Abstract
Objectives
The Renal Activity Index for Lupus (RAIL) score was developed in children with lupus nephritis (LN) as a weighted sum of six urine biomarkers (RAIL-UBMs) [neutrophil gelatinase associated lipocalin, monocyte chemotactic protein 1, ceruloplasmin, adiponectin, hemopexin and kidney injury molecule 1] measured in a random urine sample. We aimed at prospectively validating the RAIL in adults with LN.
Methods
Urine from 79 adults was collected at the time of kidney biopsy to assay the RAIL-UBMs. Using Receiver Operating Characteristic curve (ROC) analysis, we evaluated the accuracy of the RAIL to discriminate high LN-activity status [National Institutes of Health Activity Index (NIH-AI) score > 10], from low/moderate LN-activity status [NIH-AI score ≤ 10].
Results
In this mixed racial cohort, high LN-activity was present in 15 patients (19%), and 71% had proliferative LN. Use of the identical RAIL algorithm developed in children (P-RAIL) resulted only in fair prediction of LN-activity status of adults [area-under the ROC-curve (AUC) 0.62]. Alternative weightings of the six RAIL-UBMs as suggested by logistic regression yielded excellent accuracy to predict LN-activity status (A-RAIL; AUC = 0.88). Accuracy of the model did not improve with adjustment of the UBMs for urine creatinine or albumin, and was little influenced by concurrent kidney damage.
Conclusions
The RAIL-UBMs provide excellent prediction of LN-activity in adults. Age-adaption of the RAIL is warranted to optimize its discriminative validity to non-invasively predict high LN-activity status.
Keywords: systemic lupus erythematosus, lupus nephritis, adult, urinary biomarkers, renal activity index of lupus
INTRODUCTION
Kidney involvement with systemic lupus erythematosus (SLE) is associated with significant morbidity, including end-stage renal disease (1). The International Society of Nephrology/Renal Pathology Society (ISN/RPS) Classification of lupus nephritis (LN) recognizes mesangial, proliferative and membranous patterns of LN (2), with the degree or LN activity and chronicity often scored as per the LN Activity (NIH-AI) and LN Chronicity (NIH-CI) index, respectively (3). Kidney biopsies are needed to diagnose LN and to support the initiation of appropriate therapies, guided by ISN/RPS Class, NIH-AI and NIH-CI scores. Although the concept of a “liquid-biopsy”, i.e. biomarker levels measurement in body fluids, has been proposed and is, at least partially, implemented for several cancers (4), the same has not been realized for LN. Indeed, traditional blood and urine tests do not reliably reflect biopsy findings, especially in patients receiving medication (5,6).
Using a cohort of children with LN, we developed the Renal Activity Index for Lupus (RAIL) to allow for the non-invasive assessment of LN activity, based solely on the concentrations of six proteins measured in a random urine sample. The six protein urine biomarkers (RAIL-UBMs) are neutrophil gelatinase associated lipocalin (NGAL), monocyte chemotactic protein 1 (MCP-1), ceruloplasmin, adiponectin, hemopexin and kidney injury molecule 1 (KIM-1). In children, a RAIL score of 0.39 or higher correctly identified 92% of all children with high LN-activity status, defined as NIH-AI scores exceeding 10 (7).
Initial unbiased discovery of biomarkers, such as the RAIL-UBMs, is best done in children as they generally lack co-morbid conditions that could affect urine biomarker concentrations. However, prior to the potential use of a novel biomarker panel in clinical practice or research, rigorous validation of the measurement properties in other cohorts is necessary (8).
The goals of this study were to (1) prospectively validate the RAIL algorithm when used in adults with LN; and (2) explore whether alternative scoring algorithms based on the RAIL-UBMs can improve the accuracy with which LN can be measured in adult populations; and (3) in support of the biological relevance of the RAIL-UBMs, we also performed staining of kidney tissues.
MATERIAL & METHODS
Patients and samples
Seventy-nine consecutive patients enrolled in the Ohio State University (OSU) LN Registry were included in this cross-sectional prospective study. All patients fulfilled the 1997 American College of Rheumatology (ACR) classification criteria for SLE (9), and treating physicians requested a kidney biopsy as part of routine evaluation for SLE. At the time of biopsy, a random urine specimen was collected. Registry information included data on patient demographics, results of standard laboratory parameters and traditional LN measures, including complements levels C3 and C4, presence/absence of anti-dsDNA antibodies, protein to creatinine ratio from a random urine sample, and the estimated glomerular filtration rate (eGFR), using the Modification of Diet in Renal Disease Study equation (10).
The OSU Institutional Review Board approved of the OSU LN Registry and the sharing of samples and data for this study.
Renal Histology and LN activity
Two expert nephro-pathologists (SB, TN), who were blinded to patient clinical information and the results of UBM testing, independently interpreted the renal histology and scored the NIH-AI and NIH-CI indices. The NIH-AI scores range from 0 to 24 (0= inactive LN), with higher scores representing more active LN (3). Similarly, we used the NIH-CI with scores ranging 0 to 12 (0= no LN damage) and higher scores signifying more chronicity (3).
Urinary Biomarker Assays
Laboratory personnel assaying the UBMs were also blinded to patient information. Spun urine samples were stored at 0°C within 1 hour of collection and frozen at −80°C within 24 hours prior to batch processing.
Unless stated otherwise, UBMs were quantified using commercial ELISA kits as per the manufacturers’ instructions. A four parameter logistic curve-fit was used to fit the standard curve. Inter-assay and intra-assay variability of these assays is expressed in percent of the coefficient of variation [CV intra/inter]: NGAL [CV intra/inter: 1.0%/9.1%] was measured by ELISA (Human NGAL; Bioporto, Grusbakken, Denmark); MCP-1 [CV intra/inter: 5.0%/5.9%] by ELISA (R&D Systems, Minneapolis, MN). Ceruloplasmin [CV intra/inter: 4.1%/7.1%] was quantified by ELISA (Assaypro, St.Charles, MO). Adiponectin [CV intra/inter: 4.0%/9.9%] was measured using the Quantikine ELISA Human HMW Adiponectin/Acrp30 (R&D Systems, Minneapolis, MN) and Hemopexin [CV intra/inter: 4.8%/7.3%] was quantified with the AssayMax Human Hemopexin ELISA Kit (Assaypro, St. Charles, MO). The KIM-1 assay [CV intra/inter: 2%/7.8%] as constructed using commercially available reagents (Duoset DY1750, R & D Systems, Minneapolis, MN) as described previously (11). Urine creatinine [CV intra/inter: 2.4%/4.2%] measurements were made using a modified Jaffe reaction, and albumin [CV intra/inter 2.9%/5.9%] was measured by immunoturbidimetry, both on a Dimension Xpand Plus HM Clinical Analyzer (Siemens, Munich, Germany).
Concentrations of the RAIL-UBMs are reported in ng/ml: for NGAL, ceruloplasmin, adiponectin and hemopexin; in pg/ml: for MCP-1 and KIM-1. Urine levels of creatinine are presented in mg/ml and of albumin in mg/l, respectively.
Immunostaining
Section Preparation
Human kidney biopsy sections were deparaffinized, and heated with a microwave oven in 0.1 M sodium citrate buffer, pH 6.0 for 10 minutes. The endogenous peroxidase activity was ablated by incubation in 0.5% hydrogen peroxide in methanol for 10 minutes.
Immunohistochemistry
The following procedure was followed for adiponectin (ThermoFisher, Waltham, MA; MA1-054; dilution 1:50), ceruloplasmin (Abcam, Cambridge, UK; ab135649; dilution 1:50), hemopexin (LifeSpan BoiScience, Seattle, WA; LS-C341670; dilution 1:200), KIM-1 and MCP-1 (R&D Systems, Minneapolis, MN; MAB1750 and MAB2791, respectively; dilution 1:200). The sections were incubated with 0.2%Triton-X-100 in 1X PBS for 10 min, and were blocked with Avidin/Biontin blocking kit (SP-2001, Vector) at room temperature for 1 hour, primary antibody were added to the sections and incubated sections overnight at 4°C. After application of the primary antibody, signals were detected using a commercially available Vectastain ABC kit with DAB Substrate (Vector laboratories: ImmPACT DAB SK-4105) kit for peroxidase staining. The sections were counterstained with Harris hematoxylin (Sigma-Aldrich, St. Louis, MO).
Immunofluorescence
For NGAL (a gift from Dr. Jonathan Barasch, Columbia, University, New York, NY; rabbit anti-mouse, 1:500) sections were incubated with 0.2%Triton-X-100 in 1X PBS for 10 min, and were blocked with 100% goat serum at room temperature for 1 hour. The sections were incubated with primary antibody NGAL at room temperature for 1 hr. The signal was detected with secondary antibody GRCY3 (Amersham Biosciences: goat anti rabbit, fluorolink labeled), 1:2000 dilution at room temperature for 30 minutes protected from light.
RAIL algorithm
The RAIL algorithm proposed for children with LN (P-RAIL) is calculated from the log-transformed and creatinine standardized concentrations of the six RAIL-UBMs as follows: P-RAIL = − 4.29 −0.34* NGAL −0.06*ceruloplasmin + 0.89* MCP-1 + 0.18* adiponectin − 0.65 * hemopexin + 0.62 * KIM-1 (7). The P-RAIL showed outstanding accuracy [area under the receiver operating characteristic curve (AUC) > 0.92] in identifying high LN-activity status (NIH-AI score > 10) in pediatric LN. In the development dataset of children, consideration of uncorrected amounts of the RAIL-UBMs in the algorithm mentioned above was less powerful in discriminating LN-activity status (AUC =0.71). Additional details are provided elsewhere (7).
Statistical Analysis
Demographic information, biopsy findings and traditional LN measures were summarized for patients with moderate/low versus (vs.) high LN-activity status, defined as a NIH-AI score of ≤ 10 vs. more than 10. This threshold was chosen based on the distribution of the NIH-AI scores in the development data set of children with LN. Group differences were evaluated for categorical variables by Chi-Square test (or Fisher’s exact test where applicable) and for continuous variables by Student’s t-test.
Prior to statistical analyses, all RAIL-UBM levels were log-transformed to account for skewing of the values. For some analyses, standardization of values for urine creatinine levels (in mg/ml) or albumin (in mg/l) was used besides considering their uncorrected urine concentrations.
To prospectively assess the P-RAIL, we applied the P-RAIL algorithm to the study group. Then, using multivariate logistic regression, we evaluated alternative weighting of the six RAIL-UBMs included in the algorithm, i.e. whether an adult modification improves the prediction of LN-activity status (high versus moderate/low) in the study cohort (A-RAIL).
Given the absence of consensus of whether to standardize UBM levels by urine creatinine, urine albumin or simply uncorrected urine concentrations for the UBMs, we considered all three approaches in our univariate and multivariate analyses.
We also examined whether individual RAIL-UBM levels were influenced by patient demographics or NIH-CI scores (data not shown).
There were only weak associations of the individual UBMs with any of the demographic variables (age, gender, race), but KIM-1 levels and NIH-CI scores were strongly correlated. Therefore, we repeated analyses with adjustment for NIH-CI scores.
For all of the RAIL algorithms (P-RAIL and A-RAIL) sensitivity, specificity, positive and negative likelihood ratios (LR+, LR-) were calculated for their logit cut off values. Accuracy assessment was based on the AUC. Values of the AUC are considered as outstanding, excellent, good, fair, or poor if for AUC of 0.90–1.00, 0.81–0.90, 0.71–0.80, 0.61–0.70, or 0.51–0.60, respectively. The LR+ values were interpreted as large (>10), moderate (5 to 9.9), and small (2 to 4.9), respectively. Similarly, LR-values can be used to “rule out” active LN.
Statistical analyses were performed using SAS® Version 9.4 software (SAS, Cary, NC, USA). A p-value < 0.05 was considered statistically significant for interpretation.
RESULTS
Patient Characteristics
Table 1 summarizes patient demographics and relevant LN parameters for the study patients. The mean ± standard deviation (SD) age of the patients was 32.4 ± 9.8 years; the majority (79%) were female and of white or black racial backgrounds. There were 64 patients with low/moderate LN-activity status and 15 with high LN-activity. The mean ± SD of NIH-AI and NIH-CI scores were at 6.46 ± 4.96 and 3.26 ± 2.61, with medians (ranges) at 5 (0 – 19) and 3 (0 – 10), respectively. There were only 16 (20.3%) patients without features of kidney damage (NIH-CI score = 0). Treatment at the time of biopsy included steroids [n= 71, mean = 21.5 mg per day (SD = 20.5)], immunosuppressant drugs [mycophenolate mofetil (n= 39 (49.4%), cyclophosphamide (n=5 (6.3%), hydroxychloroquine (n=55 (69.6%)), azathioprine (n=19 (24.1%)) and methotrexate (n=8 (10.1%))], and renin-angiotensin-aldosterone system (RAAS) inhibiting antihypertensive agents [n = 54 (68.3%)]. There was only one patient who was not being for SLE at the time of biopsy. Besides differences in ISN/RPS Class (p=0.002), complement C3 levels (p= 0.015), serum creatinine (p=0.034), and eGFR (p=0.045), traditional measures of LN were similar in groups of patients with low/moderate vs. high LN-activity status. There were 34 (43%) patients with eGFRs in the normal range (> 90ml/min/1.73 m2 body surface area), and only three of them were found to have high LN-activity status.
TABLE 1.
Demographic Summary of Patients in the A-RAIL Study
| Variable† | LN Activity Status | ||||
|---|---|---|---|---|---|
| Moderate/low (n=64) | High (n=15) | P-value | |||
| Demographics | |||||
| Gender | (Female, %) | 78.7% | 80.0% | 0.911 | |
|
| |||||
| Race/Ethnicity (%) | White | 48.5% | 33.3% | 0.107 | |
|
|
|||||
| Black | 45.3% | 46.7% | |||
|
|
|||||
| Hispanic | 3.1% | 6.7% | |||
|
|
|||||
| Asian | 3.1% | 13.3% | |||
|
| |||||
| Age (Years) | 32.6±10.2 | 31.3±8.5 | 0.633 | ||
|
| |||||
| Laboratory testing | |||||
| Urine Protein: Creatinine ratio, mg/mg | 3.1±3.5 | 4.6±3.4 | 0.152 | ||
|
| |||||
| Serum Creatinine, mg/dl | 1.3±1.0 | 1.9±1.4 | 0.034 | ||
|
| |||||
| Glomerular filtration rate‡, ml/min/1.73 m2 | 85.5±47.1 | 58.6±37.6 | 0.045 | ||
|
| |||||
| Complement C3, mg/dl | 68.2±35.2 | 40.4±25.0 | 0.015 | ||
|
| |||||
| Complement C4, mg/dl | 13.9±9.8 | 10.5±7.3 | 0.295 | ||
|
| |||||
| Urinalysis | |||||
| Granular casts | Present, n (%) | 2 (3%) | 1 (6%) | 0.4731 | |
|
| |||||
| Hematuria (cells/HPF) | <5 | 39 (60%) | 8 (50%) | 0.229 | |
|
| |||||
| 5–10 | 11 (17%) | 1 (7%) | |||
|
| |||||
| 11–15 | 9 (15%) | 2 (14%) | |||
|
| |||||
| >15 | 5 (8%) | 4 (29%) | |||
|
| |||||
| Pyuria (cells/HPF) | < 5 | 34 (53%) | 5 (31%) | 0.190 | |
|
| |||||
| 5–10 | 7 (11%) | 0 (0%) | |||
|
| |||||
| 11–15 | 5 (8%) | 2 (15%) | |||
|
| |||||
| >15 | 18 (28%) | 8 (54%) | |||
|
| |||||
| ISN/RPS Class | II | 8 (13%) | 0 (0%) | 0.002 | |
|
| |||||
| III¶ | 6 (25%) | 1 (7%) | |||
|
| |||||
| IV# | 25 (39%) | 14 (93%) | |||
|
| |||||
| V | 15 (23%) | 0 (0%) | |||
|
| |||||
| Chronic Kidney Disease Stage‡ | CKD1 | 31 (48%) | 3 (20%) | 0.326 | |
|
| |||||
| CKD2 | 13 (20%) | 3 (20%) | |||
|
| |||||
| CKD3 | 11 (18%) | 5 (33%) | |||
|
| |||||
| CKD4 | 7 (11%) | 3 (20%) | |||
|
| |||||
| CKD5 | 2 (3%) | 1 (7%) | |||
Values are either number of patients, n (percent %) or means ± standard deviations unless indicated differently; p-values are based on independent sample t-tests.
Includes Class III/V (10.5% and 9.1% for the Moderate/low and High groups, respectively)
Includes Class IV/V (12.0% and 36.4% for the Moderate/low and High groups, respectively)
Chronic Kidney Disease (CKD) staging is based on Glomerular Filtration Rate (GFR) value using the Modification of Diet in Renal Disease (MDRD) Study equation. CKD 1, CKD 2, CKD 3, CKD 4 and CKD 5 are classified based on GFR ranges >90, 60 to 89, 30 to 59, 15 to 29 and <15 or on dialysis, respectively.
Differences in individual UBMs with levels of LN activity
Results of univariate analyses assessing for differences in the levels of the RAIL-UBMs between groups of patients with low/moderate vs. high LN-activity status are presented in Table 2. The uncorrected concentrations of individual RAIL-UBMs differed significantly with LN-activity status. On correction for urine creatinine, all the individual RAIL-UBMs continued to demonstrate significant difference between the two groups. However, when corrected for urine albumin level, only adiponectin remained significantly altered in patients with different degrees of histologic activity. The results shown in Table 2 were not corrected for concurrent renal damage (NIH-CI), and hence provide a conservative estimation of the discriminative ability of the RAIL-UBMs.
TABLE 2.
Differences in urine biomarker concentration by LN-activity status (Not adjusted for NIH-CI)‡
| Urine Biomarkers & |
Unadjusted
†
|
p-value |
Creatinine adjusted
†
|
p-value |
Albumin-adjusted
†
|
p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Moderate/low (n=64) | High (n=15) | Moderate/low (n=64) | High (n=15) | Moderate/low (n=64) | High (n=15) | ||||
| 1. NGAL | 40.8 (27.8, 59.7) | 117.1 (53.2, 257.5) | 0.021 | 0.50 (0.35, 0.71) | 1.32 (0.64, 2.71) | 0.020 | 0.09 (0.06, 0.14) | 0.15 (0.06, 0.38) | 0.310 |
|
| |||||||||
| 2. MCP-1 | 1,032 (766, 1,391) | 2,383 (1,313, 4,325) | 0.016 | 11.98 (9.29, 15.45) | 26.9 (16.2, 44.8) | 0.007 | 2.30 (1.59, 3.32) | 3.06 (1.46, 6.42) | 0.495 |
|
| |||||||||
| 3. Ceruloplasmin | 32.1 (23.1, 44.6) | 103.3 (52.5, 203.3) | 0.003 | 0.39 (0.28, 0.54) | 1.2 (0.6, 2.2) | 0.005 | 0.07 (0.05, 0.10) | 0.13 (0.06, 0.28) | 0.126 |
|
| |||||||||
| 4. Adiponectin | 16.4 (10.5, 25.8) | 133.1 (52.5, 337.9) | 0.000 | 0.18 (0.12, 0.29) | 1.50 (0.59, 3.82) | 0.000 | 0.04 (0.02, 0.05) | 0.17 (0.07, 0.41) | 0.002 |
|
| |||||||||
| 5. Hemopexin | 2,106 (1,575, 2,817) | 5,957 (3,330, 10,656) | 0.002 | 24.43 (17.73, 33.67) | 67.23 (35.41, 127.66) | 0.007 | 4.68 (3.16, 6.93) | 7.66 (3.49, 16.79) | 0.276 |
|
| |||||||||
| 6. KIM-1 | 1,571 (1,234, 2,001) | 3,781 (2,304, 6,205) | 0.003 | 19.26 (15.45, 24.00) | 42.67 (27.56, 66.06) | 0.002 | 3.40 (2.48, 4.66) | 4.86 (2.55, 9.27) | 0.332 |
Values are geometric means (95% confidence intervals) of biomarker concentrations after log transformation.
Concentrations of UBMs are not adjusted for concomitant renal damage as measured by the NIH-CI scores. Results remained congruent even after adjusting for NIH-CI scores (data not shown).
Urine biomarkers include: neutrophil gelatinase associated lipocalin (NGAL) [ng/ml], monocyte chemotactic protein 1 (MCP-1) [pg/ml], ceruloplasmin [μg/ml], adiponectin [ng/ml], hemopexin [mg/ml] and kidney injury molecule 1 (KIM-1) [pg/ml].
Performance of RAIL algorithms
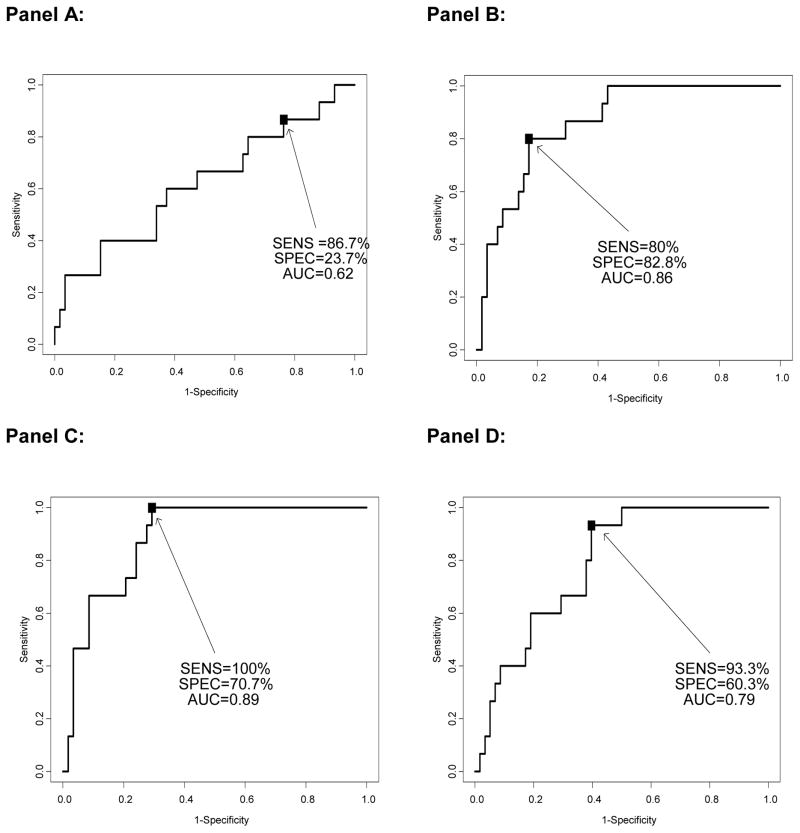
Differential weightings of the RAIL-UBMs in the A-RAIL allowed for excellent prediction of LN-activity status. Details of the performance of the RAIL-UBMs to discriminate low/moderate vs. high LN-activity status without (Table 3) and after adjusting for LN damage (Figure 1) suggest that the A-RAIL is relatively insensitive to concurrent LN damage (NIH-CI). When applied to adult patient data, the P-RAIL only had fair accuracy to discriminate adults with high LN-activity status from those with low/moderate one.
TABLE 3.
Diagnostic Accuracy of RAIL algorithms that do not adjust for concomitant kidney damage
| Measurement properties | A-RAIL
|
P-RAIL | ||
|---|---|---|---|---|
| Creatinine adjusted (1a) | Absolute amounts (1b) | Albumin adjusted (1c) | Creatinine adjusted (2) | |
| Accuracy [AUC (95% CI)]# | 0.86 (0.76, 0.95) | 0.88 (0.80, 0.96) | 0.78 (0.66, 0.90) | 0.63 (0.46, 0.79) |
| Sensitivity | 80.0% | 100.0% | 100.0% | 86.7% |
| Specificity | 76.3% | 67.8% | 44.1% | 25.9% |
| Positive likelihood ratio | 3.38 | 3.11 | 1.79 | 1.17 |
| Negative likelihood ratio | 0.26 | 0.00 | 0.00 | 0.51 |
| Logit cut | −0.97 | −1.92 | −2.06 | −1.84 |
The AUC refers to the area under the Receiver Operating Curve (ROC), expressed with its 95% confidence interval (CI).
Differential weightings for the 6 RAIL-UBM standardized by urine creatinine and uncorrected for concomitant LN chronicity are used. Weightings were derived by logistic regression using data from the 79 patients enrolled in this study. Please refer to Table 4 for details about the A-RAIL algorithms used.
As (1a) but without standardization by urine creatinine and without correction for concomitant LN chronicity.
As (1a) but RAIL-UBM levels are standardized by urine albumin and without correction for concomitant LN chronicity.
RAIL algorithm (reference 7) considering the 6 RAIL-UBM standardized by urine creatinine and uncorrected for concomitant LN chronicity. This algorithm was developed using pediatric data and samples. (− 4.29 −0.34* NGAL + 0.89* MCP-1 −0.06*ceruloplasmin + 0.18* adiponectin − 0.65 * hemopexin + 0.62 * KIM-1).
FIGURE 1. Accuracy of RAIL algorithms after adjustment for renal damage (NIH-CI).
Accuracy of the A-RAIL and P-RAIL Algorithms: Using receiver operating characteristic analysis, the accuracy of the A-RAIL and P-RAIL were assessed by determining the area under the curve (AUC), sensitivity (SENS) and specificity (SPEC) for each model at its optimal cut point. The data are adjusted for renal damage (NIH-CI). The models show the P-RAIL and A-RAIL adjusted for urine creatinine (Panel A and B, respectively), A-RAIL using the concentrations of the UBMs without adjustment, (Panel C), and A-RAIL with UBMs adjusted for urine albumin (Panel D). Please refer to Table 3 for additional details on the performance of the algorithms.
In the multivariate RAIL algorithm that is used to calculate the RAIL score, the beta coefficient for NGAL and adiponectin were larger in the A-RAIL than the P-RAIL, whereas those for MCP-1 and KIM-1 were lower; hemopexin lessened the overall RAIL score (negative beta coefficient) more so in the P-RAIL than the A-RAIL.
Different algorithms considering unadjusted, urine creatinine- or albumin-adjusted RAIL-UBMs levels of the A-RAIL and the P-RAIL developed in a previous study are presented in Table 4, with additional details provided in Supplementary Table 1.
TABLE 4.
A-RAIL and P-RAIL Algorithms used with Cut off (Logit) Values‡
| Name | Algorithms for predicting Lupus Nephritis activity† | Logit Cut-off |
|---|---|---|
| A-RAIL | ||
| Creatinine adjusted (1a) | 0.21 +0.67*NGAL +0.28*MCP1 −0.12*Ceruloplasmin +0.88*Adiponectin −0.25*Hemopexin −0.05*KIM1 | −0.97 |
| Absolute amounts (1b) | −5.05 +0.56*NGAL +0.12*MCP1 −0.29*Ceruloplasmin +0.88*Adiponectin +0.01*Hemopexin +0.02*KIM1 | −1.92 |
| Albumin adjusted (1c) | 3.47 +0.43*NGAL +0.16*MCP1 −0.23*Ceruloplasmin +0.81*Adiponectin −0.05*Hemopexin −0.62*KIM1 | −2.06 |
|
| ||
| P-RAIL | ||
| Creatinine adjusted (2) | −4.29 −0.34*NGAL +0.89* MCP1 −0.06*ceruloplasmin +0.18* Adiponectin −0.65*Hemopexin +0.62*KIM1 | −1.87 |
Parameter estimates used for the algorithms calculated using a logistic regression model with LN activity (NIH-AI) being the dichotomized outcome variable. Please refer to Supplementary Table 1 for details on the parameters ± SE values for individual biomarkers used.
Urine biomarkers include: neutrophil gelatinase associated lipocalin (NGAL) [ng/ml], monocyte chemotactic protein 1 (MCP-1) [pg/ml], ceruloplasmin [μg/ml], adiponectin [ng/ml], hemopexin [mg/ml] and kidney injury molecule 1 (KIM-1) [pg/ml].
For (1a), (1b), (1c) and (2): Please refer to Table 3 for details.
Kidney localization of the RAIL-UBMs
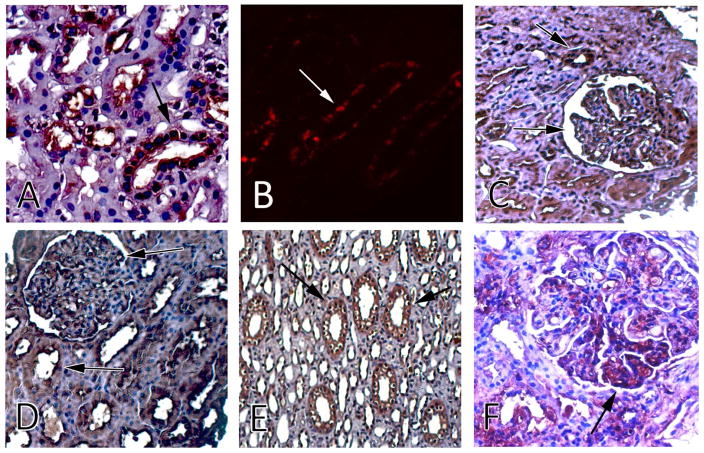
To support the biological rationale of the proposed composite biomarker, we performed immunohistochemistry on patient biopsy samples (Figure 2). NGAL, MCP-1 and ceruloplasmin, were expressed primarily in renal tubules, whereas adiponectin, hemopexin and KIM-1 localized to the glomeruli and the tubules.
Figure 2. Lupus Nephritis histology and immunostaining for the six urinary biomarkers (UBMs).
Expression of the RAIL component biomarkers in renal tissue. A) Ceruloplasmin is present in kidney tubules (DAB with H&E counterstain). B) NGAL staining of distal tubules (Cy3). C) KIM-1 shows expression in both glomeruli and tubules (DAB with H&E counterstain). D) Adiponectin was strongly expressed throughout the kidney tissue, with clear expression in glomeruli and tubules (DAB with H&E counterstain). E) MCP-1 staining of tubules (DAB with H&E counterstain). F) Hemopexin shown as dense staining within the glomerular lumen and proximal tubule cytoplasm (DAB with H&E counterstain). Representative staining images have been obtained from patients with low activity (NIH-AI ≤ 10).
DISCUSSION
In the present study, we confirm that NGAL, MCP-1, ceruloplasmin, adiponectin, hemopexin and KIM-1 form a noninvasive composite biomarker of LN histologic activity. The model was able to successfully discriminate high activity of LN from moderate/low activity with a high accuracy of 88%, using uncorrected urinary concentrations of the UBMs when their weights were appropriately adjusted for the adult patients.
In line with our study in children with LN (7), the results of this investigation confirm that LN chronicity has little impact on the accuracy of the RAIL. This is especially important for the use of the RAIL in adults with LN as they frequently are found to have not only features of LN activity, but also histological changes compatible with chronic LN damage on kidney biopsy (12,13).
The results of this study clearly support that patient age requires consideration when interpreting the levels of the RAIL-UBMs, especially in the setting of a combined interpretation as was done with the RAIL. The P-RAIL was derived using data of patients with an average age (SD) of 15.7 (3.01) years. In this study, we found that RAIL algorithms required adjustment for use in adults with a mean age of 32.4 years. The need of adjustment of renal parameters is not surprising based on known age-differences of some of the RAIL-UBMs (14), as well as some of the traditional measures of kidney function, such as GFR.
We hypothesize that the more common multi-organ involvement of SLE in children compared to adult might contribute to the observed age-dependence of the RAIL (15), as could co-morbid conditions which are often present in adults (16). Indeed about 30% of the urine proteins are thought to be filtered from the blood (17) and at least some of the more active extra-renal SLE in children might be associated with differences in RAIL-UBMs. However, our earlier research showed no relation between UBMs and extra-renal SLE (18). Given that all of the UBMs are proteins, standardization by urine albumin, the predominant protein excreted with proteinuria, has been proposed. As such, Torres-Salido et al. reported that urine NGAL was a predictor of LN flare, but only if corrected for urine albumin and not when corrected for urine creatinine or when expressed as a concentration (19). Of note, we were unable to replicate this observation in our study cohort. Interestingly, standardization by urine albumin even diminished the accuracy of the RAIL-UBMs to measure LN activity. This observation might be due to the fact that proteinuria is a reflection of both active inflammation and damage with LN, thus not an accurate reflection of LN activity, especially when patients are treated with RAAS blocking medications (6).
Other UBMs, besides the six RAIL-UBMs, are differentially excreted in active LN (20). These include, among others, α1-acid glycoprotein and transferrin. Given the high accuracy of the RAIL already, adding more urine biomarkers can only minimally improve the accuracy of the RAIL further and certainly increase cost and complexity for use of the RAIL in the future. Alternatively, one might consider fewer than six biomarkers to predict LN-activity. This could be advantageous if the RAIL moves to the clinic. We explored this possibility and found that this results in a markedly lower specificity of the RAIL for acceptable sensitivities of over 80%.
Differences in patient hydration must be a considered for urine analytes, with urine creatinine often used for standardization. However, our study did not find an improvement in the accuracy when comparing models using uncorrected UBMs versus urine creatinine adjusted UBMs. We therefore present detailed analysis that the RAIL is a robust measure of LN activity, which does not require standardization of the RAIL-UBM concentrations for urine creatinine to accurately predict LN activity.
There are some important limitations to our study. As seen commonly in clinical practice, most of our patients were being treated with steroids and other immunosuppressive therapies at the time of biopsy, which might have impacted kidney histology. However, our previous work did not demonstrate that the individual biomarker components of RAIL are robust measures of LN activity (20). Indeed the excellent performance of the RAIL as a composite biomarker in a cohort of patients on background immunosuppression and renin-angiotensin-aldosterone system blockade could be considered a real-world strength. Previous research suggests that at least some of the RAIL-UBMs may differ with patient gender (21). We did not find any significant differences between the two LN-activity groups in gender ratios or racial distribution. Further, there was no significant association of individual UBMs with the patient age, race and gender with LN-activity status. In addition, since we aimed at establishing an easy to collect and interpret urinary assay to predict LN-activity, further studies are needed to assess whether a morning urine sample would improve the predictability of RAIL algorithm. Further research in larger patient cohorts is also needed to assess whether the RAIL can also help discriminate different LN classes.
Lastly, our cross-sectional study does not provide evidence that the RAIL is a useful tool to monitor response to therapy over time or to help anticipate LN flares. However, given that all of the RAIL-UBMs have been found to differ with LN activity or even change with its course, the RAIL does hold the promise of being a powerful tool to noninvasively monitor the course of LN (18,22).
Certainly, further validation of the RAIL in longitudinal datasets will be necessary for confirmation. If confirmed in an independent cohort, the RAIL-UBMs can support the accurate, non-invasive measurement of LN activity. This is poised to constitute a major step towards a so-called “liquid biopsy” to monitor LN in the close future.
Supplementary Material
Acknowledgments
Funding acknowledgement: This study is supported by grants from the NIH U01 AR059509 to HB, P50 DK096418 to PD and HB; U01 DK096927 to BR and HB) and the Innovation Fund from Cincinnati Children’s Center for Technology Commercialization to PD and HB.
Footnotes
Competing interest: PD is a co-inventor on patent applications submitted for the use of NGAL as a biomarker of kidney injury. PD and HB are co-inventors on patent applications submitted for the use of the RAIL Score to predict lupus nephritis activity.
Contributor Information
Gaurav Gulati, Division of Immunology, Allergy and Rheumatology, Department of Internal Medicine, University of Cincinnati College of Medicine.
Michael R. Bennett, Division of Nephrology and Hypertension, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine.
Khalid Abulaban, Division of Pediatric Rheumatology, Helen DeVos Childrens Hospital, Michigan State University. Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine.
Huijuan Song, Division of Nephrology, Department of Internal Medicine, Ohio State University Wexner Medical Center.
Xiaolan Zhang, Division of Nephrology, Department of Internal Medicine, Ohio State University Wexner Medical Center.
Qing Ma, Division of Nephrology and Hypertension, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine.
Sergey V. Brodsky, Department of Pathology, Ohio State University Wexner Medical Center.
Tibor Nadasdy, Department of Pathology, Ohio State University Wexner Medical Center.
Christopher Haffner, Division of Nephrology and Hypertension, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine.
Kasha Wiley, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine.
Stacy P Ardoin, Division of Rheumatology, Department of Internal Medicine, Ohio State University Wexner Medical Center.
Prasad Devarajan, Division of Nephrology and Hypertension, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine.
Jun Ying, Department of Environmental Health, University of Cincinnati College of Medicine.
Brad H. Rovin, Division of Nephrology, Department of Internal Medicine, Ohio State University Wexner Medical Center.
Hermine I. Brunner, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine.
References
- 1.Sexton DJ, Reule S, Solid C, Chen S-C, Collins AJ, Foley RN. ESRD from lupus nephritis in the United States, 1995–2010. Clin J Am Soc Nephrol CJASN. 2015 Feb 6;10(2):251–9. doi: 10.2215/CJN.02350314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol JASN. 2004 Feb;15(2):241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 3.Austin HA, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984 Apr;25(4):689–95. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 4.Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011 Jun;11(6):426–37. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 5.Alvarado AS, Malvar A, Lococo B, Alberton V, Toniolo F, Nagaraja HN, et al. The value of repeat kidney biopsy in quiescent Argentinian lupus nephritis patients. Lupus. 2014 Jul;23(8):840–7. doi: 10.1177/0961203313518625. [DOI] [PubMed] [Google Scholar]
- 6.Mina R, Abulaban K, Klein-Gitelman M, Eberhard A, Ardoin S, Singer N, et al. Validation of the lupus nephritis clinical indices in childhood-onset systemic lupus erythematosus. Arthritis Care Res. 2015 Jul 20; doi: 10.1002/acr.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner HI, Bennett MR, Abulaban K, Klein-Gitelman MS, O’Neil KM, Tucker L, et al. Development of a Novel Renal Activity Index of Lupus Nephritis in Children and Young Adults. Arthritis Care Res. 2016 Jul;68(7):1003–11. doi: 10.1002/acr.22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh JA, Solomon DH, Dougados M, Felson D, Hawker G, Katz P, et al. Development of classification and response criteria for rheumatic diseases. Arthritis Rheum. 2006 Jun 15;55(3):348–52. doi: 10.1002/art.22003. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997 Sep;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5(2):128–34. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarr T, Dérfalvi B, Győri N, Szántó A, Siminszky Z, Malik A, et al. Similarities and differences between pediatric and adult patients with systemic lupus erythematosus. Lupus. 2015 Jul;24(8):796–803. doi: 10.1177/0961203314563817. [DOI] [PubMed] [Google Scholar]
- 13.Esdaile JM, Levinton C, Federgreen W, Hayslett JP, Kashgarian M. The clinical and renal biopsy predictors of long-term outcome in lupus nephritis: a study of 87 patients and review of the literature. Q J Med. 1989 Sep;72(269):779–833. [PubMed] [Google Scholar]
- 14.McWilliam SJ, Antoine DJ, Sabbisetti V, Pearce RE, Jorgensen AL, Lin Y, et al. Reference intervals for urinary renal injury biomarkers KIM-1 and NGAL in healthy children. Biomark Med. 2014;8(10):1189–97. doi: 10.2217/bmm.14.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mina R, Brunner HI. Update on differences between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Res Ther. 2013;15(4):218. doi: 10.1186/ar4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlsson AC, Larsson A, Helmersson-Karlqvist J, Lind L, Ingelsson E, Larsson TE, et al. Urinary kidney injury molecule-1 and the risk of cardiovascular mortality in elderly men. Clin J Am Soc Nephrol CJASN. 2014 Aug 7;9(8):1393–401. doi: 10.2215/CJN.11901113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thongboonkerd V, Malasit P. Renal and urinary proteomics: current applications and challenges. Proteomics. 2005 Mar;5(4):1033–42. doi: 10.1002/pmic.200401012. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki M, Wiers K, Brooks EB, Greis KD, Haines K, Klein-Gitelman MS, et al. Initial validation of a novel protein biomarker panel for active pediatric lupus nephritis. Pediatr Res. 2009 May;65(5):530–6. doi: 10.1203/PDR.0b013e31819e4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres-Salido MT, Cortés-Hernández J, Vidal X, Pedrosa A, Vilardell-Tarrés M, Ordi-Ros J. Neutrophil gelatinase-associated lipocalin as a biomarker for lupus nephritis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2014 Sep;29(9):1740–9. doi: 10.1093/ndt/gfu062. [DOI] [PubMed] [Google Scholar]
- 20.Brunner HI, Bennett MR, Mina R, Suzuki M, Petri M, Kiani AN, et al. Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis Rheum. 2012 Aug;64(8):2687–97. doi: 10.1002/art.34426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thrailkill KM, Moreau CS, Cockrell GE, Jo C-H, Bunn RC, Morales-Pozzo AE, et al. Disease and gender-specific dysregulation of NGAL and MMP-9 in type 1 diabetes mellitus. Endocrine. 2010 Apr;37(2):336–43. doi: 10.1007/s12020-010-9308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rovin BH, Zhang X. Biomarkers for lupus nephritis: the quest continues. Clin J Am Soc Nephrol CJASN. 2009 Nov;4(11):1858–65. doi: 10.2215/CJN.03530509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.