Abstract
Purpose
To determine correlations between gut microbiota composition and alterations in cardiorespiratory fitness and psychosocial outcomes among post-primary treatment breast cancer survivors (BCS).
Methods
Composition of the gut microbiota in BCS (n=12) was assessed at baseline (M0) and at the end of 3 months (M3) using Illumina MiSeq DNA Sequencing of the 16S rRNA gene. Gut microbiota composition was analyzed using QIIME bioinformatics software and represented through diversity metrics and taxa analyses. Cardiorespiratory fitness, fatigue, anxiety, depression, and sleep dysfunction were assessed at M0 and M3 via submaximal treadmill test, Fatigue Symptom Inventory, Hospital Anxiety and Depression Scale, and Pittsburgh Sleep Quality Index, respectively.
Results
Increased fatigue interference in BCS was associated with increased mean within-sample Shannon diversity (organism richness and evenness) (p=0.009). Weighted UniFrac analysis (shifts in taxa relative abundance) revealed significant differences in between-sample (beta) diversity for changes in fatigue interference (p=0.01) and anxiety (p=0.022), with a trend observed for fatigue intensity and sleep dysfunction (p<0.1). Unweighted UniFrac analysis (shifts in taxa types) found significant beta diversity differences for cardiorespiratory fitness (p=0.026). Prior to false discovery correction (FDR), changes in fitness, fatigue, anxiety, and sleep dysfunction were associated with the frequency of certain gut bacteria genera (e.g., Faecalibacterium, Prevotella, Bacteroides) (p<0.05).
Conclusions
Correlations may exist between alterations in gut microbiota composition and longitudinal changes in cardiorespiratory fitness, fatigue, and anxiety in BCS. Further research examining the role of the microbiota-gut-brain axis in exercise-induced effects on psychosocial outcomes in BCS is warranted.
Keywords: cancer, exercise, microbiome, oncology, physical activity, survivorship
Background
Early detection and improved therapies have contributed to the growing number of breast cancer survivors (BCS), which are projected to exceed 4 million in the U.S. by 2024 [11]. Despite increasing long-term survival rates, BCS are often plagued by persistent psychosocial distresses including anxiety, depression, fatigue, and sleep disturbances [11, 19]. While greater physical activity confers numerous health benefits [23], the mechanisms whereby exercise improves psychosocial domains have not been fully elucidated. It is possible that habitual exercise promotes psychological well-being through favorable modulations in systemic inflammation, hormone profile, and body composition [24, 27]. However, the potential mechanistic role of the gut microbiota composition corresponding to exercise-related adaptations/effects has not been studied. The link between the brain and gut microbiota, termed the microbiota-gut-brain axis [8], has garnered much attention in non-cancer populations due to its possible involvement in stress-related disorders [29]. To date, no prior investigation has evaluated whether the gut microbiota composition is associated with exercise and/or psychosocial outcomes in BCS.
As a dynamic regulator of the endocrine system, the microbiota has become a promising area of study with potential clinical applications. Emerging evidence in non-cancer animal models has demonstrated a link between exercise and gut microbiota diversity, composition, and beneficial metabolite production [3]. The two human studies available, to date, have been cross-sectional investigations linking greater microbial diversity to exercise habits and diet in professional rugby athletes [7] and to cardiorespiratory fitness in healthy adults [12]. Additional research examining the direct longitudinal effects of exercise on the gut microbiota in humans is needed. Such research in cancer survivors is particularly important because cancer-related factors increase the risk of gut dysbiosis and may influence mediators of psychosocial outcomes in response to exercise [22, 35, 37].
The hypothalamic-pituitary-adrenal (HPA) axis is active during the human stress response, with potential modulation by the enteric microbiota [18]. Limited research in humans supports a link between microbes and behavior [16, 25, 30, 34], thus necessitating further inquiry into the connection between psychosocial outcomes and gut microbiota in cancer survivors. To this end, our group performed post-hoc analyses on a subsample (n=12) of BCS participating in a larger physical activity trial to explore the associations between gut microbiota (alpha diversity, beta diversity, and taxa frequencies) and changes in anxiety, depression, fatigue, sleep quality, and cardiorespiratory fitness over a 3 month period. In this pilot, proof-of-concept study, we hypothesized that favorable changes in psychosocial factors (e.g., anxiety, depression, fatigue, sleep quality) and cardiorespiratory fitness among BCS would be correlated with gut microbiota diversity and the abundance of specific taxa.
Methods
Participants and Design
Inclusion criteria [31] included female BCS between the ages of 18 and 70 years of age with a history of ductal carcinoma in situ (DCIS), Stage I, II, or IIIA breast cancer who were English-speaking, obtained physician medical clearance for participation, and self-reported engaging in less than 30 minutes of vigorous- or 60 minutes of moderate-intensity physical activity per week on average in the previous six months at baseline. Participants were excluded if they had dementia or exhibited other medical, psychological, or social characteristics that could interfere with study participation. Additional relevant exclusion criteria included: 1) physical activity contraindication; 2) unable to ambulate; 3) recurrent or metastatic cancer; and 4) anticipated elective surgery or travel for >1 week during the first 3 months of the study. Research staff screened prospective participants via telephone using an institutional review board (IRB) approved script. Eligible individuals provided written informed consent. Although randomization was completed as previously reported [31], data for participants from both study group allocations were pooled for the purposes of this pilot study (i.e., all participants received standard American Cancer Society written materials regarding physical activity for cancer survivors with five also receiving a physical activity behavior change intervention focused on increasing physical activity to at least 150 weekly minutes).
Measurements
Participants provided a 3-day diet record and self-reported age, race, ethnicity, and medical history [i.e., breast cancer stage, months since cancer diagnosis, history of chemotherapy and/or radiation therapy, history of irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), gastric bypass, or bowel resection, menopausal status, smoking status, and recent antibiotic use]. Body mass index was calculated from the following equation [body weight (kg)/standing height (m2)]. A submaximal treadmill test was used to estimate cardiorespiratory fitness (quantified as predicted O2) [31]. Anxiety and depression were measured using the 14-item Hospital Anxiety and Depression Scale (HADS) [41], while fatigue intensity and interference were measured using the 13-item Fatigue Symptom Inventory [31]. In these instances, higher scores correspond with higher anxiety, depression, and fatigue. Sleep dysfunction was assessed using the Pittsburgh Sleep Quality Index (PSQI), with higher scores corresponding to greater subjective sleep dysfunction [5]. Data obtained at baseline and 3 months for the subsample of participants submitting fecal samples were used for this report.
Microbiome Analyses
Participants reporting antibiotic use during the week prior to sample collection were excluded from the analysis (n=3). The study group allocation (i.e., 5 participants received the intervention and 7 received usual care) was not tested in this pilot study due to the small sample size and the need for participants to experience different magnitudes of cardiorespiratory fitness change over time. Fecal wipe samples were collected and processed according to the protocol for pre-moistened wipe fecal samples outlined by Kumar et al. [20]. Briefly, samples from the assessed participants were collected and shipped overnight to the University of Alabama at Birmingham (UAB) Microbiome Resource Laboratory. Samples were stored at −80 °C until analysis. Each sample was dissolved in a buffer solution, then processed with a DNA Miniprep Kit to obtain isolated fecal DNA. Polymerase chain reaction (PCR) was used to amplify the V4 region of the 16S rRNA gene, which was then analyzed using Illumina MiSeq DNA sequencing. 250 base paired-end sequences were obtained with raw data processed, integrated, and analyzed using the Quantitative Insights into Microbial Ecology (QIIME) bioinformatics software and QWRAP program. Microbiome analyses, including quality control, OTU picking, sequence databases and sequencing primers, were performed as previously described [20].
Statistical Analyses
For the purposes of these analyses, ten BCS were assessed using fecal wipe samples from months 0 and 3. Two BCS were evaluated using samples from months 0 and 6. For samples with both month 3 and month 6 samples, we performed clustering analysis to determine whether there were any significant changes in beta diversity between month 3 and 6. There were no statistically significant differences between months 3 and 6 (PERMANOVA p > 0.05) for this sample set, therefore inclusion of 2 participants providing only a month 0 and month 6 sample was deemed acceptable. Individual (within-sample) microbial diversity, i.e., alpha diversity, was quantified as a Shannon diversity index, which assesses microbial richness and evenness within a sample. Alpha diversity analyses were also performed using chao1, observed species, PD whole tree, and Simpson indices, the results of which are included in the supplemental materials for reader perusal. Community (between-sample) similarity, i.e., beta diversity, was measured using weighted UniFrac and unweighted UniFrac metrics (both take into account organisms’ phylogenetic relationships with weighted UniFrac also taking into account the relative abundance of the organisms) [21, 36]. For statistical tests involving alpha diversity, changes in fatigue intensity, fatigue interference, and cardiorespiratory fitness were dichotomized as decreased/no change versus increased. Changes in sleep dysfunction, anxiety, and depression measures were dichotomized as decreased versus no change/increased. Dichotomization was done such that a balanced number of participants were represented in each category. To evaluate the relationship between changes in within-sample (alpha) diversities and changes in psychosocial and fitness outcomes, t-test was used to compare the mean change in alpha diversity from baseline to month 3 for each dichotomized outcome (Wilcoxon rank-sum test was used when the assumptions of t-test may not hold). For statistical tests involving beta diversity (between-sample differences), changes in psychosocial and fitness outcomes were divided into quartiles. Quartiles were calculated for each psychosocial or fitness outcome by taking the difference of the minimum and maximum values of the outcome in the cohort and dividing by four. Members of quartile 1 displayed the largest negative change in the psychosocial or fitness outcome over time, and members of quartile 4 showed the largest positive change over time (quartile boundaries are provided in the supplemental materials). Relationships between beta diversity indices of microbial composition and changes in psychosocial outcomes and cardiorespiratory fitness were visualized by principal coordinate analysis (PCoA; a measure not to be confused with principal component analysis [36]). Permutational multivariate analysis of variance (PERMANOVA) testing was used to assess the statistical significance of relationships between changes in outcomes and beta diversity at the latest time point. Taxa level analyses were performed in QIIME using Kruskal Wallis testing with false discovery rate (FDR) correction. The threshold of statistical significance for all tests performed was defined as two-sided p ≤ 0.05.
Results
Demographic characteristics for the 12 BCS who successfully returned fecal wipe samples and had not taken antibiotics are provided in Table 1. Increased fatigue interference compared with decreased/no change was associated with increased mean ± standard deviation (SD) Shannon diversity (1.096 ± 0.894 vs. −0.581 ± 0.699; p = 0.005). Group data exhibiting increased/no change in depression scores compared with decreases were not significantly associated with changes in mean Shannon diversity, although a trend towards significance was observed (0.863 ± 0.878 vs. −0.347 ± 1.152; p = 0.070). Changes in anxiety, cardiorespiratory fitness, fatigue intensity, and sleep dysfunction were not statistically associated with changes in mean Shannon diversity. In addition, number of participant comorbid medical conditions was not associated with mean alpha diversity at baseline (p = 0.91) or follow-up (p = 0.30). Results from other metrics of within-sample diversity are included in the supplemental materials.
Table 1.
Baseline participant characteristics
| Breast cancer survivors (n = 12) | |
|---|---|
| Social demographics | |
| Age, years [mean ± SD] | 55 ± 13 |
| Race [no. (%)] | |
| Caucasian | 8 (66.7%) |
| African American | 3 (25.0%) |
| Other | 1 (8.3%) |
| Body mass index, kg/m2 [mean ± SD] | 30.4 ± 5.1 |
| Waist circumference, cm [mean ± SD] | 89.1 ± 9.9 |
| Cancer status | |
| Months since diagnosis [mean ± SD] | 54 ± 56 |
| Cancer stage [no. (%)] | |
| DCISa | 2 (16.7%) |
| 1 | 4 (33.3%) |
| 2 | 4 (33.3%) |
| 3 | 2 (16.7%) |
| Chemotherapy (yes) [no. (%)] | 8 (66.7%) |
| Radiation yes [no. (%)] | 7 (58.3%) |
| Health measures | |
| Cardiorespiratory fitness, mL/kg/min [mean ± SD] | 20.2 ± 3.7 |
| Fiber intake, grams [mean ± SD] | 14.9 ± 6.4 |
| Post-menopausal (yes) [no. (%)]b | 10 (83.3%) |
| Current smoker (yes) [no. (%)] | 1 (8.3%) |
| Psychosocial measuresc | |
| Anxietyd | 6 ± 3 |
| Depressiond | 3 ± 3 |
| Fatigue interferencee | 3 ± 2 |
| Fatigue intensitye | 4 ± 1 |
| Sleep dysfunctionf | 8 ± 4 |
| Gastrointestinal history | |
| GI disease [no. (%)] | |
| Irritable bowel syndrome | 2 (16.7%) |
| Inflammatory bowel disease | 0 (0.0%) |
| Surgery [no. (%)] | |
| Gastric bypass or bowel resection | 0 (0.0%) |
DCIS = ductal carcinoma in situ,
1 participant did not report menopausal status,
Higher scores for anxiety, depression, fatigue, and sleep dysfunction indicate greater disturbances,
Anxiety and depression = 14-item Hospital Anxiety and Depression Scale (0–21 scale for each),
Fatigue = Fatigue Symptom Inventory (1–10 scale),
Sleep dysfunction = Pittsburgh Sleep Quality Index (0–21 scale).
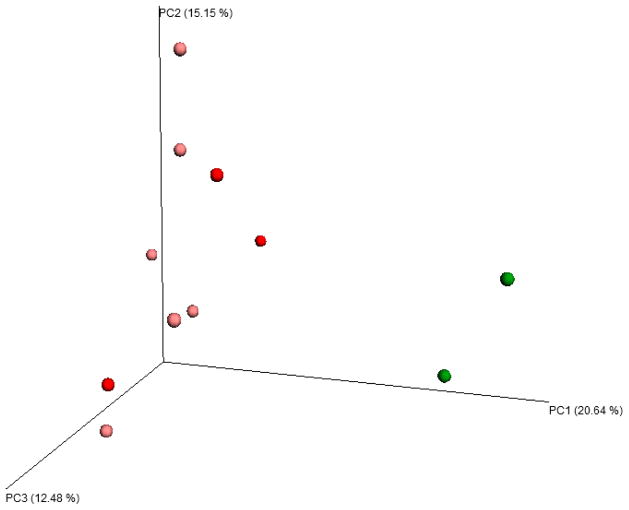
Using weighted UniFrac clustering, significant differences in beta diversity were found for anxiety (p = 0.022) and fatigue interference (p = 0.01) at the latest time point suggesting shifts in taxa relative abundance, while sleep dysfunction (p = 0.056) and fatigue intensity (p=0.086) trended toward significance. Using unweighted UniFrac clustering, significant differences in beta diversity were found for cardiorespiratory fitness (p = 0.026) at the latest time point suggesting changes in specific taxa present. There were no significant differences in beta diversity for depression or fatigue intensity (p > 0.05 for weighted and unweighted UniFrac clustering). Of note, the two participants with the lowest baseline cardiorespiratory fitness and the largest magnitude improvements in cardiorespiratory fitness showed striking microbial diversity clustering at the latest time point (Figure 1). To ensure that differences in beta diversity were due to the changes in fitness and psychosocial measures, we performed the same clustering analyses using microbiome samples from the baseline time point. We found no significant differences in beta diversity with the baseline samples using any of the clustering methods (p > 0.05). In addition, number of participant comorbid medical conditions was not associated with beta diversity at baseline (p > 0.05) or follow-up (p > 0.05). There were no statistically significant associations between cancer factors (e.g., stage and treatment type) and alpha/beta diversity at baseline (p > 0.05). Paired t-test analyses indicated there were no statistically significant changes in carbohydrate intake, fiber intake or BMI from baseline to follow-up (p > 0.05). Additionally, there was no statistically significant association between change in self-reported amount of carbohydrate or fiber consumed and change in cardiorespiratory fitness (p > 0.05).
Fig. 1.
Principal coordinate analysis: beta diversity and quartile of magnitude change in cardiorespiratory fitness. PCoA analysis of beta diversity using unweighted UniFrac clustering. Donors in light red and dark red had magnitude changes in the lower first and second quartiles, respectively. Donors in light green and dark green had magnitude changes in the upper 3rd and 4th quartiles, respectively.
Kruskal Wallis testing without FDR correction identified significant taxa differences across the beta diversity quartiles are provided in Table 2. Of note, the magnitude change in fatigue interference was associated with the frequency of genera Faecalibacterium (p = 0.033) and Prevotella (p = 0.044). Quartile of magnitude change in anxiety was associated with the frequency of genera Coprococcus (p = 0.041) and Bacteroides (p = 0.041). Quartile of magnitude change in cardiorespiratory fitness was associated with the frequency of genera Roseburia (p = 0.046) and SMB53 (p = 0.023), a subset of family Clostridiaceae. Further details of microbe composition are provided as supplementary materials available online. Kruskal Wallis testing with FDR correction yielded no statistically significant associations for bacterial taxa level analyses, likely due to the limited sample size of this pilot study. However, in the interest of outlining trends which may warrant further investigation in studies with larger sample sizes, we have provided taxa associations which were significant prior to correction.
Table 2.
Kruskal Wallis tests: taxa frequencies associated with changes in psychosocial outcomes
| Variable | Uncorrected p-value |
|---|---|
| Fatigue Interference | |
| Coprobacillus(g)a | 0.028 |
| Actinomycetales (o) | 0.033 |
| Faecalibacterium (g) | 0.033 |
| Prevotellaceae (f) | 0.044 |
| Prevotella(g) | 0.044 |
| Fatigue Intensity | |
| Beijerinckiaceae (f) | 0.013 |
| Bradyrhizobiaceae (f) | 0.013 |
| Balneiomonas (g) | 0.013 |
| Oxalobacteraceae (f) | 0.030 |
| Betaproteobacteria (c) | 0.045 |
| Burkholderiales (o) | 0.045 |
| Citrobacter (g) | 0.049 |
| Anxiety | |
| Adlercreutzia (g) | 0.015 |
| Phenylobacterium (g) | 0.028 |
| Vogesella (g) | 0.028 |
| Coprococcus(g) | 0.041 |
| Bacteroidaceae (f) | 0.041 |
| Bacteroides(g) | 0.041 |
| Mogibacteriaceae (f) | 0.043 |
| Global sleep dysfunction | |
| Paracoccus (g) | 0.013 |
| Rikenellaceae (f) | 0.027 |
| Clostridium (g) | 0.030 |
| Cardiorespiratory fitness | |
| Nocardioidaceae (f) | 0.011 |
| SMB53 (g) | 0.023 |
| Roseburia (g) | 0.046 |
Letters following bacteria name indicate the taxonomic rank as follows: (c) indicates class, (o) indicates order, (f) indicates family, and (g) indicates genus. According to convention, family and genus names are italicized.
Discussion
In this proof-of-concept pilot study, our purpose was to determine if gut microbiota diversity among post-primary treatment BCS may be related to psychosocial outcomes and cardiorespiratory fitness. We show a significant association between longitudinal changes in fatigue interference and microbial diversity and a trend toward significance for association of changes in microbial diversity and depression. Additionally, we found that the gut microbial composition was significantly associated with changes in anxiety, fatigue interference, and cardiorespiratory fitness, and a trend was detected for changes in fatigue intensity and sleep dysfunction. The significance with change in cardiorespiratory fitness was found using the unweighted UniFrac metric, which indicates shifts in, or the appearance/disappearance of, small microbial populations. In addition, the significance found for anxiety and fatigue interference using the weighted UniFrac metric is more likely to be driven by shifts in large microbial populations, due to its incorporation of information regarding relative species abundance as well as phylogeny. Therefore, our results support potential relationships between the gut microbiota diversity in post-primary treatment BCS and psychosocial and cardiorespiratory fitness outcomes.
To our knowledge, this is the first study to examine the potential associations between prospective changes in gut microbiota and alterations in psychosocial outcomes and cardiorespiratory fitness among cancer survivors of any type. It is important to evaluate changes in microbiota in understudied groups as performed in this investigation. A case-control study by Goedert, et al. [14] reported that the gut microbe in BCS differed in composition and was less diverse when compared with controls. Therefore, our report provides preliminary data in a patient group at increased risk of gut dysbiosis possibly due to cancer treatments [26] and changes in diet and physical activity behaviors post-diagnosis [15, 40].
Of note, participants in the present study exhibiting the largest improvement in cardiorespiratory fitness clustered together in their microbiota beta diversity. This finding is consistent with a recent cross-sectional study which found a correlation between cardiorespiratory fitness and microbial diversity in the gut microbiomes of 39 healthy human participants ages 18 to 35 years old [12]. This finding is also consistent with the only other publication to date testing the association between exercise and gut microbiota composition in humans (i.e., a cross-sectional study that focused on professional male rugby players) [7]. Moreover, our findings are biologically plausible given the potential for acute and chronic exercise to influence the gut microbiota through increased transit rate [9], splanchnic hypoperfusion with ischemia and subsequent reperfusion [3], and increased core body temperature [1]. Robust randomized controlled exercise training trials in humans are needed to confirm whether exercise-induced effects on the gut microbiota occur and potentially mediate changes in psychosocial outcomes. Because diet readily affects the gut microbiota, future exercise training studies should be combined with controlled feeding that also maintains energy balance. Indeed, this approach would permit a clearer assessment of the direct effects of exercise on the gut microbiota independent of potential confounding by variance in dietary intake and BMI [10, 32].
The pilot taxa analysis results described herein underscore the need for additional research into the role of the microbiota-gut-brain axis and the potential for its involvement in mediating psychosocial sequelae in BCS. Several genera found to be significantly associated with change in psychosocial symptoms levels (e.g., Bacteroides, Roseburia, Prevotella, etc.) have been associated in other studies with butyrate production and inflammatory regulation [3, 18]. In particular, our results are consistent with previous work in animal and human studies reporting associations of anxiety, depression, and fatigue with gut microbiota composition [16, 17, 28, 33, 38]. Several small-scale studies have shown beneficial effects on anxiety, depression, chronic fatigue, and sleep with probiotic consumption [25, 30, 39]. More specifically, Jiang et al. [16] found that patients with active major depressive disorder (MDD) possessed greater gut microbiota alpha diversity (Shannon index) when compared with healthy controls. In addition, a negative relationship between genus Faecalibacterium and depressive symptoms was demonstrated, as well as increased abundance of genus Roseburia and reduced abundance of genus Bacteroides in MDD patients relative to controls [16]. In our study, genera Faecalibacterium and Bacteroides were observed to correlate with quartile of magnitude changes in fatigue interference and anxiety, respectively. Since these psychosocial conditions often co-exist as symptom clusters in cancer survivors [2, 13], future research should evaluate the gut microbiota as a potential underlying mechanism for these symptom clusters.
We acknowledge our small sample size that restricted statistical power and our ability to adjust for covariates (e.g., diet and BMI). However, paired t-test analyses indicated there were no statistically significant changes in carbohydrate intake, fiber intake or BMI from baseline to follow-up. There was also no association found between change in self-reported amount of carbohydrates or fiber consumed and change in cardiorespiratory fitness. For the purposes of this pilot study, we were not able to collect detailed medical records for participants. However, we were able to take into account self-reported medication changes and number of comorbidities (i.e., comorbidity score). Accordingly, none of the study participants (n=12) reported any new or recent medication changes during the investigation, which have previously been shown to interfere with the gut microbiota. Also, the comorbidity score was not significantly associated with microbiotal alpha diversity or beta diversity at either baseline or follow-up. While this study collected data from participants regarding antibiotic use for the week prior to fecal sample collection, studies have demonstrated the long-term impact of antibiotics on the gut microbiome [6]. Therefore, future studies should collect antibiotic usage data for a minimum of three to six months prior to sample collection. Finally, our inclusion criteria excluding BCS over age 70 caused our sample to be, on average, younger than that reported in larger studies of “typical” BCS populations [4]. Nevertheless, this report provides support for investing the financial resources required to carry out randomized trials with larger sample sizes testing physical activity intervention effects on the gut microbiota composition and the potential associations between these changes and often observed improvements in psychosocial outcomes. Inclusion of metagenomics analyses in such studies were significantly advance the field.
This novel, first of its kind report in cancer survivors supports the feasibility of and need for further research testing the effects of exercise training on the gut microbiota composition and the relationships between these changes and psychosocial outcomes in BCS. This approach could have important clinical applications such as enhancing positive exercise-induced effects on psychosocial outcomes through manipulating the gut microbiota composition. In so doing, more effective interventions for reducing the psychosocial burden experienced by BCS could be developed.
Supplementary Material
Acknowledgments
We acknowledge the following funding sources: R01CA136859, U01CA136859, R25CA76023 (CaRES), R25CA047888 (Cancer Prevention and Control Training Grant), and P30DK056336 (University of Alabama at Birmingham Nutrition Obesity Research Center). We also acknowledge support from the Microbiome Resource at the University of Alabama at Birmingham: School of Medicine, Comprehensive Cancer Center (P30AR050948), Center for AIDS Research (5P30AI027767), Center for Clinical Translational Science (UL1TR000165), Multidisciplinary Clinical Research Center (P60AR064172) and Heflin Center.
Footnotes
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of Interest
Jesseca A. Paulsen and all contributing authors declare they have no personal or professional relationships that may represent a potential conflict of interest.
References
- 1.al-Majid S, McCarthy DO. Cancer-induced fatigue and skeletal muscle wasting: the role of exercise. Biol Res Nurs. 2001;2:186–197. doi: 10.1177/109980040100200304. [DOI] [PubMed] [Google Scholar]
- 2.Barsevick AM. The concept of symptom cluster. Semin Oncol Nurs. 2007;23:89–98. doi: 10.1016/j.soncn.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Bermon S, Petriz B, Kajeniene A, Prestes J, Castell L, Franco OL. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21:70–79. [PubMed] [Google Scholar]
- 4.Blanchard CM, Courneya KS, Stein K American Cancer Society’s SCS, II. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 5.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 6.Cervantes J. Use your antibiotics wisely. Consequences to the intestinal microbiome. FEMS Microbiol Lett. 2016:363. doi: 10.1093/femsle/fnw081. [DOI] [PubMed] [Google Scholar]
- 7.Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O’Reilly M, Jeffery IB, Wood-Martin R, Kerins DM, Quigley E, Ross RP, O’Toole PW, Molloy MG, Falvey E, Shanahan F, Cotter PD. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 8.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 9.Dainese R, Serra J, Azpiroz F, Malagelada JR. Effects of physical activity on intestinal gas transit and evacuation in healthy subjects. Am J Med. 2004;116:536–539. doi: 10.1016/j.amjmed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 10.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 12.Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, Ahmadi-Vand Z, Marsden KR, Gibson DL. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4:42. doi: 10.1186/s40168-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorentino L, Rissling M, Liu L, Ancoli-Israel S. The Symptom Cluster of Sleep, Fatigue and Depressive Symptoms in Breast Cancer Patients: Severity of the Problem and Treatment Options Drug Discov Today. Dis Models. 2011;8:167–173. doi: 10.1016/j.ddmod.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goedert JJ, Jones G, Hua X, Xu X, Yu G, Flores R, Falk RT, Gail MH, Shi J, Ravel J, Feigelson HS. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, Ballard-Barbash R. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36:1484–1491. [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Kang SS, Jeraldo PR, Kurti A, Miller ME, Cook MD, Whitlock K, Goldenfeld N, Woods JA, White BA, Chia N, Fryer JD. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014;9:36. doi: 10.1186/1750-1326-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knobf MT. Clinical update: psychosocial responses in breast cancer survivors. Semin Oncol Nurs. 2011;27:e1–e14. doi: 10.1016/j.soncn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R, Eipers P, Little RB, Crowley M, Crossman DK, Lefkowitz EJ, Morrow CD. Getting started with microbiome analysis: sample acquisition to bioinformatics. Curr Protoc Hum Genet. 2014;82:18 18 11–18 18 29. doi: 10.1002/0471142905.hg1808s82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAuley E, White SM, Rogers LQ, Motl RW, Courneya KS. Physical activity and fatigue in breast cancer and multiple sclerosis: psychosocial mechanisms. Psychosom Med. 2010;72:88–96. doi: 10.1097/PSY.0b013e3181c68157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8:205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 25.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 26.Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, Bruley des Varannes S, Massart S, Moreau P, Potel G, de La Cochetiere MF, Batard E, Knights D. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther. 2015;42:515–528. doi: 10.1111/apt.13302. [DOI] [PubMed] [Google Scholar]
- 27.Moylan S, Eyre HA, Maes M, Baune BT, Jacka FN, Berk M. Exercising the worry away: how inflammation, oxidative and nitrogen stress mediates the beneficial effect of physical activity on anxiety disorder symptoms and behaviours. Neurosci Biobehav Rev. 2013;37:573–584. doi: 10.1016/j.neubiorev.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 29.Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clin Ther. 2015;37:984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, Logan AC. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1:6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers LQ, McAuley E, Anton PM, Courneya KS, Vicari S, Hopkins-Price P, Verhulst S, Mocharnuk R, Hoelzer K. Better exercise adherence after treatment for cancer (BEAT Cancer) study: rationale, design, and methods. Contemp Clin Trials. 2012;33:124–137. doi: 10.1016/j.cct.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Shukla SK, Cook D, Meyer J, Vernon SD, Le T, Clevidence D, Robertson CE, Schrodi SJ, Yale S, Frank DN. Changes in Gut and Plasma Microbiome following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) PLoS One. 2015;10:e0145453. doi: 10.1371/journal.pone.0145453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, Mayer EA. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. 1401e1391–1394. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetiere MF. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40:409–421. doi: 10.1111/apt.12878. [DOI] [PubMed] [Google Scholar]
- 36.Tyler AD, Smith MI, Silverberg MS. Analyzing the human microbiome: a “how to” guide for physicians. Am J Gastroenterol. 2014;109:983–993. doi: 10.1038/ajg.2014.73. [DOI] [PubMed] [Google Scholar]
- 37.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12:282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 38.Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, Kentish S, Xie P, Morrison M, Wesselingh SL, Rogers GB, Licinio J. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamura S, Morishima H, Kumano-go T, Suganuma N, Matsumoto H, Adachi H, Sigedo Y, Mikami A, Kai T, Masuyama A, Takano T, Sugita Y, Takeda M. The effect of Lactobacillus helveticus fermented milk on sleep and health perception in elderly subjects. Eur J Clin Nutr. 2009;63:100–105. doi: 10.1038/sj.ejcn.1602898. [DOI] [PubMed] [Google Scholar]
- 40.Zhang FF, Liu S, John EM, Must A, Demark-Wahnefried W. Diet quality of cancer survivors and noncancer individuals: Results from a national survey. Cancer. 2015;121:4212–4221. doi: 10.1002/cncr.29488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.