Abstract
Entrectinib, a potent oral inhibitor of the tyrosine kinases TRKA/B/C, ROS1, and ALK, was evaluated in two Phase 1 studies in patients with advanced or metastatic solid tumors, including patients with active CNS disease. Here we summarize the overall safety and report the antitumor activity of entrectinib in a cohort of patients with tumors harboring NTRK1/2/3, ROS1, or ALK gene fusions, naïve to prior TKI treatment targeting the specific gene, and who were treated at doses that achieved therapeutic exposures consistent with the RP2D. Entrectinib was well tolerated, with predominantly Grades 1/2 adverse events that were reversible with dose modification. Responses were observed in NSCLC, colorectal cancer, mammary analog secretory carcinoma, melanoma, and renal cell carcinoma, as early as 4 weeks after starting treatment and lasting as long as > 2 years. Notably, a complete CNS response was achieved in a patient with SQSTM1-NTRK1-rearranged lung cancer.
Keywords: Entrectinib, RXDX-101, NTRK fusion, NTRK rearrangement, ROS1 fusion
INTRODUCTION
Recurrent gene fusions are oncogenic drivers of tumor growth and survival across a variety of malignancies.(1) Structurally, many of these fusions retain an intact tyrosine kinase domain fused to an upstream gene partner that promotes ligand-independent dimerization. The resultant chimeric oncoprotein initiates and sustains downstream signaling resulting in tumor growth and proliferation.(2) As molecular profiling of tumors continues to migrate toward more comprehensive platforms, such as DNA-based next-generation sequencing and RNA-based anchored multiplex polymerase chain reaction (PCR), the number of these fusion events that are detectable in the clinic continues to rise substantially(3, 4).
Most importantly, a significant proportion of recurrent gene rearrangements are clinically actionable. In patients with advanced ALK and ROS1-rearranged non-small cell lung cancer (NSCLC), three targeted therapies (crizotinib [Xalkori®], ceritinib [Zykadia™], alectinib [Alecensa®]) have been approved based on dramatic improvements in response rate and progression-free survival.(5–9). Beyond NSCLC, ALK rearrangements have also been identified in a variety of malignancies, including anaplastic large cell lymphoma, renal cell, breast, esophageal, and colorectal cancers, as have ROS1 rearrangements in colorectal, gastric, and ovarian cancers, glioblastoma multiforme, and cholangiocarcinomas.(2)
Similar to ALK and ROS1 rearrangements, recurrent gene fusions involving the genes NTRK1, NTRK2, and NTRK3 are actionable drivers of tumor growth(10, 11). These genes encode the proteins TRKA, TRKB, and TRKC, respectively, and play roles in neuronal development, cell survival, and cellular proliferation(12). In the rearranged state, the activated fusion kinases signal through the RAS-RAF-MEK-ERK, PI3K-AKT-mTOR, and PLCγ-PKC pathways, driving the initiation and progression of malignancy. These fusions have been detected in a variety of tumors, including lung(13), gastrointestinal(14–16), head and neck(1), thyroid(17–19), spitzoid cancers(20, 21), sarcomas(22–24), primary brain tumors(25–27) and AML(28, 29). While many of these events are found at a lower incidence in tumors such as lung and gastrointestinal cancers, they are found in the majority of rare tumors such as secretory breast carcinoma(30), mammary analogue secretory carcinoma (MASC)(31, 32) and congenital infantile fibrosarcoma(33), where the identification of an NTRK fusion is a defining factor for diagnosis.
The presence of recurrent gene fusions involving NTRK1, NTRK2, NTRK3, ROS1, and ALK across different tumor histologies and the growing number of events that are detected in patient samples underscore the ongoing need for routine diagnostic testing to identify gene fusions. Equally critical is the need for clinical trials that afford access to effective targeted agents regardless of histology, in what is commonly referred to as a “basket” trial design. Phase 1 trials have rapidly evolved to meet this challenge, establishing not only the recommended Phase 2 dose (RP2D) of a promising agent, but also providing meaningful efficacy data in molecularly defined subsets of patients.(34) Here, we present the combined results of two Phase 1 trials of entrectinib(16), a novel, highly-potent, orally-available, ATP-competitive tyrosine kinase inhibitor with low- to sub-nanomolar enzymatic efficacy against TRKA, TRKB, TRKC, ROS1, and ALK (IC50s of 1.7, 0.1, 0.1, 0.2, and 1.6 nM, respectively)(35). Furthermore, the drug was specifically designed to cross the blood-brain barrier in an effort to address both primary brain tumors and brain metastases in patients with NTRK1-, NTRK2-, NTRK3-, ROS1-, and ALK-rearranged cancers(36).
RESULTS
Demographics
Between October 2012 and March 2016, a total of 119 patients with advanced solid tumors were treated with entrectinib: 54 on ALKA-372-001 and 65 on STARTRK-1. The demographic features of these patients are summarized in Table 1. The median age was 55 years (range 18–80 years). The majority of patients had an ECOG performance status of 0–1 (96%, n=114/119) and received 3 or more prior treatments for their cancer (83%, n=98/119), including prior ALK/ROS1 inhibitors (27%, n=32/119) and checkpoint inhibitors (3%, n=4/119). Patients with a wide range of solid tumors, including primary brain, head and neck, sarcoma, breast, melanoma, renal cell, and ovarian tumors were treated. The most predominant tumor type was NSCLC (60%, n=71/119), followed by tumors of the gastrointestinal tract (15%, n=18/119).
Table 1. Demographics.
The clinical and pathologic features of 119 patients with advanced solid tumors who received entrectinib on either Phase 1 trial (ALKA-372-001 or STARTRK-1) are summarized. Most patients had an ECOG performance status of 0 or 1 and were heavily pre-treated with three or more prior anti-cancer therapies. Patients with a wide range of solid tumors were treated.
| ALKA-372-001 (n=54) | STARTRK-1 (n=65) | TOTAL (n=119) | |
|---|---|---|---|
| Age, years, median (range) | 53 (22–77) | 57 (18–80) | 55 (18–80) |
| Sex, male/female (%) | 44/56 | 48/52 | 46/54 |
| ECOG performance status, n (%) | |||
| 0 | 30 (56) | 22 (34) | 52 (44) |
| 1 | 21 (39) | 41 (63) | 62 (52) |
| 2 | 2 (4) | 2 (3) | 4 (3) |
| Unknown | 1 (2) | 0 | 1 (1) |
| Prior Systemic Therapies, n (%) | |||
| 0 | 0 | 6 (9) | 6 (5) |
| 1–2 | 0 | 15 (23) | 15 (13) |
| 3 – 4 | 3 (6) | 25 (39) | 28 (24) |
| > 4 | 51 (94) | 19 (29) | 70 (59) |
| Prior ROS1/ALK Inhibitors, n (%) | 10 (19) | 22 (34) | 32 (27) |
| Prior Immunotherapy, n (%) | 0 | 4 (6) | 4 (3) |
| Tumor type, n (%) | |||
| NSCLC | 35 (65) | 36 (56) | 71 (60) |
| Gastrointestinal Tract | 9 (17) | 9 (14) | 18 (15) |
| CNS | 4 (7) | 1 (2) | 5 (4) |
| Head & Neck | 1 (2) | 4 (6) | 5 (4) |
| Other* | 5 (9) | 15 (23) | 20 (17) |
Other tumor types: breast, cholangiocarcinoma, melanoma, neuroblastoma, neuroendocrine tumors, ovarian, pancreatic, prostate, renal cell carcinoma, sarcoma, squamous skin cancer, unknown primary
The 54 patients on ALKA-372-001 were treated on the following dosing schedules: 19 on Schedule A (fasted, 4 days on entrectinib and 3 days off entrectinib for 21 of 28 days), 29 on Schedule B (fed, continuous daily dosing for 28 days), and 6 on Schedule C (fed, 4 days on entrectinib and 3 days off entrectinib for 28 days). All 65 patients on STARTRK-1 received continuous daily dosing with entrectinib (daily for 28 days).
Safety Profile
The most common treatment-related adverse events of any grade were fatigue/asthenia (46%, n=55/119), dysgeusia (42%, n=50/119), paresthesias, (29%, n=34/119), nausea (28%, n=33/119), and myalgias (23%, n=27/119) (Table 2). The majority of treatment-related adverse events were Grade 1 or 2 in severity; Grade 3 events were reversible with dose modifications. Dose reduction occurred in 15% (n=18/119) of patients. No dose-limiting toxicities (DLTs) were observed on ALKA-372-001; two DLTs occurred on STARTRK-1 at a daily dose of 800 mg: Grade 3 cognitive disturbance and Grade 3 fatigue, both resolved with dose interruption. At the 800 mg dose level, one additional patient experienced Grade 4 eosinophilic myocarditis, which was the only Grade 4 treatment-related adverse event reported on either study. This event occurred after 2 doses of entrectinib; the patient was subsequently discontinued from the study and fully recovered from the event. No Grade 5 treatment-related adverse events were reported.
Table 2. Adverse Events.
Listed below are adverse events reported in at least 10% of the patients (n=119) with advanced solid tumors who received entrectinib on either Phase 1 trial (ALKA-372-001 or STARTRK-1) and that were deemed by the investigators to be related to study drug. There was only one Grade 4 treatment-related adverse event of eosinophilic myocarditis on STARTRK-1. No treatment-related Grade 5 events were reported.
| Adverse Event Term n (%) | Grade 1 | Grade 2 | Grade 3 | All Grades (n=119) |
|---|---|---|---|---|
| Fatigue/Asthenia | 28 (24) | 22 (19) | 5 (4) | 55 (46) |
| Dysgeusia | 47 (40) | 3 (3) | 0 | 50 (42) |
| Paresthesia | 34 (29) | 0 | 0 | 34 (29) |
| Nausea | 29 (24) | 4 (3) | 0 | 33 (28) |
| Myalgia | 23 (19) | 4 (3) | 0 | 27 (23) |
| Diarrhea | 19 (16) | 3 (3) | 1 (1) | 23 (19) |
| Vomiting | 19 (16) | 1 (1) | 0 | 20 (17) |
| Arthralgia | 12 (10) | 6 (5) | 1 (1) | 19 (16) |
| Dizziness | 14 (12) | 5 (4) | 0 | 19 (16) |
| Constipation | 12 (10) | 2 (2) | 0 | 14 (12) |
| Weight increased | 4 (3) | 6 (5) | 2 (2) | 12 (10) |
There were no significant differences in toxicity between patients who received intermittent dosing (Schedules A and C on ALKA-372-001) and continuous dosing (Schedule B on ALKA-372-001 and STARTRK-1) despite numerical differences between these two groups, such as a higher incidence of dysgeusia, increased blood creatinine, and weight increase in patients on continuous dosing. These are detailed in Supplemental Table 1.
The number of patients and treatment-related adverse events observed at each dose level on either trial are summarized in Supplemental Tables 2 and 3. A continuous dose of 400 mg/m2 was designated as the BSA-based RP2D based upon a review of safety and pharmacokinetics. Per protocol, the next dose tested was 800 mg (fixed dosing), which resulted in two DLTs as described above; a continuous dose of 600 mg daily was then tested and identified as the fixed-dose MTD and RP2D in adults. The treatment-related adverse events in patients who received entrectinib at the RP2D are summarized in Supplemental Table 4.
Pharmacokinetics
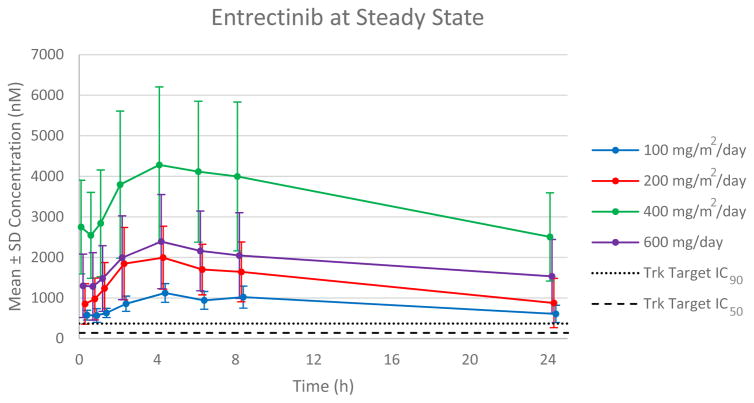
In the ALKA-372-001 study, Schedule A, when entrectinib was administered in the fasted state, exposure (Cmax and AUC) appeared to increase in a dose-proportional manner across the dose range of 100 to 800 mg/m2 with no appreciable increase in exposure observed at higher doses of 1200 or 1600 mg/m2. In Schedules B and C, entrectinib exposure increased in a less than dose-proportional manner when it was co-administered with food. In the STARTRK-1 study, entrectinib was administered with food and exposure increased in a linear manner from 100 to 400 mg/m2, and from 600 to 800 mg flat dosing (Figure 1). Steady state was reached within two weeks of continuous dosing with average accumulation of approximately 2-fold. The plasma half-life of entrectinib was estimated to be between 20–22 hours and compatible with a once daily, continuous dosing regimen (Supplemental Table 5). At the RP2D, the mean steady state Ctrough of 1590 nM was greater than 4-fold higher than that of trough concentrations observed in animal tumor models with complete tumor inhibition (corrected for plasma protein binding differences across species). (36, 37)
Figure 1. Pharmacokinetics of Entrectinib at Steady-State (Continuous Daily Dosing).
Mean steady state (Day 28) patient plasma concentration profiles at escalating dose levels were plotted over the dosing interval following once-daily continuous dosing. The target IC50 and IC90 values are based on entrectinib-induced tumor growth inhibition in mouse xenograft models of NTRK1-rearranged colorectal cancer.
Antitumor Activity
Of the 119 patients treated on either trial, 60 had a gene rearrangement involving NTRK1/2/3, ROS1, or ALK. Of the remaining 59 patients, 53 had other molecular alterations, broadly categorized as point mutations, amplifications, copy number variants, or insertions/deletions, and six patients were enrolled without a known alteration of NTRK1/2/3, ROS1, or ALK (Supplemental Table 6). No objective responses (per RECIST v1.1)(38) were observed in patients whose tumors harbored non-gene fusions involving NTRK1/2/3, ROS1, or ALK, with the exception of one patient with an ALK F1245V mutant neuroblastoma for whom a durable, confirmed PR lasted 8.3 months; this patient remained on study treatment for more than 3.5 years due to clinical benefit. Furthermore, no responses were observed in the 25 patients with recurrent gene rearrangements involving ROS1 (n=6) or ALK (n=19) who had previously received a ROS1 inhibitor (crizotinib) or ALK inhibitors (crizotinib, ceritinib, or alectinib), respectively, prior to entrectinib. Thirteen of the 19 ALK patients received more than one prior ALK inhibitor, including two patients that received more than two prior ALK inhibitors.
Phase 2-Eligible Population
Given that responses were observed only in TKI treatment-naïve patients with a fusion involving NTRK1/2/3, ROS1, or ALK, the population that would serve as the focus of later-phase development of entrectinib, an analysis was performed of patients treated on either Phase 1 trial who met criteria for what was defined as a “Phase 2-eligible population”. This included patients whose tumors harbored a recurrent gene fusion involving any of the 5 genes of interest, with a history of no prior TKI treatment targeting the fusion of interest, and who were treated on ALKA-372-001 or STARTRK-1 at doses that achieved therapeutic exposures consistent with the RP2D of 600 mg of entrectinib daily (Supplemental Figure 1).
Of the 60 patients with gene rearrangements, five patients were treated with doses that were below those which achieved therapeutic exposures consistent with the RP2D. Of the remaining 55 patients, 25 were previously treated with a TKI targeted to one of the fusions of interest (45%, n=25/55). For the purposes of this analysis, crizotinib was not considered a significant inhibitor of TRKA/B/C (IC50s of 580 and 399 nM towards TRKA and TRKB, respectively)(39), and one patient with an NTRK3 fusion who received this drug in the past was classified as ”Phase 2-eligible”.
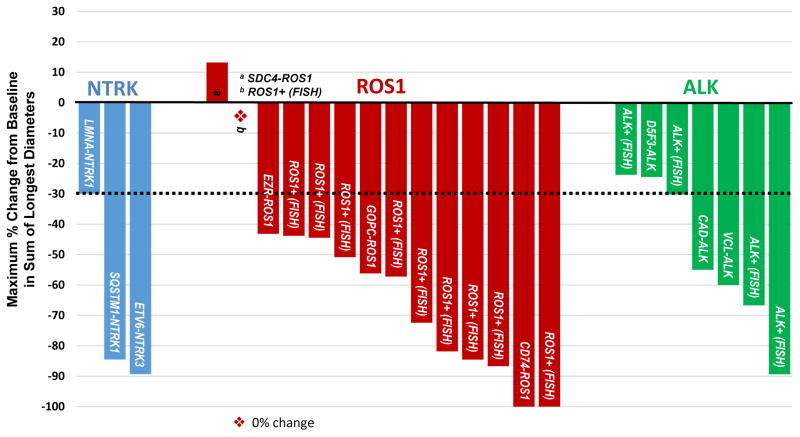
Of the resulting 30 patients comprising the “Phase 2-eligible” patient population as defined above, 25 patients were evaluable (Table 3), of whom 24 patients had extracranial solid tumors and one patient had a glioneuronal tumor. The waterfall plot for the 24 patients with extracranial solid tumors is shown in Figure 2. In three NTRK1/2/3-rearranged advanced solid tumors with RECIST-measurable disease, the ORR was 100% (95% CI: 44, 100). These included patients with NSCLC (SQSTM1-NTRK1)(40), MASC (ETV6-NTRK3)(31), and colorectal cancer (LMNA-NTRK1)(16). An additional patient with a BCAN-NTRK1-rearranged glioneuronal tumor experienced stable disease by RECIST, but further analysis via 3-dimensional volumetric assessment demonstrated a 60% reduction in total tumor burden(41). This radiographic response was accompanied by a clinical response to therapy with diminished ataxia and diplopia.
Table 3. Molecular Characteristics of the “Phase 2-Eligible” Patients.
The specific molecular profile of 25 patients with advanced solid tumors who received entrectinib on either Phase 1 trial (ALKA-372-001 or STARTRK-1) are summarized.
| No. | Gene | Tumor Type | Molecular Alteration | Diagnostic Method |
|---|---|---|---|---|
| 1 | NTRK | NSCLC | SQSTM1-NTRK1 | NGS |
| 2 | NTRK | Glioneuronal | BCAN-NTRK1 | NGS |
| 3 | NTRK | MASC | ETV6-NTRK3 | NGS |
| 4 | NTRK | mCRC | LMNA-NTRK1 | NGS |
| 5 | ROS1 | NSCLC | ROS1+ | FISH |
| 6 | ROS1 | NSCLC | ROS1+ | FISH |
| 7 | ROS1 | NSCLC | CD74-ROS1 | NGS |
| 8 | ROS1 | NSCLC | ROS1+ | FISH |
| 9 | ROS1 | NSCLC | ROS1+ | FISH |
| 10 | ROS1 | NSCLC | EZR-ROS1 | NGS |
| 11 | ROS1 | NSCLC | ROS1+ | FISH |
| 12 | ROS1 | Melanoma | GOPC-ROS1 | NGS |
| 13 | ROS1 | NSCLC | ROS1+ | FISH |
| 14 | ROS1 | NSCLC | ROS1+ | FISH |
| 15 | ROS1 | NSCLC | ROS1+ | FISH |
| 16 | ROS1 | NSCLC | ROS1+ | FISH |
| 17 | ROS1 | NSCLC | ROS1+ | FISH |
| 18 | ROS1 | NSCLC | SDC4-ROS1 | NGS |
| 19 | ALK | NSCLC | ALK+ | FISH |
| 20 | ALK | NSCLC | ALK+ | FISH |
| 21 | ALK | RCC | VCL-ALK | NGS |
| 22 | ALK | NSCLC | ALK+ | FISH |
| 23 | ALK | mCRC | CAD-ALK | NGS |
| 24 | ALK | NSCLC | ALK+ | FISH |
| 25 | ALK | Unknown Primary | D5F3-ALK | NGS |
MASC = Mammary Analog Secretory Carcinoma; RCC = Renal Cell Carcinoma
Figure 2. Best Response to Entrectinib in TKI Treatment-Naïve Extracranial Solid Tumor Patients.
Each bar represents maximal tumor regression from baseline based upon the sum of the longest diameters of target lesions (per RECIST 1.1) in the 24 “Phase 2-eligible” patients with extracranial solid tumors. The dashed line at −30% indicates the threshold for partial response. Specific molecular alterations are shown for each patient. The asterisk indicates one patient with ROS1-rearranged NSCLC, who experienced no change in tumor burden during treatment with entrectinib.
In 14 ROS1-rearranged solid tumors, the ORR was 86% (95% CI: 60, 96). These confirmed responses included 2 CRs. With the exception of one patient with a GOPC-ROS1-rearranged melanoma, all other patients who responded had ROS1-rearranged NSCLC. In seven ALK-rearranged solid tumors, the ORR was 57% (95% CI: 25, 84), and responses were observed in ALK-rearranged NSCLC, renal cell carcinoma, and colorectal cancer.
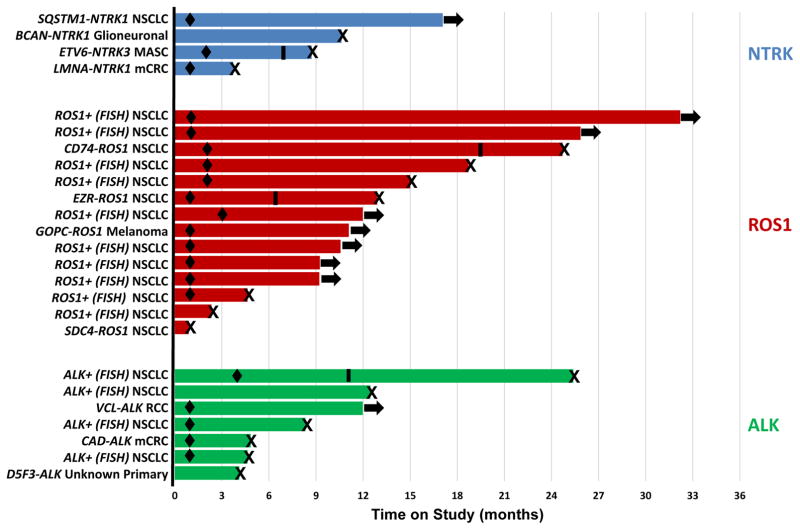
Initial responses to entrectinib were demonstrated within Cycles 1 (scans performed at 4 weeks) or 2 (scans performed at 8 weeks). Responses to entrectinib therapy were also durable, with the longest duration of clinical benefit observed in a ROS1-rearranged lung cancer patient who remains on therapy at 32 months as of the data cutoff date (Figure 3). Recognizing that different tumor types were treated, the median duration of response for ROS1- and ALK-rearranged cancers was 17.4 months (95% CI: 12.7, not reached) and 7.4 months (95% CI: 3.7, not reached), respectively. For the three responding patients with NTRK-rearranged cancers, the durations of response were 2.6 months, 4.6 months, and 15.1 months (patient ongoing as of data cutoff date), respectively.
Figure 3. Duration of Treatment.
Each bar indicates the duration of treatment for the 25 “Phase 2-eligible” patients at the time of data cutoff. Specific molecular alterations are shown next to each patient. Arrows indicate patients who were ongoing on study; X denotes patients who discontinued the study (all due to disease progression); black diamonds represent time of first response; black bars represent three patients who experienced disease progression but remained on study due to clinical benefit.
With a median duration of follow up of 15 months, a number of exploratory secondary endpoints were analyzed. Considering the variety of tumor types evaluated in this study, each with a different natural history, the median progression-free survival for patients harboring NTRK1/2/3- (n=4), ROS1- (n=14), and ALK- (n=7) rearranged malignancies was not reached (95% CI: 3.6, not reached), 19.0 months (95% CI: 6.5, not reached), and 8.3 months (95%CI: 4.6, 12.0), respectively. Among all 25 patients, the median overall survival has not been reached (95% CI: 19.0 months, not reached). The proportion of patients surviving at 12 months was 89.4% (95% CI: 75.5, 100%).
Intracranial Activity
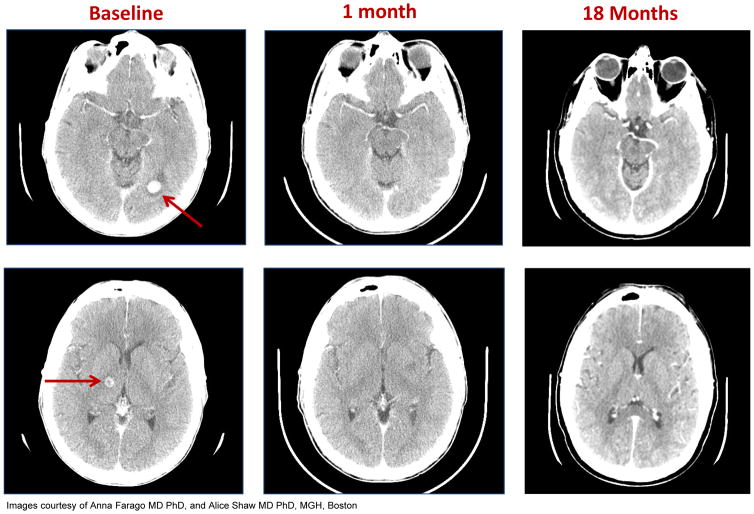
Among the 25 evaluable “Phase 2-eligible population”, 32% (n=8/25) of patients had known primary or metastatic disease involving the brain prior to treatment with entrectinib. Responses were noted in five out of the eight (63%) patients: four patients with NTRK1- (n=1), ROS1- (n=2), and ALK- (n=1) rearranged NSCLC and one additional patient with ALK-rearranged colorectal cancer; among the responders, four patients have had prior radiotherapy to the brain. Of note, the patient with SQSTM1-NTRK1-rearranged NSCLC had 15–20 brain metastases identified at baseline not previously irradiated; a complete intracranial response was achieved with entrectinib therapy that is ongoing at 15 months as of the data cutoff date (Figure 4)(40).
Figure 4. Baseline and on-study brain MRI images for a patient with SQSTM1-NTRK1-rearranged lung cancer.
Baseline head CT scans show metastases (red arrows) in the left occipital lobe (top panel) and in the right thalamus (bottom panel). Restaging head CT scans show complete response at 1 month and 18 months on entrectinib (at the time of data cutoff).
DISCUSSION
Here, we present a large multicenter safety experience in 119 patients treated with the novel pan-TRK, ROS1, and ALK inhibitor entrectinib on two Phase 1 trials. We demonstrated that the drug is safe and well-tolerated. No patients discontinued the study due to adverse events. The majority of treatment-related adverse events were Grade 1 or 2 in severity, and all were reversible with dose interruption and/or modification. Dose modification was required in only 15% of patients. Specific adverse events such as dysgeusia, sensory neuropathy, cognitive changes, and weight gain, are thought to be on-target toxicities of entrectinib mediated by TRK receptor inhibition(42). Forty-five patients were treated at the identified recommended Phase 2 dose of 600 mg daily with continuous dosing. Given that no significant differences in toxicity were observed between continuous daily dosing and intermittent dosing schedules, continuous dosing was chosen due to its ability to enable 24-hour continuous exposures above those required for complete tumor inhibition in animal tumor models, resulting in sustained target inhibition.
Entrectinib demonstrated robust antitumor activity in TKI-naïve patients harboring gene rearrangements involving NTRK, ROS1, or ALK genes. Responses were fast and durable, and clinical benefit was observed across a broad range of solid tumors regardless of histology, including NSCLC, MASC, melanoma, glioneuronal tumor, colorectal cancer, and renal cell carcinoma. The majority of responses were observed within Cycle 1 or 2, and several patients continued treatment beyond a year, with the longest response approaching 2.5 years as of the time of the data cutoff. Intriguingly, the responses in patients with NTRK-rearranged tumors are particularly notable as these provide proof-of-principle that NTRK rearrangements are clinically actionable drivers of tumor growth. We thus strongly encourage that clinicians continue to test for these alterations using comprehensive molecular profiling platforms that are poised to identify these alterations, preferably with a strategy that combines both testing at the DNA level and, potentially, the RNA level, when feasible.(13) While no responses were observed in patients with recurrent gene rearrangements who had previously received ROS1 or ALK inhibitors, further investigation will be required to determine the activity of this drug in TKI pre-treated patients considering that the drug is active preclinically against potential resistance mutations such as the ALK L1196M mutation that can emerge after crizotinib therapy in ALK-rearranged lung cancers.
Of note, entrectinib showed promising antitumor activity in the central nervous system. This becomes particularly important when we consider that cancers that can harbor NTRK, ROS1, or ALK rearrangements such as lung cancers and melanomas have a proclivity for central nervous system metastasis. Moreover, many primary adult and pediatric brain tumors such as astrocytoma, glioblastoma, and pediatric gliomas harbor NTRK1, NTRK2, NTRK3, or ROS1 fusions.(10) On STARTRK-1, a complete response was achieved in the brain in a patient with SQSTM1-NTRK1-rearranged lung cancer with an ongoing response at 15 months at the time of the data cutoff(40). Substantial reduction in disease burden was likewise noted by volumetric analysis in a BCAN-NTRK1-rearranged glioneuronal tumor(41). These cases highlight the intracranial activity of entrectinib against both metastatic disease and primary brain tumors that can otherwise result in substantial morbidity and mortality. As has been observed in ALK-rearranged lung cancers, the use of a CNS-penetrant drug like entrectinib in the first-line setting in patients with ROS1-rearranged lung cancers may potentially improve outcomes for patients compared to treatment with crizotinib, which is thought to be less CNS-penetrant.
Lastly, these studies emphasize the utility of clinical trial strategies that focus on molecular enrichment independent of tumor histology as a model for the development of promising targeted therapies, especially in patients with rare incidence of genomic aberrations. Over the last decade, expansion cohorts on Phase 1 trials have driven the accelerated approval of targeted therapies such as crizotinib for ALK- and ROS1-rearranged lung cancers.(6, 34) The same model can potentially be applied to establish preliminary efficacy across a variety of cancer types, especially as actionable drivers of interest such as NTRK rearrangements are detected at a lower frequency across multiple histologies, precluding the ability to easily accrue histology-specific cohorts on ongoing trials. Later phase clinical trials, so called “basket studies” provide a complementary approach that utilizes this paradigm. For entrectinib, a global, multicenter, Phase 2 basket study (STARTRK-2, NCT02568267) is currently accruing patients with NTRK-, ROS1-, and ALK-rearranged cancers with the intent of confirming the results generated by STARTRK-1 and ALKA.
METHODS
Patients with locally advanced or metastatic solid tumors harboring NTRK1/2/3, ROS1, or ALK molecular alterations were enrolled in one of two Phase 1 studies aimed at determining the MTD or RP2D of entrectinib: Study ALKA-372-001 (“ALKA”; EudraCT 2012-000148-88; 2 sites, Italy) and Study RXDX-101-01 (“STARTRK-1”; NCT02097810; 10 sites, United States, Korea, and Spain). Patients were enrolled in the ALKA study between October 2012 and November 2015, and in the STARTRK-1 study between July 2014 and February 2016, respectively.
Study Design
Patients were assigned sequentially to escalating dose levels of entrectinib following a standard 3+3 design. All patients received entrectinib orally and remained on study treatment until disease progression (with allowance to remain on study if the treating physician deemed the patient as continuing to derive clinical benefit), development of unacceptable toxicity, or withdrawal of consent. Fasted and fed, BSA-based and flat dosing, as well as intermittent and continuous daily dosing regimens were evaluated. Entrectinib was initially dosed by body surface area (BSA; doses ranging from 100 mg/m2 to 1600 mg/m2) and later transitioned to flat dosing (doses ranging from 600 mg to 800 mg). In addition, intermittent dosing regimens were evaluated in addition to once daily, continuous dosing. In the ALKA study, patients were enrolled across three dosing regimens: Schedule A (fasted, 4 days on/3 days off for three out of four weeks), Schedule B (fed, continuous daily dosing), and Schedule C (fed, 4 days on/3 days off). In the STARTRK-1 study, all patients received entrectinib on a fed, continuous daily dosing regimen (Supplemental Figure 2). Patients were enrolled into STARTRK-1 and ALKA Schedules B and C simultaneously after Schedule A was completed.
On both studies, the starting dose was 100 mg/m2. At least 3 patients at each dose level were monitored for dose limiting toxicities (DLTs) through Cycle 1 (Day 28 for ALKA and Day 42 for STARTRK-1). DLTs were defined as any Grade ≥ 2 CNS, Grade ≥ 3 non-hematologic, Grade ≥ 3 and/or lasting > 7 days hematologic (as well as febrile neutropenia) toxicities according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE; v4.03). Failure to recover (except alopecia) to Grade ≤ 1 or baseline after delaying the initiation of next cycle by a maximum of 14 (ALKA) or 28 (STARTRK-1) days was also considered a DLT. Patients were eligible for DLT evaluation if they experienced a DLT after at least one dose of study drug or did not experience a DLT having taken a minimum of 75% (ALKA) or 80% (STARTRK-1) of doses expected during Cycle 1. Patients who did not fulfill these requirements or who discontinued the study prior to completing the DLT evaluation period were to be replaced. The MTD was defined as the highest dose associated with first-cycle DLT in < 33% of patients. If an MTD was not reached, RP2D was to be selected based on available safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) data from different dose levels and dosing regimens tested.
Study Population
All patients had a histologically- or cytologically-confirmed diagnosis of relapsed or refractory advanced/metastatic solid tumor for whom no alternative effective standard therapy was available or for whom standard therapy was considered unsuitable or intolerable, Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2, a life expectancy of ≥ 3 months, and adequate organ function. Patients with stable asymptomatic CNS involvement were eligible.
TRKA/B/C, ROS1, or ALK molecular alterations were detected via immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), or RNA/DNA-based methods (e.g., next generation sequencing [NGS], NanoString) performed at the various local institutions or through third-party commercial diagnostic providers.
Institutional review boards and/or ethics committees approved the study, which was conducted according to the Declaration of Helsinki, the International Conference on Harmonisation, and the Guidelines for Good Clinical Practice. Data were anonymized to protect the identities of subjects involved in the research. All patients provided written informed consent.
Safety Assessments
Safety was assessed from the first dose until 30 days after the last dose of entrectinib or until the resolution or stability of any drug-related toxicity. Clinical and laboratory assessments were performed at least once weekly during the first 2 cycles of treatment: weekly and bi-weekly after ≥ 1 year of treatment (ALKA) or bi-weekly (STARTRK-1), at the end of treatment visit, and approximately 28–30 days after discontinuing study drug. Laboratory assessments included routine hematology and chemistry panels, coagulation parameters, and urinalysis. Twelve-lead single ECGs (triplicate in STARTRK-1) were obtained at baseline and around the anticipated maximal and steady-state entrectinib plasma concentrations (e.g., between 3 and 6 hours post-dose) with time-matched PK samples (various time points on Cycles 1 through 4), at the end of treatment visit, as well as whenever clinically indicated, to assess for potential QTc changes as a result of treatment with entrectinib.
Pharmacokinetics
Depending on dosing regimens, serial blood samples for PK analyses were obtained at various time points throughout Cycle 1. Samples were shipped frozen to Accelera (ALKA; Nerviano, Italy) and InVentiv (STARTRK-1; Princeton, NJ, USA) for analysis of entrectinib (and its metabolites) using a validated liquid chromatography–tandem mass spectrometry assay. PK analysis for all parameters was performed using Phoenix WinNonlin software (version 6.4.0.768, Pharsight Corporation, Mountain View, CA, USA). Parameters analyzed included: maximum observed plasma concentration (Cmax) and minimum observed plasma concentration (C24h), time of maximum observed plasma concentration (Tmax), effective half-life (t1/2), and area under the plasma concentration-time curve (AUC).
Pharmacodynamics
For all patients who provided consent, archival tumor tissue, if available, was submitted for retrospective and/or additional exploratory genomic profiling. Tissue (if clinically feasible) and blood at the time of progression in addition to monthly blood samples were collected to gain insights into potential mechanisms of resistance. Molecular alteration status of the NTRK1/2/3, ROS1, and ALK genes, among others, were collected in nucleic acids isolated from plasma using NGS for future analyses.
Tumor Assessments
CT/MRI of the brain, chest, abdomen, and pelvis, as clinically indicated, were initially performed at the end of Cycle 2 and every 8 weeks thereafter. For both studies, a protocol amendment later modified the first assessment time point to the end of Cycle 1 and every 8 weeks thereafter. All scans were read locally and tumor response evaluated according to the Response Evaluation Criteria In Solid Tumors (RECIST) v1.1.(38)
Statistical Analysis
Patients who received at least one dose of entrectinib were included in the efficacy and safety analyses, irrespective of molecular alteration. In addition, patients with evidence of a gene fusion were analyzed as a subset. Demographics, baseline characteristics, adverse events, vital signs, and clinical laboratory evaluations were summarized with descriptive statistics. Objective response was defined as confirmed complete response (CR) or partial response (PR) that persists on repeat imaging ≥ 4 weeks after initial documentation of response. Objective response rate (ORR) was calculated as the proportion of responders out of the population of patients with measurable disease at baseline. The Kaplan-Meier method was used to estimate the median, 25th, and 75th percentiles for time-to-event endpoints (duration of response [DOR], progression-free survival [PFS], and overall survival [OS]), with corresponding 95% confidence intervals. The data cutoff date for safety and efficacy analyses for both studies was September 20, 2016.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
Gene fusions of NTRK1/2/3, ROS1 and ALK (encoding TRKA/B/C, ROS1 and ALK, respectively) lead to constitutive activation of oncogenic pathways. Entrectinib was shown to be well tolerated and active against those gene fusions in solid tumors, including in patients with primary or secondary CNS disease.
Acknowledgments
FUNDING
Supported by Ignyta, Inc.
Footnotes
CONFLICTS OF INTEREST
AD has received honoraria from Ignyta. AF has consulted for AbbVie, Intervention Insights, Merrimack Pharmaceuticals, Pharmamar SA, and Takeda. JW is currently employed by Novartis. RD has research agreements with Ignyta and Loxo and intellectual property royalties and/or licensing fees from Abbott Molecular and Ariad Pharmaceuticals. SS is an advisory board member of Amgen, Bayer, Eli Lilly, Merck, Novartis, Roche, and Sanofi. GL, JC, KK, AJ, RP, DL, ECM, ZH, and PM are employees of Ignyta. EA is an employee of Nerviano Medical Sciences.
References
- 1.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nature communications. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw AT, Hsu PP, Awad MM, Engelman JA. Tyrosine kinase gene rearrangements in epithelial malignancies. Nature reviews Cancer. 2013;13(11):772–87. doi: 10.1038/nrc3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drilon A, Wang L, Arcila ME, Balasubramanian S, Greenbowe JR, Ross JS, et al. Broad, Hybrid Capture-Based Next-Generation Sequencing Identifies Actionable Genomic Alterations in Lung Adenocarcinomas Otherwise Negative for Such Alterations by Other Genomic Testing Approaches. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(16):3631–9. doi: 10.1158/1078-0432.CCR-14-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy DA, Ely HA, Shoemaker R, Boomer A, Culver BP, Hoskins I, et al. Detecting Gene Rearrangements in Patient Populations Through a 2-Step Diagnostic Test Comprised of Rapid IHC Enrichment Followed by Sensitive Next-Generation Sequencing. Applied immunohistochemistry & molecular morphology. 2016 Mar 29; doi: 10.1097/PAI.0000000000000360. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. The New England journal of medicine. 2014;371(23):2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. The New England journal of medicine. 2014;371(21):1963–71. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. The New England journal of medicine. 2014;370(13):1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ou SH, Ahn JS, De Petris L, Govindan R, Yang JC, Hughes B, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(7):661–8. doi: 10.1200/jco.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. The lancet oncology. 2016;17(2):234–42. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer discovery. 2015;5(1):25–34. doi: 10.1158/2159-8290.CD-14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1(2) doi: 10.1136/esmoopen-2015-000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nature reviews Neuroscience. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 13.Vaishnavi A, Capelletti M, Le AT, Kako S, Butaney M, Ercan D, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nature medicine. 2013;19(11):1469–72. doi: 10.1038/nm.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardini E, Bosotti R, Borgia AL, De Ponti C, Somaschini A, Cammarota R, et al. The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition. Molecular oncology. 2014;8(8):1495–507. doi: 10.1016/j.molonc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Li GG, Kim ST, Hong ME, Jang J, Yoon N, et al. NTRK1 rearrangement in colorectal cancer patients: evidence for actionable target using patient-derived tumor cell line. Oncotarget. 2015;6(36):39028–35. doi: 10.18632/oncotarget.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartore-Bianchi A, Ardini E, Bosotti R, Amatu A, Valtorta E, Somaschini A, et al. Sensitivity to Entrectinib Associated With a Novel LMNA-NTRK1 Gene Fusion in Metastatic Colorectal Cancer. Journal of the National Cancer Institute. 2016;108(1) doi: 10.1093/jnci/djv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greco A, Mariani C, Miranda C, Lupas A, Pagliardini S, Pomati M, et al. The DNA rearrangement that generates the TRK-T3 oncogene involves a novel gene on chromosome 3 whose product has a potential coiled-coil domain. Molecular and cellular biology. 1995;15(11):6118–27. doi: 10.1128/mcb.15.11.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;120(6):799–807. doi: 10.1002/cncr.28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bongarzone I, Pierotti MA, Monzini N, Mondellini P, Manenti G, Donghi R, et al. High frequency of activation of tyrosine kinase oncogenes in human papillary thyroid carcinoma. Oncogene. 1989;4(12):1457–62. [PubMed] [Google Scholar]
- 20.Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nature communications. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh I, Tee MK, Botton T, Hunter Shain A, Sparatta AJ, Gagnon A, et al. NTRK3 kinase fusions in Spitz tumours. The Journal of pathology. 2016;240(3):282–290. doi: 10.1002/path.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haller F, Knopf J, Ackermann A, Bieg M, Kleinheinz K, Schlesner M, et al. Paediatric and adult soft tissue sarcomas with NTRK1 gene fusions: a subset of spindle cell sarcomas unified by a prominent myopericytic/haemangiopericytic pattern. The Journal of pathology. 2016;238(5):700–10. doi: 10.1002/path.4701. [DOI] [PubMed] [Google Scholar]
- 23.Morosini D, Chmielecki J, Goldberg M, Ross J, Stephens P, VAM, et al. Comprehensive genomic profiling of sarcomas from 267 adolescents and young adults to reveal a spectrum of targetable genomic alterations. Journal of Clinical Oncology. 2015;33(15_suppl (May 20 Supplement)):11020. [Google Scholar]
- 24.Punnett HH, Tomczak EZ, Pawel BR, de Chadarevian JP, Sorensen PH. ETV6-NTRK3 gene fusion in metastasizing congenital fibrosarcoma. Medical and pediatric oncology. 2000;35(2):137–9. doi: 10.1002/1096-911x(200008)35:2<137::aid-mpo12>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 25.Frattini V, Trifonov V, Chan JM, Castano A, Lia M, Abate F, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nature genetics. 2013;45(10):1141–9. doi: 10.1038/ng.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones DT, Hutter B, Jager N, Korshunov A, Kool M, Warnatz HJ, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nature genetics. 2013;45(8):927–32. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nature genetics. 2014;46(5):444–50. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eguchi M, Eguchi-Ishimae M, Tojo A, Morishita K, Suzuki K, Sato Y, et al. Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25) Blood. 1999;93(4):1355–63. [PubMed] [Google Scholar]
- 29.Kralik JM, Kranewitter W, Boesmueller H, Marschon R, Tschurtschenthaler G, Rumpold H, et al. Characterization of a newly identified ETV6-NTRK3 fusion transcript in acute myeloid leukemia. Diagnostic pathology. 2011;6:19. doi: 10.1186/1746-1596-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer cell. 2002;2(5):367–76. doi: 10.1016/s1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 31.Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC) Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2016;27(5):920–6. doi: 10.1093/annonc/mdw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urano M, Nagao T, Miyabe S, Ishibashi K, Higuchi K, Kuroda M. Characterization of mammary analogue secretory carcinoma of the salivary gland: discrimination from its mimics by the presence of the ETV6-NTRK3 translocation and novel surrogate markers. Human pathology. 2015;46(1):94–103. doi: 10.1016/j.humpath.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nature genetics. 1998;18(2):184–7. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 34.Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. The lancet oncology. 2012;13(10):1011–9. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson D, Ciomei M, Banfi P, Cribioli S, Ardini E, Galvani A, et al. Inhibition of Trk-driven tumors by the pan-Trk inhibitor RXDX-101. European journal of cancer. 2014;50:101. [Google Scholar]
- 36.Ardini E, Menichincheri M, Banfi P, Bosotti R, De Ponti C, Pulci R, et al. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Molecular cancer therapeutics. 2016;15(4):628–39. doi: 10.1158/1535-7163.MCT-15-0758. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Kim ST, Kim K-M, Lee J, Russo M, Misale S, et al. Abstract A173: Potent anti-tumor activity of entrectinib in patient-derived models harboring oncogenic gene rearrangements of NTRKs. Molecular cancer therapeutics. 2015;14(12 Supplement 2):A173-A. [Google Scholar]
- 38.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer research. 2007;67(9):4408–17. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 40.Farago AF, Le LP, Zheng Z, Muzikansky A, Drilon A, Patel M, et al. Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10(12):1670–4. doi: 10.1097/01.JTO.0000473485.38553.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez-Breckenridge C, Miller J, Nayyar N, Gill C, Kaneb A, D’Andrea M, et al. Clinical and radiographic response following targeting of novel BCAN-NTRK1 fusion in glioneuronal tumors. NPJ Precision Oncology. 2017 doi: 10.1038/s41698-017-0009-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin JC, Tsao D, Barras P, Bastarrachea RA, Boyd B, Chou J, et al. Appetite enhancement and weight gain by peripheral administration of TrkB agonists in non-human primates. PloS one. 2008;3(4):e1900. doi: 10.1371/journal.pone.0001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.