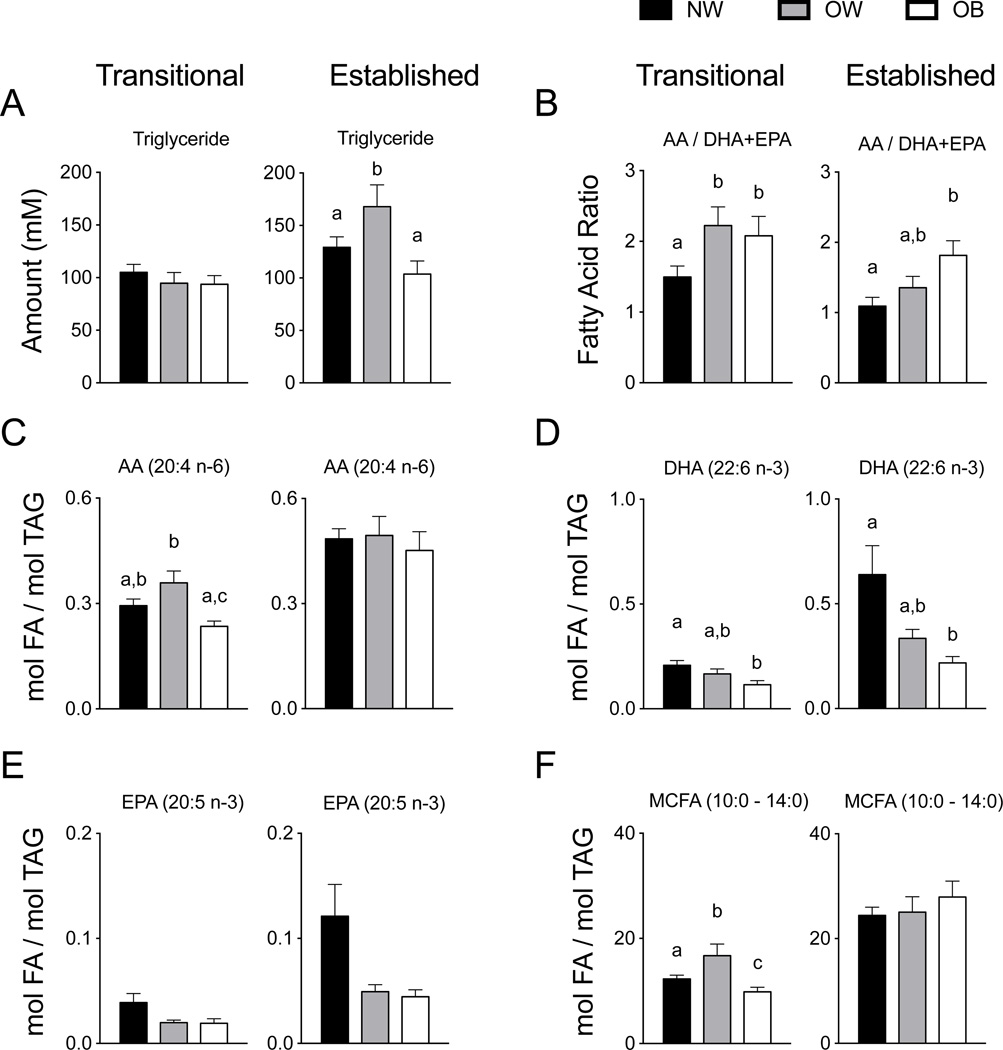
It is well established HM composition is dynamic over time, being dependent in part on stage of lactation37–40. Specifically, the fatty acid composition of breastmilk has been shown to differ by lactation stage41, 42, thus, samples included transitional (2-weeks) and established milk (4-months) to identify changes due to maternal obesity at each point individually. Transitional HM was not different in TAG amount, but in established HM the OW group had significantly greater TAG than did the NW and OB groups (P = 0.048 and 0.01) (Figure 1A), which was consistent with percentage of milk fat creamatocrit values (not shown). Transitional HM had an AA/DHA+EPA ratio 30–33% lower in the NW group than that observed in OW and OB groups (P = 0.04 and 0.05) (Figure 1B), however, the AA/DHA+EPA ratio in the NW group was lower than in the OB group in established HM (P = 0.02). The absolute amount of AA (20:4 n-6) present in the OW transitional HM was 1.6-times greater relative to the OB group (P = 0.004), but no differences were observed in the level of AA in established HM (Figure 1C). The absolute amount of DHA in transitional HM was 43% higher in the NW group than in the OB group (P = 0.008), while the OW group had DHA amounts in between NW and OB groups (Figure 1D). This pattern persisted as the absolute DHA amount in established HM was 65% greater in the NW group than in OB (P = 0.005), with the mean amount of the OW group between that of the NW and OB groups. Quantitative amounts of EPA were not significant among groups in either transitional or established HM. The quantitative amount of medium chain fatty acid (MCFA) was significantly increased in the OW group (P = 0.035) and significantly decreased in the OB group (P = 0.005) relative to the NW group (Figure 1F), however, the quantity of MCFA in established HM was not different (see Supplemental Table 1 and Supplemental Table 2 for all quantitative HM FA data).
Figure 1. Triglyceride and fatty acid composition of transitional and established human milk.
(A) In transitional milk TAG was not different by maternal prepregnancy BMI group, but in established HM the OW group had significantly greater TAG than did the NW and OB groups (P = 0.048 and 0.01). (B) The ratio of AA/DHA+EPA was significantly greater in OW and OB groups compared to NW (P = 0.03 and 0.04) in transitional HM, and in established HM the AA/DHA+EPA ratio in the OB group 41% greater than the NW group (P = 0.02). (C) The absolute amount of AA (20:4 n-6) present in transitional HM was 30–33% greater in the OW group in the NW and OB groups (P ≤ 0.01), but unchanged among groups in established HM. (D). The absolute DHA amount was decreased by 43% in the OB group relative to the NW group in transitional HM (P = 0.008), and in established HM the DHA amount was 65% less than in the NW group (P = 0.005). (E) EPA tended to be lower in the OW and OB groups in both transitional and established HM. (F) The MCFA in transitional HM was 36% greater in the OW group but 26% lower than the NW group (P = 0.035 and 0.005, respectively). MCFA, medium chain fatty acids; TAG, triglyceride; NW, normal weight n = 26; OW, overweight n = 12; OB, obese n = 10.
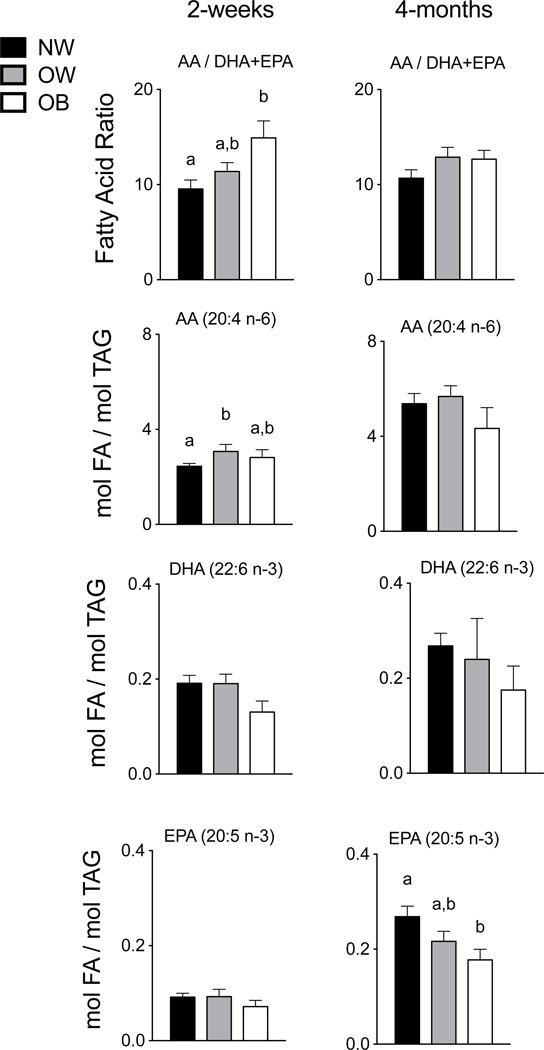
Given the differences observed by group in transitional and established HM, we investigated maternal RBC composition as a well-established marker of long-term maternal dietary fatty acid intake43. At 2-weeks postpartum, the AA/DHA+EPA FA ratio in OB maternal RBCs was 1.5-fold higher than that in the NW group (P = 0.007) (Figure 2). No difference was observed for the OW group, despite the greater AA/DHA+EPA FA ratio present in the OW group’s transitional HM. The absolute amount of AA in the RBCs of the OW group at 2-weeks postpartum was 21% greater than that detected in the NW group RBCs (P = 0.031), with no difference observed between NW and OB groups (Figure 2). At 4-months postpartum, the FA composition of maternal RBCs did not differ significantly among prepregnancy BMI groups, except for EPA that was decreased 34% in the OB relative to the NW groups (P = 0.04).
Figure 2. Maternal red blood cell membrane fatty acids at 2-weeks and 4-months postpartum.
At 2-weeks postpartum, the AA/DHA+EPA ratio observed in maternal RBCs of the OB group was 37% greater than in the NW group (P = 0.007), and the absolute amount of AA was 21% more in the OW group compared to the NW group (P = 0.031). The absolute amounts of DHA and EPA in the maternal RBC at 2-weeks postpartum were not significantly among maternal BMI groups. At 4-months postpartum, only the EPA amount was decreased in the OB group relative to the NW group (P = 0.04). NW, normal weight n = 16; OW, overweight n = 10; OB, obese n = 6.
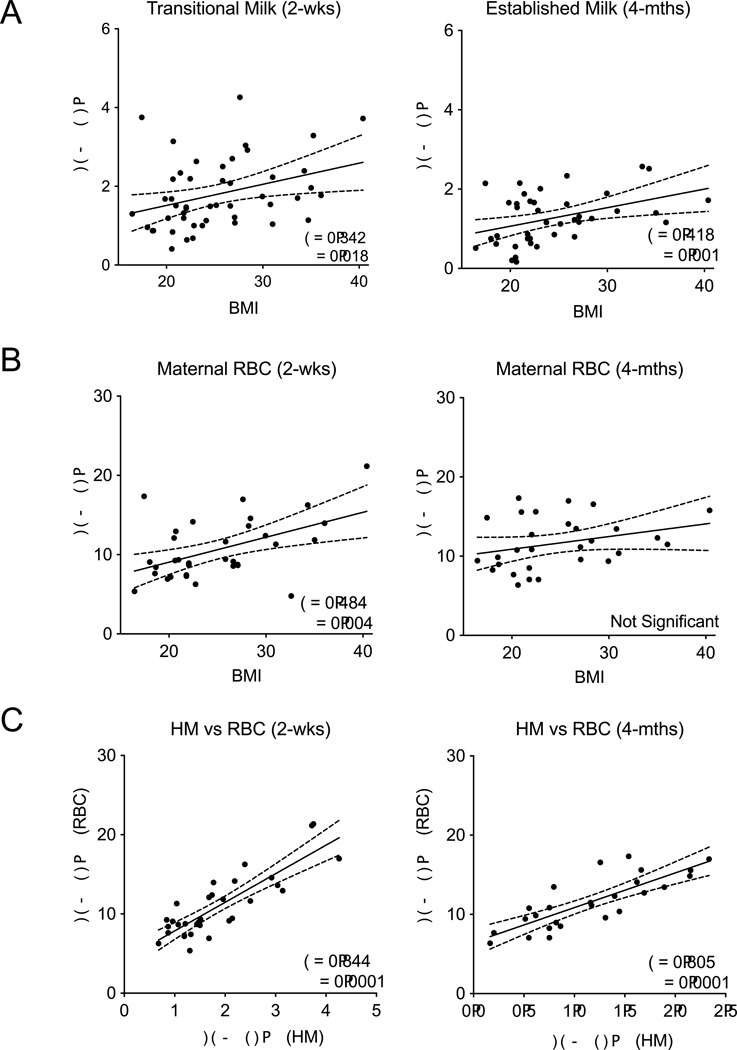
Prepregnancy BMI positively correlates with HM AA/DHA+EPA ratio
For all women (n = 48), prepregnancy BMI was moderately and significantly correlated with the AA/DHA+EPA ratio in both transitional and established HM (P = 0.018 and P = 0.001) (Figure 3A). The same was observed for the total n-6/n-3 FA ratio in transitional (P = 0.019) but not established HM (Supplementary Figure 1A). Maternal prepregnancy BMI was also moderately and significantly correlated with the AA/ DHA+EPA ratio of maternal RBCs at 2-weeks (P = 0.004) but not 4-months postpartum (Figure 3B), and the total n-6/n-3 FA ratio was not correlated with BMI at either time (Supplementary Figure 1B). The HM AA/DHA+EPA ratio was strongly and positively correlated with the maternal RBC AA/DHA+EPA ratio at 2-weeks (P = 0.0001), and 4-months postpartum (P = 0.0001) (Figure 3C). Overall, as maternal prepregnancy BMI increases, increasing amounts of n-6 fatty acids were present in the HM, and the AA/DHA+EPA ratio present in HM strongly correlated with maternal dietary FA composition.
Figure 3. Pearson correlations between maternal prepregnancy BMI and HM or maternal RBC membrane FA composition.
(A) Maternal prepregnancy BMI was significantly and positively correlated with transitional and established HM AA/EPA+DHA ratio (P = 0.018 and P = 0.001). (B) Maternal prepregnancy BMI was positively correlated with maternal RBC AA/EPA+DHA ratio at both 2-weeks (P = 0.004, n=48) but not at 4-months (n=40). D–E: Correlations between maternal prepregnancy BMI and maternal RBC FA composition. (C) The AA/EPA+DHA FA ratio in HM was highly correlated with the FA composition in RBCs at 2-weeks (P = 0.0001, n=32) and 4-months (P = 0.0001, n=26). Solid lines indicate slope and dashed lines indicate 95% CI.
Infant adipose deposition is predicted by AA/DHA+EPA ratios in established HM
Infant body composition assessed at 4-months postpartum was stratified by upper and lower AA/DHA+EPA ratio tertiles. The mean infant fat mass was significantly greater in the upper AA/DHA+EPA ratio tertile relative to the lower AA/DHA+EPA ratio tertile (P = 0.019), with no significant difference in fat-free mass (Figure 4A). Conversely, when infant body composition is grouped by maternal prepregnancy BMI, no difference in 4-month infant fat mass is observed among groups (Figure 4B). To test this observation, multivariable linear regression associated the AA/DHA+EPA FA ratio in either transitional or established HM with the postnatal change in infant adipose deposition (Δ-fat mass or Δ % body Fat) between 2-weeks and 4-months of age. The infant Δ-fat mass and the Δ-% body fat were significantly predicted by the AA/DHA+EPA ratio in established HM (P = 0.038 and P = 0.010), when controlling for GWG category, maternal prepregnancy BMI, infant sex, maternal use of fish oil supplements, birth weight, and breastfeeding exclusivity (Figure 4C). The β estimates for each association of HM AA/DHA+EPA ratio and change in infant adiposity were positive (287 g fat mass, 4.7 %BF), indicating that the greater the AA/DHA+EPA ratio in HM the greater the gain in infant adipose deposition.
Figure 4. Infant body composition at 4-months of age is predicted by the AA/DHA+EPA ratio in human milk.
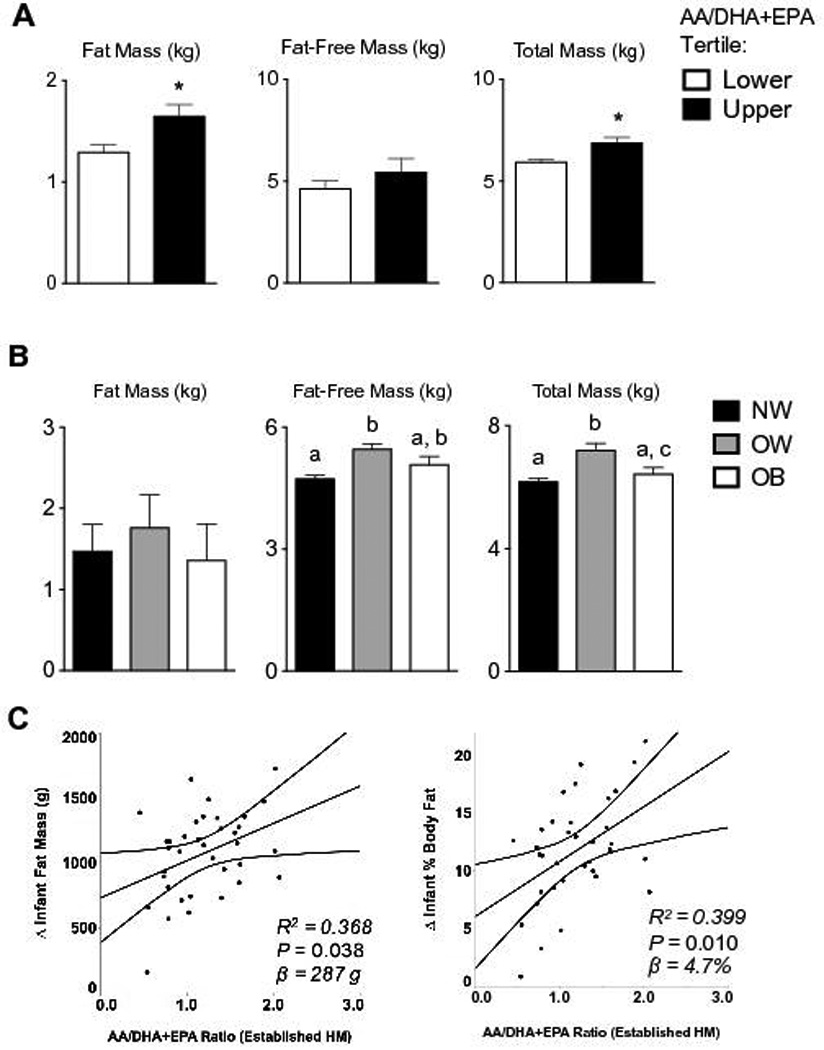
(A) Infant fat mass, fat-free mass, and total mass at 4-months of age were stratified by upper (n = 14) and lower tertile (n = 11) of established HM AA/DHA+EPA ratios. Infant fat mass, but not fat-free mass, was significantly greater in the upper AA/DHA+EPA ratio tertile relative to the lower (P = 0.019). (B) No difference in infant fat mass was observed when grouped according to maternal prepregnancy BMI. (C) The postnatal change in infant adipose deposition (Δ-fat mass or Δ % body Fat) between 2-weeks and 4-months of age were significantly associated with the established HM A/DHA+EPA ratio (P = 0.038 and P = 0.010). The AA/DHA+EPA ratio in established HM predicted 287g or 4.7 %BF increases (β estimate) in infant adiposity for each 1-unit increase in ratio (n = 40).
Discussion
Abundance of DHA in HM has been negatively associated with childhood %BF by DEXA (years 6–9), supporting potential sustained effects of HM n-3 PUFA on adipose programming44. In this well-characterized cohort of predominantly breastfed infants, our principle finding was that the upper tertile of HM AA/DHA+EPA ratio corresponds with greater infant fat mass, and this HM FA ratio predicts the amount of infant adipose deposition during the first 4-months of life. Specifically, our results indicate that every 1-unit increase in the HM AA/DHA+EPA ratio associates with a 287 g fat mass or 4.7 %BF increase (Figure 4C). No significant associations were observed for changes in total infant weight or fat-free mass (not shown), indicating increases in AA/DHA+EPA ratio may specifically affect the adipose. This finding is consistent with preterm infants who consumed DHA enriched formula and had less total and central fat deposition at 1 and 2 years of age45, implying lasting effects on adipose programming due to low n-6/n-3 FA exposures. It is worth noting that all infants in our study had normal growth patterns, and %BF was well within the normal PEA POD range at both 2-weeks and 4-months5, indicating the HM n-6/n-3 levels we observed resulted in normal adipose deposition. Nevertheless, studies will address whether increased adipose deposition following elevated n-6/n-3 FA exposures might persist or have clinical relevance to later childhood obesity risk. Collectively, our results demonstrate a high HM AA/DHA+EPA ratio is associated with adipose deposition through 4-months of life, supporting the hypothesis that HM n-6/n-3 FA ratios may facilitate adipose expansion when infants typically double their %BF.
The composition of HM directly influences infant growth, development, and health in numerous ways, and the FA constituents of HM are known to change by maternal BMI and diet18. Our data indicate maternal obesity affects transitional and established HM composition. The absolute amount of DHA present in the transitional HM of OB mothers was 43% less per mole of TAG relative to NW and OW groups (P = 0.07); this pattern persisted in established HM, where DHA in the OB group was significantly decreased 65% of the NW group (Figure 1D). The significant loss of HM DHA in the OB group was not observed in the RBC composition (Figure 2), suggesting that mammary metabolism, not supply of long-chain PUFA, might influence DHA processing or synthesis. Indeed, DHA synthesis requires seven sequential enzymes and two subcellular compartments, while AA synthesis requires only three steps. Further, MCFA that are synthesized de novo exclusively by the mammary epithelium46 were significantly lower in the OB group HM in transitional HM relative to the NW and OW groups. We have observed suppressed MCFA in diet-induced obese mice, in which maternal obesity leads to milk with lower amounts of de novo-derived lipids47, 48. Based on our findings here, and previous lactation studies in diet-induced obese mice, we conclude that maternal obesity influences the long-chain PUFA and MCFA amounts in HM.
We observed a robust correlation between HM n-6/n-3 FA ratios and n-6/n-3 FA ratios in maternal RBC that provide a consistent biomarker of maternal dietary FA intake over the preceding 90 days43, 49. The strong relationship between maternal dietary fat composition and HM FA composition was particularly evident for the AA/DHA+EPA ratio (Figure 3C), which significantly associated with measures of infant adipose deposition (Figure 4C). This observation is consistent with findings that HM n-6/n-3 ratios correlate with maternal EPA and DHA intake in the second and third trimesters and during lactation50, and that maternal consumption of DHA enriches levels in the infant during gestation and lactation51, 52. Interestingly, the relationship between maternal diet and prepregnancy BMI n-6/n-3 FA ratios was not significant at 4-months postpartum (Figure 1B and Supplemental Figure 1B), suggesting that the AA/DHA+EPA ratio in established HM is reflective of maternal adipose. Together, maternal DHA intake (from diet and/or supplements), likely lowers the HM AA/DHA+EPA FA ratio, which could offset influences driven by maternal phenotype to potentially protect infants against childhood obesity risk.
A major strength of this study was the longitudinal experimental design, which permitted assessment of the relationships with infant growth between an early and later postpartum time point. Other strengths include a cohort of healthy and predominantly breastfeeding mother/infant dyads with a large range of maternal BMIs, use of a well-established biomarker of maternal dietary FA intake (maternal RBC), controlled HM sampling procedures, and measures of infant body composition using PEA POD technology that has been validated to measure infant %BF53, 54. We devised methods for HM analyses designed to equally load the amount of TAG into lipid GC/MS, and absolute quantification for each FA species relative to its stable isotope reference standard35, 36. This study is limited by its relatively small sample size and lack of measures of HM volume intake, which would be necessary to calculate true n-6 and n-3 FA “dosages” delivered. Furthermore, we have no measures of body fat distribution or intrahepatic fat deposition, which may be affected by high n-6 FAs and could be critical for later risk of obesity and metabolic disease, but would necessitate MRI spectroscopy55. Additional limitations include the lack of follow-up data beyond 4 months when childhood obesity risk may become evident; the lack of inclusion of other factors in HM that have bioactive properties such as hormones or oligosaccharides; and the lack of information on the infant microbiome.
Cumulatively, excessive adipose tissue expansion and maladaptive programming are hallmarks of adult obesity that may be affected by early life nutritional cues. This study establishes that a high HM AA/DHA+EPA FA ratio may promote infant adipogenesis and highlights the need for additional HM and infant growth research to characterize early markers of risk associated with childhood obesity. Modeling early life nutritional exposures in the animal may provide detailed understanding of adipose tissue programming that occurs during the expansion of infant adipose.
Supplementary Material
Acknowledgments
The authors express our sincere appreciation to all study participants, those who provided critical reading, and Rachel C Janssen with manuscript preparation. MCR by 5 K12 HD057022 BIRCWH, MCR and DJL by P30-DK048520 Nutrition and Obesity Research Center Pilot and NICHD T32-HD007186. BEY by NICHD F32-HD0978068, T32-DK007658-21, Thrasher Research Fund Early Career Award, and Center for Women’s Health Research. NFK by K24-DK083772. DJL by F32-DK101179. PSM by P01-HD038129 and R01-HD075285. Lipid mass spectrometry by NIH/NCATS Colorado CTSA Grant UL1 TR001082. Other support: NIH National Center for Advancing Translational Sciences UL1 TR001082 and Colorado Clinical and Translational Science Institute grants UL1 RR025780.
Abbreviations used
- AA
arachidonic acid (20:4 n-6)
- DHA
docosahexaenoic acid (22:6 n-3)
- EPA
eicosapentaenoic acid (20:5 n-3)
- FA
fatty acid
- GWG
gestational weight gain
- HM
human milk
- LC-PUFA
long chain polyunsaturated fatty acids
- MCFA
medium chain fatty acid
- NW
normal weight
- OB
obese
- n-6/n-3
omega-6 relative to omega-3 fatty acid ratio
- OW
overweight
- % BF
percentage body fat
- PUFA
polyunsaturated fatty acid
- RBC
red blood cell
- TAG
triglyceride.
Footnotes
Disclaimers: The authors have no disclaimers
Conflict of interest: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young B, Johnson SL, Krebs NF. Biological determinants linking infant weight gain and child obesity: current knowledge and future directions. Adv. Nutr. 2012 doi: 10.3945/an.112.002238. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalano PM. Obesity and pregnancy--the propagation of a viscous cycle? The Journal of clinical endocrinology and metabolism. 2003;88(8):3505–3506. doi: 10.1210/jc.2003-031046. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Fields DA, Gilchrist JM, Catalano PM, Gianni ML, Roggero PM, Mosca F. Longitudinal body composition data in exclusively breast-fed infants: a multicenter study. Obesity (Silver Spring) 2011;19(9):1887–1891. doi: 10.1038/oby.2011.11. [DOI] [PubMed] [Google Scholar]
- 6.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123(4):1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch. Pediatr. Adolesc. Med. 2011;165(11):993–998. doi: 10.1001/archpediatrics.2011.167. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes. Rev. 2005;6(2):143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 9.Belfort MB, Rifas-Shiman SL, Rich-Edwards J, Kleinman KP, Gillman MW. Size at birth, infant growth, and blood pressure at three years of age. J. Pediatr. 2007;151(6):670–674. doi: 10.1016/j.jpeds.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;8:CD003517. doi: 10.1002/14651858.CD003517.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Section on B. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 12.Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):30–37. doi: 10.1111/apa.13133. [DOI] [PubMed] [Google Scholar]
- 13.Buyken AE, Karaolis-Danckert N, Remer T, Bolzenius K, Landsberg B, Kroke A. Effects of breastfeeding on trajectories of body fat and BMI throughout childhood. Obesity (Silver Spring) 2008;16(2):389–395. doi: 10.1038/oby.2007.57. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Kaur H, Choi WS, Huang TT, Lee RE, Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res. 2005;13(2):362–371. doi: 10.1038/oby.2005.48. [DOI] [PubMed] [Google Scholar]
- 15.Woo JG, Martin LJ. Does Breastfeeding Protect Against Childhood Obesity? Moving Beyond Observational Evidence. Curr Obes Rep. 2015;4(2):207–216. doi: 10.1007/s13679-015-0148-9. [DOI] [PubMed] [Google Scholar]
- 16.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med. 2016;50(6):761–779. doi: 10.1016/j.amepre.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Innis SM. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Maternal & child nutrition. 2011;7(Suppl 2):112–123. doi: 10.1111/j.1740-8709.2011.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storck Lindholm E, Strandvik B, Altman D, Moller A, Palme Kilander C. Different fatty acid pattern in breast milk of obese compared to normal-weight mothers. Prostaglandins, leukotrienes, and essential fatty acids. 2013;88(3):211–217. doi: 10.1016/j.plefa.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab. 2014;19(1):8–20. doi: 10.1016/j.cmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muhlhausler BS, Ailhaud GP. Omega-6 polyunsaturated fatty acids and the early origins of obesity. Curr Opin Endocrinol Diabetes Obes. 2013;20(1):56–61. doi: 10.1097/MED.0b013e32835c1ba7. [DOI] [PubMed] [Google Scholar]
- 21.White PJ, Mitchell PL, Schwab M, Trottier J, Kang JX, Barbier O, et al. Transgenic omega-3 PUFA enrichment alters morphology and gene expression profile in adipose tissue of obese mice: Potential role for protectins. Metabolism. 2015;64(6):666–676. doi: 10.1016/j.metabol.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Massiera F, Saint-Marc P, Seydoux J, Murata T, Kobayashi T, Narumiya S, et al. Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J Lipid Res. 2003;44(2):271–279. doi: 10.1194/jlr.M200346-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Massiera F, Barbry P, Guesnet P, Joly A, Luquet S, Moreilhon-Brest C, et al. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J Lipid Res. 2010;51(8):2352–2361. doi: 10.1194/jlr.M006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvheim AR, Malde MK, Osei-Hyiaman D, Lin YH, Pawlosky RJ, Madsen L, et al. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring) 2012;20(10):1984–1994. doi: 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri JM, Guesnet P. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res. 2006;45(3):203–236. doi: 10.1016/j.plipres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Kozak LP, Newman S, Chao PM, Mendoza T, Koza RA. The early nutritional environment of mice determines the capacity for adipose tissue expansion by modulating genes of caveolae structure. PloS one. 2010;5(6):e11015. doi: 10.1371/journal.pone.0011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bongiovanni KD, Depeters EJ, Van Eenennaam AL. Neonatal growth rate and development of mice raised on milk transgenically enriched with omega-3 fatty acids. Pediatric research. 2007;62(4):412–416. doi: 10.1203/PDR.0b013e31813cbeea. [DOI] [PubMed] [Google Scholar]
- 28.Heerwagen MJ, Stewart MS, de la Houssaye BA, Janssen RC, Friedman JE. Transgenic increase in N-3/n-6 Fatty Acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PloS one. 2013;8(6):e67791. doi: 10.1371/journal.pone.0067791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. The American journal of clinical nutrition. 2011;93(4):780–788. doi: 10.3945/ajcn.110.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen KM, Yaktine AL Institute of Medicine (U.S.) Weight gain during pregnancy : reexamining the guidelines. Washington, DC: National Academies Press; 2009. Committee to Reexamine IOM Pregnancy Weight Guidelines. [PubMed] [Google Scholar]
- 31.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: study methods. Pediatrics. 2008;122(Suppl 2):S28–S35. doi: 10.1542/peds.2008-1315c. [DOI] [PubMed] [Google Scholar]
- 32.Rudolph MC, Wellberg EA, Lewis AS, Terrell KL, Merz AL, Maluf NK, et al. Thyroid Hormone Responsive Protein Spot14 Enhances Catalysis of Fatty Acid Synthase in Lactating Mammary Epithelium. J Lipid Res. 2014 doi: 10.1194/jlr.M044487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence RA, Lawrence RM. Breastfeeding : a guide for the medical profession. 7th. Maryland Heights, Mo: Mosby/Elsevier; 2011. [Google Scholar]
- 34.Van Veldhoven PP, Swinnen JV, Esquenet M, Verhoeven G. Lipase-based quantitation of triacylglycerols in cellular lipid extracts: requirement for presence of detergent and prior separation by thin-layer chromatography. Lipids. 1997;32(12):1297–1300. doi: 10.1007/s11745-006-0166-1. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph MC, Wellberg EA, Lewis AS, Terrell KL, Merz AL, Maluf NK, et al. Thyroid hormone responsive protein Spot14 enhances catalysis of fatty acid synthase in lactating mammary epithelium. J Lipid Res. 2014;55(6):1052–1065. doi: 10.1194/jlr.M044487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudolph MC, Karl Maluf N, Wellberg EA, Johnson CA, Murphy RC, Anderson SM. Mammalian fatty acid synthase activity from crude tissue lysates tracing 13C-labeled substrates using gas chromatography mass spectrometry. Analytical Biochemistry. 2012;428(2):158–166. doi: 10.1016/j.ab.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen RG. Lipids in human milk. Lipids. 1999;34(12):1243–1271. doi: 10.1007/s11745-999-0477-2. [DOI] [PubMed] [Google Scholar]
- 38.German JB, Dillard CJ. Composition, structure and absorption of milk lipids: a source of energy, fat-soluble nutrients and bioactive molecules. Critical reviews in food science and nutrition. 2006;46(1):57–92. doi: 10.1080/10408690590957098. [DOI] [PubMed] [Google Scholar]
- 39.Smit EN, Martini IA, Mulder H, Boersma ER, Muskiet FA. Estimated biological variation of the mature human milk fatty acid composition. Prostaglandins, leukotrienes, and essential fatty acids. 2002;66(5–6):549–555. doi: 10.1054/plef.2002.0398. [DOI] [PubMed] [Google Scholar]
- 40.Spevacek AR, Smilowitz JT, Chin EL, Underwood MA, German JB, Slupsky CM. Infant Maturity at Birth Reveals Minor Differences in the Maternal Milk Metabolome in the First Month of Lactation. J Nutr. 2015;145(8):1698–1708. doi: 10.3945/jn.115.210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou XQ, Guo Z, Huang JH, Jin QZ, Cheong LZ, Wang XG, et al. Human milk fat globules from different stages of lactation: a lipid composition analysis and microstructure characterization. J Agric Food Chem. 2012;60(29):7158–7167. doi: 10.1021/jf3013597. [DOI] [PubMed] [Google Scholar]
- 42.Jiang J, Wu K, Yu Z, Ren Y, Zhao Y, Jiang Y, et al. Changes in fatty acid composition of human milk over lactation stages and relationship with dietary intake in Chinese women. Food Funct. 2016 doi: 10.1039/c6fo00304d. [DOI] [PubMed] [Google Scholar]
- 43.Serra-Majem L, Nissensohn M, Overby NC, Fekete K. Dietary methods and biomarkers of omega 3 fatty acids: a systematic review. The British journal of nutrition. 2012;107(Suppl 2):S64–S76. doi: 10.1017/S000711451200147X. [DOI] [PubMed] [Google Scholar]
- 44.Pedersen L, Lauritzen L, Brasholt M, Buhl T, Bisgaard H. Polyunsaturated fatty acid content of mother's milk is associated with childhood body composition. Pediatric research. 2012;72(6):631–636. doi: 10.1038/pr.2012.127. [DOI] [PubMed] [Google Scholar]
- 45.Pittaluga E, Vernal P, Llanos A, Vega S, Henrriquez MT, Morgues M, et al. Benefits of supplemented preterm formulas on insulin sensitivity and body composition after discharge from the neonatal intensive care unit. The Journal of pediatrics. 2011;159(6):926 e2–932 e2. doi: 10.1016/j.jpeds.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Smith S. Mechanism of chain length determination in biosynthesis of milk fatty acids. J Dairy Sci. 1980;63(2):337–352. doi: 10.3168/jds.S0022-0302(80)82935-3. [DOI] [PubMed] [Google Scholar]
- 47.Wahlig JL, Bales ES, Jackman MR, Johnson GC, McManaman JL, Maclean PS. Impact of high-fat diet and obesity on energy balance and fuel utilization during the metabolic challenge of lactation. Obesity. 2012;20(1):65–75. doi: 10.1038/oby.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saben JL, Bales ES, Jackman MR, Orlicky D, MacLean PS, McManaman JL. Maternal obesity reduces milk lipid production in lactating mice by inhibiting acetyl-CoA carboxylase and impairing fatty acid synthesis. PloS one. 2014;9(5):e98066. doi: 10.1371/journal.pone.0098066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, Ciraolo PJ, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112(10):4284–4291. doi: 10.1182/blood-2008-04-154112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura RY, Barbieiri P, Castro GS, Jordao AA, Jr, Perdona Gda S, Sartorelli DS. Dietary polyunsaturated fatty acid intake during late pregnancy affects fatty acid composition of mature breast milk. Nutrition. 2014;30(6):685–689. doi: 10.1016/j.nut.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC International Society for the Study of Fatty A, Lipids I. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins, leukotrienes, and essential fatty acids. 2009;80(2–3):85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Brenna JT, Lapillonne A. Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann Nutr Metab. 2009;55(1–3):97–122. doi: 10.1159/000228998. [DOI] [PubMed] [Google Scholar]
- 53.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. The American journal of clinical nutrition. 2007;85(1):90–95. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- 54.Barbour LA, Hernandez TL, Reynolds RM, Reece MS, Chartier-Logan C, Anderson MK, et al. Striking differences in estimates of infant adiposity by new and old DXA software, PEAPOD and skin-folds at 2 weeks and 1 year of life. Pediatr Obes. 2015 doi: 10.1111/ijpo.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. The Journal of pediatrics. 2013;162(5):930 e1–936 e1. doi: 10.1016/j.jpeds.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.