Abstract
Inhibitor of DNA binding (ID) proteins, including ID1-4, are transcriptional regulators involved in promoting cell proliferation and survival in various cell types. Although upregulation of Id proteins has been associated with a broad spectrum of tumors, recent studies have identified that ID3 plays a tumor suppressor role in the development of Burkitt’s lymphoma in humans and Hepatosplenic T cell lymphomas in mice. Here, we report rapid lymphoma development in Id2/Id3 double knockout (L-DKO) mice caused by unchecked expansion of either invariant Natural Killer T (iNKT) cells, or a unique subset of innate-like, CD1d-independent T cells. These populations started expansion in neonatal mice and, upon malignant transformation, caused fatality at age between 3–11 months. The malignant cells also gave rise to lymphomas upon transfer to Rag-deficient and wild-type hosts, reaffirming their inherent tumorigenic potential. Microarray analysis revealed a significantly modified program in these neonatal iNKT cells that ultimately led to their malignant transformation. The lymphoma cells demonstrated chromosome instability, along with upregulation of several different signaling pathways, including the cytokine-cytokine receptor interaction pathway, which can promote their expansion and migration. Dysregulation of genes with reported driver mutations and the NF-kB pathway were found to be shared between L-DKO lymphomas and human NKT tumors. Our work identifies a distinct premalignant state and multiple tumoriogenic pathways caused by loss function of ID2 and ID3. Thus, conditional deletion of Id2 and Id3 in developing T cells establishes a unique animal model for iNKT and relevant innate-like lymphomas.
Keywords: Thymus, lymphoma, iNKT cells, innate-like T cells, ID proteins, mouse
Introduction
A significant portion of cancer research is dedicated to the identification of underlying factors that contribute to the hallmarks of tumorigenesis[1–3] such as dysregulated proliferation and self-renewal.[4, 5] All four members (ID1-4) of the ID family of helix-loop-helix (HLH) transcription factors share the ability to promote proliferation and a stem cell-like dedifferentiated state,[6, 7] and are often upregulated in various cancer types.[8–12] ID protein activity has also been found to be directly correlated with tumor initiation, progression and sensitivity to therapy.[7] Therefore, they have been deemed as attractive tumor therapeutic targets using small-molecule inhibitors and ID-binding peptides in mouse models and cancer cell lines.[10, 13–16] In contrast, deep sequencing of human Burkitt’s lymphoma samples has revealed loss-of-function mutations in Id3 in a large subset of patients, supporting the tumor suppressor role of ID3 in some contexts.[17–19] Id3−/− mice have also been reported to develop γδ Hepatosplenic T-cell lymphoma (HSTCL) as a consequence of Vγ1.1+Vδ6.3+ γδ T cell population expansion.[20] While there are some reports suggesting a context-dependent role of ID4 in tumor progression or suppression,[21–23] there is only limited evidence in favor of a tumor suppressive role played by ID2.[24]
ID proteins are primarily considered as inhibitors of E proteins, founding members of basic-HLH transcription factors.[25] ID2 and ID3, which are highly expressed in lymphocytes, are known for their critical roles in suppressing E protein activity at various stages to allow conventional αβ T cell development.[26, 27] They have also been recently described to repress innate-like γδ and iNKT cell development.[28–37]
Innate-like T cells are unique populations of T cells that derive their name from an innate cell-like ability to quickly secrete a myriad of cytokines in response to antigen.[38, 39] These populations play key roles in providing protection against tumors and certain infectious and autoimmune diseases, even though they are usually present in negligible proportions compared to conventional T cells.[40, 41] The best characterized innate-like T cells are Vγ1.1Vδ6.3 γδ T cells and iNKT cells, but other cell types like mucosal-associated invariant T (MAIT) cells are also included in this category.[42] iNKT cells express a semi-invariant T cell receptor (TCR), Vα14Jα18, which allows them to be identified by α-GalCer-loaded CD1d tetramers (CD1dTet).[43]
In this paper, we describe a rapid generation of iNKT or innate-like lymphoid tumors upon deletion of Id2 and Id3 in thymocytes. We also delineate the expanding precursor populations and dysregulated pathways that account for the lymphoma development. Given that NKT lymphomas are extremely rare and highly lethal in humans,[44] our study provides a much needed animal model for understanding the genetic basis of similar types of tumors in humans.
Materials and Methods
Mice
Id2f/fId3f/fLckCre (L-DKO) mice were generated as previously described.[34] CD1d−/− mice were purchased from Jackson Laboratory (Strain 008881) and bred with L-DKO mice to generate Id2f/fId3f/fLckCre+CD1d−/− (TKO) mice. All mice were bred in a specific pathogen-free facility of Duke University Division of Laboratory Animal Resources, and all procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee.
Flow cytometry analysis
Staining with surface marker antibodies (Biolegend) was done before intracellular staining for PLZF antibody (eBioscience), using Foxp3 staining buffer set (eBioscience). CD1d tetramers were obtained from the Tetramer Facility of the National Institutes of Health. Flow cytometry analysis was performed on a FACSCanto II (BD Biosciences). Doublets and dead cells (7AAD+) were gated out before data analysis. Data were analyzed with FlowJo software (Tree Star).
Histopathological analysis
Tissue sections were removed immediately after sacrificing the mice, and fixed in 10% phosphate-buffered saline (PBS)-formalin. Embedding, sectioning and staining (hematoxylin and esosin, and Masson’s Trichrome) were done by the Pathology service core at Duke University.
Adoptive transfer of lymphoma cells to Rag2−/− or wild type hosts
Enlarged thymi from L-DKO donor mice were minced in PBS with 5% BCS, filtered, lysed for red blood cells using BD Pharm Lyse lysing buffer (BD Bioscience) and washed with PBS. 5×106 cells per recipient were injected into 6–8 week old Rag2−/− mice through their tail vein. 5–7 week old WT mice were sublethally irradiated with 300 rads, and injected with tumor cells 24 hours after irradiation. Recipient mice were sacrificed 4–10 weeks after transfer, and tissues were collected for FACS analysis and H&E staining.
PCR and Real Time PCR
Genotyping of mice was done as described previously.[34] Total RNA was extracted using an RNAqueous Kit (Life Technology) according to manufacturer’s protocol, and reverse transcribed into cDNA by murine leukemia virus reverse transcriptase (Life Technology). Real time PCR (RT-PCR) was performed by Fast-Start DNA master SYBR green kit and quantitative expression is normalized by β-actin. Forward (F) and reverse (R) primers are listed below, in 5′ to 3′ orientation.
Genotyping Primers:
Id2f/f (F) TGTGCATAATTAATCGCATCA; (R) TTGGGAAGTCACATTTGTAGTG
Id3f/f (F) GCTCTGAGGTCATAAATCCC; (R) CCATTTGGTTCTATGTATGCCCGTG
LckCre (F) GCAGGAAGTGGGTAACTAGACTAAC;
LckCre (R) TCTCCCACCGTCAGTACGTGAGATATC
CD1d WT (F) AGGGCTGTGTAGAACTCTGGCGCTA;
CD1d WT (R) GCAGGGAGCGGAAGGTGTAATT
CD1d KO (F) AGGGCCAGCTCATTCCTCCACT; (R) GCAGGGAGCGGAAGGTGTAATT
RT-PCR primers:
IL-4 (F) ATCATCGGCATTTTGAACGAGGTC; (R) ACCTTGGAAGCCCTACAGACGA
Cell sorting and microarray
Pre-malignant iNKT cells were sorted as TCRβ+CD1dTet+ cells from L-DKO mice at 20 days of age. Lymphoma cells were sorted from tissues of 18–37 week old mice as T cells that are CD1dTet+ or CD1dTet−. Total RNA was extracted as described for RT-PCR. mRNA expression profiling was done by the Duke Microarray Core Facility using GeneChip Mouse Genome 430A 2.0 arrays (Affymetrix). GEO data accession link http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=utczygaczzglfol&acc=GSE83761
Bioinformatics and statistical analysis
Microarray data for pre-malignant and lymphoma cells was normalized using RMA, and differential analysis was done using the limma package available through Bioconductor.[45] Publicly available, normalized Immgen data for WT cells was requested and downloaded from http://rstats.immgen.org/DataRequest/.[46] The two normalized datasets were combined according to the Emperical Bayes method using the web tool ArrayMining (www.arraymining.net).[47]
Data plotting, visualization and statistics
Gene expression (fold change) heatmaps were generated using Gene-E (http://www.broadinstitute.org/cancer/software/GENE-E/). Self-organizing Maps (SOM) were generated by the Partek Genomics Suite made available by the Duke Center for Genome and Computational Biology. Principal Component Analysis (PCA) was performed and plotted using the in-built R functions, prcomp and plot3d, in the open-source RStudio software. Gene overlaps in the form of Venn diagram was drawn using the eulerAPE software.94 Pathway analysis was done using the HOMER software.70 Survival curves and bar graphs were drawn using GraphPad Prism (GraphPad Software). Two-tailed student’s t-test was used for statistical analyses, with p values less than 0.05 considered significant.
Results
Conditional deletion of Id2 and Id3 leads to rapid lymphoma development
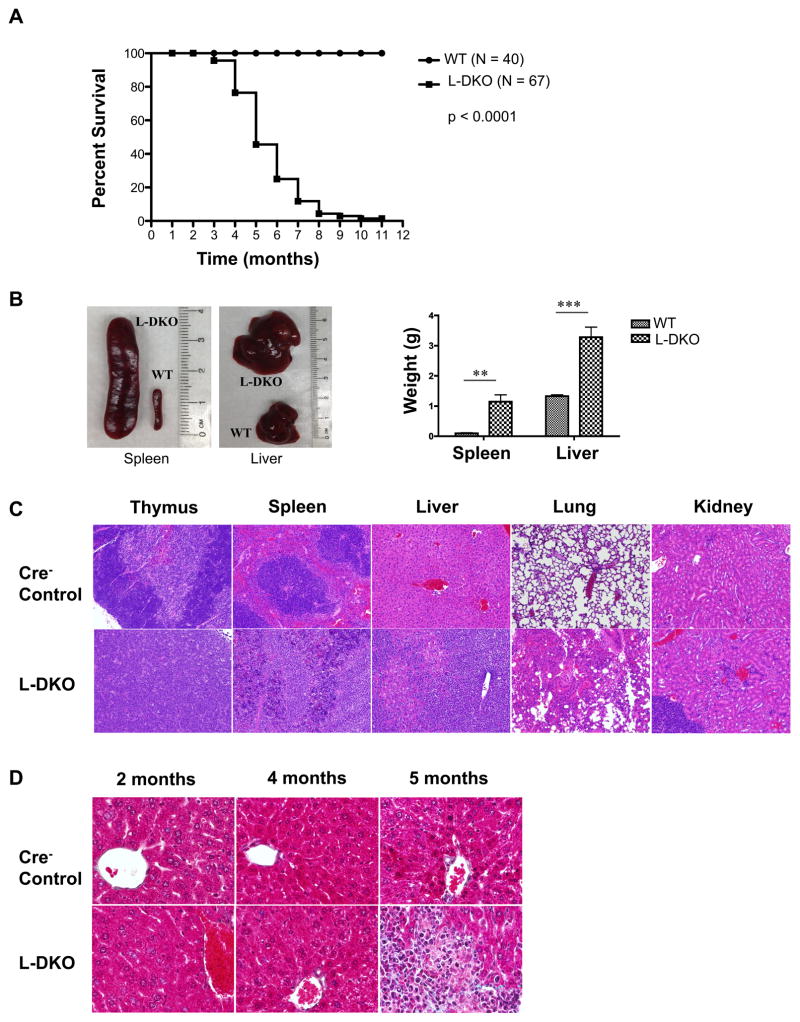
We have previously reported a dramatic expansion of iNKT cells upon Id2/Id3 conditional deletion using LckCre in L-DKO mice.[34] Surprisingly, we found that these mice also rapidly develop tumors and start dying between 3–11 months of age (Figure 1A). L-DKO mice developed lymphoma in several organs, including thymus, lymph nodes, bone marrow, spleen, liver and gut (Table I). Splenomegaly and hepatomegaly was apparent in all mutant mice analyzed in this age window, which was also reflected in the significant increase in the weight of liver and spleen of these mice as compared to control mice (Figure 1B). Histopathological H&E (Hematoxylin and eosin) staining of the thymus, spleen, liver, lung and kidney of L-DKO tumor mice revealed infiltration of lymphoma cells in these organs, and the disruption of normal tissue structures (Figure 1C). Masson’s trichrome staining, which is used to detect collagen and fibrosis in tissues, suggested a mild fibrosis in the lymphoma-infiltrated area of livers of 5 months or older L-DKO mice (Figure 1D). At this stage, the normal liver parenchyma was found to be replaced by malignant lymphocytes, which could also lead to liver dysfunction and death. Thus, we observed that conditional deletion of Id2 and Id3 led to lymphocyte infiltration and rapid development of tumors in various peripheral organs, which ultimately caused their death.
Figure 1. Deficiency of both ID2 and ID3 in developing T cells leads to lymphomagenesis in mice.
(A) Survival curve for Id2f/fId3f/fLCKCre+ (L-DKO) and control (LckCre−) mice. p value based on the Mantel-Cox test (B) Comparison of size and weight (N = 4 for WT, N = 5 for L-DKO) of spleen and liver from L-DKO and wild type control mice (C) Representative H&E staining for thymus, spleen, liver, lung and kidney from L-DKO mice and controls (100X). (D) Representative Masson’s trichrome staining for livers from L-DKO mice and controls at 2, 4 and 5 months of age (400X). N = 3 for (C) and (D).
Table I. Phenotypic characteristics of tumors in L-DKO mice.
Sex, age, organs with lymphoma, and surface markers on tumors in L-DKO mice
| Original tumor | T cell markers | Other markers for TCRβ+ cells | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| # | sex | age(w) | Involvement | TCRβ | CD4 | CD8 | CD1dtet | CD44 | CD25 | CD24 | CD69 |
| LII10^ | F | 19 | T S L G | + | 34% | − | − | ND | ND | ND | ND |
| LII30 | M | 27 | T S L | + | 78% | − | + | ND | ND | ND | ND |
| LIII10 | M | 18 | T S L G | + | 43% | − | − | int | − | − | ND |
| L60 | M | 26 | T S L | + | 79% | 9% | + | int | − | int | ND |
| LIII22 | F | 27 | T S L LN BM | + | 41% | 46% | − | int | − | − | + |
| LII65 | M | 25 | T S L BM | 10% | 10% | − | + | int | − | − | ND |
| LII66 | M | 25 | T S L | 5% | − | − | + | int | − | − | ND |
| LIII36 | F | 30 | T S L LN BM | + | 31% | 42% | − | int | − | − | ND |
| LIII46 | F | 27 | T S L LN BM | + | − | 78% | + | int | − | − | ND |
| LVI 23 | M | 24 | T S L G LN BM | + | − | − | + | int | − | − | ND |
| LV9 | M | 20 | T S L G | + | 49% | − | − | int | − | − | ND |
| LVI 7* | F | 33 | T S L G LN BM | + | − | 58% | − | int | − | − | ND |
| LV27^ | M | 25 | T S L LN BM | + | − | − | − | int | − | − | + |
| LV40 | M | 29 | T S L G LN BM | + | 90% | − | − | int | − | − | + |
| LV69* | F | 26 | T S L G LN BM | + | 43% | − | − | int | − | − | + |
| LIV9 | M | 29 | T S L LN BM | + | 78% | − | − | int | − | − | + |
| LIV11 | M | 29 | T S L G BM | + | 84% | − | − | int | − | − | + |
| LV36 | F | 34 | T S L G LN BM | + | 92% | − | int | − | − | + | |
| LIV34Δ | M | 20 | T S L G LN BM | + | 82% | − | + | int | − | int | + |
| LIV56 | F | 18 | T S L G LN BM | + | 46% | − | + | int | − | − | + |
| LV21Δ | M | 37 | T S L G LN BM | + | 70% | 20% | − | int | − | int | + |
| LIIIV5^ | F | 18 | T S L G | + | 70% | − | int | − | − | + | |
| LIIIV12^ | F | 18 | T S L G LN BM | + | 31% | 49% | − | int | − | − | + |
| LIIIV10Δ^ | M | 20 | T S L G LN BM | + | 20% | + | int | − | − | + | |
| LIIIV14* | M | 20 | T S L LN BM | + | 75% | − | int | − | − | + | |
| LIIIV57Δ^ | M | 20 | T S L G LN BM | + | 36% | + | int | − | − | + | |
| LIIIV75Δ^* | M | 19 | T S L G LN BM | + | 23% | 32% | − | int | − | int | + |
+: positive; −: negative; ND: not done
T: thymus; S: spleen; L: liver; G: gut; LN: lymph node; BM: bone marrow
mice with lymphocyte-infiltrated kidney
mice with lymphocyte-infiltrated lung
mice with lymphadenopathy
L-DKO lymphomas are derived from either CD1dTet+ iNKT or CD1dTet− innate-like T cells
Since we had previously observed neonatal expansion of iNKT cells in L-DKO mice, we hypothesized that the tumor in these mice are derived from the uncontrolled expansion of the iNKT population. However, on further assessment, we found that only 36% of L-DKO mice developed CD1dTet+TCRβ+ tumors, while the rest mostly developed CD1dTet−TCRβ+ tumors (Table I, Figure 2A).
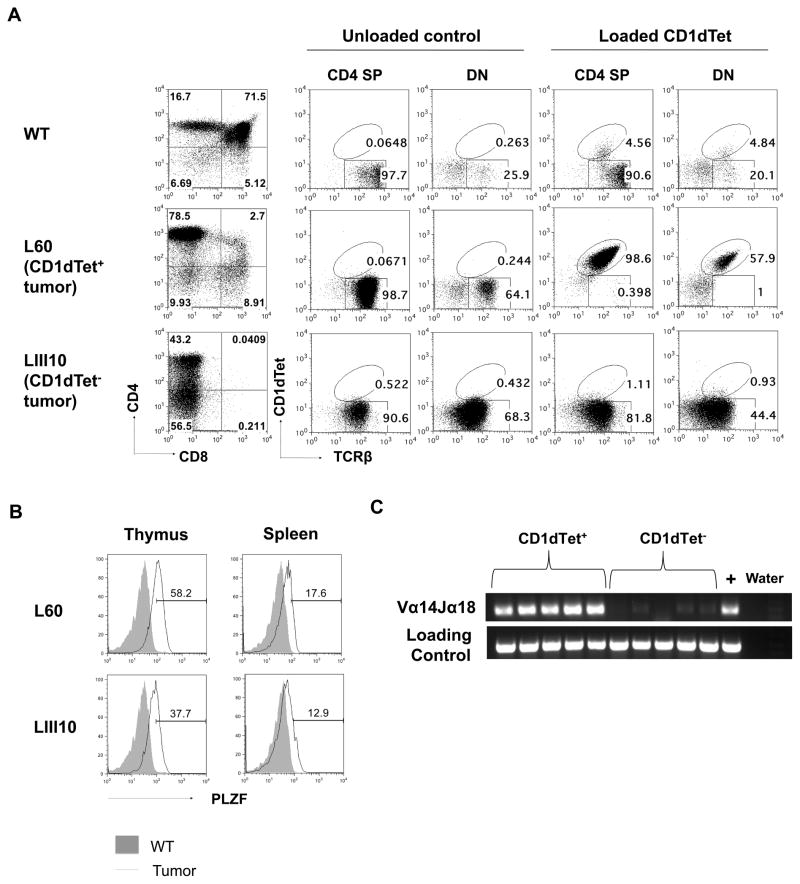
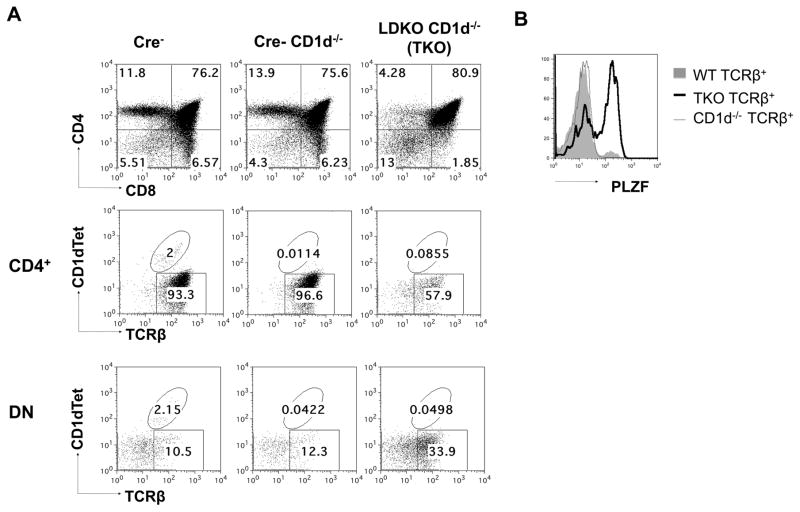
Figure 2. Lymphomas in L-DKO mice are CD1dTet+ (iNKT) or CD1dTet− in origin.
(A) Representative staining of thymocytes with CD4 and CD8 markers from a Cre− control and two L-DKO mice with either CD1dTet+ (L60) or CD1dTet− (LIII10) tumor. CD4 and DN fractions were further analyzed by staining with TCRβ and CD1dTet without or with loaded antigen. (B) Representative histogram of intracellular PLZF staining of TCRβ+ populations from mice (N = 2) with tumor shown in (A). (C) Detection of Vα14Jα18 rearrangement in CD1dTet+ or CD1dTet− lymphoma samples by PCR. CD14 was used as a loading control. (N = 5)
A recent publication from the Murre lab has also described the expansion of innate-like TFH cells and lymphoma development in Id2f/fId3f/fIL7RCre mice.[48] In order to better characterize the lymphoma populations in L-DKO mice (with a later deletion of Id2/Id3), we started by examining the expression of promyelocytic leukemia zinc finger protein (PLZF), a key transcription factor for all innate-like cells, including iNKT cells.[29, 49] High PLZF expression in the CD1dTet+TCRβ+ population verified that these were indeed NKT cells (Figure 2B, upper panel). The NKT lymphoma cells was further confirmed to be iNKT cells by their typical Vα14Jα18 rearrangement, which was not observed in the CD1dTet− cells (Figure 2C). Interestingly, the CD1dTet−TCRβ+ cells were also found to be PLZF+, distinguishing them from conventional αβ T cells (Figure 2B, lower panel). Phenotypic analysis of tumor samples from several L-DKO tumor mice also revealed that majority of lymphoma cells (both CD1dTet+ and CD1dTet−) expressed surface markers CD69 and CD44, but lacked CD25 and CD24 expression, similar to innate-like iNKT cells (Table I). These expression patterns further verified that the PLZF+CD1dTet−TCRβ+ population was innate-like. While other groups have also reported the expansion of PLZF+CD1dTet−TCRβ+ populations in the absence of ID proteins, the different stage of Id deletion and lack of comprehensive surface markers make these populations difficult to compare.[50, 51] Thus, we found that L-DKO mice develop innate-like T cell lymphomas derived from either CD1dTet+ iNKT or CD1dTet− innate-like T cells.
Malignant innate-like T cells from L-DKO mice are able to invade healthy tissues
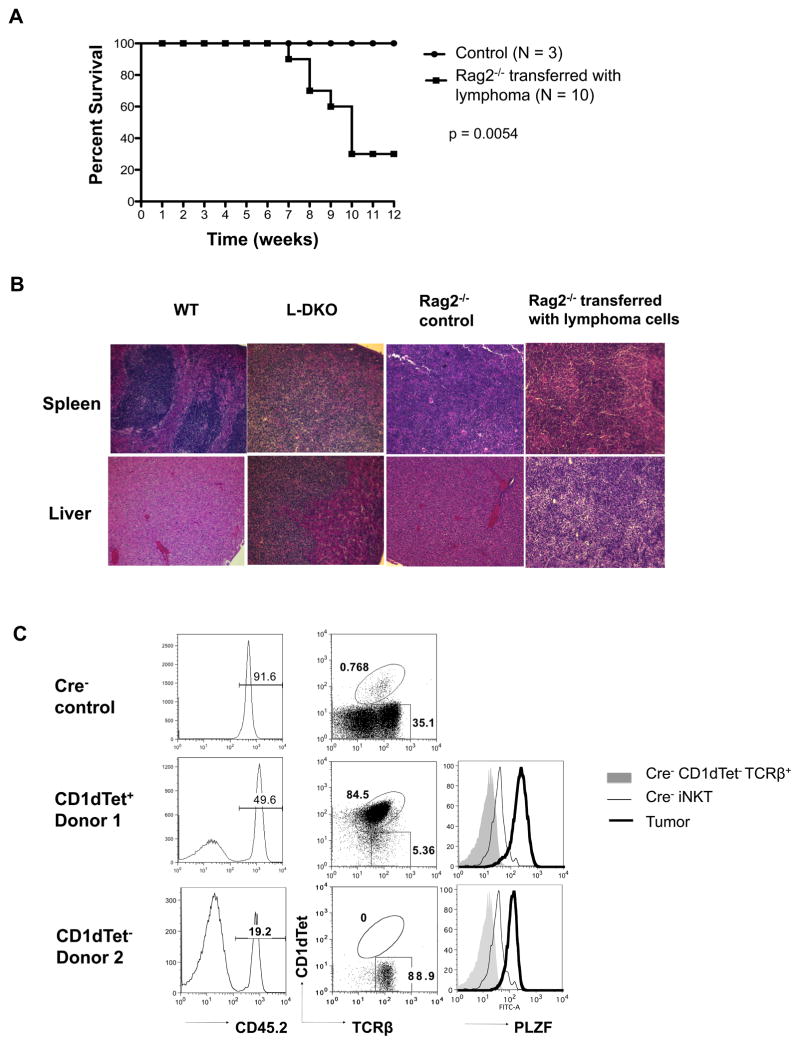
In order to evaluate the tumorigenic potential of innate-like lymphoma cells in L-DKO mice, we transferred these cells into Rag2−/− mice. We found that 70% of the Rag2−/− recipients died within 7–12 weeks of transfer of L-DKO lymphoma cells (Figure 3A). Substantial lymphocyte infiltration was observed by H&E staining of liver and spleen tissues from recipient mice (Figure 3B). It was also evident that both CD1dTet+ cells and CD1dTet− cells were capable of giving rise to secondary lymphomas in Rag2−/− hosts. These secondary lymphomas matched the original phenotype of the donor innate-like lymphoma cells, such that lymphomas derived from donor 1 cells were CD1dTet+PLZF+, whereas those from donor 2 gave rise to CD1dTet−PLZF+ lymphomas (Figure 3C). Adoptive transfer of lymphoma cells into wild type (WT) hosts also gave rise to tumors within 10 weeks (Supplementary Figure 1), indicating that these tumors have acquired the ability to evade immune surveillance. These results demonstrated the malignancy of these innate-like lymphomas derived from L-DKO mice.
Figure 3. Adoptive transfer of L-DKO lymphoma cells gives rise to tumor in Rag2-deficient recipients.
(A) Survival curve for Rag2−/− hosts after receiving 5×106 lymphoma cells from L-DKO mice (N = 10). Rag2−/− mice that were not injected with lymphoma cells were used as control (N = 3). p value based on the Mantel-Cox test (B) Representative H&E staining for spleen and liver from WT control, L-DKO mice with lymphoma, Rag2−/− control and Rag2−/− mice that received lymphoma cells (N = 5 for each host type). (C) CD45.2+ lymphoma cells in recipient mice analyzed for their CD1dTet and TCRβ expression. Intracellular PLZF expression levels in CD1dTet−TCRβ+ and CD1dtet+TCRβ+ (iNKT) control cells from Cre− mice, and CD45.2+ tumor cells from Rag2−/− recipients are shown. Data is representative of 3 analyzed recipients.
CD1dTet− innate-like T cells start expanding in neonatal L-DKO mice
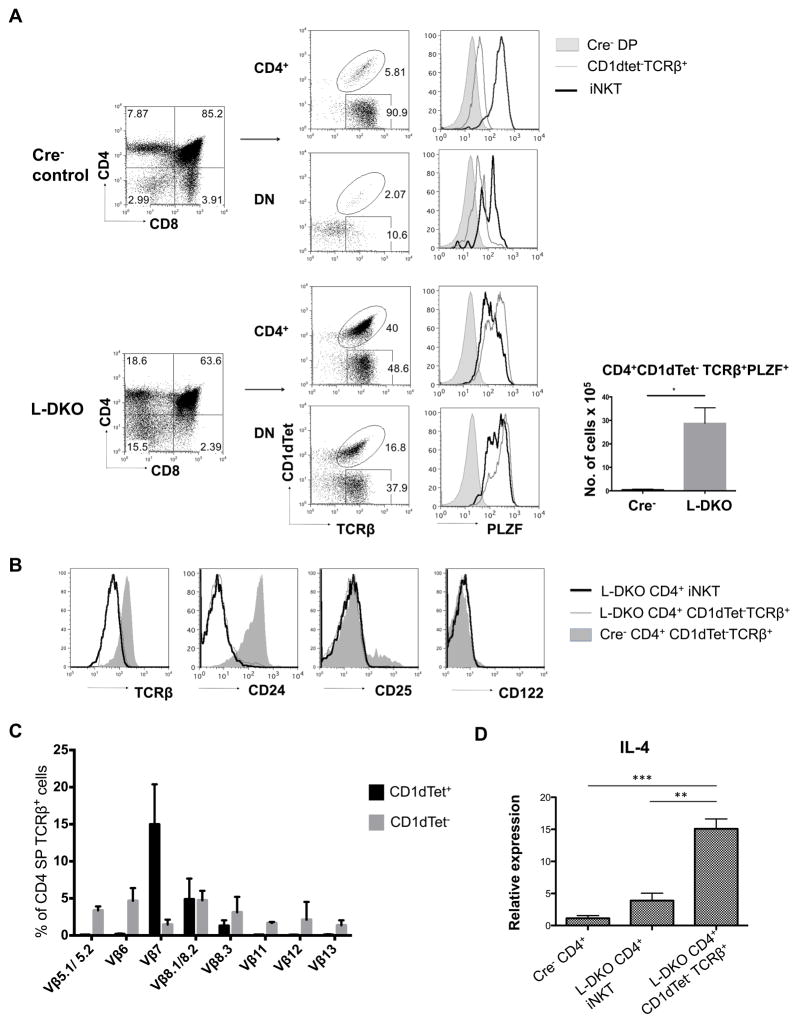
Next, we wanted to explore the possible neonatal expansion of these cells in L-DKO mice, similar to iNKT cells. After gating out γδ T cells, which aberrantly upregulate CD4 and CD8 in L-DKO mice, we found that the CD1dTet−TCRβ+ population was indeed expanded in 20-day old L-DKO mice (Figure 4A). This population had markedly upregulated PLZF expression, clearly demarcating them from conventional CD4SP and DN cells. Interestingly, the PLZF level in CD4SP CD1dTet− cells was even higher than that in iNKT cells. When compared to WT conventional CD4 T cells, we found that innate-like CD4+CD1dTet− cells had a similar, lower surface expression of TCRβ and CD24 as iNKT cells from L-DKO mice (Figure 4B). We also looked at surface expression of CD122 and CD25, which are usually upregulated in type II NKT cells. However, we found no significant upregulation of these markers among the L-DKO CD1dTet− population (Figure 4B). These cells had a broader and more evenly spread TCRβ usage than that of CD1dTet+ iNKT cells, which is more typical of type II NKT cells (Figure 4C). A unique feature of innate-like lymphocytes is their ability to produce IL4 at steady state. Q-PCR analysis revealed that the CD4+CD1dTet− population had a higher IL-4 expression at steady state than iNKT cells and conventional CD4 T cells, which indicated a possible regulatory role of this subset (Figure 4D). These observations are indicative of neonatal expansion of the innate-like CD1dTet− population, that has shared features with type II NKT cells.
Figure 4. Innate-like CD1dTet− T cells expand in the absence of ID2 and ID3.
(A) Representative flow cytometry analysis of thymocytes (TCRγδ+ cells gated out) from 20 day old L-DKO and Cre− control mice. Cells were stained for CD4 and CD8 to separate the CD4+ and DN populations, which were further analyzed with TCRβ and CD1dTet markers. Intracellular PLZF staining is shown for the corresponding CD1dTet− and CD1dTet+ (iNKT) cells from the CD4 SP and DN fractions, along with Cre− DP cells as controls. Absolute numbers of CD4+CD1dTet−TCRβ+PLZF+ cells are shown for 20 day old L-DKO and Cre− mice (N=3 for both Cre− and L-DKO mice). (B) Representative surface staining of thymocytes gated on CD4+ CD1dTet+ (iNKT) or CD4+CD1dTet−TCRβ+ cells from 20 day old L-DKO or Cre− control mice. Histograms are shown for TCRβ, CD24, CD25 and CD122 staining (N = 2). (C) TCRβ chain distribution among CD4+TCRβ+CD1dTet+ (iNKT) or CD4+TCRβ+CD1dTet− cells from 20 day old L-DKO (N = 4) and Cre− controls (N = 4) as measured by a panel of corresponding Vβ antibodies. (D) IL-4 transcript expression in sorted CD4+TCRβ+CD1dTet+ (iNKTs) and CD1dTet− cells from 20 day old Cre− or L-DKO mice, as measured by RT-PCR (N = 4).
CD1dTet− cells develop in a CD1d-independent manner, and expand to cause lymphoma in L-DKO CD1d-deficient mice
Since we found low CD122 and CD25 expression, but a diverse TCRβ repertoire among the L-DKO CD1dTet− cells, we needed to further verify if the L-DKO CD1dTet− cells belonged to the type II NKT lineage or not. It is known that all types of αβ NKT cells depend on CD1d-mediated selection for their development.[52] Therefore, we generated Id2f/fId3f/fLckCre+CD1d−/− mice (henceforth referred as triple knockout, or TKO), and found that the CD1dtet−TCRβ+ population was still existent in the absence of CD1d (Figure 5A). PLZF expression also verified that the CD1dtet−TCRβ+ cells in TKO mice were innate-like (Figure 5B). TCRα repertoire analysis indicated that CD1dTet−TCRβ+ cells from both TKO and L-DKO mice had fairly broad distribution of TCRα chains, with no clear preference for Vα14 or Vα3, unlike iNKT and type II NKT cells (Figure 6A). Similar TCRβ usage also suggested that the same CD1dTet− population was present in TKO and L-DKO mice (Figure 6B, 4C). Interestingly, one of the TKO mice demonstrated a dominant usage of Vβ7, which suggested possible clonal expansion and tumorigenic potential as early as 20 day of age. Cumulatively, these data verified that these CD1dTet− cells are innate-like, and a novel type of CD1d-independent NKT cells, similar to γδ NKT cells. We found that lymphocyte infiltration by the expanded CD1dTet− population also gave rise to tumors in TKO mice (Supplementary Figure 2).
Figure 5. Expansion of CD1d-independent, innate-like T cells in TKO mice.
(A) Representative staining of thymocytes from 20 day old Cre− control, Cre− CD1d−/− control or L-DKO CD1d−/− (TKO) mice using CD4 and CD8 markers (top panel). CD4+ and DN gated cells were further analyzed for CD1dTet and TCRβ expression. (B) Representative intracellular PLZF staining for TCRβ+ cells from 20 day old WT (Cre−), Cre- CD1d−/− and TKO mice (N = 3).
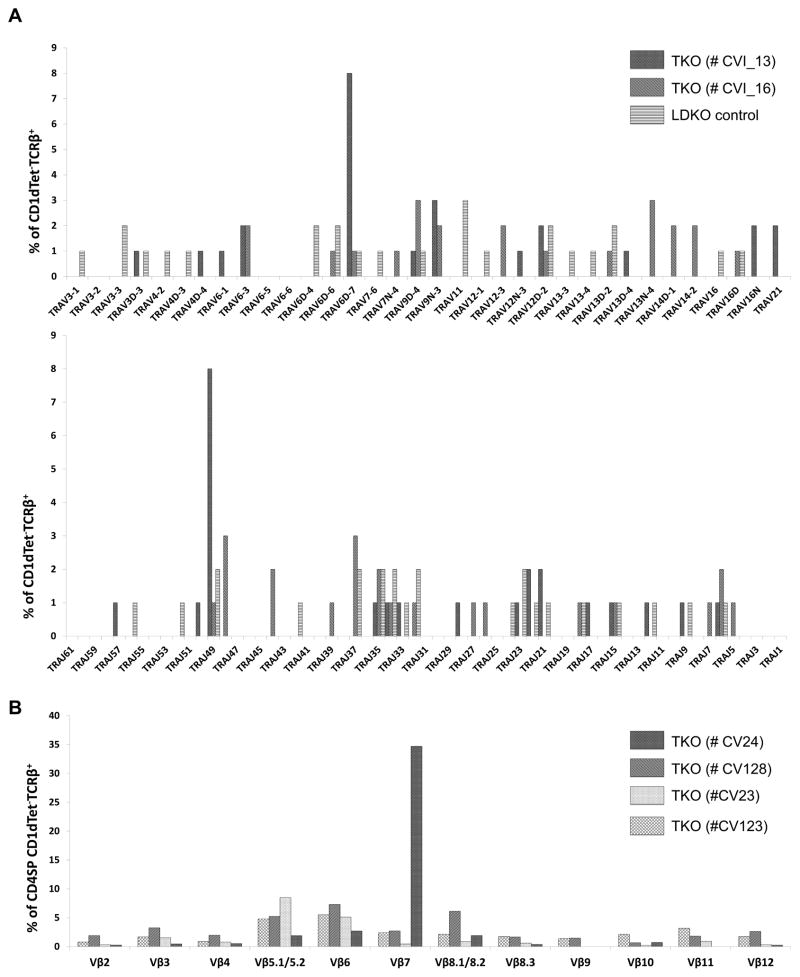
Figure 6. CD1d-independent CD1dTet−TCRβ+ T cells have broad TCRα and TCRβ repertoires.
(A) Vα and Jα repertoires (% usage) of CD1dtet−TCRβ+ T cells from 20 day old TKO mice (N=2) and L-DKO (N=1) mice as measured by 5′RACE (Invitrogen). TRAV11 and TRAV9 correspond to Vα14 and Vα3 chains respectively, according to the new HUGO Gene Nomenclature Committee. (B) TCRβ chain distribution among CD4+TCRβ+CD1dTet− cells from 20 day old TKO mice (N = 4) as measured by a panel of corresponding Vβ antibodies. Repertoire for each individual mouse is depicted by a separate pattern.
Neonatal L-DKO iNKT cells have a unique transcriptional program that promotes NKT development and expansion, and predisposes them to lymphomagenesis
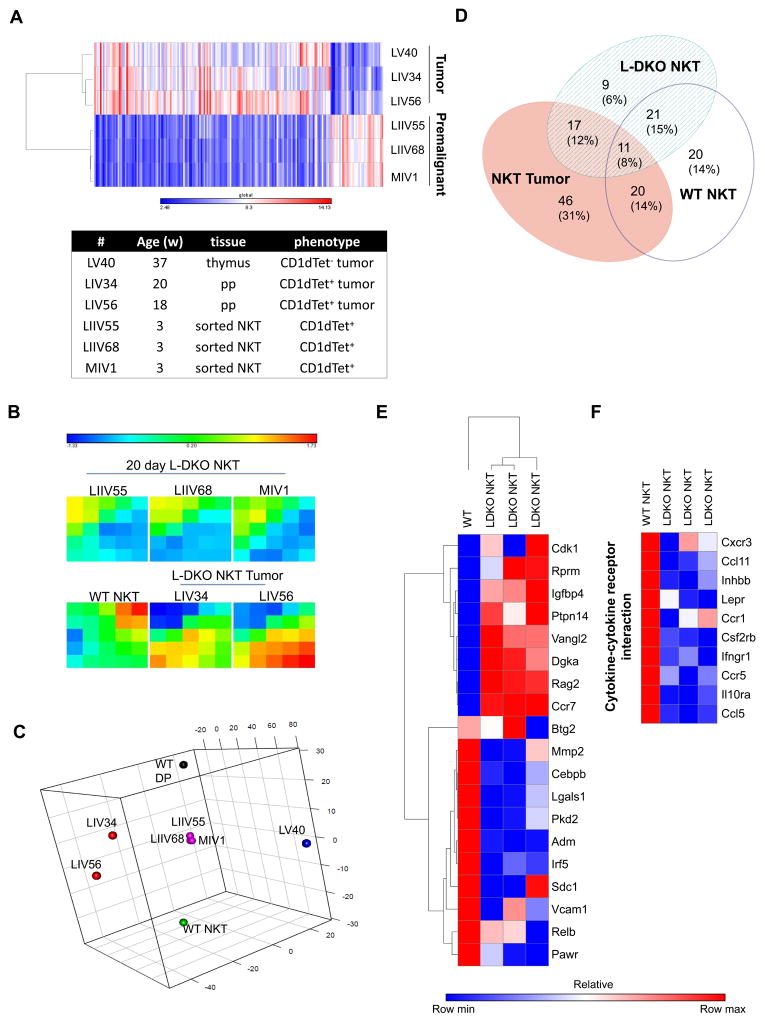
We then sought to identify the dysregulated molecular mechanisms responsible for tumor initiation and development. We did a microarray analysis to compare pre-malignant iNKT cells from 20 day old L-DKO mice, and lymphoma cells (either CD1dTet− or CD1dTet+/iNKT in origin) from L-DKO mice with well-developed tumors. We found that the tumors upregulated several genes and downregulated fewer genes, as compared to pre-malignant iNKT cells (Figure 7A). Hierarchical clustering also demonstrated a clear distinction between lymphoma and pre-malignant iNKT cells, and more variability between the tumor samples (Figure 7A). In order to allow direct comparisons of our L-DKO pre-malignant and tumor cells with control WT NKT and WT DP cells, we combined our microarray data with publicly available ImmGen data.[46] Clustering patterns showed clear segregation between the WT NKT, L-DKO neonatal and tumor samples (Figure 7B, 7C). We found that there were both shared and unique gene signatures between WT NKT, premalignant L-DKO iNKT and CD1dTet+ NKT tumors, but surprisingly there was only a moderate overlap between 20 day L-DKO NKT and WT NKT cells (Figure 7B, 7D).
Figure 7. Aberrant gene expression program in neonatal NKT cells in the absence of Id2 and Id3.
(A) Heat map with hierarchical clustering showing gene expression in sorted neonatal NKT cells from 20 day old L-DKO mice and tumor cells (CD1dTet+ and CD1dTet−) from 18–37 week old mice, as measured by mouse genome arrays. Colors represent global values of low (blue) to high (red) gene expression, with values ranging from 2.48 to 14.13 (in log2 scale, normalized values). Age of the mice, tissue origin of cells and their phenotype is also listed. (B) Self-organized map (SOM) showing gene expression in clusters of genes for tumor or NKT cells from mice listed above, or from WT control mice (combined data). Colors represent low (blue) to high (red) log2 fold change in gene expression with respect to WT DP cells. (C) Principal Component Analysis (PCA) for L-DKO NKT, NKT tumor and CD1dTet− tumor samples (described in (A)), combined with WT NKT and WT DP cells from Immgen. (D) Venn diagram represents the number and percentage of NKT-specific genes (p < 0.05 and absolute fold change greater than two in WT NKT with respect to WT DP) that are unique or shared between WT NKT, neonatal L-DKO NKT and NKT tumor cells. (E) Heat map showing hierarchical clustering and relative log fold change of gene expression in WT NKT and neonatal L-DKO NKT cells with respect to WT DP cells. Genes were selected based on expression patterns of SOM clusters (listed in Supplementary Table I). (F) Heat map showing the relative log fold change for genes involved in cytokine-cytokine receptor interaction. For (E) and (F), colors represent the lowest (blue) to highest (red) fold change of a particular gene among the different samples.
Based on the expression patterns of gene clusters in the samples, we identified genes that were differentially expressed in either WT or L-DKO iNKT cells (Figure 7B, Supplementary Table I). Interestingly, a significant fraction of genes were downregulated in L-DKO iNKT cells as compared to WT NKT cells, which included anti-proliferative and pro-apoptotic genes Pawr[53] and Lgals1[54], and tumor suppressor genes Cebpb[55] and Irf5[56] (Figure 7E). Genes implicated in cell cycle progression and metastasis, such as Vangl2 (Wnt pathway), Cdk1 (p53 pathway), Ccr7 and Igfbp4 were significantly upregulated in L-DKO iNKT cells. Dgka, which has been reported to be important for NKT development[57] as well as to promote tumorigenesis,[58] was also found to be more than 2 fold upregulated in L-DKO iNKT cells. These expression patterns supported the tumorigenic potential of these cells.
On the other hand, these premalignant cells also demonstrated the upregulation of several cell cycle arrest, tumor suppressor and anti-proliferative genes such as Rprm[59], Ptpn14[60] and Btg2[61] (Figure 7E). Other genes that commonly contribute to tumor development, or are overexpressed in tumors, such as Pkd2[62], Mmp2[63], Adm[64] and Vcam1[65], had reduced expression in these cells. Genes involved in cytokine-cytokine receptor interaction, many of which have been implicated in facilitating tumor metastasis, were also downregulated (Figure 7F).[66] This data hints towards the existence of a tumor suppression program in these cells that prevents tumorigenesis at this stage.
Rag2 was found to be upregulated by more than 3 fold in L-DKO iNKT cells, which would allow prolonged TCRα rearrangement to increase chances of the distal Vα14Jα18 rearrangement to promote iNKT cell development (Figure 7E).[67] We have previously described a block in iNKT development beyond stage 1 in L-DKO mice, which allows these cells to constantly proliferate without undergoing maturation.[34] We found downregulation of Relb in L-DKO iNKT cells, which has been described to be critical for developmental progression of NKT cells to stage 2 and 3.[68] Overall, the gene expression and pathway analysis revealed that Id2/Id3 deletion initiates a modified transcriptional program in L-DKO iNKT cells that supports their prolific expansion while maintaining a pre-malignant state.
Conditional Id2/Id3 deletion promotes acquisition of multiple tumorigenic programs
Since the tumors in L-DKO mice share lineage identity with iNKT or CD1dTet− cells that had undergone persistent expansion starting at neonatal age, it is likely that the acquisition of secondary and tertiary oncogenic mutations ultimately led to tumor development. We found a clear distinction between the tumor samples, which could indicate the existence of multiple, varying aberrant pathways that may be reflective of the history of accumulated mutations in the parent cells, which was also supported by their oligoclonal expansion patterns (Figure 7C). Karyotyping analysis of lymphoma cells from L-DKO mice also revealed aneuploidy in one of two tumor cases (Supplementary Figure 3). This could be an indication of chromosome instability (CIN), which is often linked to tumorigenesis, and may play a role in promoting lymphoma development in L-DKO mice.[69, 70]
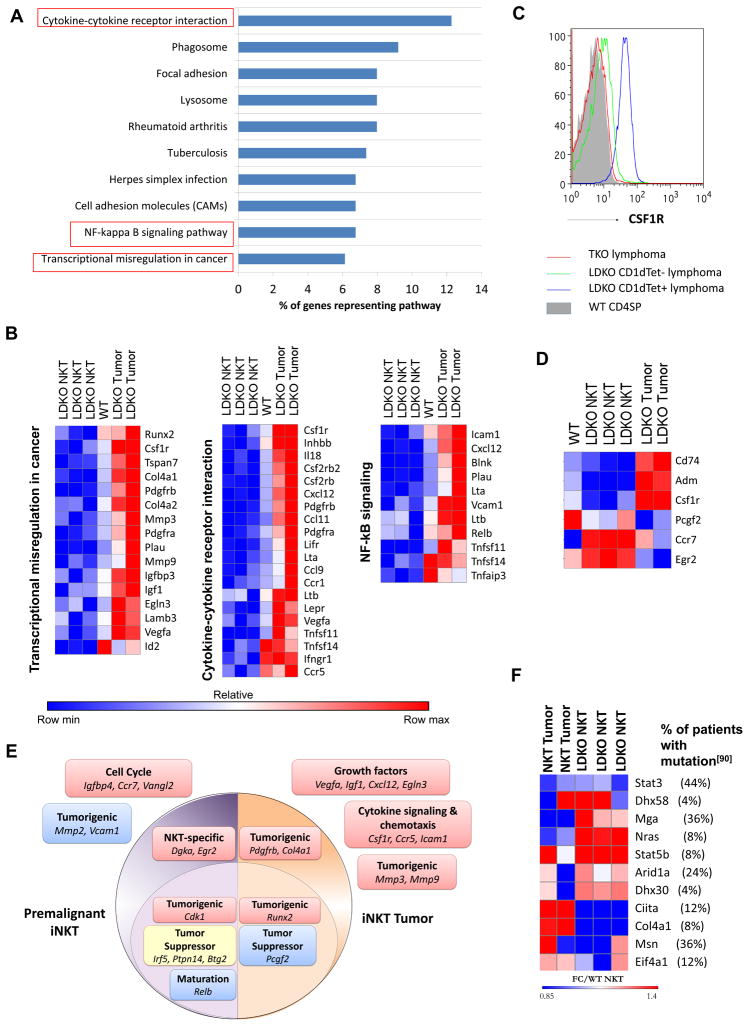
Since the CD1dTet− tumor sample was significantly distant from the CD1dTet+ tumors, it was treated as a unique outlier, and was not analyzed further. We then focused our attention to the iNKT tumor samples, and identified genes with more than 2-fold expression in L-DKO iNKT, iNKT tumors or WT NKT compared to WT DP cells (Figure 7D). Of these genes, the iNKT tumor cells had a largely unique gene profile with only a small fraction retained in common with WT NKT and premalignant L-DKO iNKT cells. We also did pathway analysis to identify the pathways over-represented in the tumor samples (Figure 8A).[71] This analysis revealed many aberrant pathways specific to the tumor samples, particularly corresponding to cytokine-cytokine receptor interaction, NF-kB signaling and transcriptional misregulation in cancer (Figure 8A, 8B). Interestingly, the cytokine-cytokine receptor pathway, which was downregulated in premalignant iNKT cells, was found to be upregulated upon tumorigenesis. This included genes such as Csf1r that aid in proliferation and can act as proto-oncogenes[72], as well as chemokines and their ligands (Cxcl12, Ccl11, Ccl9, Ccr1, Ccr5) which have been shown to contribute to metastasis.[73, 74] We noted that upregulation of Csf1r was uniquely detected in iNKT lymphomas but not in CD1dTet− lymphomas (Figure 8C). Icam1 and Cxcl12, which have been reported to regulate NKT homing to the liver and bone marrow (BM),[75, 76] were also upregulated in the tumor cells, and therefore could potentially promote accumulation of iNKT cells and tumor formation[77, 78] in these organs (Table I). Lta and Ltb have been shown to play roles in iNKT thymic emigration,[79] and were significantly upregulated in iNKT tumors. Furthermore, there were several interesting genes involved in the transcriptional misregulation of cancer, such as Runx2, Mmp3, Mmp9, Egln3 and Vegfa.[80–83] We also found upregulation of the Id2 transcript in iNKT tumors as compared to premalignant iNKT cells (Figure 8B). This could represent overexpression of Id2 exon 3, which is the only remaining exon in Id2f/f mice.[84]
Figure 8. The concurrent loss of ID2 and ID3 turns on an oncogenic program in NKT cells.
(A) Pathways overrepresented by genes with greater than 2 fold gene expression in NKT tumor samples according to gene sets annotated by Kyoto Encyclopedia of Genes and Genomes (KEGG)[97]. Percentages represent fraction of genes from each pathway that are overexpressed in the samples. (B) Heat maps showing relative log fold change of gene expression with respect to WT DP cells for genes from pathways identified in the above analysis. (C) Representative CSF1R surface staining for CD1dTet+, CD1dTet− lymphoma samples (N=4) from L-DKO, TKO and WT control mice (N=3). (D) Heat maps showing select genes with significant differential expression among premalignant and tumor samples. (E) Graphic depicting a few key overrepresented pathways in premalignant NKT cells from 20 day old L-DKO mice (purple), or in CD1dTet+ NKT lymphoma cells from older L-DKO mice (orange). Downregulation (blue), upregulation (red) or partial upregulation and downregulation (yellow) of selected genes from the pathways is also shown. (F) Fold change in gene expression with respect to WT NKT cells, of genes that are implicated in human NKT tumors,[90] and also dysregulated in L-DKO iNKT tumors. Percentages in top panel indicate the percent of patients with NKT lymphomas (total N = 25) that have mutations in the listed genes, as characterized by Jiang et al.[90]
We then identified interesting genes that were unique to L-DKO iNKT or iNKT tumor samples (Figure 8D). Genes supporting cell proliferation, such as Cd74[85] and Adm[64], were downregulated in premalignant iNKT cells but highly upregulated in iNKT tumors. The tumor suppressor, Pcgf2[86], was also significantly downregulated in iNKT tumor cells. Interestingly, Ccr7[87] and Egr2[88, 89], which have both been shown to play critical roles in NKT development, were specifically upregulated in premalignant iNKT cells. Therefore, gene expression profiling revealed several known oncogenic pathways that may contribute to the development of iNKT tumors. This analysis also indicated that there were distinct pathways involved in the pre-malignant expansion of iNKT cells and the ultimate transformation of iNKT cells leading to uncontrolled tumor growth (Figure 8E). Comparison of these identified genes and pathways, however, revealed only a limited overlap with lymphomas described in Id2f/fId3f/fIL7RCre mice (data not shown). We also found a moderate, but varying upregulation of CXCR5 in L-DKO lymphoma cells (data not shown)[48]. The phenotypic difference between NKT tumors in L-DKO mice and TFH tumors in Id2f/fId3f/fIL7RCre mice indicates that tumor types may be dictated by the timing of Id gene deletion.
Additionally, to determine similarity with human NKT tumors, we compared our L-DKO tumor data to that from a recent publication characterizing key driver mutations and pathways in patients with NKT lymphomas.[90] We found many of the mutated genes in human patients from their study[90] to also be dysregulated in the L-DKO tumor model. The shared genes had differential expression patterns in L-DKO iNKT tumor samples and neonatal iNKT cells as compared to WT NKT cells (Figure 8F). The publication also implicated the upregulation of the NF-kB pathway in driving tumorigenesis in a subset of patients with poor prognosis.[90] Several genes of the NF-kB pathway were also found to be uniquely upregulated or downregulated in the L-DKO iNKT tumors, but not in the premalignant, neonatal iNKT cells (Figure 8B). This data suggests that this is a potential mouse model to investigate mechanisms of iNKT and innate-like tumors in humans.
Discussion
A previous study of Id3−/− mice has revealed a tumor suppressor role of ID3 in the development of HSTCL-like tumors. [20] In this study, we observed iNKT and innate-like T cell tumors upon deletion of both Id2 and Id3. It is interesting to note the difference in kinetics of lymphoma development and progression in Id3−/− mice and L-DKO mice. Id3−/− mice often develop autoimmune diseases,[92] but tumor development is much more infrequent and delayed, such that these mice live for at least one year. On the other hand, L-DKO mice start dying of tumor by 3 months of age. This rapid development of αβ T cell lymphomas in Id2f/fId3f/fIL7RCre+ mice,[48] and iNKT and CD1dTet− tumors in L-DKO mice argue in favor of ID2 playing novel compensatory and non-redundant roles in the regulation and suppression of tumorigenesis of developing T cells in the murine thymus along with ID3.
Previous publications have demonstrated the role of ID proteins in suppressing the development of innate-like γδ and iNKT cell development.[29, 35] In this paper, our findings highlight the suppressive role of ID proteins in overall innate-like T cell development, such that there is also expansion of CD1dTet− innate-like T cells in L-DKO mice. Interestingly, this population bears resemblance to the CD4+PLZF+ cells reported to expand in mice deficient in Itk, Id3 or early deletion of both Id2 and Id3.[48, 50, 93] Additional characterization of surface markers and gene expression programs in these cells would be important for comparing these innate-like T cells, and for understanding the cellular origin of iNKT and innate-like T cell tumors in humans.[44, 94–96]
To delve into the mechanism(s) of tumor formation in L-DKO mice, we performed meta-analysis by combining our L-DKO microarray data with WT data. This allowed us to perform direct comparisons between our mutant cells and WT NKT cells, which were originally not included in our microarray analysis. However, this approach limits the analysis to only the common gene probes in the two microarray datasets, which can lead to the omission of some potentially interesting genes in this model. It is also important to note that the expanded iNKT cells in L-DKO mice are a heterogeneous population consisting primarily of stage 1 and stage 2 NKT cells.[34] Due to the lack of availability of an appropriate control population, we used mature NKT cells from B6 mice as reference. Despite this distinction between the populations, it is reasonable to make this comparison as a reflection of changes in transcriptional programs in NKT cells lacking Id2 and Id3 that lead to lymphoma development. With the microarray datasets combined, we were able to observe the deviation in the genetic program in L-DKO iNKT cells as compared to WT NKT cells. We found that several cell cycle genes were upregulated, while pro-apoptotic genes were downregulated in neonatal L-DKO iNKT cells. This modified program allowed their dramatic expansion, but also kept their tumorigenic potential in check. We inferred that these expanding neonatal innate-like cells are stochastically driven towards tumorigenesis via different pathways, giving rise to heterogeneous tumors in these mice.
Among the L-DKO tumors, we observed dysregulation of genes in pathways such as transcriptional misregulation in cancer and cytokine-cytokine receptor interactions, as well as others that are commonly overexpressed in various cancer types. While it is difficult to determine with certainty which genes contributed to, versus those that were upregulated as a result of lymphoma development, we verified the sharing of key genes and pathways in L-DKO tumors with human NKT tumors. Therefore, these mice serve as an appropriate mouse model to study iNKT and innate-like tumors. Furthermore, the striking resemblance between all premalignant NKT samples and the divergence of tumor samples leads us to the enticing prospect of treating tumors by identifying and targeting early tumorigenic pathways. Such a study of tumor initiation and gradual progression is only possible in a mouse model, and would be useful in determining common genes that lead to malignant transformation.
It is indeed an interesting proposition that ID2 and ID3, through their suppression of innate-like cell fate, prevent unchecked expansion of iNKT and CD1dTet− cells under WT conditions. Therefore, upon deletion of Id2 and Id3, the rapid proliferation and expansion of these cells makes them prone to accrual of additional mutations leading to tumorigenesis by various mechanisms.
Supplementary Material
Acknowledgments
We t hank Drs. A. Lasorella and A. Iavarone (Columbia University) for sharing the Id2f/f strain. We thank M. Dai (Duke University) for technical assistance in generating the initial L-DKO breeding colony and adoptive transfer of lymphoma cells, Duke Cancer Center Flow Cytometry Facility for assistance in cell sorting, Duke Cancer Center Sequencing Facility for assistance in Ion Torrent sequencing, and the NIH tetramer facility for providing the CD1d tetramer. We thank S. Sen (Carnegie Mellon University) for his assistance in Bioinformatics analysis and R implementation.
This work has been supported by National Institute of Health grants GM R01 GM059638, and 1 P01 AI102853 awarded to Dr. Yuan Zhuang.
Glossary
- ID
Inhibitor of DNA binding
- iNKT
invariant Natural Killer T cell
- CD1dTet
CD1d tetramer
- L-DKO
Id2f/fId3f/fLckCre+
- TKO
Id2f/fId3f/fLckCre+CD1d−/−
- PLZF
Promyelocytic Leukemia Zinc Finger
- WT
Wild Type
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Hainaut P, Plymoth A. Targeting the hallmarks of cancer: towards a rational approach to next-generation cancer therapy. Curr Opin Oncol. 2013;25:50–51. doi: 10.1097/CCO.0b013e32835b651e. [DOI] [PubMed] [Google Scholar]
- 3.Ellenbroek SI, van Rheenen J. Imaging hallmarks of cancer in living mice. Nat Rev Cancer. 2014;14:406–418. doi: 10.1038/nrc3742. [DOI] [PubMed] [Google Scholar]
- 4.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 5.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer. 2005;5:603–614. doi: 10.1038/nrc1673. [DOI] [PubMed] [Google Scholar]
- 7.Lasorella A, Benezra R, Iavarone A. The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Cancer. 2014;14:77–91. doi: 10.1038/nrc3638. [DOI] [PubMed] [Google Scholar]
- 8.Hasskarl J, Munger K. Id proteins--tumor markers or oncogenes? Cancer Biol Ther. 2002;1:91–96. doi: 10.4161/cbt.50. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd TG, Theriault BL, Nachtigal MW. Autocrine BMP4 signalling regulates ID3 proto-oncogene expression in human ovarian cancer cells. Gene. 2008;414:95–105. doi: 10.1016/j.gene.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Hao E, Kiselyuk A, Shapiro J, Shields DJ, Lowy A, Levine F, Itkin-Ansari P. The Id3/E47 axis mediates cell-cycle control in human pancreatic ducts and adenocarcinoma. Mol Cancer Res. 2011;9:782–790. doi: 10.1158/1541-7786.MCR-10-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien CA, Kreso A, Ryan P, Hermans KG, Gibson L, Wang Y, Tsatsanis A, Gallinger S, Dick JE. ID1 and ID3 regulate the self-renewal capacity of human colon cancer-initiating cells through p21. Cancer Cell. 2012;21:777–792. doi: 10.1016/j.ccr.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 12.DiVito KA, Simbulan-Rosenthal CM, Chen YS, Trabosh VA, Rosenthal DS. Id2, Id3 and Id4 overcome a Smad7-mediated block in tumorigenesis, generating TGF-beta-independent melanoma. Carcinogenesis. 2014;35:951–958. doi: 10.1093/carcin/bgt479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuchiya T, Okaji Y, Tsuno NH, Sakurai D, Tsuchiya N, Kawai K, Yazawa K, Asakage M, Yamada J, Yoneyama S, Kitayama J, Osada T, Watanabe T, Tokunaga K, Takahashi K, Nagawa H. Targeting Id1 and Id3 inhibits peritoneal metastasis of gastric cancer. Cancer Sci. 2005;96:784–790. doi: 10.1111/j.1349-7006.2005.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma P, Patel D, Chaudhary J. Id1 and Id3 expression is associated with increasing grade of prostate cancer: Id3 preferentially regulates CDKN1B. Cancer Med. 2012;1:187–197. doi: 10.1002/cam4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamalian L, Forootan SS, Bao ZZ, Zhang Y, Gosney JR, Foster CS, Ke Y. Inhibition of tumourigenicity of small cell lung cancer cells by suppressing Id3 expression. Int J Oncol. 2010;37:595–603. doi: 10.3892/ijo_00000708. [DOI] [PubMed] [Google Scholar]
- 16.Gray MJ, Dallas NA, Van Buren G, Xia L, Yang AD, Somcio RJ, Gaur P, Mangala LS, Vivas-Mejia PE, Fan F, Sanguino AM, Gallick GE, Lopez-Berestein G, Sood AK, Ellis LM. Therapeutic targeting of Id2 reduces growth of human colorectal carcinoma in the murine liver. Oncogene. 2008;27:7192–7200. doi: 10.1038/onc.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Love C, Sun Z, Jima D, Li G, Zhang J, Miles R, Richards KL, Dunphy CH, Choi WW, Srivastava G, Lugar PL, Rizzieri DA, Lagoo AS, Bernal-Mizrachi L, Mann KP, Flowers CR, Naresh KN, Evens AM, Chadburn A, Gordon LI, Czader MB, Gill JI, Hsi ED, Greenough A, Moffitt AB, McKinney M, Banerjee A, Grubor V, Levy S, Dunson DB, Dave SS. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44:1321–1325. doi: 10.1038/ng.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter J, Schlesner M, Hoffmann S, Kreuz M, Leich E, Burkhardt B, Rosolowski M, Ammerpohl O, Wagener R, Bernhart SH, Lenze D, Szczepanowski M, Paulsen M, Lipinski S, Russell RB, Adam-Klages S, Apic G, Claviez A, Hasenclever D, Hovestadt V, Hornig N, Korbel JO, Kube D, Langenberger D, Lawerenz C, Lisfeld J, Meyer K, Picelli S, Pischimarov J, Radlwimmer B, Rausch T, Rohde M, Schilhabel M, Scholtysik R, Spang R, Trautmann H, Zenz T, Borkhardt A, Drexler HG, Moller P, MacLeod RA, Pott C, Schreiber S, Trumper L, Loeffler M, Stadler PF, Lichter P, Eils R, Kuppers R, Hummel M, Klapper W, Rosenstiel P, Rosenwald A, Brors B, Siebert R Project IM-S. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat Genet. 2012;44:1316–1320. doi: 10.1038/ng.2469. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz R, Ceribelli M, Pittaluga S, Wright G, Staudt LM. Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Maruyama T, Zhang P, Konkel JE, Hoffman V, Zamarron B, Chen W. Mutation of inhibitory helix-loop-helix protein Id3 causes gammadelta T-cell lymphoma in mice. Blood. 2010;116:5615–5621. doi: 10.1182/blood-2010-03-274506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu L, Liu C, Vandeusen J, Becknell B, Dai Z, Wu YZ, Raval A, Liu TH, Ding W, Mao C, Liu S, Smith LT, Lee S, Rassenti L, Marcucci G, Byrd J, Caligiuri MA, Plass C. Global assessment of promoter methylation in a mouse model of cancer identifies ID4 as a putative tumor-suppressor gene in human leukemia. Nat Genet. 2005;37:265–274. doi: 10.1038/ng1521. [DOI] [PubMed] [Google Scholar]
- 22.Chen SS, Claus R, Lucas DM, Yu L, Qian J, Ruppert AS, West DA, Williams KE, Johnson AJ, Sablitzky F, Plass C, Byrd JC. Silencing of the inhibitor of DNA binding protein 4 (ID4) contributes to the pathogenesis of mouse and human CLL. Blood. 2011;117:862–871. doi: 10.1182/blood-2010-05-284638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Y, Cheung HW, von Maltzhan G, Agrawal A, Cowley GS, Weir BA, Boehm JS, Tamayo P, Karst AM, Liu JF, Hirsch MS, Mesirov JP, Drapkin R, Root DE, Lo J, Fogal V, Ruoslahti E, Hahn WC, Bhatia SN. Targeted tumor-penetrating siRNA nanocomplexes for credentialing the ovarian cancer oncogene ID4. Sci Transl Med. 2012;4:147ra112. doi: 10.1126/scitranslmed.3003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell RG, Lasorella A, Dettin LE, Iavarone A. Id2 drives differentiation and suppresses tumor formation in the intestinal epithelium. Cancer Res. 2004;64:7220–7225. doi: 10.1158/0008-5472.CAN-04-2095. [DOI] [PubMed] [Google Scholar]
- 25.Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 26.Engel I, Murre C. The function of E- and Id proteins in lymphocyte development. Nat Rev Immunol. 2001;1:193–199. doi: 10.1038/35105060. [DOI] [PubMed] [Google Scholar]
- 27.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 28.Ueda-Hayakawa I, Mahlios J, Zhuang Y. Id3 restricts the developmental potential of gamma delta lineage during thymopoiesis. J Immunol. 2009;182:5306–5316. doi: 10.4049/jimmunol.0804249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonzo ES, Sant’Angelo DB. Development of PLZF-expressing innate T cells. Curr Opin Immunol. 2011;23:220–227. doi: 10.1016/j.coi.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP, Pereira P, Nichols KE, Koretzky GA, Jordan MS, Sant’Angelo DB. Development of promyelocytic zinc finger and ThPOK-expressing innate gamma delta T cells is controlled by strength of TCR signaling and Id3. J Immunol. 2010;184:1268–1279. doi: 10.4049/jimmunol.0903218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verykokakis M, Boos MD, Bendelac A, Adams EJ, Pereira P, Kee BL. Inhibitor of DNA binding 3 limits development of murine slam-associated adaptor protein-dependent “innate” gammadelta T cells. PLoS One. 2010;5:e9303. doi: 10.1371/journal.pone.0009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verykokakis M, Krishnamoorthy V, Iavarone A, Lasorella A, Sigvardsson M, Kee BL. Essential functions for ID proteins at multiple checkpoints in invariant NKT cell development. J Immunol. 2013;191:5973–5983. doi: 10.4049/jimmunol.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B, Lin YY, Dai M, Zhuang Y. Id3 and Id2 act as a dual safety mechanism in regulating the development and population size of innate-like gammadelta T cells. J Immunol. 2014;192:1055–1063. doi: 10.4049/jimmunol.1302694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Wu D, Jiang N, Zhuang Y. Combined deletion of id2 and id3 genes reveals multiple roles for e proteins in invariant NKT cell development and expansion. J Immunol. 2013;191:5052–5064. doi: 10.4049/jimmunol.1301252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy S, Zhuang Y. Orchestration of invariant natural killer T cell development by E and Id proteins. Crit Rev Immunol. 2015;35:33–48. doi: 10.1615/critrevimmunol.2015012207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Cruz LM, Stradner MH, Yang CY, Goldrath AW. E and Id proteins influence invariant NKT cell sublineage differentiation and proliferation. J Immunol. 2014;192:2227–2236. doi: 10.4049/jimmunol.1302904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu T, Wang H, Simmons A, Bajana S, Zhao Y, Kovats S, Sun XH, Alberola-Ila J. Increased level of E protein activity during invariant NKT development promotes differentiation of invariant NKT2 and invariant NKT17 subsets. J Immunol. 2013;191:5065–5073. doi: 10.4049/jimmunol.1301546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 39.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 40.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margulies DH. The in-betweeners: MAIT cells join the innate-like lymphocytes gang. J Exp Med. 2014;211:1501–1502. doi: 10.1084/jem.2118insight3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 44.Matsuo Y, Drexler HG. Immunoprofiling of cell lines derived from natural killer-cell and natural killer-like T-cell leukemia-lymphoma. Leuk Res. 2003;27:935–945. doi: 10.1016/s0145-2126(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heng TS, Painter MW Immunological Genome Project C. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 47.Glaab E, Garibaldi JM, Krasnogor N. ArrayMining: a modular web-application for microarray analysis combining ensemble and consensus methods with cross-study normalization. BMC Bioinformatics. 2009;10:358. doi: 10.1186/1471-2105-10-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyazaki M, Miyazaki K, Chen S, Chandra V, Wagatsuma K, Agata Y, Rodewald HR, Saito R, Chang AN, Varki N, Kawamoto H, Murre C. The E-Id protein axis modulates the activities of the PI3K-AKT-mTORC1-Hif1a and c-myc/p19Arf pathways to suppress innate variant TFH cell development, thymocyte expansion, and lymphomagenesis. Genes Dev. 2015;29:409–425. doi: 10.1101/gad.255331.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, Sant’Angelo DB. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics. Immunity. 2010;33:203–215. doi: 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyazaki M, Rivera RR, Miyazaki K, Lin YC, Agata Y, Murre C. The opposing roles of the transcription factor E2A and its antagonist Id3 that orchestrate and enforce the naive fate of T cells. Nat Immunol. 2011;12:992–1001. doi: 10.1038/ni.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 53.El-Guendy N, Rangnekar VM. Apoptosis by Par-4 in cancer and neurodegenerative diseases. Exp Cell Res. 2003;283:51–66. doi: 10.1016/s0014-4827(02)00016-2. [DOI] [PubMed] [Google Scholar]
- 54.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 55.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Yanai H, Chen HM, Inuzuka T, Kondo S, Mak TW, Takaoka A, Honda K, Taniguchi T. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc Natl Acad Sci U S A. 2007;104:3402–3407. doi: 10.1073/pnas.0611559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen S, Wu J, Srivatsan S, Gorentla BK, Shin J, Xu L, Zhong XP. Tight regulation of diacylglycerol-mediated signaling is critical for proper invariant NKT cell development. J Immunol. 2011;187:2122–2129. doi: 10.4049/jimmunol.1100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dominguez CL, Floyd DH, Xiao A, Mullins GR, Kefas BA, Xin W, Yacur MN, Abounader R, Lee JK, Wilson GM, Harris TE, Purow BW. Diacylglycerol kinase alpha is a critical signaling node and novel therapeutic target in glioblastoma and other cancers. Cancer Discov. 2013;3:782–797. doi: 10.1158/2159-8290.CD-12-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohki R, Nemoto J, Murasawa H, Oda E, Inazawa J, Tanaka N, Taniguchi T. Reprimo, a new candidate mediator of the p53-mediated cell cycle arrest at the G2 phase. J Biol Chem. 2000;275:22627–22630. doi: 10.1074/jbc.C000235200. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, Yang N, Figel SA, Wilson KE, Morrison CD, Gelman IH, Zhang J. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013;32:1266–1273. doi: 10.1038/onc.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winkler GS. The mammalian anti-proliferative BTG/Tob protein family. J Cell Physiol. 2010;222:66–72. doi: 10.1002/jcp.21919. [DOI] [PubMed] [Google Scholar]
- 62.Zou Z, Zeng F, Xu W, Wang C, Ke Z, Wang QJ, Deng F. PKD2 and PKD3 promote prostate cancer cell invasion by modulating NF-kappaB- and HDAC1-mediated expression and activation of uPA. J Cell Sci. 2012;125:4800–4811. doi: 10.1242/jcs.106542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10:7621–7628. doi: 10.1158/1078-0432.CCR-04-1061. [DOI] [PubMed] [Google Scholar]
- 64.Oehler MK, Norbury C, Hague S, Rees MC, Bicknell R. Adrenomedullin inhibits hypoxic cell death by upregulation of Bcl-2 in endometrial cancer cells: a possible promotion mechanism for tumour growth. Oncogene. 2001;20:2937–2945. doi: 10.1038/sj.onc.1204422. [DOI] [PubMed] [Google Scholar]
- 65.Banks RE, Gearing AJ, Hemingway IK, Norfolk DR, Perren TJ, Selby PJ. Circulating intercellular adhesion molecule-1 (ICAM-1), E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in human malignancies. Br J Cancer. 1993;68:122–124. doi: 10.1038/bjc.1993.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J Immunol. 2003;171:2960–2969. doi: 10.4049/jimmunol.171.6.2960. [DOI] [PubMed] [Google Scholar]
- 67.D’Cruz LM, Knell J, Fujimoto JK, Goldrath AW. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat Immunol. 2010;11:240–249. doi: 10.1038/ni.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elewaut D, Shaikh RB, Hammond KJ, De Winter H, Leishman AJ, Sidobre S, Turovskaya O, Prigozy TI, Ma L, Banks TA, Lo D, Ware CF, Cheroutre H, Kronenberg M. NIK-dependent RelB activation defines a unique signaling pathway for the development of V alpha 14i NKT cells. J Exp Med. 2003;197:1623–1633. doi: 10.1084/jem.20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukasawa K. Centrosome amplification, chromosome instability and cancer development. Cancer Lett. 2005;230:6–19. doi: 10.1016/j.canlet.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 70.Nowak MA, Komarova NL, Sengupta A, Jallepalli PV, Shih Ie M, Vogelstein B, Lengauer C. The role of chromosomal instability in tumor initiation. Proc Natl Acad Sci U S A. 2002;99:16226–16231. doi: 10.1073/pnas.202617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamprecht B, Walter K, Kreher S, Kumar R, Hummel M, Lenze D, Kochert K, Bouhlel MA, Richter J, Soler E, Stadhouders R, Johrens K, Wurster KD, Callen DF, Harte MF, Giefing M, Barlow R, Stein H, Anagnostopoulos I, Janz M, Cockerill PN, Siebert R, Dorken B, Bonifer C, Mathas S. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med. 2010;16:571–579. doi: 10.1038/nm.2129. [DOI] [PubMed] [Google Scholar]
- 73.Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 75.Franitza S, Grabovsky V, Wald O, Weiss I, Beider K, Dagan M, Darash-Yahana M, Nagler A, Brocke S, Galun E, Alon R, Peled A. Differential usage of VLA-4 and CXCR4 by CD3+CD56+ NKT cells and CD56+CD16+ NK cells regulates their interaction with endothelial cells. Eur J Immunol. 2004;34:1333–1341. doi: 10.1002/eji.200324718. [DOI] [PubMed] [Google Scholar]
- 76.Emoto M, Mittrucker HW, Schmits R, Mak TW, Kaufmann SH. Critical role of leukocyte function-associated antigen-1 in liver accumulation of CD4+NKT cells. J Immunol. 1999;162:5094–5098. [PubMed] [Google Scholar]
- 77.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 78.Imai H, Sunaga N, Shimizu Y, Kakegawa S, Shimizu K, Sano T, Ishizuka T, Oyama T, Saito R, Minna JD, Mori M. Clinicopathological and therapeutic significance of CXCL12 expression in lung cancer. Int J Immunopathol Pharmacol. 2010;23:153–164. doi: 10.1177/039463201002300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franki AS, Van Beneden K, Dewint P, Hammond KJ, Lambrecht S, Leclercq G, Kronenberg M, Deforce D, Elewaut D. A unique lymphotoxin {alpha}beta-dependent pathway regulates thymic emigration of V{alpha}14 invariant natural killer T cells. Proc Natl Acad Sci U S A. 2006;103:9160–9165. doi: 10.1073/pnas.0508892103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blyth K, Vaillant F, Hanlon L, Mackay N, Bell M, Jenkins A, Neil JC, Cameron ER. Runx2 and MYC collaborate in lymphoma development by suppressing apoptotic and growth arrest pathways in vivo. Cancer Res. 2006;66:2195–2201. doi: 10.1158/0008-5472.CAN-05-3558. [DOI] [PubMed] [Google Scholar]
- 81.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 82.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouchard F, Belanger SD, Biron-Pain K, St-Pierre Y. EGR-1 activation by EGF inhibits MMP-9 expression and lymphoma growth. Blood. 2010;116:759–766. doi: 10.1182/blood-2009-12-257030. [DOI] [PubMed] [Google Scholar]
- 84.Niola F, Zhao X, Singh D, Castano A, Sullivan R, Lauria M, Nam HS, Zhuang Y, Benezra R, Di Bernardo D, Iavarone A, Lasorella A. Id proteins synchronize stemness and anchorage to the niche of neural stem cells. Nat Cell Biol. 2012;14:477–487. doi: 10.1038/ncb2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Starlets D, Gore Y, Binsky I, Haran M, Harpaz N, Shvidel L, Becker-Herman S, Berrebi A, Shachar I. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807–4816. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 86.Wang W, Yuasa T, Tsuchiya N, Ma Z, Maita S, Narita S, Kumazawa T, Inoue T, Tsuruta H, Horikawa Y, Saito M, Hu W, Ogawa O, Habuchi T. The novel tumor-suppressor Mel-18 in prostate cancer: its functional polymorphism, expression and clinical significance. Int J Cancer. 2009;125:2836–2843. doi: 10.1002/ijc.24721. [DOI] [PubMed] [Google Scholar]
- 87.Cowan JE, McCarthy NI, Parnell SM, White AJ, Bacon A, Serge A, Irla M, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G. Differential requirement for CCR4 and CCR7 during the development of innate and adaptive alphabetaT cells in the adult thymus. J Immunol. 2014;193:1204–1212. doi: 10.4049/jimmunol.1400993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, Glimcher LH. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10:306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seiler MP, Mathew R, Liszewski MK, Spooner CJ, Barr K, Meng F, Singh H, Bendelac A. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat Immunol. 2012;13:264–271. doi: 10.1038/ni.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang L, Gu ZH, Yan ZX, Zhao X, Xie YY, Zhang ZG, Pan CM, Hu Y, Cai CP, Dong Y, Huang JY, Wang L, Shen Y, Meng G, Zhou JF, Hu JD, Wang JF, Liu YH, Yang LH, Zhang F, Wang JM, Wang Z, Peng ZG, Chen FY, Sun ZM, Ding H, Shi JM, Hou J, Yan JS, Shi JY, Xu L, Li Y, Lu J, Zheng Z, Xue W, Zhao WL, Chen Z, Chen SJ. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet. 2015;47:1061–1066. doi: 10.1038/ng.3358. [DOI] [PubMed] [Google Scholar]
- 91.Bachy E, Urb M, Chandra S, Robinot R, Bricard G, de Bernard S, Traverse-Glehen A, Gazzo S, Blond O, Khurana A, Baseggio L, Heavican T, Ffrench M, Crispatzu G, Mondiere P, Schrader A, Taillardet M, Thaunat O, Martin N, Dalle S, Le Garff-Tavernier M, Salles G, Lachuer J, Hermine O, Asnafi V, Roussel M, Lamy T, Herling M, Iqbal J, Buffat L, Marche PN, Gaulard P, Kronenberg M, Defrance T, Genestier L. CD1d-restricted peripheral T cell lymphoma in mice and humans. J Exp Med. 2016;213:841–857. doi: 10.1084/jem.20150794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Belle I, Mahlios J, McKenzie A, Zhuang Y. Aberrant production of IL-13 by T cells promotes exocrinopathy in Id3 knockout mice. Cytokine. 2014;69:226–233. doi: 10.1016/j.cyto.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prince AL, Watkin LB, Yin CC, Selin LK, Kang J, Schwartzberg PL, Berg LJ. Innate PLZF+CD4+ alphabeta T cells develop and expand in the absence of Itk. J Immunol. 2014;193:673–687. doi: 10.4049/jimmunol.1302058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohshima K, Liu Q, Koga T, Suzumiya J, Kikuchi M. Classification of cell lineage and anatomical site, and prognosis of extranodal T-cell lymphoma -- natural killer cell, cytotoxic T lymphocyte, and non-NK/CTL types. Virchows Arch. 2002;440:425–435. doi: 10.1007/s00428-001-0545-1. [DOI] [PubMed] [Google Scholar]
- 95.Yu J, Mitsui T, Wei M, Mao H, Butchar JP, Shah MV, Zhang J, Mishra A, Alvarez-Breckenridge C, Liu X, Liu S, Yokohama A, Trotta R, Marcucci G, Jr, Benson DM, Loughran TP, Jr, Tridandapani S, Caligiuri MA. NKp46 identifies an NKT cell subset susceptible to leukemic transformation in mouse and human. J Clin Invest. 2011;121:1456–1470. doi: 10.1172/JCI43242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McGregor S, Shah A, Raca G, Mirza MK, Smith SM, Anastasi J, Vardiman JW, Hyjek E, Gurbuxani S. PLZF staining identifies peripheral T-cell lymphomas derived from innate-like T-cells with TRAV1-2-TRAJ33 TCR-alpha rearrangement. Blood. 2014;123:2742–2743. doi: 10.1182/blood-2014-02-555482. [DOI] [PubMed] [Google Scholar]
- 97.Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.