Abstract
SUMOylation modifies the interactome, localization, activity and lifespan of its target proteins. This process regulates several cellular machineries, including transcription, DNA damage repair, cell-cycle progression and apoptosis. Accordingly, SUMOylation is critical in maintaining cellular homeostasis and its deregulation leads to the corruption of a plethora of cellular processes that contribute to disease states.
Among the proteins involved in SUMOylation, the Protein Inhibitor of Activated STAT (PIAS) E3-ligases were initially described as transcriptional co-regulators. Recent findings also indicate that they have a role in regulating protein stability and signaling transduction pathways. PIAS proteins interact with up to 60 cellular partners affecting several cellular processes, most notably immune regulation and DNA repair, but also cellular proliferation and survival. Here we summarize the current knowledge about their role in tumorigenesis and cancer-related processes.
The process of SUMOylation
SUMOylation is an enzymatic cascade whereby Small Ubiquitin-like Modifier (SUMO) proteins are covalently bound to lysine residues (K) present on target proteins (Fig 1A). The components of the SUMOylation machinery are highly conserved and ubiquitously expressed throughout the eukaryotic kingdoms. There are several recent publications on this topic: we will review here only the essentials regarding the SUMOylation cascade (1,2).
Figure 1. Mechanism of SUMOylation.
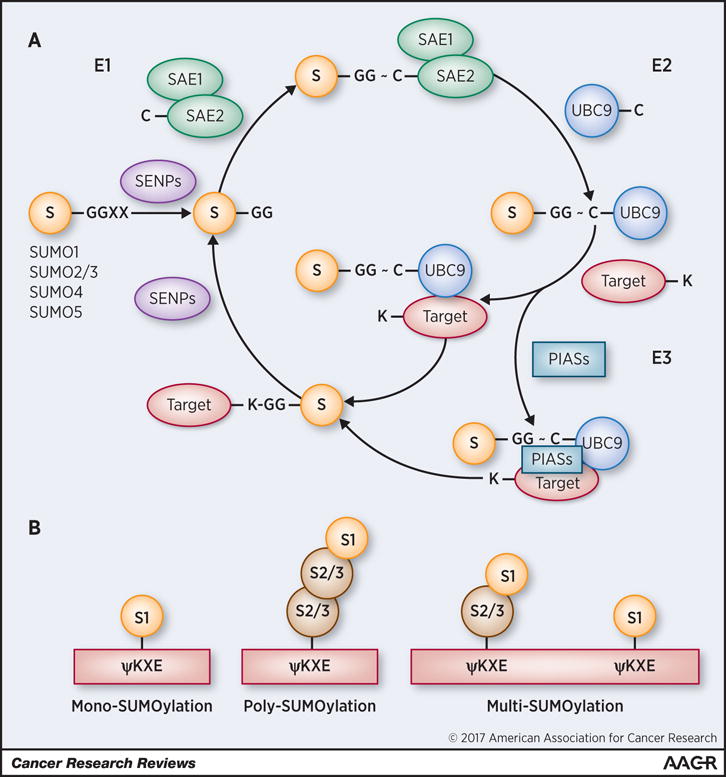
A) Sentrin-specific Proteases (SENPs) catalyze the maturation of the SUMO (S) proteins (cleavage of Gly-Gly tail, GG) that are transferred to the final substrate through an enzymatic cascade comprising the heterodimeric SAE1–SAE2 (SUMO-Activating Enzyme 1 and 2, respectively) E1-ligase and the E2-ligase UBC9. This last enzymatic step is often facilitated by an E3-ligase, which facilitates the transfer of SUMO to target proteins. Since SUMOylation is a reversible modification SENPS accounts also for the de-SUMOylation process. B) SUMO2 and SUMO3 (S2/3), can form poly-SUMO chains. SUMO1 (S1), lacking internal lysine residues, is a SUMO chain terminator. The figure depicts a classic ΨKXE SUMOylation consensus site, where Ψ is a branched aliphatic residue and X is any amino acid. Additional motifs have been identified: NDSM, amino acid-dependent SUMOylation motif; PDSM, phosphorylation-dependent SUMO-motif; pSuM, phosphorylated SUMOylation motif.
SUMOylation targets a wide variety of proteins, including transcription factors, membrane receptors and enzymes, and it is essential during embryonic development (1,2). Whole-proteomic analysis showed that approximately 2% of the entire mammalian proteome is SUMOylated (3).
In Vertebrates, five SUMO genes exist (SUMO1-5). SUMO1-3 are ubiquitously expressed while SUMO4 and SUMO5 are tissue-specific, and their functions are not completely defined yet (2,4). SUMO modifications can be distinguished in mono-, poly- and multi-SUMOylation (2,3) (Fig. 1B).
The PIAS family: classification and physiological roles
In striking contrast with the ubiquitination system, where hundreds of distinct E3-ligases mediate the recognition of specific substrates, only the PIAS family and few other SUMO E3-ligases have been described. Thus, it is not completely understood how substrate recognition is achieved in the SUMOylation system (5,6). The RING domain and the SUMO-Interacting Motif (SIM) of the SUMO E3-ligases contribute to substrate specificity and facilitate the interaction of UBC9 and SUMO with target proteins (5,6). However, it seems unlikely that this is the only mechanism mediating the specificity of substrate selection and future research will likely shed more light on this process.
PIAS orthologs can be found throughout the eukaryotic kingdoms, and Mammals have four PIAS genes (7) (Fig. 2A).
Figure 2. PIAS proteins structure and significance in cancer.
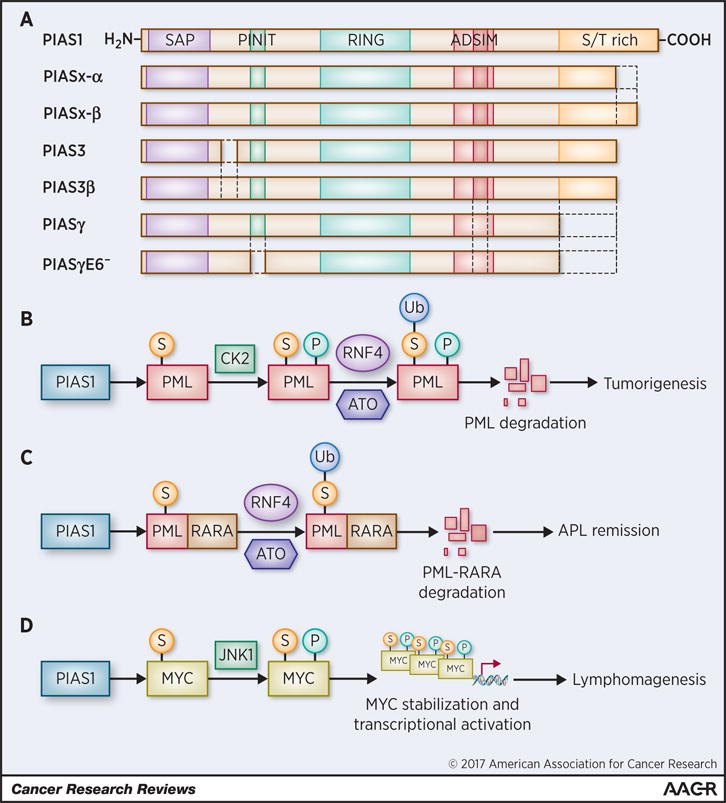
A) In Mammals, four PIAS genes exist: PIAS1, PIAS2 (also known as PIASx, with the two splice variants PIASx-α and PIASx-β), PIAS3 (with the splice variant PIAS3β) and PIAS4 (also known as PIASy, with the splice variant PIASyE6−). These genes share 40% of homology and domain organization. The N-terminal SAP domain binds to (A+T)-enriched DNA sequences and to transcriptional co-regulator through the α-helical LXXLL motif (not shown in the figure). The PINIT domain mediates nuclear localization; the RING-finger-like zinc-binding domain is required for the E3-ligase activity. In the C-terminal region are present a highly acidic region (AD) and a serine/threonine rich region (S/T rich). The AD of PIAS1, PIAS2 and PIAS3 also contains a SUMO-interacting motif (SIM), which can modulate the enzymatic activity of the protein. PIASyE6− is the splice variant of PIAS4 lacking exon 6 (i.e. the PINIT domain). B) In lung cancer cells PIAS1 SUMOylates the tumor suppressor PML, leading to the recruiting of CK2 that, in turn phosphorylates PML leading to its proteasomal degradation. ATO facilitates PIAS1-driven SUMOylation of PML and its subsequent ubiquitination by RNF4 ubiquitin E3-ligase that is recruited on SUMO chains. C) In APL PIAS1 SUMOylates PML-RARA. ATO induces PIAS1-dependent PML-RARA hyper-SUMOylation. RNF4 directs the ubiquitin-mediated degradation of hyper-SUMOylated PML-RARA causing the apoptosis of APL cells. D) PIAS1 SUMOylates MYC, recruiting JNK1 which in turn phosphorylates MYC increasing its stability and transcriptional activity. These events contribute to B-cell lymphomagenesis. S, SUMOylation; P, phosphorylation; Ub, ubiquitination.
PIAS proteins were initially identified as inhibitor of STAT transcription factors (7–9), but it is becoming clear that they regulate a broader range of proteins. For instance, PIAS family members regulate nuclear trafficking, DNA damage repair, NF-κB signaling, several transcription factors, and many nuclear receptors (7,10–13).
At the organismal level, PIAS members are involved in embryonic development, hematopoiesis, innate and adaptive immune response, spatial learning and long-term memory (14–17).
Of note, the functions of PIAS proteins are not always strictly associated with their SUMO E3-ligase activity, suggesting that their enzymatic activity might be just one of their functions. For instance, both PIAS1 and PIAS4 regulate LEF-1 and Tp53, regardless of the integrity of the RING domain, which is necessary for their SUMO E3-ligase activity (18,19).
Regulation of PIAS proteins in cancer
Several post-translational modifications modulate the function of PIAS family members, however, their mechanistic underpinnings and functional consequences are still not completely understood. cs
PIAS1 is phosphorylated by Casein Kinase 2 (CK2) in vitro on 3 conserved serine residues adjacent to the SIM domain (20). This phosphorylation event is of biological relevance since PIAS1 promotes the interaction between CK2 and PML tumor suppressor (PML), which in turn leads PML to ubiquitin mediated degradation (21). We will discuss the role of the SUMO-dependent RNF4 ubiquitin E3 ligase in this process, later in this review. Furthermore, CK2 itself undergoes SUMOylation (22). These observations suggest that PIAS1 and CK2 are part of an integrated signaling network dedicated to degradation of PML in cancer cells.
During inflammation, PIAS1 is rapidly phosphorylated on S90 by IKKα and this modification seems to promote PIAS1 E3-ligase activity (23). There is no evidence that these phosphorylation events influence the role of PIAS proteins in cancer. However, since CK2 and NF-kB pathways are often deregulated in cancer, it is likely that downstream phosphorylation of PIAS proteins contribute to tumorigenesis.
The mechanisms that regulate the transcription of PIAS family members are still largely unknown, however PIAS genes are often over-expressed in cancer (24). This area of research will likely be explored in the near future. Little is known regarding the turnover of PIAS family members and it will not be discussed here.
SUMO ligases and cancer
Several components of the SUMO machinery are highly expressed in cancer, suggesting that SUMOylation is required to initiate or sustain tumorigenesis. For example, SAE1/2 are amplified in breast, prostate and pancreatic cancers (24). Moreover, SAE1/2 is a MYC synthetic lethality in breast cancer cells (25). In this context, SAE1/2 silencing inhibits MYC SUMOylation causing the repression of a subset of MYC target genes, causing a mitotic catastrophe (25). Moreover, elevated levels of SAE1/2 are found in patients with hepatocellular carcinoma (HCC), while UBC9 is amplified or over-expressed in breast, prostate and pancreatic cancer (24).
PIAS1 and PIAS3 have been most often implicated in tumorigenesis: PIAS1 is over-expressed in prostate cancer where it suppresses p21 leading to an increased proliferation rate (26,27); PIAS1 over-expression correlates with a poor clinical outcome in multiple myeloma (28); PIAS3 is often over-expressed in colorectal cancer (CRC) (24). As described in the following sections, PIAS proteins promote tumorigenesis and cancer cell survival through the interaction with several tumor suppressors and oncogenes.
PIAS family members and the regulation of Tp53
The tumor suppressor Tp53 is a master regulator of apoptosis and senescence. Tp53 is often lost or inactivated in many types of cancer (29). In vitro assays using both cell-free and cell culture-based assays have shown that all PIAS members physically interact with Tp53 (29). SUMOylation of Tp53 primarily occurs at K386, but in its absence, other lysine residues can be SUMOylated (29). However, the functional consequence of Tp53 SUMOylation has been controversial. For example, it was reported that PIAS4-dependent SUMOylation promotes the transcriptional activity of wild-type Tp53 leading to senescence (29). However, others reported that PIAS4 ligase promotes the nuclear export of Tp53, counteracting its transcriptional activity (29).
It was also reported that both PIAS1 and PIASx-β SUMOylate Tp53. Also in this case it is still unclear whether PIAS1 and PIASx-β-dependent SUMOylation either activates or represses Tp53, and if they exert these functions independently of their E3-ligase activity (29). One possible explanation for such divergent results is that the effects of PIAS proteins on Tp53 are influenced by cellular context, post-translational modifications (PTMs) or other, yet to be identified, interacting proteins.
Interestingly, also the other Tp53 family members p63 and p73, and the Tp53 ubiquitin ligase MDM2 undergo PIAS-mediated SUMOylation. PIAS1-dependent SUMOylation negatively regulates the transcriptional activity of p73 (29). Moreover, both PIAS1 and PIASx-β SUMOylate the nuclear localization signal (NLS) of MDM2 at K182, promoting MDM2 nuclear translocation (30).
All together, these data indicate that PIAS proteins are able to modulate the activity and the fate of Tp53 and Tp53-related members, highlighting their relevance in Tp53 regulation.
PIASs and the PI3K/AKT axis
The serine/threonine kinase AKT plays a pivotal role in the regulation of several physiological and oncogenic processes (31). AKT is regulated by several PTMs, including PIAS1-dependent SUMOylation on K276. This modification is essential for AKT activation and mutation of K276 completely abrogates its kinase activity. Moreover, PIAS1 SUMOylates also AKT E17K, which is a common cancer associated mutation, more efficiently than wild type AKT. Furthermore, ablation of K276 dramatically decreases the tumorigenic activity of AKT E17K (32). Neither the activation of PI3K nor the ability of AKT to bind to the plasma membrane affects its SUMOylation. Instead, the SUMOylation of AKT is negatively regulated by PML upon nutrient starvation or wortmannin treatment (33).
By contrast, PIASx-α opposes PI3K/AKT signaling by directly SUMOylating the tumor suppressor PTEN, which inhibits PI3K/AKT signaling through its phosphatase activity. The effect of PTEN SUMOylation is a reduction of its ubiquitin-proteasomal degradation (34).
These results suggest that PIAS1 and PIASx-α have an opposite influence on the PI3K signaling axis. Future experiments will elucidate the mechanistic insights by which PIAS1 and PIASx-α control PI3K-AKT signaling pathway.
PIAS1, PML and PML-RARA
The tumor suppressor PML was one of the first proteins described to be SUMOylated (35,36). Initially identified as a component of the PML-RARA oncoprotein of t(15;17) of acute promyelocytic leukemia (APL), PML tumor suppressor is frequently lost in human cancer through aberrant ubiquitin-proteasomal degradation triggered by CK2 (37,38). PIAS1 and PIASx-α are PML SUMO E3-ligases (21). PIAS1-dependent SUMOylation drives PML to ubiquitination and degradation by promoting the recruitment of CK2 to poly-SUMOylated PML (Fig. 2B). Indeed, loss of PIAS1 in the developing embryo or silencing of PIAS1 in non-small cell lung cancer (NSCLC) cell lines leads to up-regulation of PML and decreased cell proliferation (16,21). Even though PIAS1 is not commonly mutated in cancer, it is often amplified in NSCLC cell lines and is over-expressed in NSCLC and several other cancer types (21,24). Thus, it is notable that PIAS1 and PML protein levels are inversely correlated in human primary NSCLC samples (21). All together, these observations support the conclusion that PIAS1 is oncogenic and that PML is its bona fide target.
We expect that the identification of the transcriptional and post-transcriptional mechanisms that mediate PIAS1 over-expression and substrate selection will reveal novel details regarding cancer underpinnings.
SUMOylation plays a dual role in the regulation of PML-RARA. Mono- or oligo-SUMOylation of K160 stabilizes PML-RARA and is essential for leukemogenesis. Arsenic trioxide (ATO) treatment (a major therapeutic agent in Acute Promyelocytic Leukemia, APL) induces a conformational change of both PML and PML-RARA that causes their poly-SUMOylation. Subsequently, the RNF4 E3-ubiquitin ligase binds to poly-SUMOylated PML and PML-RARA through its SUMO-interacting motifs (SIM), directing their ubiquitin-dependent proteasomal degradation (39). Although ATO does not distinguish between PML and its fusion protein, the degradation of PML-RARA causes the death of APL cells and ultimately remission in APL patients (40).
It has been demonstrated that PIAS1 is the PML-RARA SUMO E3-ligase that SUMOylates PML at K160 in basal conditions. Furthermore, PIAS1 mediates the ability of ATO to induce PML-RARA hyper-SUMOylation. Thus, PIAS1 is required for the therapeutic action of arsenic trioxide (21) (Fig.2B–2C).
These data demonstrate that the nature and the intensity of the stimulus that activates PIAS1 critically influence its biological function. We also speculate that PIAS1 may contribute to other effects of ATO such as its well-known carcinogenic effects and the induction of oxidative stress responses.
The role of PIASx-α in the regulation of PML has not been characterized yet, however unpublished data from our lab suggest that PIASx-α might be involved in the promotion of replicative senescence (AR unpublished). This observation suggests that PIAS family members have divergent biological roles even when SUMOylating a common substrate. Future investigations are needed to clarify whether this property is due to the SUMOylation of specific lysine acceptors or to the quality or composition of SUMOylated chains conjugated to the substrate.
PIAS1, MYC and lymphomagenesis
MYC exerts critical oncogenic activities in a wide variety of human cancers. MYC is strictly regulated by several post-translational modifications, including phosphorylation and ubiquitination (41). MYC family members are also SUMOylated (42). The observation that the SUMO E1 ligase is required for the viability of MYC-dependent breast cancer cells, suggests that SUMOylation is involved in the regulation of MYC dependent processes (25). However, until recently, it was unknown whether SUMOylation directly regulates MYC.
Recently, PIAS1 has been reported to account for MYC SUMOylation (43,44) (Fig.2D). K51/52 are the major MYC SUMOylation sites. SUMOylation not only prolongs MYC half-life preventing its proteasomal degradation, but also positively regulates MYC transcriptional activity. Significantly, we also demonstrated that MYC is SUMOylated in primary B-cell lymphomas (43). Furthermore, PIAS1-mediated SUMOylation is required to maintain the oncogenic activity of MYC in lymphoma cells. Accordingly, we found that PIAS1 and MYC are often co-expressed in human and murine diffuse large B-cell lymphoma (DLBCL) (43). This study also suggests that PIAS1 regulates MYC during B-cell maturation and activation in vitro. These observations are consistent with the observation that Pias1 null mice have defective differentiation of the lymphoid lineage in vivo (15).
We propose that when expressed at physiological levels, PIAS1 promotes the expansion and differentiation of B-cells through the physiological activation of MYC. Instead, in conditions of up-regulation of MYC, as it often occurs in cancer, PIAS1 promotes the supra-physiological activation of MYC, contributing not only to B-cell lymphomagenesis but also to other cancer types (43).
In this regards, it is noteworthy that PIAS1 is required also for the viability of MYC-dependent breast cancer and of lung cancer cells (43) (and AR unpublished results). These findings support the conclusion that PIAS1 is a requirement of other MYC-driven malignances. Consistent with this view, several components of the SUMOylation machinery are up-regulated by MYC in mouse and human B-cell lymphomas. Furthermore, inhibition of SUMOylation induces the apoptosis of MYC-dependent lymphoma cells (45).
González-Prieto et al. reported that PIAS1 induces MYC-degradation in a SUMO-dependent manner (44). Even though this report is consistent with the notion that PIAS1 SUMOylates MYC, it reaches a conclusion that is at odds with ours (43). We reason that this discrepancy could be explained by the fact that the experiments of Gonzales-Prieto et al. were performed mainly using ectopically expressed genes in U2OS and HeLa cell lines, which could have influenced the outcome of their experiments. Future experiments will undoubtedly further address the role of PIAS1 in the regulation of MYC. For instance, our laboratory is performing experiments with a Pias1 conditional null mouse to dissect the function of PIAS1 on tumorigenesis in vivo.
PIAS1, cell migration, EMT and metastasis
Metastasis requires complex cellular changes including cytoskeleton remodeling, loss of cell-polarity and of cell-adhesion. Focal adhesion kinase (FAK) plays a crucial role in these processes. Indeed, FAK is over-expressed in many kinds of tumors, including breast, pancreas, lung, and colon cancer (46). A crucial step in FAK activation is the auto-phosphorylation on tyrosine (Y) 397, which is required for the activation of its downstream signaling network. It was shown that PIAS1 SUMOylates FAK promoting Y397 phosphorylation and increasing its kinase activity (46). Moreover, it has been discovered that PIAS1 and FAK are co-amplified in a subset of NSCLC. Furthermore, over-expression of PIAS1 leads to FAK activation, while PIAS1 silencing leads to apoptosis and focal adhesion turnover (47).
Epithelial to Mesenchymal Transition (EMT) is an essential developmental process that is a critical factor contributing to metastasis and drug resistance. TGF-β is a key regulator of EMT: TGF-β-induced EMT is often associated to tumor progression in many types of cancers, including breast, prostate and colorectal cancer (48). TGF-β induces the degradation of PIAS1 during EMT (48). However, it was also reported that PIAS1 negatively regulates EMT by antagonizing the activity of TGF-β in a SUMO-dependent manner (48). The latter set of observations, describing PIAS1 as a metastasis-inhibitor, seem in contrast with the reports that PIAS1 promotes the activity of FAK, which is a positive regulator of EMT (46,47). We reason that the preponderance of evidence supports the conclusion that PIAS1 plays a positive role in EMT and metastasis. However, further studies are needed to solve these inconsistencies.
Conclusions
Recent evidence indicates that SUMOylation is directly involved in the regulation of oncogenic networks and in the promotion of cancer. Accordingly, several SUMO-ligases, including PIAS family members, are often over-expressed in cancer. PIAS proteins are emerging as key positive regulators of several oncogenic networks. At present, PIAS1 is the family member that has been involved more consistently in tumorigenesis through its ability to positively regulate AKT and MYC and negatively regulate PML, Tp53 and PTEN. In addition, PIAS1 is a critical determinant of the therapeutic action of arsenic trioxide. These activities coordinately regulate several oncogenic networks to cooperate toward tumorigenesis. Several compounds that target SAE1/SAE2 or UBC9 are entering clinical testing. We also reason that PIAS1 could be a worthy therapeutic target (45,49). We expect that future studies combining cancer genetics, functional studies and in vivo models will continue to increase our understanding of the role of the SUMOylation machinery in cancer and in determining the response to therapy.
Acknowledgments
This work was supported by R01CA137195, CDMRP LCRP grant LC110229, CPRIT RP140672, RP160652, NCI 5P50 CA70907-15, UT Southwestern Friends of the Comprehensive Cancer Center, the ACS Grant # 13-068-01-TBG, the Gibson Foundation, Texas 4000 (PPS), the Lymphoma Research Foundation (AR) and by Cancer Center Support Grant 2P30 CA142543-06.
Footnotes
Disclosure of potential conflicts of interest
The authors do not have conflict of interest.
References
- 1.Nacerddine K, Lehembre F, Bhaumik M, Artus J, Cohen-Tannoudji M, Babinet C, et al. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell. 2005;9:769–79. doi: 10.1016/j.devcel.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–85. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- 3.Hendriks IA, Vertegaal AC. A comprehensive compilation of SUMO proteomics. Nat Rev Mol Cell Biol. 2016;17:581–95. doi: 10.1038/nrm.2016.81. [DOI] [PubMed] [Google Scholar]
- 4.Liang YC, Lee CC, Yao YL, Lai CC, Schmitz ML, Yang WM. SUMO5, a Novel Poly-SUMO Isoform, Regulates PML Nuclear Bodies. Sci Rep. 2016;6:26509. doi: 10.1038/srep26509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yunus AA, Lima CD. Structure of the Siz/PIAS SUMO E3 ligase Siz1 and determinants required for SUMO modification of PCNA. Mol Cell. 2009;35:669–82. doi: 10.1016/j.molcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner A, Flotho A, Melchior F. The RanBP2/RanGAP1*SUMO1/Ubc9 complex is a multisubunit SUMO E3 ligase. Mol Cell. 2012;46:287–98. doi: 10.1016/j.molcel.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Rytinki MM, Kaikkonen S, Pehkonen P, Jaaskelainen T, Palvimo JJ. PIAS proteins: pleiotropic interactors associated with SUMO. Cell Mol Life Sci. 2009;66:3029–41. doi: 10.1007/s00018-009-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, et al. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–5. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Liao J, Rao X, Kushner SA, Chung CD, Chang DD, et al. Inhibition of Stat1-mediated gene activation by PIAS1. Proc Natl Acad Sci U S A. 1998;95:10626–31. doi: 10.1073/pnas.95.18.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanty Y, Belotserkovskaya R, Coates J, Polo S, Miller KM, Jackson SP. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature. 2009;462:935–9. doi: 10.1038/nature08657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang HD, Yoon K, Shin YJ, Kim J, Lee SY. PIAS3 suppresses NF-kappaB-mediated transcription by interacting with the p65/RelA subunit. J Biol Chem. 2004;279:24873–80. doi: 10.1074/jbc.M313018200. [DOI] [PubMed] [Google Scholar]
- 12.Kotaja N, Vihinen M, Palvimo JJ, Janne OA. Androgen receptor-interacting protein 3 and other PIAS proteins cooperate with glucocorticoid receptor-interacting protein 1 in steroid receptor-dependent signaling. J Biol Chem. 2002;277:17781–8. doi: 10.1074/jbc.M106354200. [DOI] [PubMed] [Google Scholar]
- 13.Morris JR, Boutell C, Keppler M, Densham R, Weekes D, Alamshah A, et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature. 2009;462:886–90. doi: 10.1038/nature08593. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Tahk S, Yee KM, Fan G, Shuai K. The ligase PIAS1 restricts natural regulatory T cell differentiation by epigenetic repression. Science. 2010;330:521–5. doi: 10.1126/science.1193787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Yee KM, Tahk S, Mackie R, Hsu C, Shuai K. PIAS1 SUMO ligase regulates the self-renewal and differentiation of hematopoietic stem cells. EMBO J. 2014;33:101–13. doi: 10.1002/embj.201283326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constanzo JD, Deng M, Rindhe S, Tang KJ, Zhang CC, Scaglioni PP. Pias1 is essential for erythroid and vascular development in the mouse embryo. Dev Biol. 2016;415:98–110. doi: 10.1016/j.ydbio.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai DJ, Hsu WL, Liu YC, Ma YL, Lee EH. Novel role and mechanism of protein inhibitor of activated STAT1 in spatial learning. EMBO J. 2011;30:205–20. doi: 10.1038/emboj.2010.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachdev S, Bruhn L, Sieber H, Pichler A, Melchior F, Grosschedl R. PIASy, a nuclear matrix-associated SUMO E3 ligase, represses LEF1 activity by sequestration into nuclear bodies. Genes Dev. 2001;15:3088–103. doi: 10.1101/gad.944801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt D, Muller S. Members of the PIAS family act as SUMO ligases for c-Jun and p53 and repress p53 activity. Proc Natl Acad Sci U S A. 2002;99:2872–7. doi: 10.1073/pnas.052559499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stehmeier P, Muller S. Phospho-regulated SUMO interaction modules connect the SUMO system to CK2 signaling. Mol Cell. 2009;33:400–9. doi: 10.1016/j.molcel.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Rabellino A, Carter B, Konstantinidou G, Wu SY, Rimessi A, Byers LA, et al. The SUMO E3-ligase PIAS1 regulates the tumor suppressor PML and its oncogenic counterpart PML-RARA. Cancer Res. 2012;72:2275–84. doi: 10.1158/0008-5472.CAN-11-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Q, Li H, Liu BQ, Huang XY, Guo L. SUMOylation-regulated protein phosphorylation, evidence from quantitative phosphoproteomics analyses. J Biol Chem. 2011;286:27342–9. doi: 10.1074/jbc.M111.220848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, et al. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129:903–14. doi: 10.1016/j.cell.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 24.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessler JD, Kahle KT, Sun T, Meerbrey KL, Schlabach MR, Schmitt EM, et al. A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis. Science. 2012;335:348–53. doi: 10.1126/science.1212728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puhr M, Hoefer J, Eigentler A, Dietrich D, van Leenders G, Uhl B, et al. PIAS1 is a determinant of poor survival and acts as a positive feedback regulator of AR signaling through enhanced AR stabilization in prostate cancer. Oncogene. 2016;35:2322–32. doi: 10.1038/onc.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoefer J, Schafer G, Klocker H, Erb HH, Mills IG, Hengst L, et al. PIAS1 is increased in human prostate cancer and enhances proliferation through inhibition of p21. Am J Pathol. 2012;180:2097–107. doi: 10.1016/j.ajpath.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Driscoll JJ, Pelluru D, Lefkimmiatis K, Fulciniti M, Prabhala RH, Greipp PR, et al. The sumoylation pathway is dysregulated in multiple myeloma and is associated with adverse patient outcome. Blood. 2010;115:2827–34. doi: 10.1182/blood-2009-03-211045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stehmeier P, Muller S. Regulation of p53 family members by the ubiquitin-like SUMO system. DNA Repair (Amst) 2009;8:491–8. doi: 10.1016/j.dnarep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Miyauchi Y, Yogosawa S, Honda R, Nishida T, Yasuda H. Sumoylation of Mdm2 by protein inhibitor of activated STAT (PIAS) and RanBP2 enzymes. J Biol Chem. 2002;277:50131–6. doi: 10.1074/jbc.M208319200. [DOI] [PubMed] [Google Scholar]
- 31.Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li R, Wei J, Jiang C, Liu D, Deng L, Zhang K, et al. Akt SUMOylation regulates cell proliferation and tumorigenesis. Cancer Res. 2013;73:5742–53. doi: 10.1158/0008-5472.CAN-13-0538. [DOI] [PubMed] [Google Scholar]
- 33.de la Cruz-Herrera CF, Campagna M, Lang V, del Carmen Gonzalez-Santamaria J, Marcos-Villar L, Rodriguez MS, et al. SUMOylation regulates AKT1 activity. Oncogene. 2015;34:1442–50. doi: 10.1038/onc.2014.48. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Yan J, Zhang J, Zhu S, Wang Y, Shi T, et al. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat Commun. 2012;3:911. doi: 10.1038/ncomms1919. [DOI] [PubMed] [Google Scholar]
- 35.Kamitani T, Nguyen HP, Kito K, Fukuda-Kamitani T, Yeh ET. Covalent modification of PML by the sentrin family of ubiquitin-like proteins. J Biol Chem. 1998;273:3117–20. doi: 10.1074/jbc.273.6.3117. [DOI] [PubMed] [Google Scholar]
- 36.Bernardi R, Pandolfi PP. A Dialog on the First 20 Years of PML Research and the Next 20 Ahead. Front Oncol. 2014;4:23. doi: 10.3389/fonc.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scaglioni PP, Yung TM, Cai LF, Erdjument-Bromage H, Kaufman AJ, Singh B, et al. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell. 2006;126:269–83. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 38.Gurrieri C, Capodieci P, Bernardi R, Scaglioni PP, Nafa K, Rush LJ, et al. Loss of the tumor suppressor PML in human cancers of multiple histologic origins. J Natl Cancer Inst. 2004;96:269–79. doi: 10.1093/jnci/djh043. [DOI] [PubMed] [Google Scholar]
- 39.Lallemand-Breitenbach V, Jeanne M, Benhenda S, Nasr R, Lei M, Peres L, et al. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008;10:547–55. doi: 10.1038/ncb1717. [DOI] [PubMed] [Google Scholar]
- 40.de The H, Le Bras M, Lallemand-Breitenbach V. The cell biology of disease: Acute promyelocytic leukemia, arsenic, and PML bodies. J Cell Biol. 2012;198:11–21. doi: 10.1083/jcb.201112044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amati B. Myc degradation: dancing with ubiquitin ligases. Proc Natl Acad Sci U S A. 2004;101:8843–4. doi: 10.1073/pnas.0403046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabo A, Doni M, Amati B. SUMOylation of Myc-family proteins. PLoS One. 2014;9:e91072. doi: 10.1371/journal.pone.0091072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabellino A, Melegari M, Tompkins VS, Chen W, Van Ness BG, Teruya-Feldstein J, et al. PIAS1 Promotes Lymphomagenesis through MYC Upregulation. Cell Rep. 2016;15:2266–78. doi: 10.1016/j.celrep.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez-Prieto R, Cuijpers SA, Kumar R, Hendriks IA, Vertegaal AC. c-Myc is targeted to the proteasome for degradation in a SUMOylation-dependent manner, regulated by PIAS1, SENP7 and RNF4. Cell Cycle. 2015;14:1859–72. doi: 10.1080/15384101.2015.1040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoellein A, Fallahi M, Schoeffmann S, Steidle S, Schaub FX, Rudelius M, et al. Myc-induced SUMOylation is a therapeutic vulnerability for B-cell lymphoma. Blood. 2014;124:2081–90. doi: 10.1182/blood-2014-06-584524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Constanzo JD, Tang KJ, Rindhe S, Melegari M, Liu H, Tang X, et al. PIAS1-FAK Interaction Promotes the Survival and Progression of Non-Small Cell Lung Cancer. Neoplasia. 2016;18:282–93. doi: 10.1016/j.neo.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Licciardello MP, Kubicek S. Pharmacological treats for SUMO addicts. Pharmacol Res. 2016;107:390–7. doi: 10.1016/j.phrs.2016.01.004. [DOI] [PubMed] [Google Scholar]


