Summary
The goal to prevent Plasmodium falciparum transmission from humans to mosquitoes requires the identification of targetable metabolic processes in the mature (stage V) gametocytes, the sexual stages circulating in the bloodstream. This task is complicated by the apparently low metabolism of these cells, which renders them refractory to most antimalarial inhibitors and constrains the development of specific and sensitive cell-based assays. Here we identify and functionally characterize the regulatory regions of the P. falciparum gene PF3D7_1234700, encoding a CPW-WPC protein and named here Upregulated in Late Gametocytes (ULG8), which we have leveraged to express reporter genes in mature male and female gametocytes. Using transgenic parasites containing a pfULG8-luciferase cassette, we investigated the susceptibility of stage V gametocytes to compounds specifically affecting redox metabolism. Our results reveal a high sensitivity of mature gametocytes to the glutathione reductase inhibitor and redox cycler drug methylene blue (MB). Using isobologram analysis, we find that a concomitant inhibition of the parasite enzyme glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase, a key component of NADPH synthesis, potently synergizes MB activity. These data suggest that redox metabolism and detoxification activity play an unsuspected yet vital role in stage V gametocytes, rendering these cells exquisitely sensitive to decreases in NADPH concentration.
Keywords: Plasmodium falciparum, malaria, gametocytes, transmission, methylene blue, transfection, luciferase
ABBREVIATED SUMMARY
The poorly understood physiology of the mature gametocytes of Plasmodium falciparum was investigated with bioluminescent parasites, revealing that these stages, known to be refractory to virtually all antimalarial drugs, are exquisitely vulnerable if their ability to contrast oxidative stress is impaired.
Introduction
Malaria is the most prevalent and fatal mosquito-borne parasitic disease. An estimated 3.3 billion people are at risk of being infected by one of the five species of human malaria parasites, of which the most lethal is Plasmodium falciparum. In 2015, estimates are that 212 million cases of malaria occurred globally leading to 429,000 deaths, with 92% of the cases in Africa and 70% of the deaths in children under 5 years (WHO World Malaria Report 2016).
The transmission of Plasmodium from infected humans to mosquitoes is mediated by sexual stage gametocytes, whose maturation occurs in human red blood cells (RBCs). P. falciparum gametocytes progress through five stages of maturation (I–V) in ~10 days (Hawking et al., 1971). Immature gametocytes are sequestered in internal organs, including the bone marrow (Joice et al., 2014), and only stage V gametocytes circulate and are transmissible to the mosquito vector during the blood meal.
P. falciparum gametocyte maturation is accompanied by profound physiological and morphological changes including the upregulation of the expression of ~200 gametocyte-specific genes (Young et al., 2005) and proteins (Silvestrini et al., 2010) as well as changes in gametocyte cell-mechanical properties (Tiburcio et al., 2015). The elaboration of subcellular structures and the process of hemoglobin digestion accompany progression of gametocytogenesis from stage I to IV, whereas the final stage of maturation appears to be comparatively less active. Besides the fact that stage V gametocytes persist in circulation or in culture for several days with little, if any, further morphological differentiation, the notion that this stage is comparatively metabolically less active derives from the observation that hemoglobin digestion has ceased (Hanssen et al., 2012) and that most drugs and compounds killing the asexual and the immature sexual stages are no longer active against stage V gametocytes (Adjalley et al., 2011; Plouffe et al., 2016). At present the only exception is primaquine (Smithuis et al., 2010), an approved 8-aminoquinoline recommended by WHO as a gametocytocidal adjunct to first-line artemisinin-based combination therapies (ACT). Primaquine, however, has toxicity issues and can cause hemolysis in individuals harboring certain forms of glucose-6-phosphate dehydrogenase (G6PD) deficiency (Butterworth et al., 2013; Chen et al., 2015).
The apparently lower metabolic activity of P. falciparum stage V gametocytes is a major obstacle in the search for novel drugs that can efficiently kill these stages and block malaria transmission. This feature also challenges the development of cell-based drug discovery assays that combine a reliable readout for stage V gametocyte viability with an easy and inexpensive high-throughput screening protocol.
The current notion that stage V gametocytes have a low metabolic activity calls for a deeper investigation of this still obscure aspect of Plasmodium biology, an objective that requires sensitive and robust cell-based assays. Of several recently developed assays (Birkholtz et al., 2016), the few specifically designed for P. falciparum mature gametocytes are based on high-content imaging of fluorescent gametocytes and gametes or time-lapse imaging of male gamete exflagellation (Ruecker et al., 2014; Lucantoni et al., 2015; Miguel-Blanco et al., 2015).
Here, we leveraged the unsurpassed sensitivity, versatility and scalability of cell-based assays using bioluminescent reporters to produce transgenic P. falciparum lines in which a luciferase reporter is driven by a promoter highly upregulated in mature gametocytes. Transcriptomic analyses show that transcript upregulation in mature gametocytes generally occurs through a steady mRNA accumulation rather than an abrupt transcriptional switch from stage IV to stage V (Young et al., 2005; Alano, 2007). Furthermore, several late-stage gametocyte transcripts are not translated until gametes are formed in the mosquito midgut (Paton et al., 1993; Mair et al., 2006). Nevertheless, in this study we report the identification of gene regulatory regions that combine a high level of transcription and a higher expression in stage V gametocytes compared to earlier non-transmissible stages. Transgenic parasites engineered to upregulate the expression of a luciferase reporter in stage V gametocytes were used to quantitatively investigate how compounds targeting key pathways of the parasite redox metabolism affected this stage of gametocyte development. This work identified a highly synergistic activity of a redox cycler drug, methylene blue (MB), and a selective inhibitor of the parasite enzyme glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase (PfGluPho), revealing that P. falciparum mature gametocytes are highly sensitive to perturbations in redox equilibrium.
Results and Discussion
Identification of P. falciparum regulatory sequences upregulating gene expression in stage V gametocytes
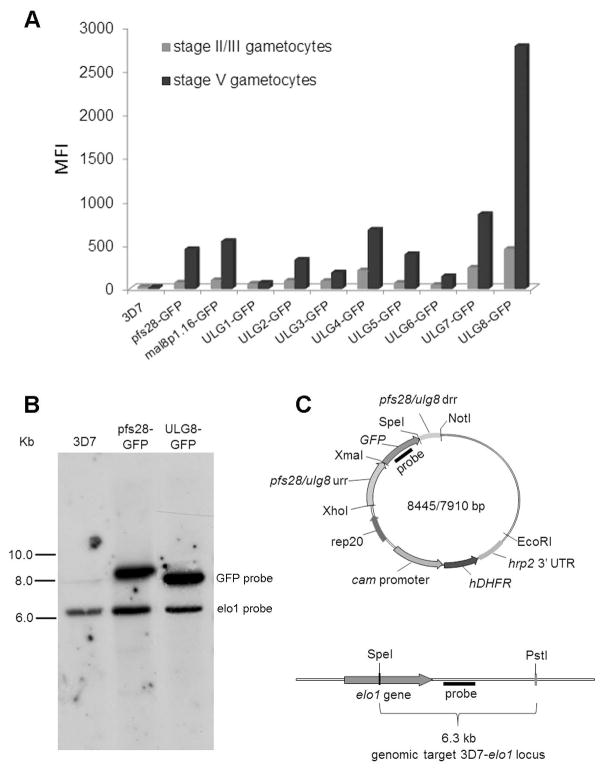
Quantitative RNA expression profiles from time courses of P. falciparum asexual and sexual development (Le Roch et al., 2003; Young et al., 2005) were examined to select candidate gene regulatory regions that efficiently activated gene expression in stage V gametocytes (Fig. S1). Pairs of upstream and downstream regulatory regions from eight genes whose transcripts accumulated in late stage gametocytes, defined as Upregulated in Late Gametocytes (ULGs), were amplified with specific primers (Table S1) and cloned to drive expression of a GFP reporter gene in plasmids pULG-GFP 1 through 8. Flanking regions were also cloned from genes pfs28 and mal8p1.16, the only genomic sequences so far used to upregulate reporter gene expression in late stage gametocytes (Adjalley et al., 2011; Eksi et al., 2008). Each of these ten plasmids (Fig. S2, Table S2) was introduced by electroporation into P. falciparum clone 3D7. A preliminary inspection of GFP expression in gametocytes at different stages of maturation showed that in none of the ten transgenic lines was the expression of the GFP reporter specifically restricted to the stage V gametocytes. A quantitative comparison of GFP expression by flow cytometry between synchronous stage II–III and stage V gametocytes from the ten transgenic lines showed that the regulatory regions of gene pfULG8 outperformed the other ULG sequences and the reference genes pfs28 and mal8p1.16, as pfULG8 regulatory elements yielded a high level of GFP expression in stage V gametocytes. Expression was six–fold higher expression as compared to the immature gametocytes (Fig. 1A; Fig. S3).
Figure 1. Development of the P. falciparum line 3D7/pfULG8-GFP upregulating GFP expression in stage V gametocytes.
A: Histograms representing the mean fluorescent intensity (MFI) of the GFP reporter expressed under control of the pfULG 1–8, the pfs28 and the mal8p1.16 regulatory regions in stage II/III and in stage V gametocytes (representative of two biological replicates). B. Southern blot analysis of genomic DNA from lines 3D7wt, 3D7/pfULG8-GFP and 3D7/pfs28-GFP. Equal amounts of SpeI+PstI digested genomic DNAs from the indicated parasite lines were electrophoresed and hybridized with probes specific for the GFP coding sequence and the single copy gene pfelo1 (see panel C). Autoradiographs from the two hybridizations were combined in the panel and hybridization bands specific for each probe are indicated. C. Diagram of the plasmid containing the GFP coding sequence flanked by the pfs28 or the pfULG8 regulatory regions (above) and of the chromosomal locus of the single copy gene pfelo1 (below) are shown to indicate position of the GFP and the pfelo1 specific probes, as are the positions of the SpeI and the PstI restriction sites used in the hybridization experiments in panel B. urr: upstream regulatory region; drr: downstream regulatory region.
Southern blot analysis was performed to compare the gene copy number of the GFP reporter in the 3D7/pfs28 and in the 3D7/pULG8-GFP parasite lines. Intensity of the signal obtained with a GFP-specific probe was similar between these lines, indicating that the higher GFP expression in the 3D7/pULG8-GFP gametocytes was not due to pULG8-GFP plasmid amplification of but rather to the elevated activity of the pfULG8 regulatory elements (Fig. 1B).
We then used the pfULG8 upstream and downstream regulatory regions to drive expression of the Phyrophorus plagiophtalamus CBG99 luciferase, which provides exquisite sensitivity (Cevenini et al., 2014). This reporter cassette was integrated into the dispensable pfcg6 parasite chromosomal locus (Nkrumah et al., 2006), yielding the parasite line NF54-cg6-pULG8-CBG99 (Fig. S4). Three independent comparisons of luciferase activity between stage V and stage II/III gametocytes of this line showed a 3.8 ± 0.4 higher reporter gene expression in the late stages, confirming the ability of the pfULG8 regulatory sequences to upregulate gene expression in mature gametocytes using an independent reporter that had been chromosomally integrated.
Reporter genes driven by pfULG8 regulatory sequences escape translational repression and sex-specific expression control but maintain upregulation in stage V gametocytes
The pfULG8 gene (PlasmoDB ID PF3D7_1234700) is a member of the P. falciparum gene family encoding the CPW-WPC proteins. An integrated transcriptomic and proteomic analysis of P. falciparum gene expression recently showed that transcripts from the nine CPW-WPC genes predominantly accumulate in female gametocytes and are subject to translational repression (TR) (Lasonder et al., 2016), i.e. these transcripts are produced in gametocytes but are translated only in the mosquito parasite stages. First described for the mRNA of the Plasmodium berghei 21kDa ookinete surface protein (Paton et al., 1993), TR was shown to control several rodent malaria gametocyte transcripts through RNA binding proteins recognizing motifs in the mRNA untranslated regions (Mair et al., 2006; Mair et al., 2010). In P. falciparum, the TR machinery was reported to rely on interactions of the parasite RNA binding protein Pumilio family 2 (Puf2) with specific sequences in the untranslated regions of transcripts, such as UGUAAUUA or UGUUAAUA in the pfs25 and pfs28 mRNAs (Miao et al., 2013).
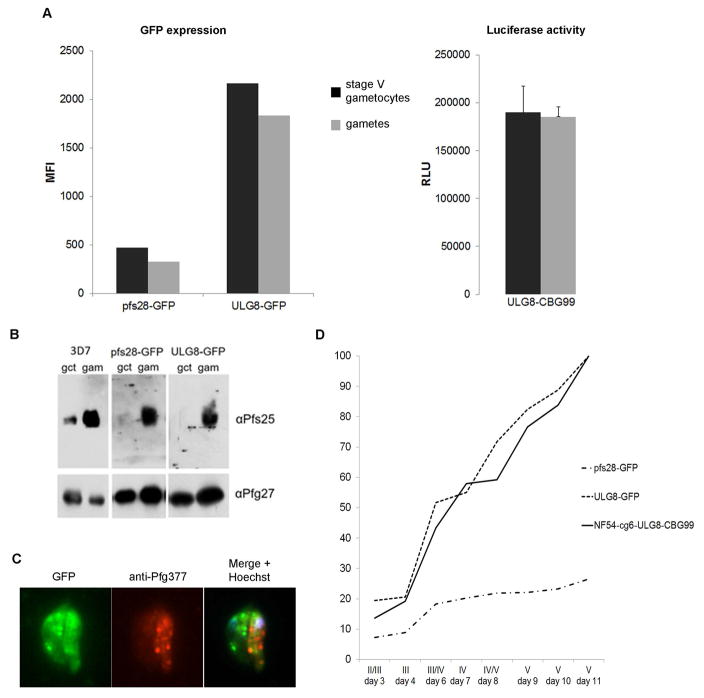
Our results show that the GFP and the CBG99 luciferase mRNAs regulated by the pfULG8 and the pfs28 regulatory sequences are not under TR, as they are efficiently translated in the transgenic gametocytes. This occurs despite the presence of one copy of the TR-responsive motif UGUAAUUA in the pfULG8 5′UTR located ~380 nucleotides upstream of the reporter gene start codon, and in the pfs28 3′UTR located ~90 nucleotides downstream of the GFP stop codon (Fig. S5). To directly measure whether these transcripts are subject to TR control, GFP fluorescence and CBG99 luciferase activity were measured in the lines 3D7/pULG8-GFP, 3D7/pfs28-GFP and NF54-cg6-ULG8-CBG99 as they transitioned from mature gametocytes to gametes. Percent of gametocyte ‘rounding up’, measured 15 min after induction, was 85, 80 and 83%, respectively, indicating that efficiency of gamete activation was similar in all lines. Results clearly showed that expression of GFP under the control of either the pfs28 or the pfULG8 plasmid-borne regulatory sequences and the activity of the luciferase under the control of the chromosomally-integrated pfULG8 regulatory sequences were virtually identical before and after induction of gamete formation (Fig. 2A). This confirms that the pfs28 and pfULG8 untranslated sequences are unresponsive to TR regulation in the three parasite lines.
Figure 2. Regulation of reporter gene expression by the pfULG8 flanking regions.
A: GFP and luciferase activity before and after induction of gamete formation. Histograms represent values of mean fluorescence Intensity (MFI, left panel) and of relative light units (RLU, right panel) from gametocytes and gametes 6h post induction of the parasite lines indicated (n = 1). Error bars in RLU histograms represent SD from three technical replicates. B: Translational repression of the Pfs25 gamete/zygote surface protein in wild-type and transgenic parasites. Western blot analysis with Pfs25-specific antibodies on gametocytes (gct) and on gametes 6h post induction (gam) from the indicated parasite lines. Antibodies specific for the gametocyte-specific cytoplasmic protein Pfg27 were used on the same lanes as loading control. C: Staining of 3D7/pfULG8-GFP gametocytes with antibodies to the female-specific marker Pfg377. Representative image of two 3D7/pfULG8-GFP stage IV gametocytes expressing GFP, one positive to the anti-Pfg377 antibody (female) and one negative (male). D: Time course analysis of GFP fluorescence (MFI) and bioluminescence (RLU) in the course of gametocytogenesis of the indicated lines.
It has earlier been proposed that TR regulated mRNAs produced by episomal plasmids might deregulate the TR machinery by titrating components of the TR apparatus (Miao et al., 2013). To examine this possibility in the 3D7/pULG8-GFP and 3D7/pfs28-GFP lines, we measured the production of the endogenous Pfs25 gamete/ookinete protein in mature gametocytes and in gametes 6h post-activation. Having observed, as above, no differences in efficiency of gamete activation, the similarly strong increase in Pfs25 protein production during gametogenesis in both the transgenic lines and the non-recombinant parasite lines (Fig. 2B) showed that TR is normally functioning and indicated that production of the reporter proteins in gametocytes is not due to a deregulated TR apparatus.
These results are in contrast with the recent report that pfs28 upstream and downstream untranslated regions mediate a 50–fold increase in the activity of a plasmid-borne firefly luciferase reporter 24h after induction of gametogenesis (Rao et al., 2016). This discrepancy is possibly due to the different time after induction of gametogenesis when reporter activity was measured in the other report (Rao et al., 2016) (24h) and in our experiments (6h). The increase of Pfs25 production 6h after gamete formation (Fig. 2B) clearly indicates that this is an appropriate time to investigate this regulatory mechanism.
To investigate whether escape from TR control of the pfULG8 driven reporter mRNAs concomitantly affected the female-specific expression of the endogenous pfULG8 transcript (Lasonder et al., 2016), we labeled 3D7/pULG8-GFP stage IV gametocytes with antibodies specific for the female-specific marker Pfg377 (Severini et al., 1999). Two populations of GFP-fluorescent gametocytes were identified, one positive and one negative for Pfg377, respectively present in a 10:1 ratio (Fig. 2C), which clearly indicated that GFP is produced in both female and male gametocytes. The expression of the pfULG8-driven GFP in male gametocytes was unambiguously confirmed by observing GFP fluorescence associated with the 3D7/pULG8-GFP male gamete exflagellation centers (Movie S1).
Finally, the expression profile of the GFP and the CBG99 reporters under control of the pfULG8 regulatory sequences was characterized in a daily time course of sexual development of the transgenic gametocytes. GFP fluorescence and bioluminescence of synchronous 3D7/pULG8-GFP and NF54-cg6-ULG8-CBG99 gametocytes from stage II to stage V were measured for 11 days, confirming the previously observed five-fold increase in the activity of both reporters between stage III (day 4) and stage V (day 11) gametocytes (Fig. 2D).
This analysis in conclusion indicates that the pfULG8 regulatory regions control the upregulation of a fluorescent or a bioluminescent reporter, maintained as an episomal or a chromosomally integrated construct. The cloned pfULG8 regulatory regions however no longer mediated female-specific expression and translational repression of the reporter genes, as described for the endogenous pfULG8 gene, either because these sequences were separated from the natural pfULG8 locus or because specific motifs were not included in the reporter cassettes. As a result, the pfULG8 regulatory regions induce production of the reporter proteins in gametocytes of both sexes.
The pfULG8 driven luciferase reporter as a novel tool to investigate stage V gametocyte physiology
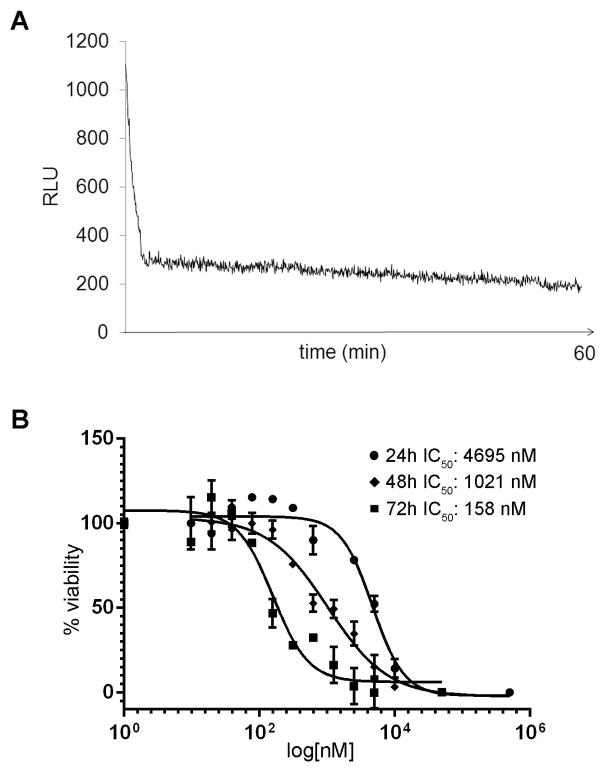
The increased expression of the CBG99 luciferase during late sexual development made it possible to develop a sensitive, quantitative and potentially scalable cell-based assay to investigate the physiology of P. falciparum stage V gametocytes. A kinetic analysis of CBG99 bioluminescence produced by the NF54-cg6-ULG8-CBG99 stage V gametocytes showed that luciferase activity was stable for at least one hour, with a Coefficient of Variation of 8% between 3 and 40 minutes from substrate addition (Fig 3A), making this readout suitable for cell-based drug assays. To validate this assay, we measured the activity of methylene blue (MB), described to kill all gametocyte stages (Adjalley et al., 2011) on NF54-cg6-ULG8-CBG99 synchronous stage V gametocytes. Dose-response curves on parasites exposed to MB for 24, 48 and 72h were readily obtained with an excellent assay Z′ factor (Zhang et al., 1999) of 0.88, confirming the potency of MB in killing mature gametocytes and showing that this increased with the time of drug exposure (Fig 3B). Importantly, the assay was able to detect MB gametocytocidal activity after only 24h of drug exposure, which is rarely observed in cell-based assays on stage V gametocytes even after treatment with potent gametocytocidal compounds such as epoxomicin (D’Alessandro et al., 2016).
Figure 3. Luciferase reporter activity in NF54-cg6-ULG8-CBG99 stage V gametocytes.
A: Kinetics of luciferase activity of the CBG99 luciferase reporter produced in stage V gametocytes of the NF54-cg6-ULG8-CBG99 line. B: Dose-response curves and IC50 values of methylene blue (MB) in 24, 48 and 72h treatments of stage V gametocytes of the NF54-cg6-ULG8-CBG99 line.
Alteration of stage V gametocyte redox metabolism
The ability of MB to readily kill stage V gametocytes is intriguing as its mechanism of action in asexual parasites was proposed to act as an inhibitor of P. falciparum glutathione reductase (GR; Färber et al., 1998) and as a redox cycling substrate of this enzyme, which uses NADPH to catalyze MB reduction (Buchholz et al., 2008). Although redox activity in gametocytes has been described and exploited in a colorimetric cell-based assay (Tanaka et al., 2011), the low signal to background ratio usually recorded in these assays (e.g. 3, Tanaka et al., 2013) suggests a low level of this metabolic activity in gametocytes.
To gain insights in these processes in mature gametocytes, we tested a panel of inhibitors of several enzymes active in redox metabolism, either alone or in combination with MB for activity against stage V gametocytes. These included uncompetitive inhibitors of both GRs from the parasitized RBCs (M5 in Salmon-Chemin et al., 2001; Davioud-Charvet et al., 2001; Biot et al., 2004; and 3f in Müller et al., 2011), antimalarial NADPH consuming redox cyclers (1c, 3c in Müller et al., 2011 and 17e in Cesar-Rodo et al., 2016), the glutathione depletor paracetamol (Forrest et al., 1982), and one specific inhibitor (ML304) of the bi-functional parasite enzyme glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase (PfGluPho) (Maloney et al., 2012) (Table 1). Dose-response experiments were performed on synchronized late sexual stages of NF54-cg6-ULG8-CBG99 with the compounds tested individually or in the presence of MB, with an incubation of 24h to identify fast-acting compounds. Results showed that none of the compounds used alone was active as it failed to produce a dose-response curve or showed an IC50 higher than our arbitrarily threshold of 20 μM. In contrast, when tested in combination with MB, the PfGluPho inhibitor ML304 was able to dramatically potentiate MB activity (Table 1). Four independent experiments showed that the fold decrease in MB IC50 achieved by 1 μM ML304 was 13.7±6.2.
Table 1.
Change in MB IC50 in 24h treatments of stage V gametocytes in co-incubation with redox perturbing compounds.
| Compound code | Description and role on redox | IC50 MB alone*/IC50 MB + compound (1 μM) | IC50 of compound alone |
|---|---|---|---|
| Benzylmenadione 1c (Müller et al., 2011) | Plasmodione (redox cycler and ROS inducer) | 0.95 | >20μM |
| Benzoylmenadione 3c (Müller et al., 2011) | Plasmodione metabolite I (redox cycler or GR substrate) | 0.95 | >20μM |
| Benzylmenadione 17e (Cesar Rodo et al., 2016) | 6-Fluoro- plasmodione (analogue 17e of plasmodione) | 0.74 | >20μM |
| M5 (Salmon-Chemin et al., 2001; Davioud-Charvet et al., 2001; Biot et al., 2004) | Menadione derivative (GR inhibitor) | 0.60 | >20μM |
| Benzylmenadione 3f (Müller et al., 2011) | Menadione derivative (GR inhibitor) | 1.03 | >20μM |
| paracetamol (Forrest et al., 1982) | Acetaminophen (glutathione depletor) | 0.41 | >20μM |
| ML304 (Maloney et al., 2012) | (R)-N-((1-ethylpyrrolidin-2-yl)methyl)-4-methyl-11-oxo-10,11-Dihydrodibenzo[b,f][1,4]thiazepine-8-carboxamide (PfGluPho inhibitor) | 17.88 | >20μM |
The reference IC50 for a 24h treatment with MB alone is 4.65 μM.
No decrease in the MB IC50 was instead observed if stage V gametocytes were incubated for 24h with 20 μM ML304 and the compound was washed out before an additional 24h treatment with MB alone (data not shown). This indicates that ML304 potentiation of MB activity requires simultaneous presence of both compounds. Further studies showed that ML304, inactive in a 24h treatment (IC50 = 56 μM), had noticeable inhibitory activity after longer incubation times (Fig. S6).
Synergy of MB and the PfGluPho inhibitor ML304 reveals a key role for NADPH production in P. falciparum mature gametocyte metabolism
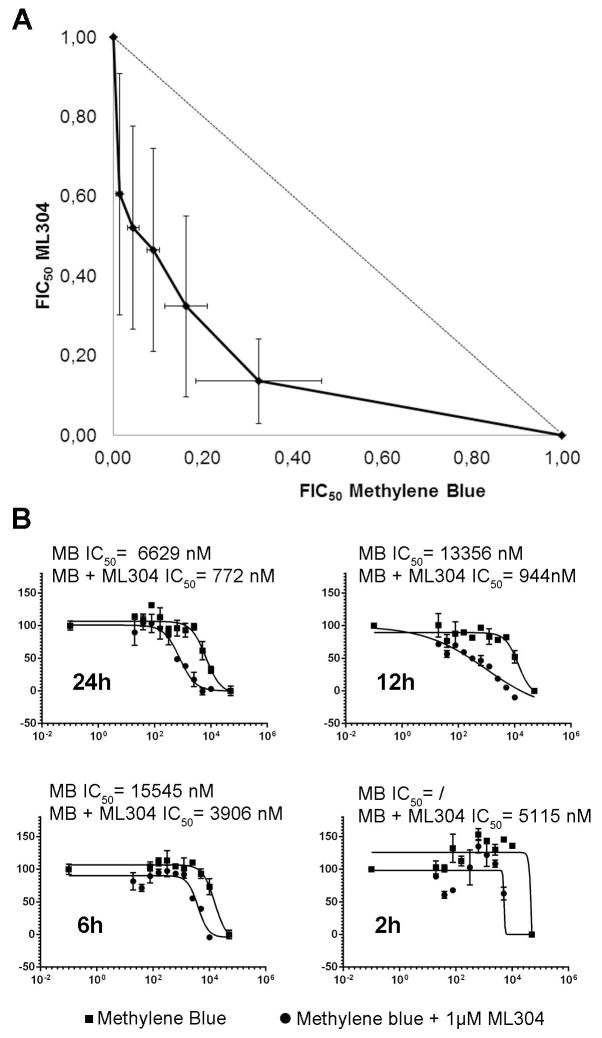
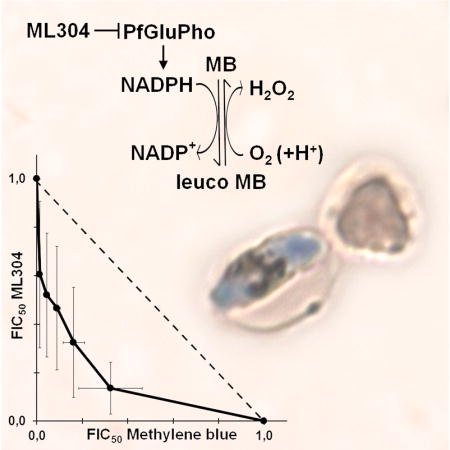
The ability of ML304 to potentiate MB activity against the late sexual stages was further investigated in an isobologram analysis on stage V gametocytes. The IC50 values of both compounds, determined in a 48h treatment, were used to design the protocol for the isobologram analysis (Moura et al., 2009). The result of three independent determinations showed a remarkable synergy of MB and ML304 against mature gametocytes (Fig. 4A). The IC50 values of MB in the presence or absence of 1 μM ML304 were measured after 2, 6, 12, and 24h of incubation on stage V gametocytes. Results showed that ML304 was able to potentiate MB activity even after a 2h treatment and lowered the IC50 value of MB within a nM range after only 12h of co-incubation (Fig. 4B).
Figure 4. Synergy of MB and ML304 in the inhibition of stage V gametocyte viability.
A: Isobologram analysis of the interaction of methylene blue (MB) and ML304 on stage V gametocytes. Determination of the values of the fractional IC50 of MB and ML304 is detailed in the Experimental Procedures. Error bars are SD from three independent biological replicates. B: Potentiation of MB activity against stage V gametocytes by ML304. Dose-response curves and IC50 values of MB in the absence or presence of 1μM ML304 were determined at decreasing time of drug treatments on NF54-cg6-ULG8-CBG99 stage V gametocytes. x axes: Log10 of inhibitor concentration (nM); y axes: percentage viability.
These results evoke a consideration of the target of ML304. This compound is a specific inhibitor of the parasite bifunctional enzyme PfGluPho, which catalyzes the first two steps of the pentose phosphate pathway (PPP) that produces >80% of the NADPH in infected erythrocytes, as determined in P. falciparum asexual stages (Atamna et al., 1994). PfGluPho is structurally different from the two separate isofunctional human host enzymes and has recently been validated as an antimalarial drug target (Allen et al., 2015). As the redox cycling activity of MB leads to a depletion of intracellular NADPH levels, the most likely explanation for the observed synergy of MB and ML304 is that the consumption of the parasite reducing power caused by MB is augmented by the concomitant inhibition of PfGluPho-dependent NADPH production by ML304 (Fig. 5).
Figure 5. Synergy of MB and the PfGluPho inhibitor ML304 in decreasing NADPH levels.
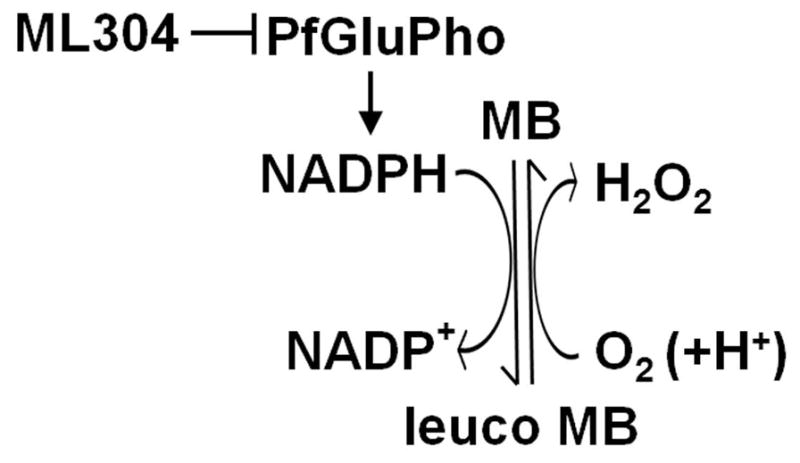
The diagram proposes how the reduction of activity of the parasite enzyme PfGluPho inhibited by ML304 cooperates with the cycles of reduction of MB via the glutathione reductase and spontaneous oxidation in reducing NADPH levels (modified from Buchholz et al., 2008).
In asexual blood stages NADPH depletion is detrimental, as this molecule is used by the parasite to counteract the oxidative stress caused by hemoglobin digestion and by GR to reduce glutathione disulfide (GSSG) to GSH, which participates in multiple cellular detoxification pathways (Jortzik et al., 2012). In this respect, the potent activity of MB and the synergy with ML304 against stage V gametocytes is surprising as hemoglobin digestion, active in all the immature sexual stages, is reported to cease at this mature stage (Hanssen et al., 2012). This suggests that antioxidant defense depending on reducing equivalents provided by NADPH plays a previously unsuspected crucial role in transmissible gametocytes. Since these parasite stages are metabolically less active than asexual parasites or immature gametocytes a particular susceptibility to oxidative challenge was not anticipated. It will be of great interest to study this phenomenon in more detail by systematically following redox parameters and NADPH levels under various conditions.
Our work supports a re-evaluation of MB as a valuable dual-active drug able to inhibit both asexual parasite growth and mature gametocyte viability in order to meet the urgent need for novel interventions to block parasite transmission. The focus on MB, a clinically licensed drug with nanomolar antimalarial activity (Schirmer et al., 2011), is motivated by several considerations. MB has potent low nanomolar activity against asexual stage parasites (Vennerstrom et al., 1995) and gametocytes in vitro (Adjalley et al., 2011; Kasozi et al., 2011), reduces gametocyte carriage rates in vivo (Coulibaly et al., 2009), is relatively safe in clinical trials that included G6PD-deficient children (Müller et al., 2013; Meissner et al., 2005; Mandi et al., 2005) and, importantly, has failed so far to select in vitro for resistant parasites (D. Fidock, personal communication). MB dual activity against asexual and sexual blood stages therefore makes it an excellent candidate as an effective and timely drug that meets the needs of the present malaria elimination/eradication goals and, in the short term, could help address the threat of ACT resistance while new generation anti-transmission drugs are validated and licensed.
This work shows that combining two pro-oxidant compounds such as MB and ML304 effectively inhibits stage V gametocytes within a few hours. Synergy between antimalarial drugs is rarely observed (Co et al., 2009), and this is, to our knowledge, the first to be reported for gametocytocidal compounds. The evidence that potentiation of the MB redox cycler activity significantly increases the inhibition of mature gametocyte metabolism opens the horizon of obtaining novel redox cycler drugs with improved activity, safety and selectivity. This can be achieved by exploring selected compound libraries, by rationally designing ad hoc modifications of the MB chemical scaffolds or by hybridizing the MB phenothiazinium pharmacophore with that of yet to be identified synergistic compounds specific to mature gametocytes.
Experimental Procedures
Inhibitors
Methylene blue trihydrate and paracetamol were purchased from Sigma. The carboxylic acids GR inhibitors M5 (Salmon-Chemin et al., 2001; Davioud-Charvet et al., 2001; Biot et al., 2004) and benzoylmenadione 3f (Müller et al., 2011), plasmodione 1c (Müller et al., 2011), 6-fluoro-plasmodione 17e (Cesar Rodo et al., 2016), and benzoylmenadione 3c (the putative metabolite I of plasmodione) (Müller et al., 2011) were freshly prepared as cited. Anthony Pinkerton, Sanford-Burnham Center for Chemical Genomics at Sanford-Burnham Medical Research Institute, La Jolla, CA kindly provided the compound ML304 (Maloney et al., 2012). Stock solutions of all the compounds were prepared in dimethyl sulfoxide (DMSO) and stored in aliquots at −20°C.
Plasmid construction
The multistep cloning strategies to obtain the pULG-GFP vectors, carrying a Green Fluorescent Protein (GFP) reporter flanked by the Upregulated in Late Gametocytes (ULG) regulatory regions (Figure S1) and plasmid PCR2.1-attP-ULG8-CBG99 (Figure S2) are described in the Supplementary Data section.
Parasite culture and transfection
The P. falciparum 3D7A (Walliker et al., 1987) and NF54attB (Nkrumah et al., 2006; Adjalley et al., 2011) lines were cultured in human 0+ erythrocytes, kindly provided by Prof. G. Girelli, ‘Sapienza’ University of Rome, at 5% hematocrit under 5% CO2, 2% O2, 93% N2 (Trager et al., 1976). Cultures were grown in RPMI 1640 medium (Gibco) supplemented with 25 mM Hepes, 50 μg/ml hypoxanthine, 0.25 mM NaHCO3, 50 μg/ml gentamicin sulfate and 10% pooled heat-inactivated O+ human serum. Ring stage parasites (3–5% parasitemia) were transfected via electroporation with 80–100 μg of plasmid DNA using a BioRad electroporator with 0.31 kV voltage, 960 μF capacitance and resistance set to infinity (Fidock et al., 1997).
To obtain the transgenic lines expressing GFP under the ULG, pfs28 and mal8p1.16 regulatory sequences from episomal plasmids, P. falciparum 3D7A was transfected with the appropriate plasmids as described above and selected by adding 2.5 nM WR99210 after an initial growth of 24h in drug-free medium.
The P. falciparum parasite line NF54attB, containing a Bbx1 attB site in the cg6 gene (Nkrumah et al., 2006) was used to produce the transgenic line NF54-cg6-ULG8-CBG99. The pCR2.1-attP-ULG8-CBG99 plasmid (Figure S2) was co-transfected with the pINT plasmid (Adjalley et al., 2011) expressing the integrase gene required for stable integration of the CBG99 luciferase-expressing plasmid (Figure S2). Double selection started 24h after transfection by adding 250 μg/ml G418 and 2.5 nM WR99210. After 6 days parasites were treated only with 2.5 nM WR99210 and 3 days later they were allowed to recover in drug-free medium.
Quantification and time course of GFP expression in gametocytes development
Parasites from the ten P. falciparum 3D7 transgenic lines expressing GFP under the ULG, pfs28 and mal8p1.16 regulatory sequences from episomal plasmids were induced to produce gametocytes by parasite overgrowth. After 48h N-acetyl-glucosamine (NAG) treatment to eliminate residual asexual parasites, stage I/II gametocytes were partially purified from uninfected erythrocytes via 60% Percoll gradient centrifugation (Kariuki et al., 1998) and incubated in complete medium. Aliquots of 105 purified stage II/III and stage V gametocytes were collected, centrifuged, resuspended in 200μl of 1× PBS and GFP expression was evaluated by flow cytometric analyses on a FACSCalibur™ instrument (BD Biosciences) using the CellQuest (BD) and FlowJo (Tree Star) software.
In the time course analysis, parasites from the 3D7/pfULG8-GFP and 3D7/pfs28-GFP lines were induced to gametocyte production as above and aliquots of 105 purified gametocytes were collected and analyzed daily for 11 days (from stage II/III to stage V) by flow cytometric analyses on a FACSCalibur™ instrument (BD Biosciences) using the CellQuest (BD) and FlowJo (Tree Star) software.
Quantification of GFP expression and of luciferase activity in stage V gametocytes and in gametes
Gametocytes of the 3D7/pfULG8-GFP and 3D7/pfs28-GFP lines were produced and purified as above. 105 stage V gametocytes were activated to form gametes by adding 20μM Xanthurenic Acid (XA) at room temperature; after 6h GFP expression was quantified by flow cytometry from equal number of stage V gametocytes and gametes.
Gametocytes and gametes at 6h post induction from the NF54-cg6-ULG8-CBG99 were obtained as above and aliquots of 105 parasites were collected and frozen. Cell pellets were resuspended in 100μl 1× PBS with Complete™ protease inhibitors (Roche); 100μl of BriteLite Plus™ substrate (Perkin Elmer) was added and luciferase activity was measured for 30 seconds on a Lumat LB 9501 Tube Luminometer.
Western blot analysis
Stage V gametocytes from 3D7 wild-type, 3D7/pfULG8-GFP and 3D7/pfs28-GFP lines were purified on a Percoll gradient; half of them were activated to transform into gametes and collected 6h after induction. Protein extracts from 106 stage V gametocytes and gametes were obtained by lysis in 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1 mM PMSF and Complete protease inhibitor Cocktail (Roche) and separated by 4–12% gradient Bis-Tris gel electrophoresis (Novex). Proteins were electroblotted onto a nitrocellulose filter (Protran, 0.2 μm) and membranes were incubated for 1h with the rabbit anti-Pfs25 antibody (MRA-38, from MR4) diluted 1:1000. Horseradish peroxidase-conjugated (HRP) secondary antibodies against rabbit IgGs were diluted 1:30,000 and chemioluminescent reactions were revealed with the Super Signal West Substrate kit (Pierce). The same membrane was saturated with H2O2 for 30 minutes and incubated for 1h with the rat anti-Pfg27 antibody (Olivieri et al., 2009) diluted 1:1,000. Horseradish peroxidase-conjugated (HRP) secondary antibodies against rat IgGs were diluted 1:30,000 and chemioluminescent reactions were revealed as above.
Immunofluorescence analysis
3D7/pfULG8-GFP stage V gametocytes were fixed on a glass smear with paraformaldehyde 4% – glutaraldehyde 0.075% for 10 minutes and permeabilized with 0.1% Triton X-100 (Tonkin et al., 2004). After 30 min preincubation in 1% BSA, parasites were incubated for 1h with an anti-377B rabbit antiserum (1:800) (Alano et al., 1995). After incubation and washes in 1×PBS, parasites were incubated with affinity purified, rhodamine-conjugated secondary antibody against rabbit IgGs (1:800) and 1μg/ml Hoechst 33258. After final washes, samples were observed in a Leica DFC340 FX camera through a Leica PL FLUOTAR 100× objective in a microscope equipped with filters BP 340–380 (Hoechst), BP 515–560 (rhodamine) and BP 470–490 (fluorescein).
Southern blot analysis
Digestion, blotting and hybridization of parasite genomic DNA with the cg6 and GFP specific probes are described in the Supplementary Information. To compare the gene copy number of the GFP reporter in the 3D7/pfs28 and in the 3D7/pULG8-GFP parasite lines we performed Southern blot analysis using pfcg6- and a gfp- radiolabeled probes (Fig. 1). Genomic DNA was isolated from the parental 3D7 and the recombinant 3D7/pfs28 and 3D7/pULG8-GFP strains, digested with SpeI and PstI, electrophoresed on a 0.8% agarose gel, and transferred onto a Nytran nylon membrane. Hybridization of the membrane was performed at 54°C with a 150 bp 32P-labeled gfp probe that was PCR amplified from the pfs28-GFP plasmid DNA using primers #43 and #44 (Table S1). After autoradiography, the membrane was stripped into boiling 0.1% SDS and hybridized at 54°C with a 639 bp 32P -labeled elo1 probe that was PCR amplified from 3D7 genomic DNA using primers #45 and #46 (Table S1).
Time course of CBG99 luciferase activity in gametocyte development
A NF54-cg6-ULG8-CBG99 culture was induced to gametocyte production via parasite overgrowth. After 48h N-acetyl-glucosamine (NAG) treatment to eliminate residual asexual parasites, stage I/II gametocytes were partially purified from uninfected erythrocytes via 60% Percoll gradient centrifugation (Kariuki et al., 1998) and incubated in complete medium. Aliquots of 105 purified gametocytes were collected and frozen daily for 11 days. Bioluminescence of the gametocyte samples was determined as described above.
Luciferase assays on stage V gametocytes
NF54-cg6-ULG8-CBG99 cultures induced to produce gametocytes were treated with NAG for 96h, after which gametocytes were purified from uninfected erythrocytes on MACS Separation Columns CS (Miltenyi Biotec) and allowed to mature to stage V over the following 8 days. To calculate IC50 values, compounds (Table 1) were serially diluted across ten twofold dilutions and dispensed in 96-well plates in a final volume of 100μl/well. Synchronous 8×104 stage V gametocytes were resuspended in 100μl of complete medium and incubated with the compounds at 37°C for the time indicated. To calculate the IC50 of MB in the presence of a fixed dose of different compounds, MB was dispensed in 96-well plates as described above. Gametocytes were dispensed as described above with 1 μM of the different compounds and incubated with MB at 37°C. Cell viability was evaluated by adding a non lysing formulation of 0.5 mM D-Luciferin substrate (Cevenini et al., 2014) and measuring luciferase activity for 1 second on a Varioskan™ Flash Multimode Reader (Thermo Scientific). The percent viability was calculated as a function of drug concentration. Curve fitting was obtained by non-linear regression analysis (GraphPad Prism 6.0).
Isobologram analysis
MB and ML304 interactions were assessed over a range of concentrations by a fixed-ratio method based on their IC50 values (Moura et al., 2009). For each compound the IC50 value was determined after a 48h treatment on mature gametocytes and stock solutions were prepared at 16 times the IC50 of each compound. Solutions were combined at MB:ML304 ratios of 10:0, 9:1, 7:3, 5:5, 3:7, 1:9 and 0:10. These starting mixes were serially diluted across ten two–fold dilutions. Cell viability was evaluated by measuring luciferase activity of each sample as described in the Methods section. The percentage of viability was calculated as a function of drug concentration and curve fitting was obtained by non-linear regression analysis (GraphPad Prism 6.0). Fractional IC50 values were calculated on the basis of the IC50 values obtained per assay for each compounds as described (Moura et al., 2009).
Supplementary Material
Acknowledgments
This work was supported by the Bill & Melinda Gates Foundation grants OPP1040398 and OPP1040394 to D.A.F. and P.A. respectively, the US National Institutes of Health (R01 AI103058), the Laboratoire d’Excellence ParaFrap (grant LabEx ParaFrap ANR-11-LABX-0024 to E.D.-C., and the German Research Foundation, DFG SPP 1710 (BE 1540/23-1), to K.B.
The authors acknowledge Dr. G. Girelli, Blood Transfusion Centre ‘Sapienza’ Unversity of Rome, for the gift of human erythrocytes; Dr. C. Scagnolari, ‘Sapienza’ University of Rome and Dr. M. Sgarbanti, Istituto Superiore di Sanità, Rome for the use of the luminometers; Dr. A. Pinkerton, Sanford-Burnham Center for Chemical Genomics at Sanford-Burnham Medical Research Institute, La Jolla, CA for kindly providing compound ML304 and BEI Resources/MR4 for providing the anti-Pfs25 antibody (MR-38).
Footnotes
Author contributions: GS, ED-C, KB, AC, DAF, PA conceived and designed the study; GS TRSK, RB, GC, MMC, LC, ED-C, KB, AC, DAF, PA acquired and/or analyzed the data; GS, ED-C, KB, DAF, PA wrote the manuscript. All authors approved the final manuscript.
Competing interests: The authors declare no competing financial interests.
References
- Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci U S A. 2011;108:E1214–1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano P. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol Microbiol. 2007;66:291–302. doi: 10.1111/j.1365-2958.2007.05904.x. [DOI] [PubMed] [Google Scholar]
- Alano P, Read D, Bruce M, Aikawa M, Kaido T, Tegoshi T, Bhatti S, Smith DK, Luo C, Hansra S, Carter R, Elliott JF. COS cell expression cloning of Pfg377, a Plasmodium falciparum gametocyte antigen associated with osmiophilic bodies. Mol Biochem Parasitol. 1995;74:143–56. doi: 10.1016/0166-6851(95)02491-3. [DOI] [PubMed] [Google Scholar]
- Allen SM, Lim EE, Jortzik E, Preuss J, Chua HH, MacRae JI, Rahlfs S, Haeussler K, Downton MT, McConville MJ, Becker K, Ralph SA. Plasmodium falciparum glucose-6-phosphate dehydrogenase 6-phosphogluconolactonase is a potential drug target. FEBS J. 2015;282:3808–23. doi: 10.1111/febs.13380. [DOI] [PubMed] [Google Scholar]
- Atamna H, Pascarmona G, Ginsburg H. Hexose-monophosphate shunt activity in intact Plasmodium falciparum-infected erythrocytes and in free parasites. Mol Biochem Parasitol 1994. 1994;67:79–89. doi: 10.1016/0166-6851(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Biot C, Bauer H, Schirmer RH, Davioud-Charvet E. 5-Substituted tetrazoles as bioisosters of carboxylic acids. Bioisosterism and mechanistic studies on glutathione reductase inhibitors as antimalarials. J Med Chem. 2004;47:5972–83. doi: 10.1021/jm0497545. [DOI] [PubMed] [Google Scholar]
- Birkholtz LM, Coetzer TL, Mancama D, Leroy D, Alano P. Discovering new transmission-blocking antimalarial compounds: challenges and opportunities. Trends Parasitol. 2016;32:669–81. doi: 10.1016/j.pt.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Buchholz K, Schirmer RH, Eubel JK, Akoachere MB, Dandekar T, Becker K, Gromer S. Interactions of methylene blue with human disulfide reductases and their orthologues from Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52:183–91. doi: 10.1128/AAC.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth AS, Skinner-Adams TS, Gardiner DL, Trenholme KR. Plasmodium falciparum gametocytes: with a view to a kill. Parasitology. 2013;140:1718–34. doi: 10.1017/S0031182013001236. [DOI] [PubMed] [Google Scholar]
- Cesar Rodo E, Feng L, Jida M, et al. A Platform of regioselective methodologies to access polysubstituted 2-methyl-1,4-naphthoquinone derivatives: scope and limitations. Eur J Org Chem. 2016;11:1982–93. [Google Scholar]
- Cevenini L, Camarda G, Michelini E, Siciliano G, Calabretta MM, Bona R, et al. Multicolor bioluminescence boosts malaria research: quantitative dual-color assay and single-cell imaging in Plasmodium falciparum parasites. Anal Chem. 2014;86:8814–21. doi: 10.1021/ac502098w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, Poirot E, Newman M, Kandula D, Shah R, Hwang J, et al. An assessment of the supply, programmatic use, and regulatory issues of single low-dose primaquine as a Plasmodium falciparum gametocytocide for sub-Saharan Africa. Malar J. 2015;14:204. doi: 10.1186/s12936-015-0714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co EM, Dennull RA, Reinbold DD, Waters NC, Johnson JD. Assessment of malaria in vitro drug combination screening and mixed-strain infections using the malaria Sybr green I-based fluorescence assay. Antimicrob Agents Chemother. 2009;53:2557–63. doi: 10.1128/AAC.01370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly B, Zoungrana A, Mockenhaupt FP, Schirmer RH, Klose C, Mansmann U, et al. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomised controlled trial. PLoS One. 2009;4:e5318. doi: 10.1371/journal.pone.0005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessandro S, Camarda G, Corbett Y, Siciliano G, Parapini S, Cevenini L, et al. A chemical susceptibility profile of the Plasmodium falciparum transmission stages by complementary cell-based gametocyte assays. J Antimicrob Chemother. 2016;71:1148–58. doi: 10.1093/jac/dkv493. [DOI] [PubMed] [Google Scholar]
- Davioud-Charvet E, Delarue S, Biot C, Schwöbel B, Böhme CC, Müssigbrodt A, et al. A prodrug form of a Plasmodium falciparum glutathione reductase inhibitor conjugated with a 4-anilinoquinoline. J Med Chem. 2001;44:4268–76. doi: 10.1021/jm010268g. [DOI] [PubMed] [Google Scholar]
- Eksi S, Suri A, Williamson KC. Sex- and stage-specific reporter gene expression in Plasmodium falciparum. Mol Biochem Parasitol. 2008;160:148–51. doi: 10.1016/j.molbiopara.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Färber PM, Arscott LD, Williams CH, Jr, Becker K, Schirmer RH. Recombinant Plasmodium falciparum glutathione reductase is inhibited by the antimalarial dye methylene blue. FEBS Lett. 1998;422:311–4. doi: 10.1016/s0014-5793(98)00031-3. [DOI] [PubMed] [Google Scholar]
- Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci USA. 1997;94:10931–6. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest JA, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet. 1982;7:93–107. doi: 10.2165/00003088-198207020-00001. [DOI] [PubMed] [Google Scholar]
- Hanssen E, Knoechel C, Dearnley M, Dixon MW, Le Gros M, Larabell C, Tilley L. Soft X-ray microscopy analysis of cell volume and hemoglobin content in erythrocytes infected with asexual and sexual stages of Plasmodium falciparum. J Struct Biol. 2012;177:224–32. doi: 10.1016/j.jsb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawking F, Wilson ME, Gammage K. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1971;65:549–559. doi: 10.1016/0035-9203(71)90036-8. [DOI] [PubMed] [Google Scholar]
- Joice R, Nilsson SK, Montgomery J, Dankwa S, Egan E, Morahan B, et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med. 2014;6:244. doi: 10.1126/scitranslmed.3008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jortzik E, Becker K. Thioredoxin and glutathione systems in Plasmodium falciparum. Int J Med Microbiol. 2012;302:187–94. doi: 10.1016/j.ijmm.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Kariuki MM, Kiaira JK, Mulaa FK, Mwangi JK, Wasunna MK, Martin SK. Plasmodium falciparum: purification of the various gametocyte developmental stages from in vitro-cultivated parasites. Am J Trop Med Hyg. 1998;59:505–8. doi: 10.4269/ajtmh.1998.59.505. [DOI] [PubMed] [Google Scholar]
- Kasozi DM, Gromer S, Adler H, Zocher K, Rahlfs S, Wittlin S, et al. The bacterial redox signaller pyocyanin as an antiplasmodial agent: comparisons with its thioanalog methylene blue. Redox Rep. 2011;16:154–65. doi: 10.1179/174329211X13049558293678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasonder E, Rijpma SR, van Schaijk BC, Hoeijmakers WA, Kensche PR, Gresnigt MS, et al. Integrated transcriptomic and proteomic analyses of Plasmodium falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. 2016;44:6087–101. doi: 10.1093/nar/gkw536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–8. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- Lucantoni L, Silvestrini F, Signore M, Siciliano G, Eldering M, Dechering KJ, et al. A simple and predictive phenotypic High Content Imaging assay for Plasmodium falciparum mature gametocytes to identify malaria transmission blocking compounds. Sci Rep. 2015;5:16414. doi: 10.1038/srep16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, et al. Regulation of sexual 28 development of Plasmodium by translational repression. Science. 2006;313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair GR, Lasonder E, Garver LS, Franke-Fayard BM, Carret CK, Wiegant JC, et al. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 2010;6:e1000767. doi: 10.1371/journal.ppat.1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P, Hedrick M, Peddibhotla S, Hershberger P, Milewski M, Gosalia P, et al. Probe reports from the NIH Molecular Libraries Program [Internet] Bethesda (MD): National Center for Biotechnology Information (US); 2012. A 2nd selective inhibitor of Plasmodium falciparum glucose-6-phosphate dehydrogenase (PfG6PDH) - probe 2. [PubMed] [Google Scholar]
- Mandi G, Witte S, Meissner P, Coulibaly B, Mansmann U, Rengelshausen J, et al. Safety of the combination of chloroquine and methylene blue in healthy adult men with G6PD deficiency from rural Burkina Faso. Trop Med Int Health. 2005;10:32–8. doi: 10.1111/j.1365-3156.2004.01356.x. [DOI] [PubMed] [Google Scholar]
- Meissner PE, Mandi G, Witte S, Coulibaly B, Mansmann U, Rengelshausen J, et al. Safety of the methylene blue plus chloroquine combination in the treatment of uncomplicated falciparum malaria in young children of Burkina Faso. Malar J. 2005;4:45. doi: 10.1186/1475-2875-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Fan Q, Parker D, Li X, Li J, Cui L. Puf mediates translation repression of transmission-blocking vaccine candidates in malaria parasites. PLoS Pathog. 2013;9:e1003268. doi: 10.1371/journal.ppat.1003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Blanco C, Lelièvre J, Delves MJ, Bardera AI, Presa JL, López-Barragán MJ, et al. Imaging-based high-throughput screening assay to identify new molecules with transmission-blocking potential against Plasmodium falciparum female gamete formation. Antimicrob Agents Chemother. 2015;59:3298–305. doi: 10.1128/AAC.04684-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura PA, Dame JB, Fidock DA. Role of Plasmodium falciparum digestive vacuole plasmepsins in the specificity and antimalarial mode of action of cysteine and aspartic protease inhibitors. Antimicrob Agents Chemother. 2009;53:4968–78. doi: 10.1128/AAC.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Johann L, Jannack B, Brückner M, Lanfranchi DA, Bauer H, et al. Glutathione reductase-catalyzed cascade of redox reactions to bioactivate potent antimalarial 1,4-naphthoquinones--a new strategy to combat malarial parasites. J Am Chem Soc. 2011;133:11557–71. doi: 10.1021/ja201729z. [DOI] [PubMed] [Google Scholar]
- Müller O, Mockenhaupt FP, Marks B, Meissner P, Coulibaly B, Kuhnert R, et al. Haemolysis risk in methylene blue treatment of G6PD-sufficient and G6PD-deficient West-African children with uncomplicated falciparum malaria: a synopsis of four RCTs. Pharmacoepidemiol Drug Saf. 2013;22:376–85. doi: 10.1002/pds.3370. [DOI] [PubMed] [Google Scholar]
- Nkrumah LJ, Muhle RA, Moura PA, Ghosh P, Hatfull GF, Jacobs WR, Jr, Fidock DA. Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nat Methods. 2006;3:615–21. doi: 10.1038/nmeth904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri A, Camarda G, Bertuccini L, van de Vegte-Bolmer M, Luty AJ, Sauerwein R, Alano P. The Plasmodium falciparum protein Pfg27 is dispensable for gametocyte and gamete production, but contributes to cell integrity during gametocytogenesis. Mol Microbiol. 2009;73:180–93. doi: 10.1111/j.1365-2958.2009.06762.x. [DOI] [PubMed] [Google Scholar]
- Paton MG, Barker GCH, Matsuoka J, Ramesar CJ, Janse AP, Waters A, Sinden RE. Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol Biochem Parasitol. 1993;59:263–276. doi: 10.1016/0166-6851(93)90224-l. [DOI] [PubMed] [Google Scholar]
- Plouffe DM, Wree M, Du AY, Meister S, Li F, Patra K, et al. High-throughput assay and discovery of small molecules that interrupt malaria transmission. Cell Host Microbe. 2016;19:114–26. doi: 10.1016/j.chom.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss J, Maloney P, Peddibhotla S, Hedrick MP, Hershberger P, Gosalia P, et al. Discovery of a Plasmodium falciparum glucose-6-phosphate dehydrogenase 6-phosphogluconolactonase inhibitor (R,Z)-N-((1-ethylpyrrolidin-2-yl)methyl)-2-(2-fluorobenzylidene)-3-oxo-3,4-dihydro-2H-benzo[b][1,4]thiazine-6-carboxamide (ML276) that reduces parasite growth in vitro. J Med Chem. 2012;55:7262–72. doi: 10.1021/jm300833h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PN, Santos JM, Pain A, Templeton TJ, Mair GR. Translational repression of the cpw-wpc gene family in the malaria parasite Plasmodium. Parasitol Int. 2016;65:463–71. doi: 10.1016/j.parint.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, et al. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother. 2014;58:7292–302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon-Chemin L, Buisine E, Yardley V, Kohler S, Debreu MA, Landry V, et al. 2- and 3-substituted-1,4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from Trypanosoma cruzi: synthesis and correlation between redox-cycling activities and in vitro cytotoxicity. J Med Chem. 2001;44:548–65. doi: 10.1021/jm001079l. [DOI] [PubMed] [Google Scholar]
- Schirmer RH, Adler H, Pickhardt M, Mandelkow E. Lest we forget you--methylene blue. Neurobiol Aging. 2011;32:2325.e7–16. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Severini C, Silvestrini F, Sannella A, Barca S, Gradoni L, Alano P. The production of the osmiophilic body protein Pfg377 is associated with stage of maturation and sex in Plasmodium falciparum gametocytes. Mol Biochem Parasitol. 1999;100:247–52. doi: 10.1016/s0166-6851(99)00050-x. [DOI] [PubMed] [Google Scholar]
- Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, et al. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2010;9:1437–48. doi: 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithuis F, Kyaw MK, Phe O, Win T, Aung PP, Oo AP, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis. 2010;10:673–81. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka TQ, Williamson KC. A malaria gametocytocidal assay using oxidoreduction indicator, alamarBlue. Mol Biochem Parasitol. 2011;177:160–3. doi: 10.1016/j.molbiopara.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka TQ, Dehdashti SJ, Nguyen DT, McKew JC, Zheng W, Williamson KC. A quantitative high throughput assay for identifying gametocytocidal compounds. Mol Biochem Parasitol. 2013;188:20–5. doi: 10.1016/j.molbiopara.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibúrcio M, Sauerwein R, Lavazec C, Alano P. Erythrocyte remodeling by Plasmodium falciparum gametocytes in the human host interplay. Trends Parasitol. 2015;31:270–8. doi: 10.1016/j.pt.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Tonkin CJ, van Dooren GG, Spurck TP, Struck NS, Good RT, Handman E, et al. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol Biochem Parasitol. 2004;137:13–21. doi: 10.1016/j.molbiopara.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vennerstrom JL, Makler MT, Angerhofer CK, Williams JA. Antimalarial dyes revisited: xanthenes, azines, oxazines, and thiazines. Antimicrob Agents Chemother. 1995;39:2671–7. doi: 10.1128/aac.39.12.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walliker D, Quakyi IA, Wellems TE, McCutchan TF, Szarfman A, London WT, et al. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987;236:1661–6. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Young JA, Fivelman QL, Blair PL, de la Vega P, Le Roch KG, Zhou Y, et al. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report. 2016 ( http://www.who.int/malaria/publications/world_malaria_report/en/)
- Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.