Abstract
Tobacco smoke exposure has been associated with risk of childhood acute lymphoblastic leukemia (ALL). Understanding the relationship between tobacco exposures and specific mutations may yield etiologic insights. We carried out a case-only analysis to explore whether prenatal and early-life tobacco smoke exposure influences the formation of leukemogenic genomic deletions. Somatic copy-number of 8 genes frequently deleted in ALL (CDKN2A, ETV6, IKZF1, PAX5, RB1, BTG1, PAR1 region, and EBF1) was assessed in 559 pre-treatment tumor samples from the California Childhood Leukemia Study. Parent and child passive tobacco exposure was assessed using interview-assisted questionnaires as well as DNA methylation in aryl-hydrocarbon receptor repressor (AHRR), a sentinel epigenetic biomarker of exposure to maternal smoking during pregnancy. Multivariable Poisson regressions were used to test association between the smoking exposures and total number of deletions. Deletion burden varied by subtype, with a lower frequency in high-hyperdiploid and higher frequency in ETV6-RUNX1 fusion ALL. Total number of deletions per case was positively associated with tobacco smoke exposure, in particular for maternal ever-smoking (ratio of means, RM=1.31; 95% CI: 1.08–1.59), maternal smoking during pregnancy (RM=1.48; 95% CI: 1.12–1.94), and during breastfeeding (RM=2.11; 95% CI: 1.48–3.02). The magnitude of association with maternal ever-smoking was stronger in male children compared with females (Pinteraction=0.04). Total number of deletions was also associated with DNA methylation at the AHRR epigenetic biomarker (RM=1.32; 95% CI: 1.02–1.69). Our results suggest that prenatal and early-life tobacco smoke exposure increase the frequency of somatic deletions in children who develop ALL.
Keywords: Tobacco smoke exposure, childhood acute lymphoblastic leukemia, gene deletion frequency, epigenetic biomarker, AHRR
Introduction
Acute lymphoblastic leukemia (ALL) is the most common cancer in children under 15 years of age (1). A small proportion of cases can be explained by well-established factors such as congenital cancer predisposition syndromes or exposure to ionizing radiation. In addition, several environmental exposures have been associated with increased ALL risk, including tobacco smoke, pesticides, paint, and air pollution (2, 3). Despite high cure-rates for ALL, survivors face long-term treatment-related morbidities and mortality (4), therefore prevention remains an important goal. Chromosomal abnormalities that initiate ALL, including high-hyperdiploidy and ETV6-RUNX1 fusion, have been shown to occur prenatally (5, 6), with subsequent mutations and copy-number alterations (CNAs) acquired as “second hits” that produce overt leukemia (7). Delineating the role of environmental modifiers of these genetic events will be important in ALL prevention efforts.
Though the mutational landscape of childhood ALL is simpler than that of most tumor types, deletions of genes involved in cell-cycle control, B-lymphocyte development, and hematopoiesis are the most frequent secondary CNAs (8–10). Assessment of large numbers of B-cell ALL (B-ALL) cases revealed the most commonly deleted genes in these pathways include CDKN2A (~27%), ETV6 (~22%), PAX5 (~19%), IKZF1 (~13%), BTG1 (~6%), PAR1 (~5%), RB1 (~5%), and EBF1 (~2%) (11, 12). Previous studies have also demonstrated that illegitimate recombination-activating gene (RAG)-mediated recombination events underlie genomic deletions in both B-ALL (11) and T-ALL (12). This intrinsic process normally involves recombination of variable (V), diversity (D), and joining (J) gene regions through initiation of the RAG1 and RAG2 proteins, in order to generate the immunoglobulins and T-cell receptors required for a highly diversified repertoire of antigen-receptors [reviewed in Fugmann et al. (13)]. Occasionally this V(D)J recombination goes awry, with erroneous repair of DNA double-strand breaks or off-target recombination leading to chromosomal rearrangements that may result in hematopoietic malignancies (11, 14, 15). In addition to the reported role of early-life infections (16), there is strong evidence that tobacco smoke exposure during fetal development can lead to an increased frequency of off-target V(D)J recombination-induced deletions (17). Therefore, we explored the hypothesis that tobacco smoke exposure during gestation and early life may be associated with an increased frequency of childhood ALL-associated deletions, and hence may have an etiologic role in leukemogenesis.
Case-control studies of self-reported tobacco smoking have revealed inconsistent associations with ALL risk, with most studies reporting no association with maternal smoking but some reporting association with paternal smoking (3, 18–21). However, such analyses may be confounded by recall bias and often have not considered germline or somatic genetics. In the current study, we carried out a case-only analysis of the effects of tobacco smoke exposure on the frequency of somatic deletions in childhood ALL cases from the California Childhood Leukemia Study (CCLS). Approximately half of our cases were of Latino (i.e. Hispanic) ethnicity, thus providing an opportunity to carry out the first comparison of the frequency of common childhood ALL gene deletions in this group compared with non-Latino cases.
Methods
Ethics Statement
This study was approved and reviewed by the Institutional Review Boards at the University of California, Berkeley, the California Department of Public Health (CDPH), and all participating hospitals. Informed consent was obtained from all study participants.
Study Population
The CCLS is a case-control study conducted from 1995 to 2015 and designed to identify risk factors for childhood leukemia. Incident cases of childhood leukemia under 15 years of age were identified and rapidly ascertained from pediatric hospitals across California, generally within 72 hours of diagnosis. Hospitals collected pre-treatment bone marrow and peripheral blood samples according to study protocols. The study population is described in further detail elsewhere (3). Control subjects recruited by CCLS were not used for this current analysis because this is a case-only study. Eligibility criteria for CCLS included: child’s age-at-diagnosis less than 15 years, no prior cancer diagnosis, residence in the study area at the time of diagnosis, and at least one biological parent available to speak English or Spanish. Additional eligibility for inclusion in this analysis was completed interviews and availability of diagnostic bone marrow samples for analysis of deletions. There were 559 ALL cases with available covariate information and diagnostic bone marrow (i.e. tumor) samples for MLPA assays (see details below), including 500 B-ALL and 46 T-ALL cases (immunophenotype was unknown for 13 cases). Demographic attributes (eg. child’s sex, age-at-diagnosis, race/ethnicity, household income) and cytogenetic subtypes were representative of the overall set of ALL cases in the CCLS (frequencies within 7% of larger case series) (3). Of the 559 cases, 52.6% were Latino, 29.3% were non-Latino white, and 17.7% were of other non-Latino races/ethnicities (including African-American, Native American, Asian, and mixed/other groups).
Determination of immunophenotype and cytogenetic profiles
Flow cytometry profiles were used to determine immunophenotype for ALL cases. Those expressing CD2, CD3, CD4, CD5, CD7, or CD8 (≥20%) were classified as T-lineage and those positive for CD10 or CD19 (≥20%) were classified as B-lineage, as described previously (22). High-hyperdiploidy (HD) was determined using FISH or G-banding, and ETV6-RUNX1 translocations were identified by FISH probes for chromosome 12p13 (ETV6) and 21q22 (RUNX1) loci (3).
Multiplex ligation-dependent probe amplification (MLPA)
Somatic copy-number of genes commonly deleted in ALL – CDKN2A, ETV6, IKZF1, PAX5, BTG1, EBF1, RB1, and genes within the pseudoautosomal region (PAR1) of the sex chromosomes (CRLF2, CSF2RA, IL3RA) – was assessed by MLPA using the SALSA MLPA probemix P335-B1 ALL-IKZF1 (MRC Holland, The Netherlands). MLPA was performed as previously described for 559 ALL samples with covariate data and sufficient bone marrow DNA available (23). Analysis of MLPA data was carried out using the Coffalyser.NET fragment analysis software (MRC Holland), and is further described in the Supplemental Material.
Self-reported tobacco smoke exposure assessment
Tobacco smoke exposure data were obtained via in-home visits and computer-assisted telephone interviews (CATI) from 1995–2015, as previously described (3). We categorized tobacco smoke exposures during particular times of the parent’s life, in relation to the child who developed leukemia: maternal ever-smoking, during preconception, during pregnancy, and during breastfeeding; paternal ever-smoking and during preconception; and finally child’s passive smoke exposure. Parental “ever-smoking” was defined as having smoked at least 100 cigarettes, pipes or cigars before the child’s diagnosis. Additional smoking-related variables refer only to whether the mother/father ever smoked during the time period described, irrespective of quantity. Preconception smoking was defined as smoking during the three months prior to the child’s conception. Child’s postnatal passive smoke exposure was defined as presence of a regular smoker (e.g. the mother, father, or other individual) in the household up to the child’s third birthday or ALL diagnosis (whichever came first). Tobacco smoke exposure was also assessed as a continuous variable, with estimates reported for each 5-unit increase in number of cigarettes per day (CPD).
Tobacco smoke epigenetic biomarker analysis
Neonatal genome-wide DNA methylation data were available for a subset of B-ALL cases (n=198). Germline (i.e. non-tumor) DNA was extracted from neonatal dried bloodspots and bisulfite-treated, before being assayed using Illumina HumanMethylation450 Beadchip arrays (450K arrays), with data normalized to remove batch and plate-position effect as previously described (24). Genotype data were also available at SNP rs148405299, a recently identified methyl-quantitative trait locus (methyl-QTL) for methylation at the AHRR CpG cg05575921 (24). Decreased methylation at cg05575921 is strongly associated with in utero exposure to maternal smoking (24, 25), and not paternal or maternal preconception smoking or mother’s exposure to secondhand smoke (26).
Statistical analysis
The number of ALL-associated deletions was not normally distributed, thus non-parametric tests were used to examine bivariate associations between the number of deletions and demographic characteristics and cytogenetic subtypes. Pearson’s chi-square tests were used to assess frequency of specific gene deletions (CDKN2A, ETV6, etc.) across ALL immunophenotypes and cytogenetic subtypes, and across the different ethnicities. Mutual exclusivity analysis was carried out for each possible gene pair (n=28) using Gitools software, as described in the Supplemental Material.
To analyze the relationship between self-reported tobacco smoke exposures and the number of deletions in the tumor, we carried out multivariable Poisson regression analyses with the number of deletions as the outcome, using the ratio of means (RM) as the measure of association. Covariates included in the final model were age-at-diagnosis (continuous), sex, household education (highest education among mother and father), and paternal age (continuous) based on a p-value cut off of 0.20 from bivariate tests of each covariate with the number of deletions (Table 1). Race/ethnicity, maternal age, and income were considered but not included in the final model as the p-values in bivariate tests exceeded the cut-off. Education and income were highly correlated, but education was used as it was more significantly associated with number of deletions. Correlation between the tobacco exposure variables was tested using Spearman’s rank correlation coefficient.
Table 1.
Characteristics of ALL cases and bivariate associations with number of deletions, from the California Childhood Leukemia Study, 1995–2015.
| Variable | N (%)a | Mean (SD) deletions | Mean rank | Test | P-value |
|---|---|---|---|---|---|
| Total | 559 (100) | ||||
| Age-at-diagnosis (years) | 559 (100) | ||||
| 0–1.9 | 41 (7.3) | 0.9 (1.1) | 247 | ||
| 2–5.9 | 329 (58.9) | 1.0 (1.0) | 269 | ||
| 6–9.9 | 106 (19.0) | 1.1 (1.1) | 292 | ||
| 10–14.9 | 83 (14.8) | 1.3 (1.1) | 325 | KW | 0.009 |
| Mean (SD) | 5.6 (3.5) | rho=0.17 b | S | <0.0001 | |
|
| |||||
| Sex | 559 (100) | ||||
| Female | 244 (43.6) | 1.0 (1.1) | 269 | ||
| Male | 315 (56.4) | 1.1 (.1) | 289 | WMW | 0.15 |
|
| |||||
| Race/ethnicitya | 557 (100) | ||||
| Latino | 294 (52.8) | 1.0 (1.0) | 280 | ||
| Non-Latino White | 164 (29.4) | 1.0 (1.1) | 273 | ||
| Non-Latino Other | 99 (17.8) | 1.1 (1.0) | 287 | KW | 0.78 |
|
| |||||
| Highest parental education | 559 (100) | ||||
| None or elementary school | 39 (7.0) | 1.3 (1.1) | 339 | ||
| High school or similar | 172 (30.8) | 1.1 (1.0) | 302 | ||
| Some college or similar | 155 (27.7) | 0.9 (1.0) | 257 | ||
| Bachelor’s degree or higher | 193 (34.5) | 1.0 (1.2) | 267 | KW | 0.003 |
|
| |||||
| Income | 559 (100) | ||||
| <$15,000 | 96 (17.2) | 1.2 (1.2) | 301 | ||
| $15,000–29,999 | 111 (19.9) | 1.0 (0.9) | 279 | ||
| $30,000–44,999 | 84 (15.0) | 0.9 (0.9) | 264 | ||
| $45,000–59,999 | 83 (14.8) | 1.2 (1.3) | 297 | ||
| $60,000–74,999 | 34 (6.1) | 1.1 (1.0) | 291 | ||
| $75,000+ | 151 (27.0) | 0.9 (1.0) | 266 | KW | 0.39 |
|
| |||||
| Maternal age (years) a | 558 (100) | ||||
| Mean (SD) | 28.2 (6.2) | rho=0.02b | S | 0.70 | |
|
| |||||
| Paternal age (years) | 559 (100) | ||||
| Mean (SD) | 30.9 (7.2) | rho=0.06 b | S | 0.13 | |
|
| |||||
| Immunophenotypea | 546 (100) | ||||
| B-cell | 500 (91.6) | 1.0 (1.1) | 273 | ||
| T-cell | 46 (8.4) | 1.0 (0.9) | 279 | WMW | 0.78 |
|
| |||||
| High hyperdiploid (B-ALL) a | 463 (100) | ||||
| No | 310 (67.0) | 1.4 (1.1) | 275 | ||
| Yes | 153 (33.0) | 0.4 (0.6) | 144 | WMW | <0.0001 |
|
| |||||
| ETV6-RUNX1 (B-ALL) a | 454 (100) | ||||
| No | 338 (74.4) | 0.9 (1.1) | 209 | ||
| Yes | 116 (25.6) | 1.4 (1.1) | 281 | WMW | <0.0001 |
Some variables were missing observations: Race/ethnicity, maternal age, and immunophenotype were missing for 2, 1, and 13 cases respectively. High hyperdiploidy and ETV6-RUNX1 were missing 37 and 46 observations respectively within the B-ALL case group.
rho is the Spearman rank correlation coefficient.
KW = Kruskall-Wallis test; WMW = Wilcoxon-Mann-Whitney test; S = Spearman rank correlation.
Independence of maternal and paternal associations with number of deletions was tested using multivariable Poisson regression for maternal ever-smoking and paternal ever-smoking adjusted for each other, paternal preconception smoking adjusted for maternal ‘prenatal’ (preconception and/or pregnancy) smoking, and for maternal smoking during preconception, pregnancy, and breastfeeding all adjusted for paternal preconception smoking.
We also explored the effects of cumulative tobacco exposures. Total number of exposures was calculated using the four bivariate exposures: paternal and maternal smoking during preconception, maternal smoking during pregnancy, and child’s postnatal passive smoking (which includes maternal breastfeeding smoking). Multivariable Poisson regression was used to test the association between number of deletions and number of tobacco exposures as both continuous and categorical variables. The joint effects of paternal preconception plus maternal ‘prenatal’ smoking, maternal ‘prenatal’ smoking plus child’s postnatal passive smoking, and paternal preconception plus child’s postnatal passive smoking were also tested using multivariable Poisson regression.
Additional analyses were restricted to B-ALL and stratified by the two main cytogenetic subtypes of ALL, i.e. HD and ETV6-RUNX1 fusion. Multiplicative interactions were also tested between each tobacco smoke exposure and age-at-diagnosis, sex, and Latino ethnicity (compared with non-Latino whites). The likelihood ratio test was carried out comparing each model with and without the interaction terms and using 0.05 as the p-value threshold for statistical significance. To further assess the impact of maternal smoking during breastfeeding, we restricted analysis to mothers who breastfed and evaluated a potential trend by duration of breastfeeding. Interactions between maternal smoking during breastfeeding and duration of breastfeeding were tested using duration of breastfeeding as binary (<6 months vs. ≥6 months) and continuous (in weeks) variables.
Logistic regression analysis was carried out to test the association between self-reported maternal smoking during pregnancy (yes/no) and DNA methylation at AHRR CpG cg05575921 in a subset of cases (n=198). This model was adjusted for gestational age, sex, race/ethnicity, estimated cell-mixture, methylation array batch number and genotype at the methyl-QTL SNP rs148405299, as previously described (24). Multivariable Poisson regression analysis was carried out to test the association between DNA methylation at cg05575921 (in beta-values) and total number of deletions in each case (as the outcome), adjusted for the same variables listed above. In a previous study of maternal smoking during pregnancy and neonatal methylation levels, neonates of mothers with undetectable cotinine levels had a median AHRR cg05575921 beta-value of 0.88 compared with a median of 0.78 for neonates of mothers with high cotinine levels (27). Therefore, estimates in our regression analysis were calculated for a 0.1 beta-value decrease at cg05575921, to reflect a biologically relevant difference. This analysis was also carried out separately in males (n=107) and females (n=91). All statistical analyses were performed using SAS software or the R statistical environment.
Results
Demographic and subtype-specific characteristics of deletions in childhood ALL
In 559 ALL cases, we identified 206 (36.9%) with zero deletions and 353 (63.1%) with at least 1 deletion across the 8 genes tested. Mean number of deletions across cases was 1.0 (standard deviation [SD]: 1.1), with a maximum of 5 genes deleted in only 5 (0.9%) cases (Table S1). Demographic characteristics of the cases are presented in Table 1. Mean age-at-diagnosis was 5.6 years (range: 0.3–14.9), and a highly significant positive association was seen between age-at-diagnosis and number of deletions in the child’s leukemia in the bivariate tests (P<0.0001) (Table 1). This association was also observed in all multivariable analyses with tobacco smoke exposures (P-value ranges: 0.0002 to 0.003). Total number of deletions was similar between Latinos and non-Latinos (Figures S1–3), and there was no association with sex or parental age at child’s birth (Table 1). There was a significant inverse association between number of deletions and household education (P=0.003) in the bivariate tests, though there was no association with parental income (P=0.39). The association between household education and deletion frequency remained significant or marginally significant in the multivariable analyses (P-value ranges: 0.01 to 0.06).
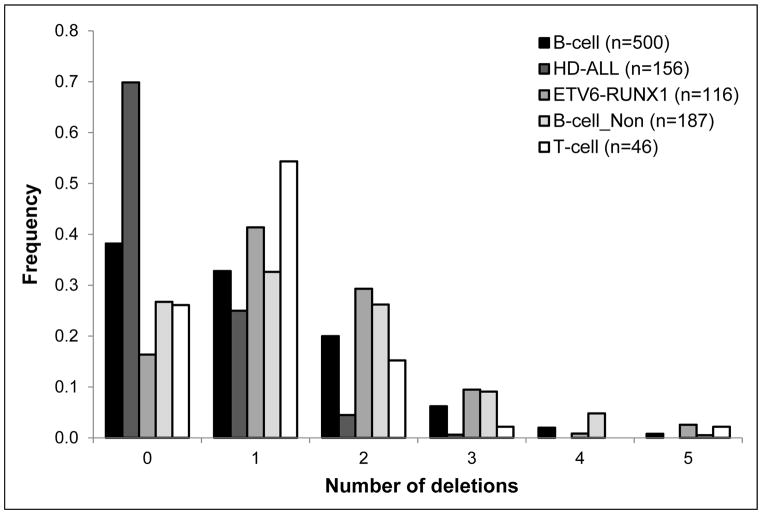
There was no significant difference in deletion burden between B-ALL and T-ALL but there was a significant difference between the two main B-ALL cytogenetic subtypes (HD and ETV6-RUNX1 fusion) (Figure 1, Table 1). HD-ALL cases had a significantly lower number of deletions compared with non-HD-ALL cases (P<0.0001), whereas ETV6-RUNX1 fusion cases had a significantly higher number of deletions compared with non-ETV6-RUNX1 cases (P<0.0001) (Figure 1, Table 1). After excluding ETV6, the number of deletions in ETV6-RUNX1 fusion cases was no longer significantly higher (P=0.35), thus this difference was driven by deletion of the non-translocated copy of ETV6.
Figure 1. Frequency of ALL cases from the California Childhood Leukemia Study with varying numbers of deletions, by subtype.
Deletions were assessed in diagnostic leukemia DNA samples by MLPA. The majority of cases were B-cell ALL (n=500), of which the most common cytogenetic subtypes were high hyperdiploidy, HD-ALL (n=156), and ETV6-RUNX1 fusion (n=116). There were 187 B-cell cases that were neither HD nor ETV6-RUNX1 fusion (“B-cell_Non”). A small number of T-cell cases (n=46) were also included.
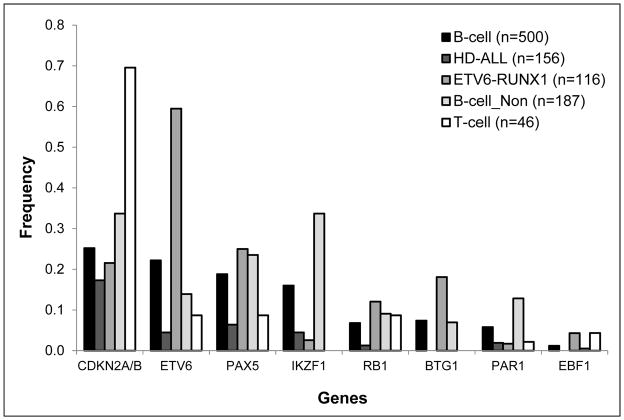
The most frequently deleted genes were CDKN2A (28.8%), ETV6 (21.3%), PAX5 (17.7%), and IKZF1 (14.8%) (Figure 2). There was variation in particular gene frequencies when stratifying by immunophenotype and by cytogenetic subtype (Figure 2). Loss of ETV6 (P=4.5×10−30) and BTG1 (P=3.6×10−8) were more frequent in ETV6-RUNX1 fusion cases, and loss of IKZF1 (P=7.1×10−18) and PAR1 (P=3.4×10−6) were more frequent in the non-HD, non-ETV6-RUNX1 B-ALL group (Figure 2). In T-ALL, loss of CDKN2A (69.6%) was significantly more frequent than in B-ALL (25.2%) (P=2.2×10−10). Gene deletion frequencies were similar in Latinos compared with non-Latinos (Table S2) (Figures S4–6).
Figure 2. Deletion frequency of each gene by subtype, in 559 ALL cases from the California Childhood Leukemia Study.
Deletion status was assessed by MLPA using the childhood ALL probeset, with multiple probes in CDKN2A/B, ETV6, PAX5, IKZF1, RB1, BTG1, PAR1region, and EBF1.
Loss of CDKN2A was consistently mutually exclusive of loss of ETV6, with P=2.9×10−11 in B-ALL and P=2.1×10−6 in T-ALL. In B-ALL, loss of IKZF1 was mutually exclusive of both CDKN2A (P=6.3×10−5) and ETV6 deletions (P=1.2×10−7). Significant exclusivity was also found between CDKN2A deletions and loss of RB1 (P=0.01) and PAR1 (P=0.03), between ETV6 deletions and loss of PAX5 (P=0.003) and PAR1 (P=4.2×10−4), and between PAX5 deletions and loss of BTG1 (P=0.03), PAR1 (P=0.003), and EBF1 (P=0.03). In non-HD, non-ETV6-RUNX1 B-ALL, there was significant co-deletion of PAX5 with CDKN2A (P=0.005), IKZF1 with BTG1 (P=0.04), and BTG1 with EBF1 (P=0.003).
Self-reported tobacco smoke exposure and deletion burden
Tobacco smoke exposure as a categorical variable was consistently associated with a higher number of ALL-associated deletions in multivariable models, including significant associations for maternal ever-smoking (RM: 1.31, 95% CI: 1.08–1.59) and for paternal smoking during preconception (RM: 1.27, 95% CI: 1.05–1.53) (Table 2). A greater effect size was seen for maternal smoking during pregnancy (RM: 1.48, 95% CI: 1.12–1.94) and during breastfeeding (RM: 2.11, 95% CI: 1.48–3.02). Breastfeeding itself was not associated with number of deletions and, among mothers who did breastfeed, the duration of the breastfeeding period did not affect the association between maternal smoking and number of deletions (data not shown). Similar associations were observed when treating smoke exposure as a continuous variable (Table 2) and when analyses were restricted to B-ALL only (Table S3). Stratifying by cytogenetic subtype, significant associations were seen for ETV6-RUNX1 fusion but not HD-ALL cases (Table S3). A proportion of cases with zero deletions was positive for each of the binary smoking variables, although frequencies were consistently lower than for cases with multiple deletions (Table S4).
Table 2.
Association between tobacco smoke exposures and the number of deletions in the child’s leukemia using multivariable Poisson regressions, the California Childhood Leukemia Study, 1995–2015.
| Binary exposures | # Cases | RM | 95% CI | P-valuea |
|---|---|---|---|---|
| Paternal, everb | ||||
| No | 318 | 1 (ref) | ||
| Yes | 213 | 1.18 | (0.99, 1.40) | 0.06 |
| Maternal, everb | ||||
| No | 444 | 1 (ref) | ||
| Yes | 110 | 1.31 | (1.08, 1.59) | 0.007c |
| Paternal, preconception | ||||
| No | 397 | 1 (ref) | ||
| Yes | 133 | 1.27 | (1.05, 1.53) | 0.01 c |
| Maternal, preconception | ||||
| No | 486 | 1 (ref) | ||
| Yes | 68 | 1.36 | (1.07, 1.72) | 0.01 c |
| Maternal, pregnancy | ||||
| No | 510 | 1 (ref) | ||
| Yes | 44 | 1.48 | (1.12, 1.94) | 0.009 c |
| Child, postnatal passive | ||||
| No | 435 | 1 (ref) | ||
| Yes | 113 | 1.36 | (1.12, 1.65) | 0.002 c |
| Maternal, breastfeeding | ||||
| No | 538 | 1 (ref) | ||
| Yes | 16 | 2.11 | (1.48, 3.02) | <.0001 c |
|
| ||||
| Continuous exposures | # Cases | RM | 95% CI | P-value |
|
| ||||
| Paternal, preconception (CPD) | 517 | 1.08 | (1.02, 1.14) | 0.009 c |
| Maternal, preconception (CPD) | 554 | 1.07 | (0.99, 1.17) | 0.10 |
| Maternal, pregnancy (CPD) | 553 | 1.22 | (1.00, 1.49) | 0.05 |
| Maternal, breastfeeding (CPD) | 530 | 1.74 | (1.30, 2.32) | 0.0002 c |
P-values adjusted for age-at-diagnosis, sex, paternal age, and household education;
Paternal and maternal “ever” smoking was defined as having smoked at least 100 cigarettes, pipes, or cigars before the child’s diagnosis. Additional binary variables only account for whether the mother or father smoked at all during the time period described.
Significant at P<0.05.
RM = ratio of the means; CPD = cigarettes, pipes, or cigars per day in 5 unit increments.
All tobacco smoking exposures were significantly correlated, with strongest correlations between maternal variables (correlation coefficient rho range = 0.32–0.99). Child’s postnatal passive smoking was moderately correlated with maternal smoking variables (rho range = 0.34–0.63) and paternal smoking variables (rho range = 0.36–0.50). Correlation between maternal and paternal smoking variables was relatively low (rho range = 0.12–0.37) (Table S5).
In the multivariable Poisson regressions adjusting paternal smoking variables for maternal variables and vice-versa, the ratios of means were not altered by >10%, supporting independent associations (Table S6). Analysis of cumulative tobacco exposures revealed a highly significant association between total number of tobacco exposures and number of deletions, with RM = 1.14 (95% CI: 1.06–1.22) for each additional exposure (P=4.0×10−4) (Table 3). The cumulative effect of tobacco exposures was also supported by the significant association of the combinations of paternal preconception and maternal prenatal smoking (RM: 1.55, 95% CI: 1.16–2.07), and child’s postnatal passive smoking with both paternal preconception smoking (RM: 1.48, 95% CI: 1.16–1.88) and maternal prenatal smoking (RM: 1.50, 95% CI: 1.18–1.92), whereas these exposures alone were not significantly associated with number of deletions (Table S7).
Table 3.
Association between cumulative tobacco smoke exposures and the number of deletions in the child’s leukemia using multivariable Poisson regressions, the California Childhood Leukemia Study, 1995–2015.
| Cumulative exposuresa | # Cases | RM | 95% CI | P-valueb |
|---|---|---|---|---|
| Number of exposures (continuous) | 518 | 1.14 | (1.06, 1.22) | 0.0004c |
| Number of exposures (categorical) | ||||
| 0 | 349 | 1 (ref) | ||
| 1 | 83 | 1.14 | (0.90, 1.44) | 0.27 |
| 2 | 34 | 1.18 | (0.84, 1.67) | 0.34 |
| 3 | 22 | 1.60 | (1.10, 2.31) | 0.01c |
| 4 | 30 | 1.64 | (1.18, 2.28) | 0.003c |
Cumulative tobacco exposures calculated from the bivariate exposures: paternal smoking during preconception, maternal smoking during preconception, maternal smoking during pregnancy, and child’s postnatal passive smoking.
P-values adjusted for age-at-diagnosis, sex, paternal age, and household education;
Significant at P<0.05.
RM = ratio of the means.
We also tested for multiplicative interactions between the effect of the binary smoking exposures and child’s age-at-diagnosis, sex, and race/ethnicity on the number of deletions detected. No significant interactions were found between any of the smoking variables and race/ethnicity. Paternal preconception smoking displayed a statistical interaction with age-at-diagnosis (as a continuous variable) (Pinteraction = 0.03), with a stronger effect on the number of deletions in younger children, with a decrease in the estimate by, on average, 5.2% (95% CI: 0.3–9.8%, p-value: 0.04) for every one year increase in age-at-diagnosis. The impact of paternal smoking during preconception leveled after age 6 (Figure S7), therefore we also modeled the interaction with child’s age as a binary variable to summarize the risk estimate for children ≤6 years of age. The RM for paternal smoking during preconception was 1.48 (95% CI: 1.17–1.87) in children age ≤6 years and 1.02 (0.74, 1.40) for children ≥7 years of age (Pinteraction = 0.057).
The effect of parental smoking on deletions differed by child’s sex. For maternal ever-smoking, the RM and 95% CI in females was 1.01 (0.73–1.39) and in males was 1.54 (1.21–1.96) (Pinteraction = 0.04), with a trend in the same direction seen for paternal ever-smoking (Pinteraction = 0.16) (Table S8). The association of maternal smoking during preconception also differed between females (RM=0.96; 95% CI: 0.64–1.45) and males (RM=1.66; 95% CI: 1.25–2.21) (Pinteraction = 0.03). A similar trend was seen for maternal smoking during pregnancy (Pinteraction = 0.089) and during breastfeeding (Pinteraction = 0.24) though these did not reach significance (Table S8).
Methylation at AHRR CpG cg05575921 and deletion burden
To assess validity of the self-reported data on maternal smoking during pregnancy, we assessed the relationship with DNA methylation at cg05575921 in a subset of B-ALL cases with available neonatal methylation data (n=198). We found a highly significant association between decreased methylation at cg05575921 and maternal smoking during pregnancy (P=5.0×10−4), with a 0.1 decrease in beta-value associated with a 6.3-fold increased odds of exposure (95% CI: 2.34–19.23). This was in the expected direction, as reduced methylation at cg05575921 is associated with increased tobacco smoke exposure. Reduced methylation at AHRR CpG cg05575921 was also associated with increased total number of deletions in the 198 B-ALL cases, with RM = 1.32 (95% CI: 1.02–1.69) (Table 4), thus validating the association first identified using self-reported smoking exposure data with an orthogonal epigenetic biomarker. When stratified by sex, this association became stronger in males (RM=1.45; 95% CI: 1.05–2.00) but was non-significant in females (RM=1.28; 95% CI: 0.82–2.00), however the interaction between sex and number of deletions was not significant in the multivariable Poisson regression model (Pinteraction = 0.90).
Table 4.
Association between neonatal DNA methylation at the AHRR CpG cg05575921 and the number of deletions in 198 B-ALL cases by sex using multivariable Poisson regression, the California Childhood Leukemia Study, 1995–2015.
| Biomarker of maternal smoking during pregnancy | B-ALL cases | RM b | 95% CI | P-value a |
|---|---|---|---|---|
| AHRR cg05575921 methylation | Total (n = 198) | 1.32 | (1.02,1.69) | 0.031c |
| Males only (n = 107) | 1.45 | (1.05, 2.00) | 0.023c | |
| Females only (n = 91) | 1.28 | (0.82, 2.00) | 0.277 |
Poisson regression model adjusted for cell-mixture, sex, gestational age, ethnicity, DNA methylation array batch number and genotype at SNP rs148405299.
RM = ratio of the means, calculated for a 0.1 beta-value decrease in cg05575921 methylation.
Significant at P<0.05.
Discussion
In this study, we carried out copy-number analysis of common ALL gene deletions in 559 cases from the CCLS and describe the first comparison of the frequency of these common CNAs in Latino children with ALL compared with non-Latino children. Furthermore, this is the first reported association study of an environmental exposure – tobacco smoke – and frequency of somatic CNAs in ALL, which was performed using a case-only comparison with data from parental interviews and from an unbiased epigenetic biomarker of tobacco smoke exposure.
Using a well-established MLPA assay for assessment of common childhood ALL gene deletions (9), we found a similar frequency of deletions and a similar proportion of ALL cases with at least one deletion as previously reported (9, 28). To investigate a potential environmental etiology of these deletions, we assessed self-reported tobacco exposure data and found significant associations between maternal smoking ever, during pregnancy, and during breastfeeding, paternal preconception smoking, and child’s postnatal passive smoking and the total number of deletions per case. We also found evidence of dose-response relationships, with highly significant associations with the continuous variables “paternal smoking during preconception” and, in particular, for “maternal smoking during breastfeeding”, with a 74% increase in mean deletion number for every 5 cigarettes smoked per day during this period. Additional analyses adjusting maternal smoking variables for paternal smoking, and vice-versa, supported that these were independent associations. It was interesting to note that both prenatal and postnatal tobacco exposures showed positive associations with tumor deletions. Indeed, cases whose mothers smoked during breastfeeding harbored, on average, over twice as many deletions as cases whose mothers did not smoke during this period, though the number of exposed cases was small. Furthermore, the significant cumulative effect of tobacco exposures on frequency of gene deletions and the joint effects of maternal and paternal prenatal smoking with child’s postnatal passive smoking support that children heavily exposed to tobacco over long periods of time are at greatest risk of developing somatic gene deletions. We also found intriguing interactions, including between paternal preconception smoking and child’s age-at-diagnosis, with a significant effect on deletion number seen only in cases 6 years of age or younger, which includes the peak age of incidence of ALL. This interaction was not observed for maternal smoking. Our results suggest that paternal preconception smoking, which is known to cause oxidative damage to sperm DNA (29), may lead to a higher propensity of leukemia gene deletions in children with earlier-onset leukemias. However, this finding will need confirmation in other studies before this scenario is established.
There was also a significant interaction between child’s sex and both maternal ever-smoking and maternal smoking during preconception, with an association with increased number of deletions in male cases only. Males also showed a trend towards higher mean number of deletions than females (Table 1), though this was not significant. Male fetuses grow more rapidly than females (30), which may lead to increased vulnerability of developing lymphocytes to clastogenic toxins. Studies have also shown sexual dimorphism in inflammatory responses to infection, with greater inflammation seen in males compared with females (31). Furthermore, mothers carrying male fetuses produce more proinflammatory cytokines than mothers carrying females (32). It is possible, therefore, that sex-specific immune responses to tobacco smoke, such as the propagation of preleukemic clones with subsequent generation of “second-hit” deletions, may contribute to the increased incidence of ALL seen in males compared with females (33, 34).
Case-control studies of self-reported tobacco smoke exposures have reported significant association between paternal preconception smoking and childhood ALL risk (19, 35). Indeed, a case-control study of CCLS participants found that the joint effect of paternal prenatal smoking combined with child’s postnatal passive smoking was associated with childhood ALL risk (3). This association was stronger in ETV6-RUNX1 fusion ALL but was not significant in HD-ALL. We also found our tobacco exposure results were no longer significant when restricted to HD-ALL, perhaps due to the lower frequency of deletions in this subtype, though numbers of cases in these stratified analyses were small. In addition to the possible mutagenic effects of paternal preconception smoking, children may be exposed to third-hand smoke due to residues that persist in the home (3). Further, it has been shown that the effects of paternal preconception smoking on childhood ALL risk are similar to the effects of paternal smoking during pregnancy (20). Therefore, children whose fathers smoked in preconception may also have been exposed to paternal smoking during pregnancy and postnatally, which are likely the more critical time windows given the timing of deletions in ALL development (9).
In contrast to paternal smoking, case-control studies have revealed little evidence of a role for maternal smoking during pregnancy (3, 20, 21) or during breastfeeding (36) in ALL etiology. One of the main weaknesses in case-control studies using interview data is the potential for parental recall and response bias, and this may vary for reports of maternal versus paternal smoking behaviors. Given the case-only design of this study, differential recall bias (between exposed and unexposed) was unlikely to affect results. Moreover, in a subset of cases with available neonatal methylation data, we found that self-reported maternal smoking during pregnancy was highly significantly associated with neonatal methylation levels at the AHRR CpG cg05575921, which previously has been strongly associated with sustained maternal smoking during pregnancy (24–26). We used this epigenetic biomarker to biologically validate the association between prenatal tobacco smoke exposure and increased number of deletions in the leukemia. The association between AHRR methylation and number of deletions became more significant when stratified by sex, with a significant effect seen in males but not females, further supporting the male-specific association seen in our analyses of parentally-reported smoking data. This interaction did not reach significance, perhaps due to small sample size, thus further work is required to elucidate the potential sex-specific effects of tobacco exposure. Furthermore, the association between AHRR methylation and deletion number may underestimate the true effect, given that neonatally-assessed DNA methylation at cg05575921 does not capture postnatal tobacco exposure. Only a small proportion of variation in cg05575921 methylation is explained by prenatal smoking exposure (24), and neonatal methylation at AHRR has been associated with maternal BMI, birth weight, and gestational age (although cg05575921 was not specifically examined (37)). It is possible, therefore, that there may be additional untested environmental exposures that confound the association between cg05575921 methylation and number of deletions in our ALL cases.
The clastogenic effects of tobacco smoke exposure are well-established (38–41). As deletion breakpoint locations were not provided by MLPA, we could not explore the molecular mechanisms underlying the formation of deletions in our ALL cases. However, sequencing analyses have demonstrated that erroneous V(D)J recombination appears to underlie the majority of deletions in ALL (11, 12). In support of our findings, an increased frequency of off-target V(D)J recombination-mediated genomic deletions was found in cord blood T lymphocytes from children of mothers exposed to active or passive tobacco smoke during pregnancy compared with children of unexposed mothers (17, 42). Such deletions may arise via increased inflammation and leukocytosis caused by chemicals within tobacco smoke (reviewed in Holt (43)). Alternatively, it has been proposed that tobacco-induced hypomethylation may lead to an increase in off-target V(D)J recombination-mediated deletions (17, 44).
Our study comprises the largest assessment of somatic CNAs in ALL in Latino children. Frequency of deletions was similar in Latino ALL cases compared with non-Latino white cases. Given that the number of non-Latino children included in this study was relatively small, we also compared frequency of gene deletions in our Latino cases with previously published data from 1427 non-Latino white cases from the UK (9), and again found no significant differences (data not shown). Variation in gene deletion frequency is, thus, unlikely to contribute to the increased risk of ALL in Latino children (33, 45), nor in the poorer survival associated with this group (34, 46).
Though we found no association with ethnicity, we did confirm the positive association between number of deletions and age-at-diagnosis (9, 23), and also confirmed the low frequency of deletions in HD-ALL and the high frequency, particularly of ETV6 and BTG1, in ETV6-RUNX1 fusion ALL (9, 11). Furthermore, we found a much higher prevalence of CDKN2A deletions in T-ALL cases relative to B-ALL cases, with a frequency similar to that reported by Mullighan et al. (2008) (8) and somewhat higher than the ~50% detected by Sulong et al. (2009) (47), though the larger sample size in the latter study (n=266) may reflect a more accurate proportion. Deletions of genes involved in the same leukemogenic pathways were mutually exclusive, for example the two tumor suppressor genes CDKN2A and RB1, and with genes involved in hematopoiesis and B-cell development, including ETV6, PAX5, IKZF1, and the PAR1 region (corresponding with CRLF2 rearrangement). However, deletions of genes with different functions were also significantly mutually exclusive, particularly loss of CDKN2A with both ETV6 and IKZF1, supporting that disruption of different pathways can lead to ALL. Though we did not have cytogenetic data pertaining to the BCR-ABL1 fusion, BCR-ABL1-like, or intrachromosomal amplification of chromosome 21 (iAMP21) subtypes of B-ALL, the significantly higher frequency of loss of IKZF1 and PAR1, and co-deletion of CDKN2A with PAX5 in the “non-HD, non-ETV6-RUNX1” cases aligns with previous observations of genomic alterations in these high-risk ALL subgroups (28, 48–50).
It is important to note that the MLPA assay used in this study does not provide genome-wide copy number information, albeit the 8 genes included are those most frequently lost in ALL (9, 10). Some cases may, therefore, harbor additional deletions in genes that were not investigated, though the relative infrequency of these deletions makes them unlikely to affect our results. MLPA may not detect deletions restricted to minor subclones, although these are more likely to arise later during tumor evolution and may have a different etiology than earlier deletions. We also do not know the timing of deletion events relative to the onset of overt leukemia, and hence the relationship of this timing to the tobacco exposure. An additional caveat is that there may be unmeasured environmental exposures that could confound our results, for example unmeasured factors associated with socioeconomic status not captured in our models by household education.
In summary, we provide evidence that increased tobacco smoke exposure increases the generation of somatic ALL-associated driver deletions. To our knowledge, this is also the first reported application of an epigenetic biomarker to assess the effects of an environmental exposure on leukemogenic alterations. Outcome data were not available, thus future studies should explore whether tobacco exposure may be associated with higher-risk ALL, given that these tend to harbor a higher frequency of deletions than standard-risk ALL (9, 28). In addition, sequencing analysis of ALL deletion breakpoints will be required to determine whether tobacco smoke exposure is associated specifically with RAG recombination-mediated deletions. Our findings should be added to an already compelling list of reasons for minimizing the prenatal and early life tobacco smoke exposure of children.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the National Institute of Environmental Health Sciences grants R01ES009137 (C.M., MK, S.S, A.Y.K, R.M.-C, L.Z, X.S, H.M.S, and J.L.W.) and P01ES018172 (C.M., S.S., A.Y.K, L.Z. and J.L.W.), the National Cancer Institute grants R01CA155461 (J.L.W.) and P30CA82103 (R.R.), the Tobacco-Related Disease Research Program grant 18CA-0127 (J.L.W.), the Alex’s Lemonade Stand Foundation ‘A’ Awards (A.J.D., K.M.W.), U.K. Children with Cancer Foundation (C.M.), the Swiss National Science Foundation grant P2LAP3_158674 (S.G.), the Sutter-Stöttner Foundation (S.G.), and the SICPA Foundation (S.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its subsidiary Institutes and Centers.
The authors gratefully acknowledge the families for their participation. We also thank the clinical investigators at the following collaborating hospitals for help in recruiting patients: University of California Davis Medical Center (Dr. Jonathan Ducore), University of California San Francisco (Drs. Mignon Loh and Katherine Matthay), Children’s Hospital of Central California (Dr. Vonda Crouse), Lucile Packard Children’s Hospital (Dr. Gary Dahl), Children’s Hospital Oakland (Drs. James Feusner and Carla Golden), Kaiser Permanente Roseville (formerly Sacramento) (Drs. Kent Jolly and Vincent Kiley), Kaiser Permanente Santa Clara (Drs. Carolyn Russo, Alan Wong, and Denah Taggart), Kaiser Permanente San Francisco (Dr. Kenneth Leung), and Kaiser Permanente Oakland (Drs. Daniel Kronish and Stacy Month). We thank Robin Cooley and Steve Graham at the California Biobank Program, California Department of Public Health, for assistance in retrieval of neonatal dried bloodspot specimens for CCLS subjects.
Footnotes
Conflicts of interest statement: The authors declare no potential conflicts of interest.
References
- 1.Linet MS, Ries LA, Smith MA, Tarone RE, Devesa SS. Cancer surveillance series: Recent trends in childhood cancer incidence and mortality in the united states. J Natl Cancer Inst. 1999;91:1051–8. doi: 10.1093/jnci/91.12.1051. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead TP, Metayer C, Wiemels JL, Singer AW, Miller MD. Childhood leukemia and primary prevention. Curr Probl Pediatr Adolesc Health Care. 2016;46:317–52. doi: 10.1016/j.cppeds.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metayer C, Zhang L, Wiemels JL, Bartley K, Schiffman J, Ma X, et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiol Biomarkers Prev. 2013;22:1600–11. doi: 10.1158/1055-9965.EPI-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mody R, Li S, Dover DC, Sallan S, Leisenring W, Oeffinger KC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. Blood. 2008;111:5515–23. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panzer-Grumayer ER, Fasching K, Panzer S, Hettinger K, Schmitt K, Stockler-Ipsiroglu S, et al. Nondisjunction of chromosomes leading to hyperdiploid childhood B-cell precursor acute lymphoblastic leukemia is an early event during leukemogenesis. Blood. 2002;100:347–9. doi: 10.1182/blood-2002-01-0144. [DOI] [PubMed] [Google Scholar]
- 6.Wiemels JL, Cazzaniga G, Daniotti M, Eden OB, Addison GM, Masera G, et al. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354:1499–503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 7.Bateman CM, Colman SM, Chaplin T, Young BD, Eden TO, Bhakta M, et al. Acquisition of genome-wide copy number alterations in monozygotic twins with acute lymphoblastic leukemia. Blood. 2010;115:3553–8. doi: 10.1182/blood-2009-10-251413. [DOI] [PubMed] [Google Scholar]
- 8.Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322:1377–80. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwab CJ, Chilton L, Morrison H, Jones L, Al-Shehhi H, Erhorn A, et al. Genes commonly deleted in childhood B-cell precursor acute lymphoblastic leukemia: Association with cytogenetics and clinical features. Haematologica. 2013;98:1081–8. doi: 10.3324/haematol.2013.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–64. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 11.Papaemmanuil E, Rapado I, Li Y, Potter NE, Wedge DC, Tubio J, et al. RAG-mediated recombination is the predominant driver of oncogenic rearrangement in ETV6-RUNX1 acute lymphoblastic leukemia. Nat Genet. 2014;46:116–25. doi: 10.1038/ng.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendes RD, Sarmento LM, Cante-Barrett K, Zuurbier L, Buijs-Gladdines JG, Povoa V, et al. PTEN microdeletions in T-cell acute lymphoblastic leukemia are caused by illegitimate RAG-mediated recombination events. Blood. 2014;124:567–78. doi: 10.1182/blood-2014-03-562751. [DOI] [PubMed] [Google Scholar]
- 13.Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG. The RAG proteins and V(D)J recombination: Complexes, ends, and transposition. Annu Rev Immunol. 2000;18:495–527. doi: 10.1146/annurev.immunol.18.1.495. [DOI] [PubMed] [Google Scholar]
- 14.Aplan PD, Lombardi DP, Ginsberg AM, Cossman J, Bertness VL, Kirsch IR. Disruption of the human SCL locus by “illegitimate” V-(D)-J recombinase activity. Science. 1990;250:1426–9. doi: 10.1126/science.2255914. [DOI] [PubMed] [Google Scholar]
- 15.Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of ikaros. Nature. 2008;453:110–4. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 16.Swaminathan S, Klemm L, Park E, Papaemmanuil E, Ford A, Kweon SM, et al. Mechanisms of clonal evolution in childhood acute lymphoblastic leukemia. Nat Immunol. 2015;16:766–74. doi: 10.1038/ni.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finette BA, O’Neill JP, Vacek PM, Albertini RJ. Gene mutations with characteristic deletions in cord blood T lymphocytes associated with passive maternal exposure to tobacco smoke. Nat Med. 1998;4:1144–51. doi: 10.1038/2640. [DOI] [PubMed] [Google Scholar]
- 18.Stjernfeldt M, Berglund K, Lindsten J, Ludvigsson J. Maternal smoking during pregnancy and risk of childhood cancer. Lancet. 1986;1:1350–2. doi: 10.1016/s0140-6736(86)91664-8. [DOI] [PubMed] [Google Scholar]
- 19.Milne E, Greenop KR, Scott RJ, Bailey HD, Attia J, Dalla-Pozza L, et al. Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol. 2012;175:43–53. doi: 10.1093/aje/kwr275. [DOI] [PubMed] [Google Scholar]
- 20.Orsi L, Rudant J, Ajrouche R, Leverger G, Baruchel A, Nelken B, et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: The ESTELLE study. Cancer Causes Control. 2015;26:1003–17. doi: 10.1007/s10552-015-0593-5. [DOI] [PubMed] [Google Scholar]
- 21.Klimentopoulou A, Antonopoulos CN, Papadopoulou C, Kanavidis P, Tourvas AD, Polychronopoulou S, et al. Maternal smoking during pregnancy and risk for childhood leukemia: A nationwide case-control study in greece and meta-analysis. Pediatr Blood Cancer. 2012;58:344–51. doi: 10.1002/pbc.23347. [DOI] [PubMed] [Google Scholar]
- 22.Aldrich MC, Zhang L, Wiemels JL, Ma X, Loh ML, Metayer C, et al. Cytogenetics of hispanic and white children with acute lymphoblastic leukemia in california. Cancer Epidemiol Biomarkers Prev. 2006;15:578–81. doi: 10.1158/1055-9965.EPI-05-0833. [DOI] [PubMed] [Google Scholar]
- 23.Walsh KM, de Smith AJ, Welch TC, Smirnov I, Cunningham MJ, Ma X, et al. Genomic ancestry and somatic alterations correlate with age at diagnosis in hispanic children with B-cell acute lymphoblastic leukemia. Am J Hematol. 2014;89:721–5. doi: 10.1002/ajh.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonseth S, de Smith AJ, Roy R, Zhou M, Lee ST, Shao X, et al. Genetic contribution to variation in DNA methylation at maternal smoking sensitive loci in exposed neonates. Epigenetics. 2016:0. doi: 10.1080/15592294.2016.1209614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, et al. DNA methylation in newborns and maternal smoking in pregnancy: Genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98:680–96. doi: 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joubert BR, Haberg SE, Bell DA, Nilsen RM, Vollset SE, Midttun O, et al. Maternal smoking and DNA methylation in newborns: In utero effect or epigenetic inheritance? Cancer Epidemiol Biomarkers Prev. 2014;23:1007–17. doi: 10.1158/1055-9965.EPI-13-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joubert BR, Haberg SE, Nilsen RM, Wang X, Vollset SE, Murphy SK, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2012;120:1425–31. doi: 10.1289/ehp.1205412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moorman AV, Enshaei A, Schwab C, Wade R, Chilton L, Elliott A, et al. A novel integrated cytogenetic and genomic classification refines risk stratification in pediatric acute lymphoblastic leukemia. Blood. 2014;124:1434–44. doi: 10.1182/blood-2014-03-562918. [DOI] [PubMed] [Google Scholar]
- 29.Fraga CG, Motchnik PA, Wyrobek AJ, Rempel DM, Ames BN. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat Res. 1996;351:199–203. doi: 10.1016/0027-5107(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen JF. Ultrasound evidence of sexual difference in fetal size in first trimester. Br Med J. 1980;281:1253. doi: 10.1136/bmj.281.6250.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–27. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enninga EA, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol. 2015;73:251–62. doi: 10.1111/aji.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the united states, 2001–2007. Blood. 2012;119:34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004) Cancer. 2008;112:416–32. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 35.Ji BT, Shu XO, Linet MS, Zheng W, Wacholder S, Gao YT, et al. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst. 1997;89:238–44. doi: 10.1093/jnci/89.3.238. [DOI] [PubMed] [Google Scholar]
- 36.Chang JS, Selvin S, Metayer C, Crouse V, Golembesky A, Buffler PA. Parental smoking and the risk of childhood leukemia. Am J Epidemiol. 2006;163:1091–100. doi: 10.1093/aje/kwj143. [DOI] [PubMed] [Google Scholar]
- 37.Burris HH, Baccarelli AA, Byun HM, Cantoral A, Just AC, Pantic I, et al. Offspring DNA methylation of the aryl-hydrocarbon receptor repressor gene is associated with maternal BMI, gestational age, and birth weight. Epigenetics. 2015;10:913–21. doi: 10.1080/15592294.2015.1078963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama T, Kaneko M, Kodama M, Nagata C. Cigarette smoke induces DNA single-strand breaks in human cells. Nature. 1985;314:462–4. doi: 10.1038/314462a0. [DOI] [PubMed] [Google Scholar]
- 39.Mohtashamipur E, Steinforth T, Norpoth K. Comparative bone marrow clastogenicity of cigarette sidestream, mainstream and recombined smoke condensates in mice. Mutagenesis. 1988;3:419–22. doi: 10.1093/mutage/3.5.419. [DOI] [PubMed] [Google Scholar]
- 40.Jalili T, Murthy GG, Schiestl RH. Cigarette smoke induces DNA deletions in the mouse embryo. Cancer Res. 1998;58:2633–8. [PubMed] [Google Scholar]
- 41.Huang YT, Lin X, Liu Y, Chirieac LR, McGovern R, Wain J, et al. Cigarette smoking increases copy number alterations in nonsmall-cell lung cancer. Proc Natl Acad Sci U S A. 2011;108:16345–50. doi: 10.1073/pnas.1102769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grant SG. Qualitatively and quantitatively similar effects of active and passive maternal tobacco smoke exposure on in utero mutagenesis at the HPRT locus. BMC Pediatr. 2005;5:20. doi: 10.1186/1471-2431-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holt PG. Immune and inflammatory function in cigarette smokers. Thorax. 1987;42:241–9. doi: 10.1136/thx.42.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Storb U, Arp B. Methylation patterns of immunoglobulin genes in lymphoid cells: Correlation of expression and differentiation with undermethylation. Proc Natl Acad Sci U S A. 1983;80:6642–6. doi: 10.1073/pnas.80.21.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeil DE, Cote TR, Clegg L, Mauer A. SEER update of incidence and trends in pediatric malignancies: Acute lymphoblastic leukemia. Med Pediatr Oncol. 2002;39:554, 7. doi: 10.1002/mpo.10161. discussion 552–3. [DOI] [PubMed] [Google Scholar]
- 46.Goggins WB, Lo FF. Racial and ethnic disparities in survival of US children with acute lymphoblastic leukemia: Evidence from the SEER database 1988–2008. Cancer Causes Control. 2012;23:737–43. doi: 10.1007/s10552-012-9943-8. [DOI] [PubMed] [Google Scholar]
- 47.Sulong S, Moorman AV, Irving JA, Strefford JC, Konn ZJ, Case MC, et al. A comprehensive analysis of the CDKN2A gene in childhood acute lymphoblastic leukemia reveals genomic deletion, copy number neutral loss of heterozygosity, and association with specific cytogenetic subgroups. Blood. 2009;113:100–7. doi: 10.1182/blood-2008-07-166801. [DOI] [PubMed] [Google Scholar]
- 48.Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: A genome-wide classification study. Lancet Oncol. 2009;10:125–34. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22:153–66. doi: 10.1016/j.ccr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrison CJ, Moorman AV, Schwab C, Carroll AJ, Raetz EA, Devidas M, et al. An international study of intrachromosomal amplification of chromosome 21 (iAMP21): Cytogenetic characterization and outcome. Leukemia. 2014;28:1015–21. doi: 10.1038/leu.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.