Abstract
Background
Medication nonadherence contributes to hospitalizations in recently discharged patients with heart failure (HF). We aimed to test the feasibility of telemonitoring medication adherence in patients with HF.
Methods and Results
We randomized 40 patients (1:1) hospitalized for HF to 30 days of loop diuretic adherence monitoring with telephonic support for nonadherence or to passive adherence monitoring alone. Eighty-three percent of eligible patients agreed to participate. The median age of patients was 64 years, 25% were female, and 45% were Hispanic. Overall, 67% of patients were nonadherent (percent of days correct number of doses taken <88%). There were no differences between intervention and passive monitoring group patients, respectively, in adherence (median correct dosing adherence 82% versus 73%; p = 0.41) or in the proportion readmitted within 30 days (30% versus 20%; p = 0.72). Eighty-eight percent of patients rated the wireless electronic adherence device as somewhat or very easy to use, and 88% agreed to use it again.
Conclusions
Adherence telemonitoring was acceptable to most patients with HF. Diuretic nonadherence was common even when patients knew they were being monitored. Future studies should assess whether adherence telemonitoring can improve adherence and reduce readmissions among patients with HF.
Keywords: Heart failure, adherence, telemonitoring, readmissions, self-care, interventions
Introduction
Heart failure (HF) is a major contributor to morbidity, mortality, and health care costs in the United States.1 Nearly 27% of Medicare patients admitted to the hospital for HF are readmitted within 30 days.2 To date, interventions aimed at reducing HF readmissions have had mixed results.3,4
Medication adherence is crucial to controlling symptoms, delaying disease progression, and preventing hospitalization in HF.5,6 Unfortunately, up to 50% of patients with HF have suboptimal adherence, suggesting that adherence interventions could reduce readmissions.5,7
Telemonitoring, defined as the use of communication technology to monitor clinical status in chronically ill patients, has not consistently improved outcomes in patients with HF.8-11 To our knowledge, however, telemonitoring HF medication adherence has yet to be investigated. Thus, we conducted a pilot randomized trial evaluating the feasibility of adherence telemonitoring in recently hospitalized patients with HF.
Methods
Design and Setting
We used a randomized design to compare adherence telemonitoring with passive adherence monitoring alone. Patients were enrolled from New York-Presbyterian Hospital in Upper Manhattan. Enrollment occurred from December 2014 to August 2015.
Participants
Patients were eligible if they were age 21 years or older; spoke English or Spanish; and were discharged home on a loop diuretic. Patients were excluded for the following criteria: New York Heart Association class IV HF symptoms12; discharge to an institutional setting; inability to self-administer medications; terminal non-cardiovascular illness; or participation in another clinical trial. The study was approved by the Institutional Review Board of Columbia University Medical Center, and is registered at ClinicalTrials.gov (NCT02378571). All participants provided written informed consent.
Randomization and Allocation Concealment
Participants were randomly assigned in a 1:1 ratio to the intervention or passive monitoring group. Randomization was generated using the Proc SurveySelect statement in SAS with METHOD=Simple Random Sampling. Study team members who enrolled participants and assessed outcomes were blinded to group assignments.
Study Protocol
All participants were provided with a GlowCap® system (Vitality, Inc., Los Angeles, CA) which includes a pill bottle cap that records the date and time when the bottle is opened. After each opening, the cap uses a wireless protocol to send data about the date and time of openings to the communication hub. These data are available on an online portal.
Participants were instructed to fill the bottle with their loop diuretic medication and take their diuretic exclusively from this bottle for 30 days following discharge. Participants were informed that bottle openings would be wirelessly recorded and, depending upon group assignment, they might be contacted by the study team if missed doses or extra openings were observed.
Intervention
For participants in the intervention group, a licensed clinical social worker reviewed adherence data on a daily basis during the first 7 days after discharge and weekly thereafter, and contacted participants who were nonadherent for two or more days per week. During these phone calls, the social worker inquired about consequences of nonadherence, and assessed and responded to reasons for missed doses. At least three attempts were made to contact nonadherent participants.
Passive Monitoring
For participants assigned to passive monitoring, adherence data were recorded but not monitored by the study team.
Outcomes
The primary outcome was adherence to loop diuretics in the 30 days after discharge, calculated as the percent of days on which the correct number of doses was taken as prescribed, irrespective of dose timing. Patients were classified as nonadherent if adherence was <88%.13
Secondary outcomes included 30-day all-cause readmission and attendance at follow-up clinic appointments. Participants were interviewed by telephone at 30 days to determine if readmissions or clinic visits had occurred. Medical records were reviewed to confirm responses. Participants were also queried to assess acceptability of adherence telemonitoring.
Statistical Analyses
Descriptive statistics were used to summarize participants’ characteristics at baseline. The Mann-Whitney U test was used to compare adherence percentages between groups, and the Chi square or Fisher exact test, when appropriate, was used to compare proportions of participants in the intervention and control groups who used the study device, were nonadherent, were readmitted, or attended a follow-up clinic visit. Analyses were performed using SPSS (version 21.0, IBM Corp., Armonk, NY).
Results
Participant Characteristics
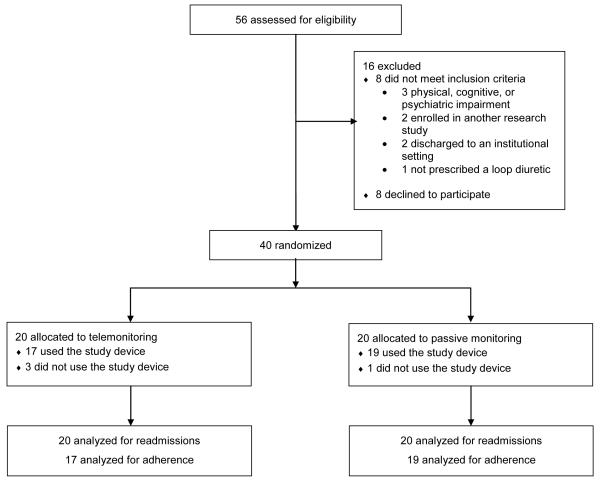
Of 56 patients screened, 8 were ineligible and 8 declined to participate (Figure). Baseline characteristics of the 40 enrolled participants are shown in Table 1.
Figure.
CONSORT (Consolidated Standards of Reporting Trials) diagram of the TElemonitoring Adherence to Medications in Heart Failure Patients (TEAM-HF) trial
Table 1.
Baseline characteristics of participants
| Characteristic | Intervention (n = 20) |
Passive Monitoring (n = 20) |
Total (n = 40) |
|---|---|---|---|
| Age (years), median (IQR*) | 68 (49-79) | 62 (52-75) | 64 (50-77) |
| Female, n (%) | 5 (25) | 5 (25) | 10 (25) |
| Black race, n (%) | 5 (25) | 6 (30) | 11 (28) |
| Hispanic ethnicity, n (%) | 8 (40) | 10 (50) | 18 (45) |
| Preferred language Spanish, n (%) | 5 (25) | 4 (20) | 9 (23) |
| Partner status, n (%) | |||
| Single | 2 (10) | 3 (15) | 5 (13) |
| Partnered/Married | 11 (55) | 11 (55) | 22 (55) |
| Widowed | 2 (10) | 2 (10) | 4 (10) |
| Divorced/Separated | 5 (25) | 4 (20) | 9 (23) |
| Employment status, n (%) | |||
| Working full or part time | 7 (35) | 2 (10) | 9 (23) |
| Unemployed or laid off | 3 (15) | 8 (40) | 11 (28) |
| Retired | 10 (50) | 8 (40) | 18 (45) |
| Insurance, n (%)* | |||
| Medicare | 11 (55) | 9 (45) | 20 (50) |
| Medicaid | 6 (30) | 9 (45) | 15 (38) |
| Private | 12 (60) | 6 (30) | 18 (45) |
| Uninsured | 0 (0) | 2 (10) | 2 (5) |
| NYHA classification, n (%) | |||
| Class I | 0 (0) | 1 (5) | 1 (3) |
| Class II | 10 (50) | 9 (45) | 19 (48) |
| Class III | 10 (50) | 10 (5) | 20 (50) |
| Ejection fraction, median (IQR) | 25% (20-38%) | 25% (15- 40%) |
25% (15- 40%) |
| Charlson Comorbidity Index14, median (IQR) | 3 (2-4) | 3 (2-5) | 3 (2-4) |
| Length of stay (days), median (IQR) | 4 (2-9) | 6 (3-13) | 5 (3-11) |
| Total number of discharge medications, median (IQR) |
9(6-11) | 8 (5-15) | 8 (6-11) |
Abbreviations: IQR, Interquartile range; NYHA, New York Heart Association
Percentages do not add up to 100% because some participants had more than one type of insurance
Electronic Adherence
Four participants (10%) did not use the study device (3 intervention group versus 1 passive monitoring group, p = 0.61). The remaining 36 participants had adherence monitored for a median of 29 days (IQR 20-30). Across both groups, median correct dosing adherence was 81% (IQR 47-94%), and 33% were classified as adherent (i.e. had adherence ≥88%). There were no significant differences between intervention and passive monitoring groups, respectively, in median correct dosing adherence (82% versus 73%; p = 0.41) or the proportion that was classified as adherent (29% versus 37%; p = 0.64; Table 2). This remained true when lowering the adherence threshold to 80% (59% versus 47%, p = 0.49).
Table 2.
Comparison of medication adherence, clinic attendance, and readmissions among patients randomized to adherence telemonitoring versus passive monitoring*
| Characteristic | Adherence Telemonitoring (n = 20) |
Passive Monitoring (n = 20) |
P-Value |
|---|---|---|---|
| Adherent to diuretics (≥88%), n (%) | 5 (29.4) | 7 (36.8) | 0.64 |
| Adherent to diuretics (≥80%), n (%) | 10 (58.8) | 9 (47.4) | 0.49 |
| Correct dosing adherence†, median % | 81.8 | 73.3 | 0.41 |
| Attended clinic appointment within 30 days, n (%) | 19 (95) | 16 (80) | 0.34 |
| Rehospitalized within 30 days, n (%) | 6 (30) | 4 (20) | 0.72 |
| Rehospitalized for HF within 30 days, n (%) | 5 (25) | 3 (15) | 0.70 |
For medication adherence outcomes, n = 17 for adherence telemonitoring group and n = 19 for passive monitoring group.
Indicates the percent of days monitored on which patients took the correct number of doses.
Of the 17 intervention group participants who used the study device, 12 (71%) were flagged for nonadherence at least once; the study team successfully contacted 11 of these (92%). Reasons for nonadherence are described in Table 3.
Table 3.
Reasons for medication nonadherence among nonadherent participants in the intervention group (n = 12)*
| Reason for Medication Nonadherence | Number (%) of Participants |
|---|---|
| True nonadherence | |
| Ran out of pills | 1 (8) |
| Out of usual routine | 1 (8) |
| Side effects | 1 (8) |
| Did not know correct dose | 1 (8) |
| Pseudo- or uncertain nonadherence | |
| Denied nonadherence | 5 (42) |
| “Pocket dosing” † | 2 (17) |
| Did not fill electronic pill bottle with pills from new refill | 1 (8) |
| Monitoring stopped early due to readmission | 1 (8) |
| Stopped using device due to unstable housing situation | 1 (8) |
| Unable to be contacted by intervention team | 1 (8) |
Number of reasons for nonadherence add up to greater than 12 because participants could state multiple reasons at each nonadherence phone call.
Pocket dosing refers to taking doses outside the GlowCap® system when away from home.
Clinical Outcomes
Ten participants (25%) were readmitted within 30 days (6 intervention group versus 4 passive monitoring group; p = 0.72). Eight of these readmissions (80%) were for HF (5 intervention group versus 3 passive monitoring group; p = 0.70). Five participants (12%) did not attend a follow-up clinic visit (1 intervention group versus 4 passive monitoring group; p = 0.34).
Feasibility
Thirty-six (90%) participants completed a follow-up assessment. Eighty-eight percent of these participants rated the electronic pill bottle as somewhat or very easy to use, and 88% agreed to use it again if asked by their provider. A minority expressed concerns about privacy and security related to adherence telemonitoring (23% and 43%, respectively). All or nearly all wanted their adherence data sent to themselves (100%), their families (85%), or their providers (98%).
Discussion
This study demonstrated the feasibility of adherence telemonitoring in recently hospitalized patients with HF. Eighty-three percent of eligible patients agreed to participate, and only 10% were unable to be contacted within one month. The wireless adherence monitoring system had a high degree of usability, resulting in 90% of participants having analyzable adherence data during the monitoring period.
This study had several strengths. First, we tested the feasibility of adherence telemonitoring as a means of reducing hospital readmission rates, which to our knowledge has not previously been investigated. Second, adherence data were collected passively, requiring no involvement from participants aside from taking their diuretic from the electronic pill bottle. Third, it involved racially and ethnically diverse group of participants.
There were also limitations. Electronic adherence monitoring cannot confirm whether the correct number of pills was taken per dosing, nor whether doses were ingested. The intervention was not targeted at nonadherent patients, who had the most to gain from adherence telemonitoring. About one third of participants expressed concerns about privacy and security, which could pose a barrier to the use of the device in larger studies. Most importantly, this study was a pilot, and was not statistically powered to test the effect of adherence telemonitoring on medication adherence or readmissions.
In summary, our results suggest that adherence telemonitoring is acceptable to most patients with HF, nonadherence is common even when patients know they are being monitored, and it is feasible to remotely track adherence. Future studies should test whether adherence telemonitoring can improve medication adherence and reduce readmissions in patients with HF.
Acknowledgements
This work was supported by the New York-Presbyterian Hospital 2014 Translational Grants Program. Dr. Kronish received support from NHLBI (K23 098359) and the New York State Department of Health Empire Clinical Research Investigator Program. Dr. Ye received support from NIH (K23 HL121144).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N. Engl. J. Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann. Intern. Med. 2011;155(8):520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 4.Feltner C, Jones CD, Cene CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann. Intern. Med. 2014;160(11):774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 5.Wu JR, Moser DK, Lennie TA, Burkhart PV. Medication adherence in patients who have heart failure: a review of the literature. Nurs. Clin. North Am. 2008;43(1):133–153. vii–viii. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald AA, Powers JD, Ho PM, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J. Card. Fail. 2011;17(8):664–669. doi: 10.1016/j.cardfail.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Avorn J. Noncompliance with congestive heart failure therapy in the elderly. Arch. Intern. Med. 1994;154(4):433–437. [PubMed] [Google Scholar]
- 8.Inglis SC, Clark RA, McAlister FA, et al. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane database of systematic reviews. 2010;(8):CD007228. doi: 10.1002/14651858.CD007228.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N. Engl. J. Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koehler F, Winkler S, Schieber M, et al. Impact of Remote Telemedical Management on Mortality and Hospitalizations in Ambulatory Patients With Chronic Heart Failure: The Telemedical Interventional Monitoring in Heart Failure Study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 11.Ong MK, Romano PS, Edgington S, et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients With Heart Failure: The Better Effectiveness After Transition-Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA internal medicine. 2016;176(3):310–318. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Criteria Committee of the New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed Little, Brown, & Co; Boston: 1994. [Google Scholar]
- 13.Wu JR, Moser DK, De Jong MJ, et al. Defining an evidence-based cutpoint for medication adherence in heart failure. Am. Heart J. 2009;157(2):285–291. doi: 10.1016/j.ahj.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]