Abstract
The adult human skeleton is a multifunctional organ undergoing continuous remodeling. Homeostasis of bone mass in a healthy adult requires an exquisite balance between bone resorption by osteoclasts and bone formation by osteoblasts; disturbance of such balance is the root cause for various bone disorders including osteoporosis. To develop effective and safe therapeutics to modulate bone formation, it is essential to elucidate the molecular mechanisms governing osteoblast differentiation and activity. Due to their specialized function in collagen synthesis and secretion, osteoblasts are expected to consume large amounts of nutrients. However, studies of bioenergetics and building blocks in osteoblasts have been lagging behind those of growth factors and transcription factors. Genetic studies in both humans and mice over the past 15 years have established Wnt signaling as a critical mechanism for stimulating osteoblast differentiation and activity. Importantly, recent studies have uncovered that Wnt signaling directly reprograms cellular metabolism by stimulating aerobic glycolysis, glutamine catabolism as well as fatty acid oxidation in osteoblast-lineage cells. Such findings therefore reveal an important regulatory axis between bone anabolic signals and cellular bioenergetics. A comprehensive understanding of osteoblast metabolism and its regulation is likely to reveal molecular targets for novel bone therapies.
Keywords: Wnt, Metabolism, mTORC1, mTORC2, Glucose, Glutamine, Fatty acids, Osteoblast, Bone
Introduction
The mammalian skeleton not only provides support and protection, but also performs endocrine functions. The homeostasis of adult bone mass under healthy conditions is maintained through the exquisite balance of bone resorption by osteoclasts and bone formation by osteoblasts. With aging or under pathological conditions bone resorption dominates over formation, resulting in osteopenia (low bone mass) or in the more severe cases, osteoporosis. Conversely, conditions that favor bone formation over resorption lead to high bone mass diseases such as sclerosteosis. Because osteoblasts are the chief cell type producing bone materials, elucidating the mechanisms that regulate osteoblast differentiation and activity is critical not only for understanding bone physiology but also for designing effective bone therapeutics. Extensive studies in the area during the past several decades have mostly focused on endocrine or paracrine signaling as well as transcriptional regulation [1, 2]. Those studies have uncovered the critical roles of growth factors such as Wnt proteins and transcription factors including Runx2, Osterix, and ATF4 during osteoblast differentiation [3–8]. However, relatively little is understood about how osteoblasts fulfill their key function of active protein synthesis and matrix secretion, a process highly demanding not only in building blocks but also in energy [9]. Recent studies have discovered that the potent bone anabolic signal Wnt directly reprograms multiple aspects of cellular metabolism integral to osteoblast differentiation and activity. This review summarizes those recent advances.
Wnt signaling
Wnt proteins are a family of secreted glycoproteins that are critical regulators of osteoblast differentiation and activity in both mice and humans [10–16]. Wnt signals are transduced by a family of seven-pass transmembrane G-protein coupled receptors of the frizzled (Fzd) family and a co-receptor of the arrow/Lrp family (e.g., Lrp5 and Lrp6) or a Ryk or Ror transmembrane tyrosine kinase [17, 18]. The binding of a given Wnt to a Fzd receptor and coreceptor activates multiple distinct intracellular signaling cascades, historically divided into the canonical β-catenin-dependent pathway and noncanonical β-catenin-independent pathways [19]. The best characterized is the canonical Wnt pathway, which results in the stabilization and translocation of β-catenin into the nucleus. β-catenin (encoded by Catnnb1) is an important transcriptional co-activator that regulates gene transcription in response to Wnt signaling. Normally, in cells not exposed to ligand, cytoplasmic levels of β-catenin are kept low through interactions with the β-catenin destruction complex [20]. The binding of Wnt to a Fzd receptor complex results in phosphorylation of the Lrp co-receptors and recruitment and tethering of GSK-3β and Axin to the ligand-receptor complex. This complex is subsequently endocytosed and inhibited through sequestration into multivesicular endosomes resulting in the stabilization and accumulation of cytoplasmic β-catenin [21]. Stabilized β-catenin translocates into the nucleus and interacts with the Lymphoid-enhancing factor/T cell factor (Lef/Tcf) family of high mobility group (HMG)-type transcription factors to stimulate expression of target genes including Lef1, Tcf7, Nkd2, and Axin2 [22–26]. Additionally, Wnt signaling can activate multiple signaling cascades independent of β-catenin. Here, Fzd seems to function more as a G-protein coupled receptor, activating intracellular cascades involving the GTPases Rho and Rac, the calcium calmodulin dependent kinase 2 (CaMK2), c-Jun N-terminal kinase (JNK) and p38, phospholipase-C, protein kinase C (PKC), protein kinase A (PKA), PI3 K/AKT, and mTOR [11, 27–33]. The pathway activated by a Wnt ligand is determined by many factors including specific ligand-receptor interactions, distinct receptor/co-receptor pairs, or the presence of intracellular proteins that regulate β-catenin activation [34–37]. Wnt signaling is also regulated by a number of secreted extracellular antagonists. These include Dickkopf (e.g., Dkk1 and Dkk2) and Sclerostin (Sost) proteins that bind to the extracellular domains of Lrp5 or Lrp6 and interfere with their interaction with Wnt proteins [38–41]. In addition, the secreted frizzled related proteins (sFRPs) bind directly to Wnt ligands and thus inhibit the formation of Wnt–Fzd complexes [42–44]. Overall, Wnt signaling is tightly controlled at multiple levels to ensure its proper activity during normal development and tissue homeostasis.
Wnt signaling in bone
The importance of Wnt signaling during bone formation has been well documented [45]. The original discovery came from human genetic studies where inactivating mutations in the Wnt co-receptor Lrp5 results in osteoporosis pseudoglioma syndrome while gain-of-function mutations causes osteosclerosis [15, 46, 47]. Moreover, mutations in either coding or regulatory sequences of Sost cause high bone mass in sclerosteosis or Van Buchem disease, respectively [48–50]. Subsequent genome-wide association studies in humans strongly support a role for Wnt signaling regulating bone mineral density (BMD) [51–54]. More recently, exome sequencing in humans identified multiple mutations in Wnt1 associated with early onset osteoporosis and osteogenesis imperfecta [55, 56]. In addition, missense mutations in Wnt16 are associated with decreased forearm and hip BMD and increased fracture risk [57].
In keeping with those findings in humans, genetic studies in mice have established a causal relationship between Wnt signaling and bone formation. Mice lacking Lrp5 either globally or in osteoblasts are characterized by osteopenia whereas expression of mutant Lrp5 alleles associated with human high-bone-mass syndromes increases bone mass in mice [58–60]. Activation of Wnt signaling through deletion of Sfrp1, Sost or a single allele of Dkk1 increases osteoblast number and activity [61–63]. In addition, multiple Wnts, including Wnt1, Wnt7b, Wnt10b, and Wnt16 and the Frizzled receptors Fzd7 and Fzd9, have been shown to regulate bone formation [10, 11, 57, 64–66]. Moreover, targeted deletion of Gpr177 (also known as Wntless—Wls, required for the secretion of all Wnt ligands) inhibits bone formation in mice [67, 68]. It is worth noting that the bone phenotypes resulting from loss of individual Wnt ligands or Fzd receptors are less severe than those reported in Gpr177 knockout mice, indicating significant functional redundancy among Wnt ligands and Fzd receptors. This viewpoint is consistent with the fact that osteoblast-lineage cells express multiple Wnt ligands during development [13, 69].
Genetic studies in mice have highlighted the importance of β-catenin in mediating Wnt signaling in bone formation. Multiple studies demonstrate that β-catenin is required for bone formation and acts at multiple stages of osteoblast differentiation to regulate both osteoblast and osteoclasts [12, 13, 16, 70–74]. The direct target genes of β-catenin during osteoblast differentiation are not fully elucidated, but β-catenin together with Tcf1 has been shown to stimulate Runx2 transcription directly [75]. In mature osteoblasts, Opg, encoding an anti-osteoclastogenic factor, is known to be a direct target of β-catenin [71]. While the importance of β-catenin in bone is well established, the contribution of β-catenin-independent Wnt signaling to bone formation is becoming increasingly clear. For example, Wnt7b can stimulate osteoblast differentiation through activation of PKCdelta [11]. Multiple Wnt proteins also activate the serine threonine kinase mammalian target of rapamycin complex 1 (mTORC1) which promotes protein synthesis and bone formation [29, 76, 77]. The stimulatory effect of mTORC1 in bone formation is further supported by genetic studies that either abolish or enhance mTORC1 activity in the mouse [78, 79]. In addition, Wnt activates mTORC2 that is required for the optimal bone accrual in response to mechanical loading or an anti-sclerostin neutralizing antibody [80–82]. Because the mTOR pathways are central to nutrient sensing and metabolic regulation, Wnt signaling has emerged as an important mechanism for modulating cellular metabolism in osteoblasts.
Glucose metabolism in osteoblasts
Glucose is the primary energy source for most mammalian cell types. Glucose is transported into the cell via the Glut family of facilitative glucose transporters. The Gluts transport glucose down a concentration gradient independent of ATP [83, 84]. Inside the cell, glucose is phosphorylated by hexokinase (Hk) to form glucose-6-phosphate (G6P). G6P can be converted to glycogen for storage or metabolized by disparate pathways including hexosamine biosynthetic pathway (HBP), pentose phosphate pathway (PPP), and glycolysis [85]. The HBP is used to produce uridine diphosphate N-acetylglucosamine (UDPGlcNAc) for protein glycosylation. The PPP is important to generate NADPH and ribose-5-phosphate important for nucleotide synthesis. Glycolysis occurs in the cytosol and produces 2 molecules of pyruvate, 2 ATP, and 2 reducing equivalents in the form of NADH per glucose molecule. Pyruvate can be converted into lactate by the enzyme lactate dehydrogenase (Ldh) independent of oxygen. This reaction regenerates oxidized NAD (NAD+) that is necessary for further glycolysis. Alternatively, pyruvate can be decarboxylated to form acetyl-CoA by the enzyme pyruvate dehydrogenase (Pdh). Pyruvate oxidation in the tricarboxylic acid (TCA) cycle produces the most ATP per glucose molecule through oxidative phosphorylation (OXPHOS). Importantly, TCA intermediates are often extracted from the cycle (cataplerosis) and used for lipid and amino acid biosynthesis, redox regulation, and epigenetic regulation of gene expression [86–88]. The TCA cycle intermediates are replenished through metabolism of amino acids or fatty acids, a process known as anaplerosis. Thus, glucose is not only an important energy source but also a critical provider of building blocks for biosynthetic reactions.
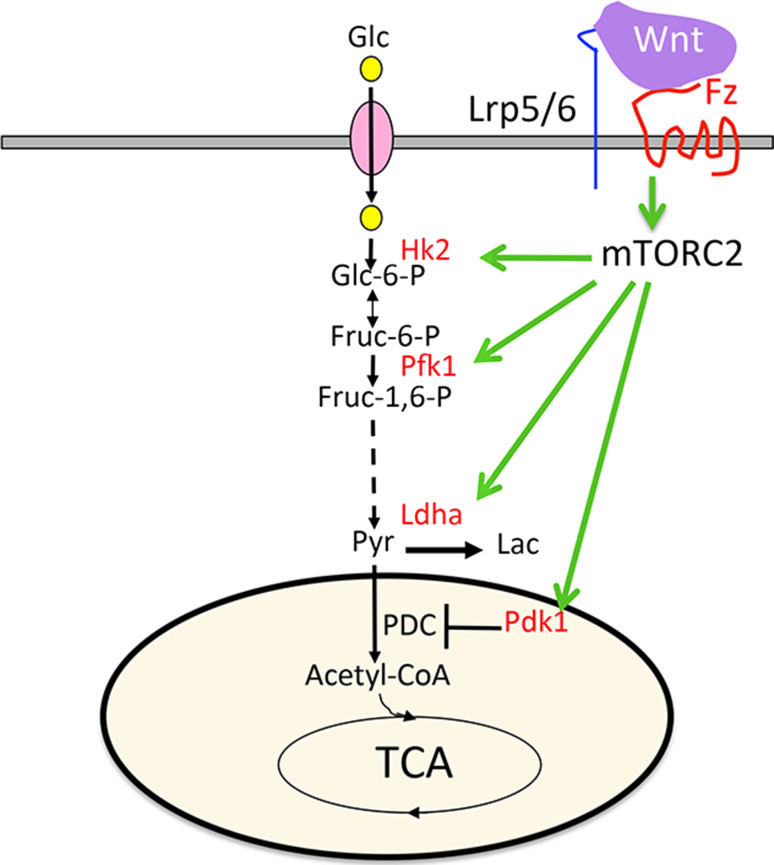
Glucose is an important nutrient for osteoblasts. Osteoblasts express the glucose transporter Glut1 and rapidly consume glucose in response to a variety of signals [81, 89–94]. Recent work has revealed a feed-forward mechanism between Glut1 and Runx2 expression, highlighting a critical role for glucose metabolism in osteoblast differentiation [93]. Mature osteoblasts are known to possess numerous mitochondria and exhibit active OXPHOS [95–97]. Interestingly, however, aerobic glycolysis appears to be the dominant mode of glucose utilization in osteoblasts. For example, early studies using both bone slices and isolated osteoblasts reveal that osteoblasts rapidly take up glucose and metabolize it primarily into lactate [98–101]. More recent studies have confirmed aerobic glycolysis as a predominant mode of glucose metabolism in primary calvarial osteoblasts despite increased OXPHOS as the cells further differentiate to form mineralized nodules in response to ascorbic acid and β-glycerophosphate [96, 102]. Functionally, stimulating glycolysis through activation of Hif1α signaling in preosteoblasts increases bone formation in vivo, indicating that reprogramming glucose metabolism is sufficient to promote osteoblast differentiation [103]. Moreover, Hif1α activation has been shown to promote bone healing partly through reprogramming of glucose metabolism, highlighting the clinical implications of understanding metabolic regulation in osteoblasts [104]. Recent studies have demonstrated that aerobic glycolysis is directly stimulated in response to osteogenic signals such as PTH and Wnt [81, 105]. For example, Wnt rapidly increases Glut1 and Hk2 protein levels to increase glucose consumption. Wnt further promotes aerobic glycolysis by upregulating Ldha and Pdk1 to favor lactate over acetyl-CoA production from pyruvate (Fig. 1). Consistent with these observations, osteoblasts from mice expressing the human Lrp5 high bone mass mutation show increased glucose consumption and expression of glycolytic enzymes. Conversely, Lrp5 −/− mice have decreased glycolytic enzyme expression and lower serum lactate levels. Mechanistically, Wnt can induce glycolysis independent of β-catenin activity, instead through mTORC2 signaling [81]. It should be noted that β-catenin has been shown to induce transcription of Pdk1 in cancer cells, indicating an additional mechanism for Wnt to stimulate aerobic glycolysis [105]. Direct inhibition of mTORC2 signaling by deletion of Rictor reduced both physiological bone formation and Wnt-induced bone formation in response to an anti-sclerostin neutralizing antibody [80, 107]. It is important to note that increased aerobic glycolysis contributes to Wnt-induced osteoblast differentiation in vitro as Ldha or Pdk1 knockdown impaired induction of osteoblast differentiation marker genes [81]. Likewise, dichloroacetate, which inhibits Pdk1 and promotes glucose metabolism through the TCA cycle, reduced bone formation in vivo in response to Hif1a overexpression or anabolic PTH treatment [91, 103]. Overall, both Wnt and PTH stimulate aerobic glycolysis as a mechanism to promote bone anabolism.
Fig. 1.
Wnt signaling promotes aerobic glycolysis through mTORC2 activation. Wnt signaling through Frizzled (Fz) and Lrp5/6 induces mTORC2 activity downstream of PI3K-Rac1 signaling whereas mTORC2 activation acutely increases the protein abundance of metabolic enzymes (in red) without changing their mRNA levels. Glc glucose, Glc-6-P glucose 6-phosphate, Fruc-6-P fructose 6-phosphate, Fruc-1,6-P fructose 1,6-bisphosphate, Pyr pyruvate, Lac lactate, Hk2 hexokinase 2, Pfk1 phosphofructokinase 1, Ldha lactate dehydrogenase A, Pdk1 pyruvate dehydrogenase kinase 1, PDC pyruvate dehydrogenase complex, TCA tricarboxylic acid cycle. See original reference for details [81]
The reasons for osteoblasts to prefer aerobic glycolysis are not fully understood at present. From the bioenergetics viewpoint, aerobic glycolysis is a less efficient means of producing ATP compared to metabolism through the TCA cycle and OXPHOS. Cancer cells display a similar metabolic reprogramming, which is postulated to provide nucleotides, amino acids, and lipids needed to support cell division [106]. Mature osteoblasts, however, generally exhibit little proliferation in vivo [107]. Increased aerobic glycolysis may help reducing reactive oxygen species or generate more amino acids to support protein synthesis in osteoblasts. Moreover, glycolytic changes could directly exert epigenetic regulation to influence osteoblast differentiation. We have recently demonstrated a link between increased aerobic glycolysis and gene suppression in response to Wnt. Increased aerobic glycolysis limits the amount of citrate exiting the TCA cycle, resulting in decreased nuclear levels of both citrate and acetyl-CoA. This leads to a large scale decrease in histone acetylation and suppression of adipogenic or chondrogenic transcription factors, thus favoring osteogenic differentiation over the alternative fates in the multipotent progenitors [108]. Further studies are warranted to elucidate the full mechanism whereby aerobic glycolysis promotes the osteoblast phenotype.
Amino acid metabolism in osteoblasts
Amino acids are not only the building block of proteins, but also an important energy source. The cellular amino acid pool is derived from multiple sources including import of extracellular amino acids, degradation of intracellular protein, and de novo synthesis. Based on their mode of catabolism, amino acids can be categorized as glucogenic or ketogenic or both. Whereas ketogenic amino acids are broken down into acetyl-coA or acetoacetate, glucogenic amino acids are broken down into either pyruvate, or different TCA intermediates including oxaloacetate, α-ketoglutarate, fumarate, and succinyl-coA. Thus, amino acids can contribute directly to ATP production via the TCA cycle and OXPHOS. Indeed, amino acid catabolism through the TCA cycle is required in many contexts including cancer cell proliferation, pluripotent progenitor maintenance, and differentiation [109–113].
Initial studies in bone explants and calvarial osteoblasts focused on amino acid uptake. These studies defined multiple transport systems in bone, including system A, system L, and system ASC, and suggested differences in amino acid transport between adult and fetal bone [114–119]. Amino acid uptake is regulated in osteoblasts, being stimulated by cAMP and various growth factors and hormones [118–124]. More recent studies have implicated individual amino acid transporters in bone biology. For example, the cystine/glutamate antiporter xCT normally suppresses osteoblast differentiation likely through decrease of glutathione production [125–127]. Amino acid uptake is also regulated transcriptionally by the transcription factor Atf4, a critical transcription factor that can be activated by unfolded protein in the endoplasmic reticulum (ER) or amino acid depletion [7, 128, 129]. Atf4 stimulates osteoblast differentiation in part through increasing amino acid import to support collagen synthesis [130–132]. The importance of amino acid import is highlighted by the observation that a high protein diet or amino acid supplementation corrects differentiation defects and bone loss in Atf4 −/− osteoblasts [130]. Besides direct contribution to protein translation, amino acids also act as a growth signal to activate mTORC1, a critical regulator of osteoblast differentiation and bone formation [29, 76, 79, 133–138]. Thus, amino acids regulate bone formation through multiple mechanisms.
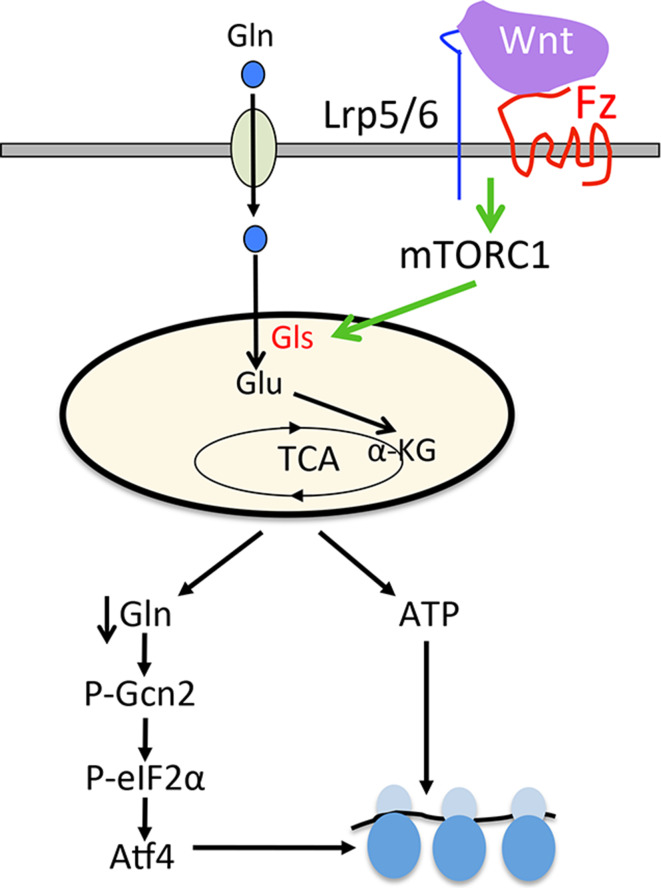
The amino acid glutamine has emerged as an important regulator of osteoblasts. Glutamine is the most abundant free amino acid in circulation and is not only an important oxidative fuel, but also a precursor for the synthesis of non-essential amino acids, nucleotides, and the anti-oxidant glutathione. Initial studies in isolated calvaria and long bones demonstrated an active consumption and metabolism of glutamine [139]. Glutamine was later shown to be required in calvarial osteoblasts for matrix mineralization [140]. Decreased glutamine consumption by bone marrow stromal cells has been linked with impaired osteoblast differentiation associated with aging [141]. Moreover, increased glutathione production from glutamine in response to Hif1α has been shown to improve cell survival and bone repair in a critical-size tibial defect model [104]. In differentiating osteoblasts, glutamine anaplerosis fulfills part of the energetic requirement of bone formation in response to Wnt signaling. Wnt increases glutamine anaplerosis into the TCA cycle by rapidly increasing glutaminase (Gls) protein levels and activity (Fig. 2). Strikingly, increased glutamine anaplerosis reduces intracellular glutamine levels, leading to activation of Gcn2. This results in Atf4 activation which stimulates both the uptake and the de novo synthesis of amino acids to promote protein synthesis. Mechanistically, this cascade of events is dependent on mTORC1 activity downstream of Wnt. Importantly, pharmacological inhibition of Gls reduces bone formation in the Lrp5 A214V/+ high bone mass mouse model demonstrating a critical role for glutamine anaplerosis to support excessive bone anabolism [133]. Whether Gls and glutamine anaplerosis are required for physiological bone formation remains unknown as a systematic analysis of the bones is yet to be performed with the Gls knockout mice [142].
Fig. 2.
Wnt signaling stimulates glutamine catabolism through the TCA cycle. Wnt signaling through Frizzled (Fz) and Lrp5/6 activates mTORC1 in a PI3K-Akt dependent manner; mTORC1 increases the protein abundance of glutaminase (Gls) and stimulates glutamine oxidation for ATP production. The increase in glutamine consumption results in lower intracellular glutamine levels that activate the Gcn2-Atf4 stress pathway and up-regulate the transcription of genes important for protein translation. Gln glutamine, Glu glutamate, α-KG α-ketoglutarate. See original reference for details [76, 77]
Fatty acid metabolism in osteoblasts
Lipids are another important carbon and energy source in mammalian cells. Lipids can be synthesized de novo or acquired either as free fatty acids that are taken up by cell surface transporters or as lipoprotein particles bound by LDL receptor family members. Once transported into the cell, fatty acids can be metabolized in the mitochondrial matrix through β-oxidation that sequentially cleaves off two carbons as acetyl-coA that enters the TCA cycle. Fatty acids are first transported into the mitochondria by the carnitine shuttle. Here the rate limiting enzyme carnitine palmitoyltransferase 1 (CPT1) generates acyl-carnitine by transferring the acyl group of a long-chain fatty acyl-CoA to the hydroxyl group of carnitine. Acyl-carnitine is then shuttled inside the mitochondria in exchange for carnitine and converted back to acyl-CoA on the inner mitochondrial membrane by CPT2. Inside the mitochondrial matrix, acyl-coA can then undergo β-oxidation. Even chain fatty acids can be completely oxidized into acetyl-CoA whereas odd chain fatty acids ultimately form acetyl-CoA and succinyl-CoA which enter the TCA cycle. Complete oxidation of lipids by β-oxidation yields the most ATP per molecule compared to glucose or amino acids.
The role and regulation of lipid metabolism in osteoblasts is understudied. However, it has been reported that bone takes up the second highest amount of postprandial lipoproteins behind liver in the mouse [143]. Moreover, lipid supplementation of serum-free medium was sufficient to support proliferation of osteoblastic cells in vitro [144]. Whether this requirement reflects an energetic or synthetic need for lipids was not clear, but another study estimated that fatty acid oxidation provided 40–80% of the energy derived from glucose in rat calvarial osteoblasts [145]. Furthermore, fatty acid oxidation increases during osteoblast differentiation in both murine and porcine models, implicating lipid metabolism in energy production [146, 147]. Interestingly, recent studies have implicated Wnt signaling in the regulation of lipid metabolism in bone. Osteoblasts lacking the Wnt co-receptor Lrp5 exhibit decreased both expression of lipid metabolism genes and lipid oxidation. Conversely, expression of the Lrp5 G171V allele or stimulation with the ligand Wnt10b increases lipid metabolism gene expression and stimulates lipid oxidation in bone. Mechanistically, the regulation by Wnt appears to be β-catenin dependent as GSK3β inhibition or β-catenin overexpression is sufficient to stimulate lipid oxidation in osteoblasts [147]. It appears that Wnt promotes fatty acid oxidation to fuel the TCA cycle and OXPHOS while also stimulating aerobic glycolysis and glutamine anaplerosis. Further studies are warranted to determine the physiological importance of lipid oxidation for osteoblast differentiation and bone formation in vivo.
Summary
Here, we have highlighted data exploring the role and molecular regulation of cellular metabolism in osteoblasts by Wnt signaling. Recent evidence has indicated that Wnt signaling stimulates aerobic glycolysis, glutamine anaplerosis, and β-oxidation of fatty acids in osteoblast-lineage cells. It is likely that β-oxidation and glutamine anaplerosis contribute to ATP production via OXPHOS to sustain protein synthesis during bone formation. The mechanism through which aerobic glycolysis contributes to the osteoblast phenotype is likely multifaceted and certainly warrants further investigation. Similarly, future studies on the role of the various metabolic pathways during physiological bone formation are necessary. It is tempting to speculate that metabolic dysregulation at the cellular level might be involved in various bone pathologies such as ectopic ossification, vascular calcification, skeletal aging or diabetes-associated bone disorders. The potential link with diabetes is of particular clinical significance as patients with either type I or type II diabetes exhibit increased risk in bone fracture [148, 149]. Although the mechanisms for diabetes-related bone fragility are undoubtedly complex, pharmaceutical targeting of osteoblast metabolism may be a promising direction towards novel therapies to improve bone health in diabetic patients.
Acknowledgements
Original work in the Long lab was supported by NIH grants R01 AR060456 (FL) and F32 AR060674 (CMK).
References
- 1.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2012;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 2.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 3.Ducy P, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/S0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee B, et al. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet. 1997;16(3):307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- 5.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin–Lowry syndrome. Cell. 2004;117(3):387–398. doi: 10.1016/S0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Z, Ethen NJ, Williams BO. WNT signaling in bone development and homeostasis. Wiley Interdiscip Rev Dev Biol. 2014;3(6):489–500. doi: 10.1002/wdev.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J. 1995;312(Pt 1):163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102(9):3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu X, et al. Noncanonical Wnt signaling through G protein-Linked PKCdelta activation promotes bone formation. Dev Cell. 2007;12(1):113–127. doi: 10.1016/j.devcel.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day TF, et al. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Hu H, et al. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132(1):49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 14.Babij P, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18(6):960–974. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 15.Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–523. doi: 10.1016/S0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 16.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133(16):3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 17.Lu W, et al. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119(1):97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 18.He X, et al. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131(8):1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 19.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 20.Kimelman D, Xu W. Beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25(57):7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 21.Taelman VF, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143(7):1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behrens J, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 23.Hovanes K, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28(1):53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 24.Zeng W, et al. naked cuticle encodes an inducible antagonist of Wnt signalling. Nature. 2000;403(6771):789–795. doi: 10.1038/35001615. [DOI] [PubMed] [Google Scholar]
- 25.Roose J, et al. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285(5435):1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- 26.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen AE, Ginty DD, Fan CM. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433(7023):317–322. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- 28.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17(2):295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoki K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 30.Sheldahl LC, et al. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol. 1999;9(13):695–698. doi: 10.1016/S0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- 31.Sheldahl LC, et al. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol. 2003;161(4):769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, et al. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133(2):340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamanaka H, et al. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3(1):69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hino S, et al. Inhibition of the Wnt signaling pathway by Idax, a novel Dvl-binding protein. Mol Cell Biol. 2001;21(1):330–342. doi: 10.1128/MCB.21.1.330-342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37(5):537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Amerongen R, et al. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev Biol. 2012;369(1):101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao B, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 39.Semenov MV, et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11(12):951–961. doi: 10.1016/S0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 40.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 41.Bourhis E, et al. Wnt antagonists bind through a short peptide to the first beta-propeller domain of LRP5/6. Structure. 2011;19(10):1433–1442. doi: 10.1016/j.str.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Rattner A, et al. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94(7):2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finch PW, et al. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci USA. 1997;94(13):6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leyns L, et al. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88(6):747–756. doi: 10.1016/S0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maupin KA, Droscha CJ, Williams BO. A comprehensive overview of skeletal phenotypes associated with alterations in Wnt/b-catenin signaling in humans and mice. Bone Res. 2013;1(1):27–71. doi: 10.4248/BR201301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 47.Little RD, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Bezooijen RL, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunkow ME, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balemans W, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39(2):91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richards JB, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371(9623):1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivadeneira F, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41(11):1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Meurs JB, et al. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299(11):1277–1290. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Estrada K, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fahiminiya S, et al. Mutations in WNT1 are a cause of osteogenesis imperfecta. J Med Genet. 2013;50(5):345–348. doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- 56.Laine CM, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013;368(19):1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng HF, et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012;8(7):e1002745. doi: 10.1371/journal.pgen.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kato M, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157(2):303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riddle RC, et al. Lrp5 and Lrp6 exert overlapping functions in osteoblasts during postnatal bone acquisition. PLoS One. 2013;8(5):e63323. doi: 10.1371/journal.pone.0063323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui Y, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17(6):684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 62.Morvan F, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21(6):934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 63.Bodine PV, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18(5):1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 64.Albers J, et al. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J Cell Biol. 2013;200(4):537–549. doi: 10.1083/jcb.201207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albers J, et al. Control of bone formation by the serpentine receptor Frizzled-9. J Cell Biol. 2011;192(6):1057–1072. doi: 10.1083/jcb.201008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joeng KS, et al. The swaying mouse as a model of osteogenesis imperfecta caused by WNT1 mutations. Hum Mol Genet. 2014;23(15):4035–4042. doi: 10.1093/hmg/ddu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maruyama T, Jiang M, Hsu W. Gpr177, a novel locus for bone mineral density and osteoporosis, regulates osteogenesis and chondrogenesis in skeletal development. J Bone Miner Res. 2013;28(5):1150–1159. doi: 10.1002/jbmr.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhong Z, et al. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci USA. 2012;109(33):E2197–E2204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Witte F, et al. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns. 2009;9(4):215–223. doi: 10.1016/j.gep.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Chen J, Long F. beta-catenin promotes bone formation and suppresses bone resorption in postnatal growing mice. J Bone Miner Res. 2013;28(5):1160–1169. doi: 10.1002/jbmr.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glass DA, 2nd, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 72.Hill TP, et al. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8(5):727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 73.Holmen SL, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280(22):21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 74.Song L, et al. Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res. 2012;27(11):2344–2358. doi: 10.1002/jbmr.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaur T, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 76.Chen J, et al. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 2014;10(1):e1004145. doi: 10.1371/journal.pgen.1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karner CM, et al. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J Clin Investig. 2015;125(2):551–562. doi: 10.1172/JCI78470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riddle RC, et al. Tsc2 is a molecular checkpoint controlling osteoblast development and glucose homeostasis. Mol Cell Biol. 2014;34(10):1850–1862. doi: 10.1128/MCB.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J, Long F. mTORC1 signaling promotes osteoblast differentiation from preosteoblasts. PLoS One. 2015;10(6):e0130627. doi: 10.1371/journal.pone.0130627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun W, et al. Rictor is required for optimal bone accrual in response to anti-sclerostin therapy in the mouse. Bone. 2016;85:1–8. doi: 10.1016/j.bone.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Esen E, et al. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17(5):745–755. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J, et al. mTORC2 signaling promotes skeletal growth and bone formation in mice. J Bone Miner Res. 2015;30:369–378. doi: 10.1002/jbmr.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bell GI, et al. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993;268(26):19161–19164. [PubMed] [Google Scholar]
- 84.Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219(3):713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 85.Esen E, Long F. Aerobic glycolysis in osteoblasts. Curr Osteoporos Rep. 2014;12(4):433–438. doi: 10.1007/s11914-014-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsukada Y, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439(7078):811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 87.Tahiliani M, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brommage R, Neuman WF. Mechanism of mobilization of bone mineral by 1,25-dihydroxyvitamin D3. Am J Physiol. 1979;237(2):E113–E120. doi: 10.1152/ajpendo.1979.237.2.E113. [DOI] [PubMed] [Google Scholar]
- 90.Cohn DV, Forscher BK. Effect of parathyroid extract on the oxidation in vitro of glucose and the production of 14CO-2 by bone and kidney. Biochim Biophys Acta. 1962;65:20–26. doi: 10.1016/0006-3002(62)90145-2. [DOI] [PubMed] [Google Scholar]
- 91.Esen E, et al. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. J Bone Miner Res. 2015;30(11):1959–1968. doi: 10.1002/jbmr.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmid C, Steiner T, Froesch ER. Parathormone promotes glycogen formation from [14C]glucose in cultured osteoblast-like cells. FEBS Lett. 1982;148(1):31–34. doi: 10.1016/0014-5793(82)81236-2. [DOI] [PubMed] [Google Scholar]
- 93.Wei J, et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;161(7):1576–1591. doi: 10.1016/j.cell.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zoidis E, Ghirlanda-Keller C, Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem. 2011;348(1–2):33–42. doi: 10.1007/s11010-010-0634-z. [DOI] [PubMed] [Google Scholar]
- 95.Passi-Even L, Gazit D, Bab I. Ontogenesis of ultrastructural features during osteogenic differentiation in diffusion chamber cultures of marrow cells. J Bone Miner Res. 1993;8(5):589–595. doi: 10.1002/jbmr.5650080510. [DOI] [PubMed] [Google Scholar]
- 96.Komarova SV, Ataullakhanov FI, Globus RK. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol. 2000;279(4):C1220–C1229. doi: 10.1152/ajpcell.2000.279.4.C1220. [DOI] [PubMed] [Google Scholar]
- 97.Klein BY, et al. Induction of osteoprogenitor cell differentiation in rat marrow stroma increases mitochondrial retention of rhodamine 123 in stromal cells. J Cell Biochem. 1993;53(3):190–197. doi: 10.1002/jcb.240530303. [DOI] [PubMed] [Google Scholar]
- 98.Borle AB, Nichols N, Nichols G., Jr Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem. 1960;235:1206–1210. [PubMed] [Google Scholar]
- 99.Cohn DV, Forscher BK. Aerobic metabolism of glucose by bone. J Biol Chem. 1962;237:615–618. [PubMed] [Google Scholar]
- 100.Felix R, Neuman WF, Fleisch H. Aerobic glycolysis in bone: lactic acid production by rat calvaria cells in culture. Am J Physiol. 1978;234(1):C51–C55. doi: 10.1152/ajpcell.1978.234.1.C51. [DOI] [PubMed] [Google Scholar]
- 101.Neuman WF, Neuman MW, Brommage R. Aerobic glycolysis in bone: lactate production and gradients in calvaria. Am J Physiol. 1978;234(1):C41–C50. doi: 10.1152/ajpcell.1978.234.1.C41. [DOI] [PubMed] [Google Scholar]
- 102.Guntur AR, et al. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology. 2014;155(5):1589–1595. doi: 10.1210/en.2013-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Regan JN, et al. Up-regulation of glycolytic metabolism is required for HIF1alpha-driven bone formation. Proc Natl Acad Sci USA. 2014;111(23):8673–8678. doi: 10.1073/pnas.1324290111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stegen S, et al. HIF-1alpha promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab. 2016;23(2):265–279. doi: 10.1016/j.cmet.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pate KT, et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33(13):1454–1473. doi: 10.15252/embj.201488598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Owen M, Macpherson S. Cell population kinetics of an osteogenic tissue. II. J Cell Biol. 1963;19:33–44. doi: 10.1083/jcb.19.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karner CM et al (2016) Wnt signaling reduces nuclear acetyl-coA levels to suppress gene expression during osteoblast differentiation. J Biol Chem 291(25):13028–13039 [DOI] [PMC free article] [PubMed]
- 109.DeBerardinis RJ, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Green CR, et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol. 2016;12(1):15–21. doi: 10.1038/nchembio.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shiraki N, et al. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 2014;19(5):780–794. doi: 10.1016/j.cmet.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 112.Tonjes M, et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat Med. 2013;19(7):901–908. doi: 10.1038/nm.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J, et al. Dependence of mouse embryonic stem cells on threonine catabolism. Science. 2009;325(5939):435–439. doi: 10.1126/science.1173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adamson LF, Ingbar SH. Further studies of amino acid transport by embryonic chick bone. J Biol Chem. 1967;242(11):2646–2652. [PubMed] [Google Scholar]
- 115.Finerman GA, Rosenberg LE. Amino acid transport in bone. Evidence for separate transport systems for neutral amino and imino acids. J Biol Chem. 1966;241(7):1487–1493. [PubMed] [Google Scholar]
- 116.Hahn TJ, Downing SJ, Phang JM. Amino acid transport in adult diaphyseal bone: contrast with amino acid transport mechanisms in fetal membranous bone. Biochim Biophys Acta. 1969;183(1):194–203. doi: 10.1016/0005-2736(69)90143-6. [DOI] [PubMed] [Google Scholar]
- 117.Kim SG, et al. Differential expression and functional characterization of system L amino acid transporters in human normal osteoblast cells and osteogenic sarcoma cells. Anticancer Res. 2006;26(3A):1989–1996. [PubMed] [Google Scholar]
- 118.Phang JM, Downing SJ. Amino acid transport in bone: stimulation by cyclic AMP. Am J Physiol. 1973;224(1):191–196. doi: 10.1152/ajplegacy.1973.224.1.191. [DOI] [PubMed] [Google Scholar]
- 119.Yee JA. Effect of parathyroid hormone on amino acid transport by cultured neonatal mouse calvarial bone cells. J Bone Miner Res. 1988;3(2):211–218. doi: 10.1002/jbmr.5650030214. [DOI] [PubMed] [Google Scholar]
- 120.Adamson LF, Ingbar SH. Selective alteration by triiodothyronine of amino acid transport in embryonic bone. Endocrinology. 1967;81(6):1362–1371. doi: 10.1210/endo-81-6-1362. [DOI] [PubMed] [Google Scholar]
- 121.Adamson LF, Ingbar SH. Some properties of the stimulatory effect of thyroid hormones on amino acid transport by embryonic chick bone. Endocrinology. 1967;81(6):1372–1378. doi: 10.1210/endo-81-6-1372. [DOI] [PubMed] [Google Scholar]
- 122.Baum BJ, Shteyer A. Characteristics of a neutral amino acid transport system (system A) in osteoblastic rat osteosarcoma cells. Exp Cell Res. 1987;169(2):453–457. doi: 10.1016/0014-4827(87)90205-9. [DOI] [PubMed] [Google Scholar]
- 123.Hahn TJ, Downing SJ, Phang JM. Insulin effect on amino acid transport in bone. Biochim Biophys Acta. 1969;184(3):675–677. doi: 10.1016/0304-4165(69)90292-X. [DOI] [PubMed] [Google Scholar]
- 124.Hahn TJ, Downing SJ, Phang JM. Insulin effect on amino acid transport in bone: dependence on protein synthesis and Na+ . Am J Physiol. 1971;220(6):1717–1723. doi: 10.1152/ajplegacy.1971.220.6.1717. [DOI] [PubMed] [Google Scholar]
- 125.Takarada-Iemata M, et al. Glutamate preferentially suppresses osteoblastogenesis than adipogenesis through the cystine/glutamate antiporter in mesenchymal stem cells. J Cell Physiol. 2011;226(3):652–665. doi: 10.1002/jcp.22390. [DOI] [PubMed] [Google Scholar]
- 126.Uno K, et al. A negative correlation between expression profiles of runt-related transcription factor-2 and cystine/glutamate antiporter xCT subunit in ovariectomized mouse bone. J Pharmacol Sci. 2011;115(3):309–319. doi: 10.1254/jphs.10310FP. [DOI] [PubMed] [Google Scholar]
- 127.Uno K, et al. Negative regulation of osteoblastogenesis through downregulation of runt-related transcription factor-2 in osteoblastic MC3T3-E1 cells with stable overexpression of the cystine/glutamate antiporter xCT subunit. J Cell Physiol. 2011;226(11):2953–2964. doi: 10.1002/jcp.22642. [DOI] [PubMed] [Google Scholar]
- 128.Harding HP, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 129.Han J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15(5):481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Elefteriou F, et al. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 2006;4(6):441–451. doi: 10.1016/j.cmet.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saito A, et al. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem. 2011;286(6):4809–4818. doi: 10.1074/jbc.M110.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang P, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22(11):3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Duran RV, et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47(3):349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 134.Huang B, et al. mTORC1 prevents preosteoblast differentiation through the notch signaling pathway. PLoS Genet. 2015;11(8):e1005426. doi: 10.1371/journal.pgen.1005426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jewell JL, et al. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347(6218):194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nicklin P, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xian L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18(7):1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zoncu R, et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334(6056):678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Biltz RM, et al. Glutamine metabolism in bone. Miner Electrolyte Metab. 1983;9(3):125–131. [PubMed] [Google Scholar]
- 140.Brown PM, Hutchison JD, Crockett JC. Absence of glutamine supplementation prevents differentiation of murine calvarial osteoblasts to a mineralizing phenotype. Calcif Tissue Int. 2011;89(6):472–482. doi: 10.1007/s00223-011-9537-6. [DOI] [PubMed] [Google Scholar]
- 141.Huang T et al (2016) Aging reduces an ERRalpha-directed mitochondrial glutaminase expression suppressing glutamine anaplerosis and osteogenic differentiation of mesenchymal stem cells. Stem Cells. doi:10.1002/stem.2470. [DOI] [PubMed]
- 142.Masson J, et al. Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci. 2006;26(17):4660–4671. doi: 10.1523/JNEUROSCI.4241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Niemeier A, et al. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43(2):230–237. doi: 10.1016/j.bone.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 144.Catherwood BD, et al. Growth of rat osteoblast-like cells in a lipid-enriched culture medium and regulation of function by parathyroid hormone and 1,25-dihydroxyvitamin D. J Bone Miner Res. 1988;3(4):431–438. doi: 10.1002/jbmr.5650030410. [DOI] [PubMed] [Google Scholar]
- 145.Adamek G, et al. Fatty acid oxidation in bone tissue and bone cells in culture. Characterization and hormonal influences. Biochem J. 1987;248(1):129–137. doi: 10.1042/bj2480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chiu KM, et al. Carnitine and dehydroepiandrosterone sulfate induce protein synthesis in porcine primary osteoblast-like cells. Calcif Tissue Int. 1999;64(6):527–533. doi: 10.1007/s002239900644. [DOI] [PubMed] [Google Scholar]
- 147.Frey JL, et al. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. 2015;35(11):1979–1991. doi: 10.1128/MCB.01343-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Napoli N et al (2016) Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. doi:10.1038/nrendo.2016.153 [DOI] [PubMed]
- 149.Lecka-Czernik B, Rosen CJ. Energy excess, glucose utilization, and skeletal remodeling: new insights. J Bone Miner Res. 2015;30(8):1356–1361. doi: 10.1002/jbmr.2574. [DOI] [PubMed] [Google Scholar]