Abstract
Background/Objectives
The intergenerational association of obesity may be driven by mother-to-newborn transmission of microbiota at birth. Yet Cesarean delivery circumvents newborn acquisition of vaginal microbiota, and has been associated with greater childhood adiposity. Here we examined the independent and joint associations of maternal pre-pregnancy BMI (kg/m2) and delivery mode with childhood overweight or obesity.
Subjects/Methods
We prospectively followed 1,441 racially and ethnically diverse mother-child dyads in the Boston Birth Cohort until age 5y (range 2.0—8.0y). We used logistic regression to examine the independent and joint associations of delivery mode (Cesarean and vaginal delivery) and pre-pregnancy BMI with childhood overweight or obesity (age-sex specific BMI≥85th percentile).
Results
Of 1,441 mothers, 961 delivered vaginally and 480 by Cesarean. Compared to vaginally delivered children, Cesarean delivered children had 1.4 (95% CI 1.1–1.8) times greater odds of becoming overweight or obese in childhood, after adjustment for maternal age at delivery, race/ethnicity, education, air pollution exposure, pre-pregnancy BMI, pregnancy weight gain, and birth weight. Compared to children born vaginally to normal weight mothers, after multivariable adjustment, odds of childhood overweight or obesity were highest in children born by Cesarean delivery to obese mothers (OR 2.8, 95% CI 1.9–4.1), followed by children born by Cesarean delivery to overweight mothers (OR 2.2, 95% CI 1.5–3.2), then children born vaginally to obese mothers (OR 1.8, 95% CI 1.3–2.6), and finally children born vaginally to overweight mothers (OR 1.7, 95% CI, 1.2–2.3).
Conclusions
In our racially and ethnically diverse cohort, Cesarean delivery and pre-pregnancy overweight and obesity were associated with childhood overweight or obesity. Needed now are prospective studies that integrate measures of the maternal and infant microbiome, and other potentially explanatory covariates, to elucidate the mechanisms driving this association and to explore whether exposure to vaginal microbiota in Cesarean delivered newborns may be an innovative strategy to combat the intergenerational cycle of obesity.
Keywords: Delivery mode, Cesarean delivery, Vaginal Delivery, Obesity, Body Mass Index, Pregnancy
Introduction
An increasing number of women who become pregnant are overweight or obese (OWOB) 1–3. Women carrying excess weight into pregnancy are at higher risk of delivering offspring that will become OWOB4–7. These women are also at higher risk of delivering by Cesarean section (C-section)8, and a systematic review found that, compared to women who deliver vaginally, those who deliver by C-section have a 30% higher odds of having OWOB children9. To date, no studies have examined the joint contribution of maternal OWOB and C-section delivery on the offspring’s risk of developing OWOB.
Human gut microbiota can transfer the obesity phenotype from humans to germ-free mice10, suggesting a causal effect of gut microbiota on obesity. However, whether the vaginal microbiota of obese mothers is associated with offspring risk of obesity is unknown. We recently observed that maternal OWOB was associated with altered gut microbiota composition in neonates delivered vaginally, yet an association between maternal OWOB and gut microbiota was not observed in neonates delivered by C-section11. Due to differential sharing of microbiota at birth during labor, delivery mode should also modify the intergenerational association between mother and child weight status. In the present study, we prospectively examined the independent and joint association of maternal pre-pregnancy OWOB and mode of delivery with offspring risk of OWOB in childhood.
Subjects and Methods
Participants and Data Collection Procedures
The Boston Birth Cohort (BBC) was initially designed as a molecular epidemiologic study on determinants of low birth weight and preterm birth12. Participants were recruited at birth at the Boston Medical Center (BMC) from 1998 to 2014. Multiple gestation pregnancies, pregnancies resulting from in-vitro fertilization, deliveries resulting from maternal trauma, and infants born <23 completed gestation weeks or with major birth defects were not included.
After consent was obtained from all participating mothers, we conducted a face-to-face interview 24 to 72 hours postpartum using a standardized questionnaire. We also abstracted clinical data, including prenatal care data, pregnancy complications, labor and delivery course, and birth outcomes, from maternal and infant electronic medical records (EMR). All enrolled children who continued to seek postnatal care at BMC were then followed.
From the 2,045 mothers and children who had complete information on height and weight, we excluded 535 preterm deliveries (<37 weeks), 58 with pre-pregnancy underweight (BMI < 18.5 kg/m2), because of inadequate power in this category, and 11 missing information on delivery mode (Supplemental Figure 1). These exclusions left us with 1,441 mother-child dyads for the current analysis. This sample size afforded us greater statistical power than our previous publication on delivery mode and childhood obesity13. The institutional review board of Johns Hopkins Bloomberg School of Public Health and Boston University Medical Center approved the study.
Exposures
The primary exposures for this study were pre-pregnancy BMI and mode of delivery. Mode of delivery (Cesarean vs. vaginal) recorded from EMR. Pre-pregnancy height and weight, ascertained from the maternal postpartum interview, was used to calculate pre-pregnancy BMI. We categorized pre-pregnancy BMI as normal weight (18.5 ≤ BMI < 25.0 kg/m2); overweight (25.0 ≤ BMI < 30.0 kg/m2); and obese (30.0 kg/m2 ≤ BMI).
Outcomes
The primary outcome for this study was childhood OWOB measured at age ~5 years (median=4.8 y; interquartile range 3.2 to 6.3y; range: 2.0 to 8.0y). To derive childhood BMI, height and weight were abstracted from EMRs. We defined OWOB using age and sex specific BMI z scores ≥85th percentile, according to Center for Disease Control growth charts.
Covariates
We extracted maternal age at delivery and birth weight directly from EMRs, and maternal race/ethnicity and maternal education level from standardized questions. To further characterize participants socioeconomic status, we estimated maternal exposure to ambient air pollution, in the form of particulate matter (PM2.5), in the 2nd trimester by obtaining data from air monitors near their residence, as described previously14. We calculated gestational weight gain from EMR weight measurement data or, if EMR was missing, from the standardized questionnaire self-report weight data. When using EMR data, gestational weight gain was defined as the weight difference between earliest weight measurement during first trimester and latest weight measurement before delivery. The end-of-pregnancy weight measurements were restricted to weight measured within 3 weeks of delivery. We considered the EMR weight measurement as missing if it occurred outside this time range. We replaced the missing earliest weight measurement data with the self-reported pre-pregnancy weight for participants that only had the last pregnancy weight measurement available in EMR.
Data analysis
We described maternal and child characteristics using percentages (%) for categorical variables, means and standard deviation (SD) for normally distributed continuous variables, and median (1st quartile (Q1), 3rd quartile (Q3)) for non-normally distributed continuous variables. We used multivariable logistic regression model to examine the associations of delivery mode and pre-pregnancy BMI with our primary outcome, childhood overweight or obesity. We further used generalized linear models to evaluate associations with childhood BMI z-scores.
To assess confounding, we began with an unadjusted model and then added covariates known to be associated with our exposure (either pre-pregnancy BMI or delivery mode) and childhood overweight or obesity, not including covariates that were on the causal pathway between our exposure and outcome. For pre-pregnancy BMI analyses, we included maternal age at delivery (quintiles), maternal race (black, not black or unknown), and maternal education (middle school and below; high school; college and above). For delivery mode analyses, we included maternal age at delivery (quintiles), maternal race/ethnicity (black, not black or unknown), maternal education (middle school and below; high school; college and above), 2nd trimester exposure to air pollution in the form of ambient PM2.5 (PM2.5>=12μg/m3 or not), maternal pre-pregnancy BMI (normal weight, overweight, obese), gestational weight gain (inadequate, normal, excess weight gain) and birth weight (quartiles). We further considered but did not include in the final model maternal smoking status during pregnancy (never smoked; quit before pregnancy; continued to smoke in pregnancy), diabetes (no; gestational diabetes; pre-pregnancy diabetes) maternal marriage status (married; other; unknown), prenatal and intra-partum antibiotics (use antibiotics during pregnancy; not; unknown), and household income (household income >=$50000 per year; less than $50000; unknown); none of these variables significantly changed the relationship of maternal pre-pregnancy BMI or delivery mode with childhood OWOB. We also conducted sensitivity analyses after excluding women who developed pre-eclampsia or gestational diabetes.
We evaluated effect modification on the multiplicative scale by including cross-product terms for mode of delivery and pre-pregnancy BMI in multivariable models. We considered a two-sided alpha of < 0.05 as evidence of statistical significance. Data management and analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA). Code is available upon request. Figures were generated by Sigmaplot for Windows Version 12.5 (Build 12.5.0.38, Systat Software, Inc).
Results
Baseline characteristics for mother-child dyads according to delivery mode and maternal pre-pregnancy BMI are provided in Table 1 (characteristics according to mode of delivery alone are provided in Supplemental Table 1). Of the 1,441 mothers in our sample, 480 (33.3%) delivered by Cesarean section, and 396 (27.4%) were overweight and 362 (25.1%) obese. Mothers with greater pre-pregnancy BMI had lower gestational weight gain, higher prevalence of gestational diabetes, and were more likely to deliver children with greater birth weight to deliver by C-section. Compared to children delivered vaginally, those delivered by C-section were more likely to be girls and have greater birth weight. Mothers who delivered by C-section were more likely to be older, and have greater pre-pregnancy BMI, gestational weight gain and prevalence of gestational diabetes.
Table 1.
Characteristics of mother-child pairs (n=1441) from the Boston Birth Cohort by delivery mode and pre-pregnancy BMI category.
| Vaginal Delivery | Cesarean Delivery | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | Normal weight (n=506) | Overweight (n=251) | Obese (n=204) | Normal weight (n=177) | Overweight (n=145) | Obese (n=158) |
|
|
||||||
| Child | ||||||
|
|
||||||
| Male, % | 52.0 | 51.8 | 56.9 | 49.2 | 39.3 | 52.5 |
|
|
||||||
| Hispanic, % | 20.5 | 13.7 | 14.4 | 20.0 | 16.8 | 13.9 |
|
|
||||||
| Gestational age, weeks, median (Q1,Q3) | 39.4 (38.4, 40.3) | 39.6 (38.6, 40.4) | 39.7 (38.7, 40.6) | 39.3 (38.4, 40.1) | 39.4 (38.4, 40.4) | 39.0 (38.6, 40.0) |
|
|
||||||
| Birth weight, g, mean±SD | 3146.4±493.9 | 3286.7±482.6 | 3334.7±573.8 | 3217.1±567.2 | 3357.7±559.4 | 3436.9±626.6 |
|
|
||||||
| Ever breastfed in the 1st year, % | 61.9 | 68.9 | 63.2 | 64.4 | 68.3 | 63.3 |
|
|
||||||
| Mother | ||||||
|
|
||||||
| Age at birth, y, median (Q1, Q3) | 25.5 (21.6, 30.3) | 29.5 (24.5, 35.0) | 27.9 (23.2, 32.1) | 29.1 (25.3, 33.2) | 31.4 (25.1, 35.3) | 30.5 (25.3, 34.9) |
|
|
||||||
| * Low education achievement, % | 36.1 | 35.7 | 35.3 | 43.9 | 35.4 | 41.1 |
|
|
||||||
| Household income <=$50,000, % | 47.8 | 52.2 | 50.0 | 50.8 | 40.0 | 43.7 |
|
|
||||||
| Pre-pregnancy BMI, kg/m2, median (Q1,Q3) | 22.2 (20.6, 23.4) | 27.4 (25.8, 28.3) | 33.6 (31.4, 38.0) | 22.8 (21.3, 24.0) | 27.4 (26.1, 28.4) | 34.0 (32.0, 38.9) |
|
|
||||||
| Gestational weight gain, lbs, median (Q1,Q3) | 30.0 (23.0, 38.0) | 25.0 (15.4, 37.0) | 22.0 (15.0, 30.0) | 33.5 (26.0, 40.0) | 31.0 (23.0, 43.0) | 30.0 (18.0, 45.0) |
|
|
||||||
| Gestational diabetes,% | 0.6 | 3.2 | 4.9 | 1.7 | 9.7 | 11.5 |
|
|
||||||
| Maternal smoking, % | 8.5 | 7.2 | 9.3 | 8.5 | 8.3 | 12.7 |
|
|
||||||
| Ambient PM2.5 in the 2nd trimester, median (Q1, Q3) | 10.8 (9.3, 12.4) | 11.1 (9.2, 12.6) | 10.8 (8.8, 12.8) | 10.4 (8.5, 12.3) | 10.8 (9.3, 12.2) | 10.5 (8.8, 12.3) |
Participants whose education level included illiteracy, primary school or middle school.
Normally distributed continuous data are described as mean (standard deviation) and non-normally distributed continuous data are described as median (1st quartile [Q1], 3rd quartile [Q3])
Of the children in our sample, 601 (47.2%) were overweight or obese at follow up. In Table 2 we show unadjusted and multivariable adjusted associations of delivery mode with childhood overweight or obesity. Before adjustment for potential confounders, C-section delivery compared to vaginal delivery was associated with 1.5 (95% CI: 1.2, 1.8) times greater odds of childhood overweight or obesity. This association was slightly attenuated but remained statistically significant (OR 1.4; 95% CI: 1.1, 1.8) after adjustment for potential confounders. Overall, compared to children born to normal weight mothers, those born to overweight and obese mothers had, respectively, 1.7 (95% CI: 1.3, 2.2) and 2.1 (95% CI: 1.6, 2.7) times greater odds of becoming overweight or obese, after multivariable adjustment.
Table 2.
Odds ratios (OR) and 95% confidence intervals (95% CI) for childhood overweight or obesity according to pre-pregnancy BMI and delivery mode before and after adjustment for potential confounders.
| Maternal pre-pregnancy BMI | n | Cases (%) | Crude | Adjusteda | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR (95%CI) | p | OR (95%CI) | p | |||
|
|
||||||
| Normal (18.5 – 25 kg/m2) | 683 | 224 (32.8) | 1.0 (Ref.) | Ref. | 1.0 (Ref.) | Ref. |
| Overweight (25.0–30 kg/m2) | 396 | 188 (47.5) | 1.9 (1.4–2.4) | <0.001 | 1.7 (1.3–2.3) | <0.001 |
| Obesity (≥30 kg/m2) | 362 | 189 (52.2) | 2.2 (1.7–2.9) | <0.001 | 2.1 (1.6–2.8) | <0.001 |
| Crude | Adjustedb | |||||
|
|
||||||
| Delivery mode | n | Cases (%) | OR (95%CI) | p | OR (95%CI) | p |
|
|
||||||
| Vaginal Delivery | 961 | 370 (38.5) | 1.0 (Ref.) | Ref. | 1.0 (Ref.) | Ref. |
| Cesarean Delivery | 480 | 231 (48.1) | 1.5 (1.2–1.8) | <0.001 | 1.4 (1.1–1.8) | 0.005 |
Adjusted for maternal age at delivery, maternal race/ethnicity, and maternal education.
Adjusted for maternal age at delivery, maternal race/ethnicity, maternal education, maternal exposure to ambient PM2.5 exposure in the 2nd trimester, maternal pre-pregnancy BMI, maternal pregnancy weight gain, and birth weight
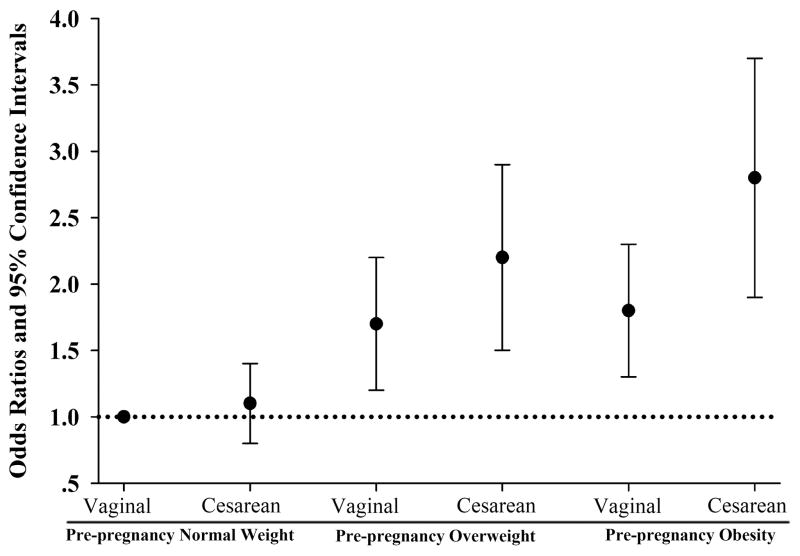
In Table 3 we present the joint effects of maternal pre-pregnancy BMI status and mode of delivery on childhood OWOB, before and after multivariable adjustment. Compared to vaginally-delivered children from normal weight mothers (reference), vaginally-delivered children from overweight and obese mothers had, respectively, 1.7 (95% CI: 1.2, 2.3) and 1.8 (95% CI: 1.3, 2.6) times greater odds of OWOB, and C-section delivered children from overweight and obese mothers had, respectively, 1.9 (95% CI: 1.2, 3.1) and 2.5 (95% CI: 1.5, 3.9) times greater odds of OWOB, after adjustment for confounders. These associations are depicted in Figure 1.
Table 3.
Odds ratios (OR) and 95% confidence intervals (95% CI) for childhood overweight or obesity according to maternal pre-pregnancy BMI status and delivery mode, modeled independently and jointly.
| Pre-pregnancy BMI | Cesarean section | n | Cases (%) | Crude | Adjusteda | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| OR (95%CI) | p | OR (95%CI) | p | ||||
|
|
|||||||
| Normal (18.5–25 kg/m2) | No | 506 | 161 (31.8) | 1.0 (Ref.) | Ref. | 1.0 (Ref.) | Ref. |
| Normal (18.5–25 kg/m2) | Yes | 177 | 63 (35.6) | 1.2 (0.8–1.7) | 0.357 | 1.1 (0.8–1.7) | 0.459 |
| Overweight (25.0–30 kg/m2) | No | 251 | 113 (45.0) | 1.8 (1.3–2.4) | <0.001 | 1.7 (1.2–2.3) | 0.001 |
| Overweight (25.0–30 kg/m2) | Yes | 145 | 75 (51.7) | 2.3 (1.6–3.3) | <0.001 | 2.2 (1.5–3.2) | <0.001 |
| Obesity (≥30 kg/m2) | No | 204 | 96 (47.1) | 1.9 (1.4–2.7) | <0.001 | 1.8 (1.3–2.6) | <0.001 |
| Obesity (≥30 kg/m2) | Yes | 158 | 93 (58.9) | 3.1 (2.1–4.4) | <0.001 | 2.8 (1.9–4.1) | <0.001 |
| Interaction | 0.549 | 0.569 | |||||
Adjusted for maternal age at delivery, maternal race/ethnicity, and maternal education.
Figure 1.
Odds ratios and 95% confidence intervals of childhood overweight or obesity according to joint categories of maternal pre-pregnancy body weight status and delivery mode.
In Supplemental Tables 2–4 we present associations with continuous BMI z score as the outcome and our findings are consistent with our primary outcome of childhood OWOB. Finally, in Supplemental Tables 5–6 we present results for sensitivity analyses in which we examined the individual and joint effects on maternal pre-pregnancy BMI and delivery mode with the odds of childhood OWOB after excluding women who developed pre-eclampsia or gestational diabetes. These findings were also consistent with the findings from our primary analyses.
Discussion
In this multiethnic urban birth cohort study, compared to vaginally-delivered children, C-section delivered children had higher odds of developing OWOB, after adjusting for key confounders. Furthermore, compared to children born to normal weight mothers, children born to overweight or obese mothers had higher odds of developing OWOB, but this intergenerational association of OWOB was attenuated if the children were delivered vaginally.
Our findings on the association of delivery mode with childhood OWOB are consistent with a growing body of literature9,13,15. A meta-analysis that found Cesarean delivery increases odds of childhood overweight or obesity by approximately 30%9—a magnitude of association comparable to our study. Our study had the added strength of adjustment for a number of confounders, including maternal pre-pregnancy BMI and gestational weight gain, and our findings were robust to exclusion of women who developed gestational diabetes or pre-eclampsia.
Similar to previous reports, in our study maternal pre-pregnancy BMI was strongly positively associated with childhood OWOB. A meta-analysis found that pre-pregnancy overweight or obesity was associated with 2.0 (95% CI: 1.8, 2.1) and 3.1 (2.7, 3.5) times greater odds of childhood overweight and obesity7. To the best of our knowledge, we are the first study to rigorously examine whether this association is modified by delivery mode. The association between maternal and child OWOB tended to be slightly stronger among C-section delivered children than among vaginally delivered children, although the test for statistical interaction on the multiplicative scale was not significant (p=0.57).
The observation that among overweight or obese women, vaginally delivered children tended to have lower odds of developing overweight or obesity than C-section delivered children suggests that among other possibilities the vertical transmission of microbiota during vaginal delivery may reduce the transmission of obesity risk to the next generation. Previously, we found that maternal overweight and obesity was associated with the neonatal gut microbiome in vaginally delivered neonates, but not C-section delivered neonates11. Taken collectively, these findings suggest that the mother-to-newborn transmission of vaginal and intestinal microbiota during vaginal delivery may serve to improve extra-uterine metabolic homeostasis of the newborn delivered to an overweight or obese mother. Due to their mothers’ weight status, these children may already be genetically programmed for obesity. The differential acquisition of microbiota by the newborn may (a) directly modify caloric extraction from the infants diet or (b) provide a hormetic stimulation that has a ‘priming’ effect on early development and immune responses16,17. C-section delivered newborns may miss this potentially protective microbial exposure. While it is intriguing to think that priming newborns to obese microbiota may contribute to long-term risks of metabolic disease, this hypothesis needs to be tested in a longitudinal birth cohort study that has collected offspring stool and measures of body composition from birth into childhood or in a clinical trial that tests whether exposure to vaginal microbiota prevents OWOB in children delivered by C-section.
There are limitations of the current study that merit mention. First, we did not have data on indication for Cesarean delivery. When we excluded women who developed gestational diabetes or pre-eclampsia, two common indications for Cesarean delivery, our effect estimates were similar. Further, the most common reasons for medically necessary Cesarean delivery, fetal intolerance of labor or failure to progress, are not known risk factors for obesity and thus unlikely to confound the association between Cesarean delivery and offspring obesity. Nevertheless, future research is needed to delineate whether the obesity risk associated with Cesarean delivery depends on whether women went into labor or whether membranes were ruptured. Another limitation to our study is that we relied on self-report of maternal pre-pregnancy height and weight when maternal height and weight from EMR was not available. However, misclassification was unlikely because in the BBC there was high correlation (Pearson r =0.91) between maternal self-report and EMR recorded BMI in a subset of 738 mothers who had height and weight during preconception or within 6 wks of gestation in their EMR18. Finally, as this is an observational study, we cannot rule out the possibility for residual or unmeasured confounding.
The major strengths of the current study lay in the prospective collection of data in a multi-ethnic, urban sample of mother-child dyads. Furthermore, the comprehensive set of covariates, collected through questionnaire and EMRs from birth through childhood, allowed us to control for many potential confounding factors for which other studies have not been able to adjust.
Conclusion
We found that the exposures of Cesarean (versus vaginal) delivery and maternal pre-pregnancy overweight and obesity (versus normal weight) are each, independent of each other, associated with higher odds of offspring obesity in this multiethnic study of mother-child dyads from Boston. Our findings suggest that, even among overweight and obese mothers, Cesarean delivery may predispose offspring to greater risk of obesity in childhood. Future studies, which directly measure the maternal and infant microbiomes and other potential explanatory factors, are needed to understand the drivers of this association, and to explore the possibility that exposure to vaginal microbiota in Cesarean delivered newborns may provide an innovative opportunity to combat the vicious cycle of intergenerational obesity.
Supplementary Material
Acknowledgments
The Boston Birth Cohort (the parent study) is supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605), and the National Institutes of Health (NIH) grants (R21ES011666, R21HD066471, and R01HD041702). The follow-up study is supported in part by the Maternal and Child Health Bureau grant (R40MC27443) and the NIH grants (U01AI090727, R21AI079872, 2R01HD041702 and R01HD086013).
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Barros FC, Victora CG. Maternal-child health in Pelotas, Rio Grande do Sul State, Brazil: major conclusions from comparisons of the 1982, 1993, and 2004 birth cohorts. Cad Saude Publica. 2008;24(Suppl 3):S461–467. doi: 10.1590/s0102-311x2008001500012. [DOI] [PubMed] [Google Scholar]
- 2.Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG. 2007;114:187–194. doi: 10.1111/j.1471-0528.2006.01199.x. [DOI] [PubMed] [Google Scholar]
- 3.Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004–2005. Matern Child Health J. 2009;13:614–620. doi: 10.1007/s10995-008-0388-3. [DOI] [PubMed] [Google Scholar]
- 4.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 5.Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140:387–398. doi: 10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- 6.Widen EM, Whyatt RM, Hoepner LA, Mueller NT, Ramirez-Carvey J, Oberfield SE, et al. Gestational weight gain and obesity, adiposity and body size in African-American and Dominican children in the Bronx and Northern Manhattan. Matern Child Nutr. 2015 doi: 10.1111/mcn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8:e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007;8:385–394. doi: 10.1111/j.1467-789X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 9.Li HT, Zhou YB, Liu JM. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes (Lond) 2013;37:893–899. doi: 10.1038/ijo.2012.195. [DOI] [PubMed] [Google Scholar]
- 10.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller NT, Shin H, Pizoni A, Werlang IC, Matte U, Goldani MZ, et al. Birth mode-dependent association between pre-pregnancy maternal weight status and the neonatal intestinal microbiome. Sci Rep. 2016;6:23133. doi: 10.1038/srep23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 13.Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond) 2015;39:665–670. doi: 10.1038/ijo.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nachman RM, Mao G, Zhang X, Hong X, Chen Z, Soria CS, et al. Intrauterine Inflammation and Maternal Exposure to Ambient PM during Preconception and Specific Periods of Pregnancy: The Boston Birth Cohort. Environ Health Perspect. 2016 doi: 10.1289/EHP243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardi JR, Pinheiro TV, Mueller NT, Goldani HA, Gutierrez MR, Bettiol H, et al. Cesarean delivery and metabolic risk factors in young adults: a Brazilian birth cohort study. Am J Clin Nutr. 2015;102:295–301. doi: 10.3945/ajcn.114.105205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics. 2016;137:1–10. doi: 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.