X-linked transmission of ataxia (spinocerebellar ataxia, X-linked; SCAX) is relatively rare. A heterozygous (p.G1107D) mutation in isoform 3 of the plasma membrane calcium-transporting ATPase (ATP2B3) was previously reported to disrupt calcium export and lead to SCAX1 (OMIM #302500) in a boy and his uncle in a single family.1 This protein is a member of the calmodulin-activated plasma membrane ATPase family, extruding excess calcium from the cell to maintain homeostasis. ATP2B3 is highly expressed within the presynaptic region of cerebellar granule cells.1
The original article implicating ATP2B3 in SCAX1 predicted this point mutation would impair interactions with calmodulin. Confirmatory in vitro experiments demonstrated decreased calcium clearance from the cell in mutant constructs. Subsequently, a single patient with X-linked ataxia harboring both a hemizygous ATP2B3 (p.R482H) mutation and LAMA1 compound heterozygous mutations was reported.2 Although a deleterious effect of the ATP2B3 (p.R482H) mutation was shown in vitro, incomplete penetrance for the ATP2B3 mutation was observed, and in the proband, a digenic effect of the ATP2B3 and LAMA1 mutations was postulated.
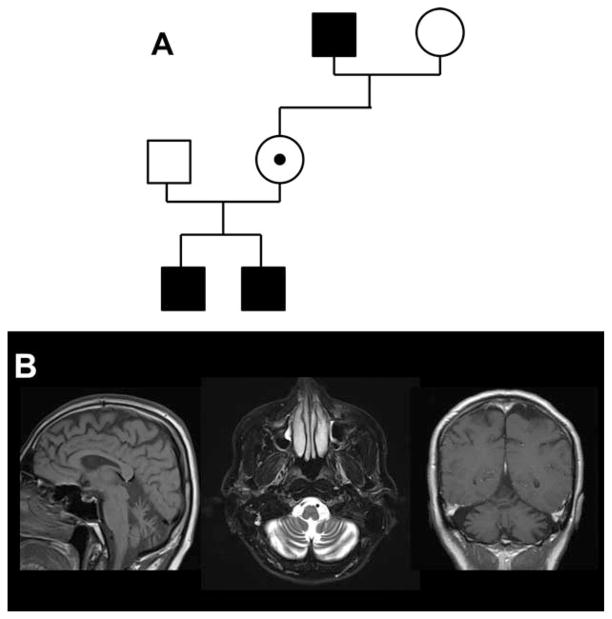
In the present family (Fig. 1A), a maternal grandfather exhibited ataxia and dysarthria as a child. No dementia was evident by MMSE. These symptoms were relatively stable for many years. This grandfather’s daughter was healthy and exhibited no signs of ataxia on examination. However, her 2 boys developed early childhood ataxia noted between their first and second birthdays. Over time, the boys exhibited increasingly prominent hypotonia, mild titubation, dysmetria, dysdiadochokinesis, and an ataxic gait. The pedigree thus suggested a nondegenerative, early-onset X-linked ataxia. Motion analysis laboratory evaluation indicated the presence of concurrent dystonia in the older boy (Supporting Information Video). His gait improved with levodopa administration, leading to a decrease in the number of falls sustained per week. Thus far, the boys’ course has been stable.
FIG. 1.
Familial cerebellar degeneration. A: Pedigree demonstrating X-linked inheritance of ataxia. B: MRI of brain demonstrates volume loss of the cerebellar hemispheres and vermis. [Color figure can be viewed at wileyonlinelibrary.com]
Family members were enrolled into our institutional review board–approved protocol, and whole exome sequencing of the affected individuals disclosed a hemizygous c.3321G>A (p.G1107D) mutation in ATP2B3. This was confirmed by Sanger sequencing. The unaffected mother was a heterozygous carrier for this mutation. ExAc Browser review (http://exac.broadinstitute.org; rs397514619) indicated a single heterozygous allele out of 28,066 alleles (allele frequency = 3.563e-05). No homozygous or hemizygous alleles were detected. A brain MRI disclosed isolated volume loss of the cerebellar vermis and hemispheres (Fig. 1B).
Thus we present a 3-generation pedigree also harboring the (p.G1107D) mutation originally reported by Zanni and colleagues.1 Onset was in childhood in the hemizygous maternal grandfather and his 2 hemizygous grandsons, whereas a heterozygous daughter remained unaffected. This second unrelated family demonstrating X-linked recessive inheritance of an ATP2B3 mutation further validates the role of this calcium transporter as an important cause of X-linked ataxia and indicates that levodopa-responsive dystonia may also be a feature of this disease. Finally, in light of the recent identification of HPCA gene mutations in dystonia, 3 the identification of dystonia in ATP2B3 patients further implicates dysregulated calcium homeostasis in dystonia.
Supplementary Material
Acknowledgments
Funding agencies: The TGen Foundation, the Phoenix Children’s Hospital Foundation, and grants from the Child Neurology Foundation (Shields Award to M.C.K.), Doris Duke Charitable Foundation (Clinical Scientist Development Award to M.C.K.) and the Dystonia Medical Research Foundation (M.C.K.).
We gratefully acknowledge the participation of the index family in our studies; without their support, this work would not have been possible. This study was supported by the TGen Foundation, the Phoenix Children’s Hospital Foundation, and by grants from the Child Neurology Foundation (Shields Award to MCK) and the Dystonia Medical Research Foundation (MCK). The authors also acknowledge the contributions of the C4RCD Research Group. This group includes the clinical and laboratory research team involved in patient enrollment, sample processing, exome sequencing, data processing, preparation of variant annotation files, data analysis, validation of data, and return of research data to families. Candidate genes are identified and discussed at data analysis meetings of the entire group. The following members of the group (listed in alphabetical order) have contributed significantly to this work: Newell Belnap (nbelnap@tgen.org), Megan Russell (mrussell@tgen.org), Amanda Courtright (acourtright@tgen.org), Matt de Both (mdeboth@tgen.org), Ana Claasen (aclaasen@tgen.org), Ahmet Kurdoglu (akurdoglu@tgen.org; akurdoglu@ashiondx.com), Sampathkumar Rangasamy (srangasamy@tgen.org), Ryan Richholt (rrichholt@tgen.org), Isabelle Schrauwen (ischrauwen@tgen.org), Ashley L. Siniard (asiniard@tgen.org), Szabolics Szelinger (sszelinger@tgen.org).
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Zanni G, Calì T, Kalscheuer VM, et al. Mutation of plasma membrane Ca2+ ATPase isoform 3 in a family with X-linked congenital cerebellar ataxia impairs Ca2+ homeostasis. Proc Natl Acad Sci USA. 2012;109(36):14514–14519. doi: 10.1073/pnas.1207488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calì T, Lopreiato R, Shimony J, et al. A novel mutation in isoform 3 of the plasma membrane Ca2+ pump impairs cellular Ca2+ homeostasis in a patient with cerebellar ataxia and laminin subunit 1α mutations. J Biol Chem. 2015;290(26):16132–16141. doi: 10.1074/jbc.M115.656496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlesworth G, Angelova PR, Bortolome-Robledo F, et al. Mutations in HPCA cause autosomal recessive primary isolated dystonia. Am J Hum Genet. 2015;96:657–665. doi: 10.1016/j.ajhg.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.