Abstract
Elevated relative cerebral blood volume (rCBV) on perfusion MRI and increased uptake on 11C-methionine-PET (Met-PET) can be used to diagnose and guide biopsy of brain tumors but are not specific. We report increased uptake on Met-PET associated with 4 developmental venous anomalies (DVAs) in 3 children with brain tumors, which could potentially mimic tumor and misdirect biopsy. Because DVAs are not readily visible on CT, prevention of misdirected biopsy in patients with focally elevated 11C-methionine uptake and rCBV relies on close correlation with contrast-enhanced anatomic MRI to exclude DVA or other non-neoplastic etiology.
Keywords: DVA, methionine, PET, MRI, CBV, pediatric
Uptake of amino acid radiotracers such as 11C-methionine by metabolically active brain or tumor tissue depends first on regional cerebral blood flow (CBF) and then crossing the blood brain barrier (BBB), either by active endothelial transport or passively via BBB disruption, and consequently may be increased in areas of increased cellular proliferation or metabolism, increased CBF or disruption of the BBB, which may occur in tumors but also infectious, inflammatory, ischemic or traumatic brain lesions.(1–4) Similarly, though elevated rCBV is associated with increased capillary volume in tumors, (4, 5) rCBV increases with CBF in normally perfused brain, and is elevated due to compensatory vasodilation in early ischemia/stroke.(6, 7) Developmental venous anomalies, the most common intracranial vascular abnormality and more common in children with brain tumors,(8) consist of abnormally dilated veins converging on a larger draining vein, and have been associated with parenchymal signal abnormalities, elevated rCBV, mean transit time and venous stasis.(9–11) Though the precise mechanism is unknown, our observations in this series suggest that increases in rCBV due to increased regional vascular density or venous congestion associated with DVAs may facilitate active or passive transendothelial transport of radiotracer, followed by preferential uptake of 11C-Met by the more metabolically active cortex in the venous drainage territories, accounting for the disproportionately cortical uptake we observed. Thus, observation of elevated 11C-Met uptake and rCBV, particularly in a somewhat discrepant distribution, should prompt close review of contrast-enhanced MRI to evaluate for a potentially vascular etiology prior to biopsy.
Case 1.
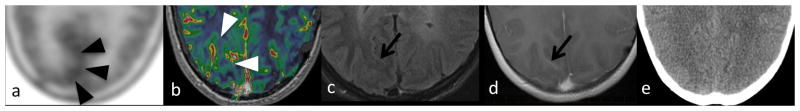
A 16-year-old girl with residual posterior fossa low grade glioma. (a) Met-PET showed increased lesion-to-contralateral-brain ratio (LBR=1.11, black arrowheads) superficial to (b) elevated rCBV (1.78, white arrowheads) in the drainage territory of a right occipital DVA (arrows), which is subtle on(c) FLAIR and visible on (d) post-contrast T1WI, but not visible on (e) CT.
Case 2.
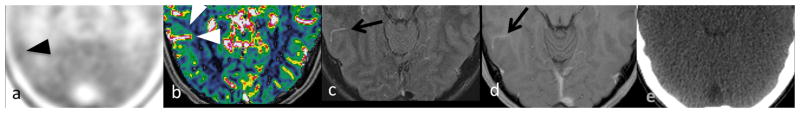
(a) Met-PET to evaluate possible residual right frontal anaplastic astrocytoma in a 16-year-old boy showed increased 11C-methionine uptake (LBR=1.19, black arrowheads) superficial to elevated (b) rCBV (1.44, white arrowheads) surrounding a right temporal DVA (arrow), which was visible on both (c) FLAIR and (d) T1WI with contrast but not (e) CT.
Case 3.
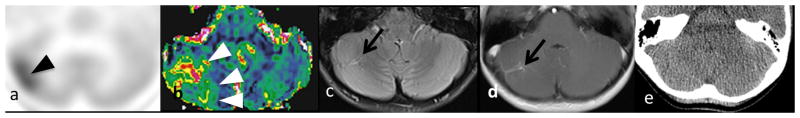
A 9-year-old girl with constitutional mismatch repair deficiency and multifocal low-grade gliomas. (a) 1C-methionine uptake (black arrowheads) and (b) rCBV (white arrowheads) were elevated in the drainage territories of a right cerebellar (arrow, LBR=1.63, rCBV=3.17) DVA, which is visible on (c) FLAIR and (d) T1WI with contrast, but not (e) CT.
Case 4.
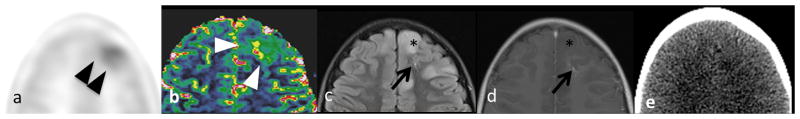
Same patient as Case 3. Elevated (a) 11C-methionine uptake (black arrowheads) and (b) rCBV (white arrowheads) in the drainage territory of a left frontal (arrow, LBR=1.17, rCBV=4.2) DVA, but not in adjacent non-enhancing left frontal tumor (*). Again, the DVA is visible on (c) FLAIR and (d) T1WI with contrast, but not (e) CT.
Acknowledgments
The authors wish to thank Scott Snyder, PhD and the St Jude Molecular Imaging Research laboratory staff for supplying 11C-methionine for these studies.
Funding: Supported in part by Grant No. P30 CA021765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Hutterer M, Nowosielski M, Putzer D, et al. [18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 2013;15(3):341–51. doi: 10.1093/neuonc/nos300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs A. Amino acid uptake in ischemically compromised brain tissue. Stroke. 1995;26(10):1859–66. doi: 10.1161/01.str.26.10.1859. [DOI] [PubMed] [Google Scholar]
- 3.Kawai N, Okauchi M, Miyake K, et al. 11C-methionine positron emission tomography in nontumorous brain lesions. No Shinkei Geka. 2010;38(11):985–95. [PubMed] [Google Scholar]
- 4.Sadeghi N, Salmon I, Decaestecker C, et al. Stereotactic comparison among cerebral blood volume, methionine uptake, and histopathology in brain glioma. AJNR American journal of neuroradiology. 2007;28(3):455–61. [PMC free article] [PubMed] [Google Scholar]
- 5.Hakyemez B, Erdogan C, Ercan I, Ergin N, Uysal S, Atahan S. High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin Radiol. 2005;60(4):493–502. doi: 10.1016/j.crad.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Karonen JO, Vanninen RL, et al. Cerebral hemodynamics in human acute ischemic stroke: a study with diffusion- and perfusion-weighted magnetic resonance imaging and SPECT. J Cereb Blood Flow Metab. 2000;20(6):910–20. doi: 10.1097/00004647-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29(3):231–40. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- 8.Jones BV, Linscott L, Koberlein G, Hummel TR, Leach JL. Increased Prevalence of Developmental Venous Anomalies in Children with Intracranial Neoplasms. AJNR American journal of neuroradiology. 2015;36(9):1782–5. doi: 10.3174/ajnr.A4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larvie M, Timerman D, Thum JA. Brain metabolic abnormalities associated with developmental venous anomalies. AJNR American journal of neuroradiology. 2015;36(3):475–80. doi: 10.3174/ajnr.A4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linscott LL, Leach JL, Zhang B, Jones BV. Brain parenchymal signal abnormalities associated with developmental venous anomalies in children and young adults. AJNR American journal of neuroradiology. 2014;35(8):1600–7. doi: 10.3174/ajnr.A3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Zipfel GJ, Hildebolt C, Derdeyn CP. Hemodynamic effects of developmental venous anomalies with and without cavernous malformations. AJNR American journal of neuroradiology. 2013;34(9):1746–51. doi: 10.3174/ajnr.A3516. [DOI] [PMC free article] [PubMed] [Google Scholar]