Abstract
World-wide methamphetamine (meth) use is increasing at a rapid rate; therefore, it has become increasingly important to understand the synaptic changes and neural mechanisms affected by drug exposure. In rodents, 6-hr access to contingent meth results in an escalation of drug intake and impaired cognitive sequelae typically associated with changes within the corticostriatal circuitry. There is a dearth of knowledge regarding the underlying physiological changes within this circuit following meth self-administration. We assessed pre- and postsynaptic changes in glutamate transmission in the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc) following daily 6-hr meth self-administration. In the mPFC, meth caused postsynaptic adaptations in ionotropic glutamate receptor distribution and function, expressed as a decrease in AMPA/NMDA ratio. This change was driven by an increase in NMDA receptor currents and an increase in GluN2B surface expression. In the NAc, meth decreased the paired-pulse ratio and increased the frequency of spontaneous excitatory postsynaptic currents with no indication of postsynaptic changes. These changes in mPFC synapses and NAc activity begin to characterize the impact of meth on the corticostriatal circuitry.
Keywords: Methamphetamine, self-administration, paired pulse ratio, AMPA/NMDA, protein expression, corticostriatal circuitry
Introduction
Methamphetamine (meth) is a highly abused psychostimulant, and repeated cycles of meth use leads to chronic relapse that is characteristic of addiction. While the underlying pathology of addiction and relapse remains poorly understood, it is clear that a change in synaptic plasticity within the reward circuit is one of the fundamental mechanisms underlying this disorder (Luthi and Luscher 2014). One component of the complex reward circuitry involves the nucleus accumbens (NAc) and its inputs from the medial prefrontal cortex (mPFC). It is hypothesized that addictive drugs hijack this circuit (Hyman et al. 2006). Glutamatergic signaling in the NAc controls drug-seeking behaviors in animal models of addiction (Kalivas 2009; Self 2004); the NAc has a large population of glutamatergic synapses that impinge upon medium spiny neurons and are subject to lasting molecular and cellular changes in response to addictive drugs (Nestler 2001; Lee and Dong 2011). Furthermore, NAc glutamatergic projections originate in the mPFC (as well as the hippocampus and basal amygdala, among other brain areas), and dysfunction of the mPFC is credited with the loss of control over drug taking behavior (Goldstein and Volkow 2011).
While considerable efforts have occurred to understand the mechanisms underlying meth addiction, relapse, and cognitive dysfunction, very little is known about the synaptic and cellular mechanisms in play. Synaptic physiology data from meth-experienced rats are scarce, but it has been reported that experimenter delivered meth (1 mg/kg, 7days) decreases calcium currents and excitatory transmission in the mPFC four days after drug treatment (González et al. 2015). Additionally, a sensitizing regimen of amphetamine increases AMPA/NMDA ratios and frequency and amplitude of miniature excitatory postsynaptic currents (mEPSc) in the shell of the nucleus accumbens (Jedynak et al. 2016). These examples begin to shed light on cortico-accumbal synaptic function following meth in mice; however, these studies do not relate physiological changes to an addiction model. Using a contingent model of meth self-administration, Graves and colleagues (Graves et al. 2015) showed that meth decreased the intrinsic excitability of medium spiny neurons in the NAc shell, and Scheyer and colleagues (Scheyer et al. 2016) demonstrated that calcium permeable (CP) AMPA receptors are accumulated in the nucleus accumbens core as early as one week after meth access is discontinued (see discussion).
These later findings are important because self-administration models rely on contingent drug delivery, thereby increasing the face and construct validity of the model relative to experimenter delivered drug models. In recent years, self-administration models that employ longer access to a drug also have received growing attention, likely owing to the noted differences between long- and short-access models (Ahmed and Koob 1998; Peters et al. 2015; Reichel et al. 2012). Long-access leads to a highly reproducible escalation of drug intake that is thought to reflect a more “addicted” state, versus short-access models that are thought to reflect “recreational” drug use (Ahmed and Koob 1998). Regarding glutamatergic synaptic function, rats given extended access to meth self-administration had elevated basal glutamate levels in the NAc during abstinence from the drug (Lominac et al. 2012). In the dorsal striatum, meth self-administration (9 hrs, 10 days) did not impact mRNA expression of glutamate receptors until presentation of a drug associated context at 30 days post meth (Li et al. 2015). We have shown that long-access meth self-administration (6 hrs per day) increased the proportion of burst firing pyramidal cells compared to tonic firing cells in mPFC (Parsegian et al. 2011); however, it is not known if this increased burst firing results frompresynaptic and/or postsynaptic changes. To test whether meth self-administration impacts pre- and/or postsynaptic plasticity, we recorded from pyramidal cells in the mPFC and medium spiny neurons (MSNs) in the NAc core in brain slice preparations of the same rats using whole-cell and field recordings. Based on our findings in the mPFC, we also quantified surface expression of NMDA receptors.
We found that long-access meth self-administration decreased AMPA/NMDA ratios in the mPFC, accompanied by an increase in NMDA receptor currents and surface expression of the GluN2B subunit of the NMDA receptor. Concurrently, in the NAc there was a significant increase in glutamate release probability as well as an increase in the frequency of sEPSCs. Together, these results show that meth elicits different long-term synaptic changes in the reward associated brain regions, suggesting that altered synaptic function is one of the fundamental mechanisms affected by drugs of addiction.
Methods
Animal housing
Sprague Dawley male rats weighing 225–250 g (n=27) were obtained from Charles River and individually housed on 12 hour reversed light/dark cycle with free access to water and food, until the beginning of self-administration (see below). All procedures were conducted in accordance with the ‘Guide for the Care and Use of Laboratory Rats’ (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 2011) and were approved by the IACUC of the Medical University of South Carolina.
Catheter implantation surgery
Rats were anesthetized with intraperitoneal injections of ketamine (66 mg/kg; VedcoInc, St Joseph, MO, USA), xylazine (1.3 mg/kg; Lloyd Laboratories, Shenandoah, IA, USA), and equithesin (0.5 ml/kg; sodium pentobarbital 4 mg/kg, chloral hydrate 17 mg/kg, and 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10 % ethanol solution). Ketorolac (2.0 mg/kg, intraperitoneal; Sigma, St. Louis, MO, USA) was given just prior to surgery as an analgesic. One end of a silastic catheter was inserted 33 mm into the external right jugular and secured with 4.0 silk sutures. The other end ran subcutaneously and exited from a small incision just below the scapula. That end was attached to an infusion harness (Instech Solomon, Plymouth Meeting, PA, USA) that provided access to an external port for IV drug delivery. Following that surgical procedure, rats were given a subcutaneous injection of an antibiotic solution Cefazolin (10 mg/0.1 ml; Schein Pharmaceuticals, Florham Park, NJ, USA) and were allowed to recover for 5 days.
Meth Self-administration
Self-administration occurred in chambers (30×20×20 cm, Med Associates, St. Albans, VT) housed inside sound-attenuating cubicles that were fitted with a fan, metal arm, and spring leash attached to a swivel (Instech); two retractable levers; two stimulus lights; a speaker for tone delivery; and a house light. Tygon® tubing extended through the leash and connected to a 10 ml syringe mounted on an infusion pump outside the cubicle. After 5 days of recovery, rats were assigned to meth or yoked-saline control groups. Methamphetamine hydrochloride (Sigma, St. Louis, MO, USA), dissolved in sterile saline, was administered daily during 1-h sessions for seven days on an FR1 schedule of reinforcement, followed by 6-h sessions for 14 days. The house light signaled the beginning of a session and remained on throughout the session. A response on the active lever resulted in a 2-s infusion (20 µg/50 µl bolus) and presentation of a stimulus complex that consisted of a 5-s tone (78 dB, 4.5 kHz) and a white stimulus light over the active lever, followed by a 20-s time out. Responses during the time out and on the inactive lever were recorded, but they had no scheduled consequences. Yoked controls received a 50 µl saline infusion whenever the matched subject received a meth infusion. Following the self-administration sessions, rats were provided 15–30 g of chow per day with free access to drinking water in their respective home cage. Eight days after the last self-administration session, rats were killed for end point measures of electrophysiology or immunoblotting.
Brain Slice Preparation
Rats were anesthetized using isoflurane (Minrad Inc. Bethlehem, PA, USA); each was decapitated, and the brain was removed quickly. Coronal slices containing mPFC (300µm) or NAc (230 or 400µm) were cut in ice-cold high-sucrose solution containing (in mM): sucrose, 200; KCl, 1.9; Na2HPO4, 1.2; NaHCO3, 33; MgCl2, 6; CaCl2, 0.5; D-Glucose, 10; ascorbic acid, 0.4 using a Leica VT 1200 S vibratome. Slices were incubated at 31°C for at least 1 h before recordings; the incubation medium contained (in mM): NaCl, 120; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 25; MgCl2, 4; CaCl2, 1; D-Glucose, 10; and ascorbic acid, 0.4, aerated with 5% CO2/95%O2. Following incubation, slices were transferred to a submerged chamber and superfused at room temperature with oxygenated artificial cerebrospinal fluid (aCSF) (in mM): NaCl, 126; KCl, 2.5; NaH2PO4, 1.4; NaHCO3, 25; CaCl2, 2.0; MgCl2, 1.3; D-Glucose, 10; and ascorbic acid, 0.4.
Voltage clamp recordings
Picrotoxin (50 µM) was included in the perfusion solution to block GABAA receptors. For voltage-clamp recordings, electrodes (2.5–3.5 MΩ resistance in situ) were filled with a solution containing (in mM): CsCl, 130; HEPES, 10; MgCl2, 2; EGTA, 0.5; Na2ATP, 2; Na-GTP, 0.3; QX-314, 2; phosphocreatine, 10; and 285 mOsmols. Series resistances (10–25 MΩ) and input resistances were continually monitored throughout the experiment via a −5 mV (50 ms) hyperpolarizing pulse. Neurons were clamped at −70 mV, and spontaneous excitatory postsynaptic currents (sEPSCs) and paired pulse responses were recorded. For AMPA/NMDA experiments, EPSCs were evoked using a bipolar concentric electrode placed within 300–400 µm of the recording electrode. Neurons were clamped at +40 mV, and currents were recorded for 5 min at 0.033 Hz; then the NMDA receptor antagonist AP-5 (D(−)-2-amino-5-phosphonovaleric acid (50 µM) was applied to isolate the AMPA currents. After 5 minutes, eEPSCs were recorded again for 5 minutes. The NMDA current was obtained by digital subtraction (ITotal − IAMPA), and the ratio was calculated with the following formula: IAMPA/ITotal − IAMPA. Paired pulse ratio (PPR) experiments were performed using an ISI of 50 msec, at 0.033 Hz. Recordings were made using a Multiclamp 700B amplifier (Axon Instruments, CA) that was connected to a computer running Windows XP and Axograph × software. All recordings were obtained from pyramidal neurons in layers V or VI of the prelimbic cortex and medium spiny neurons of the NAc core, which were identified using infrared-differential interference contrast optics and video-microscopy.
Population spikes (PS) recordings
A glass recording electrode filled with rACSF (1–2 MΩ resistance in situ) was placed in close proximity to a concentric bipolar stimulation electrode. Stimulation duration was 0.1 msec. Signals were amplified 500 times, acquired at 10 kHz, and filtered at 2 KHz. An input/output response curve was generated by stepwise increases in stimulation intensity from 10 µA to 100 µA at 0.066 Hz. The stimulation intensity that resulted in 50 percent of the maximum output amplitude was used for baseline recordings. For PPR experiments, the ISI was set to 50 ms stimulating at 0.033 Hz. PS recordings were 30 minutes in duration, and the average amplitude of the last 5 minutes was used for analysis and statistical comparison. Recordings were made using a Axopatch 200B amplifier (Axon Instruments, CA) that was connected to a computer running Windows XP and Axograph × software.
Tissue biotinylation and surface-protein expression
Following long access self-administration, rats were sacrificed, and brain tissue was removed and dissected on ice. mPFC tissue was rapidly transferred to tubes, and surface biotinylation was performed as previously described (Schwendt et al. 2012). In brief, 300 µm slices were made from 2 mm tissue punches of the mPFC area using a tissue chopper, then incubated with 1 mg/ml Sulfo-NHS-Biotin for 30 min at 4 °C with mild shaking. The reaction was quenched by 100 mM glycine in PBS (pH 8.0). Tissue was then washed in PBS and was sonicated in RIPA buffer. Following incubation, insoluble fragments were removed by centrifugation at 10,000×g for 10 min at 4 °C. After centrifugation, 500 µg of sample was combined with NeutrAvidin agarose resin to isolate the biotinylated proteins, then further incubated for 2 h at 4 °C with concurrent rotation. Biotinylated samples were eluted with laemmli sample buffer for 30 min at 37 °C. A capillary-based size sorting automated Western system (O'Neill et al. 2006), ‘WES’ by ProteinSimple (Bio-Techne) was used to quantify surface receptor protein levels as previously described (Beccano-Kelly et al. 2014; Scofield et al. 2015). During the process of loading the WES plate, biotinylated lysates (1 µg/µl stocks) were diluted to 0.2 µg/µl by combining 2.5 µl lysate, 2.5 µl master mix (proprietary ProteinSimple solution), and 7.5 µl 0.1 × sample buffer (as instructed by ProteinSimple protocol) for a total volume 12.5 µl. The reaction mix was denatured with heat at 70°C for 10 min. 5 µl of the reaction mix was then pipetted into the WES plate, resulting in a final concentration of 1 µg in the capillary. Lysate mixture, primary and secondary antibodies, chemiluminescent substrate, and wash buffer were then dispensed into designated wells on the WES microplate. Stacking matrix load time was set at 20 s, and sample load time was set at 10 s. Data were analyzed with Compass software (ProteinSimple) using the ‘Dropped Lines’ function for quantitative analysis of GluN1,GluN2A, GluN2B and with α-tubulin as a loading control. The primary antibodies used were GluN2B (mouse, BD Transduction Laboratories, 610416, 1:1000), GluN2A (rabbit, Millipore, 07-632, 1:50), GluN1 (mouse, BD Biosciences, 556308, 1:1000), and α-tubulin (mouse, Sigma-Aldrich, T6074, 1:50). Surface (biotinylated) GluN2B, GluN2A, and GluN1 signals were normalized to that of tubulin and expressed as a percentage of averaged saline values (percent control). We selected tubulin as a loading control because the molecular weight would not interfere with the main proteins we assessed: GluN2b, GluN2a, or GluN1. Although tubulin is not membrane bound, the sensitivity of our capillary-based system was able to detect a small but analyzable peak, thus providing a suitable for control against all 3 NMDA proteins.
Data analysis
PPR, input/output, and AMPA/NMDA peak amplitude values were exported directly from Axograph X. For sEPSCs, data files were exported from Axograph X, and signals were analyzed using Minianalysis® software by Synaptosoft version 6.0.7. The threshold for sEPSCs was selected as 5 times the RMS noise, measured in a 125 msec episode. The period to search the maximum peak was set at 7 msec, and the time for baseline before a peak was 9 msec.
For protein expression analysis, surface (biotinylated) receptors (GluN2B, GluN2A, and GluN1) were normalized to α-tubulin and were expressed as a percent of control values. All data were analyzed and plotted using GraphPad Prism version 6.05, GraphPad Software, Inc. Statistical comparisons were made using unpaired 2-tailed t-tests or analyses of variance (ANOVAs), followed by Sidak’s post-hoc test when appropriate.
Results
Escalation of methamphetamine self-administration during long access conditions
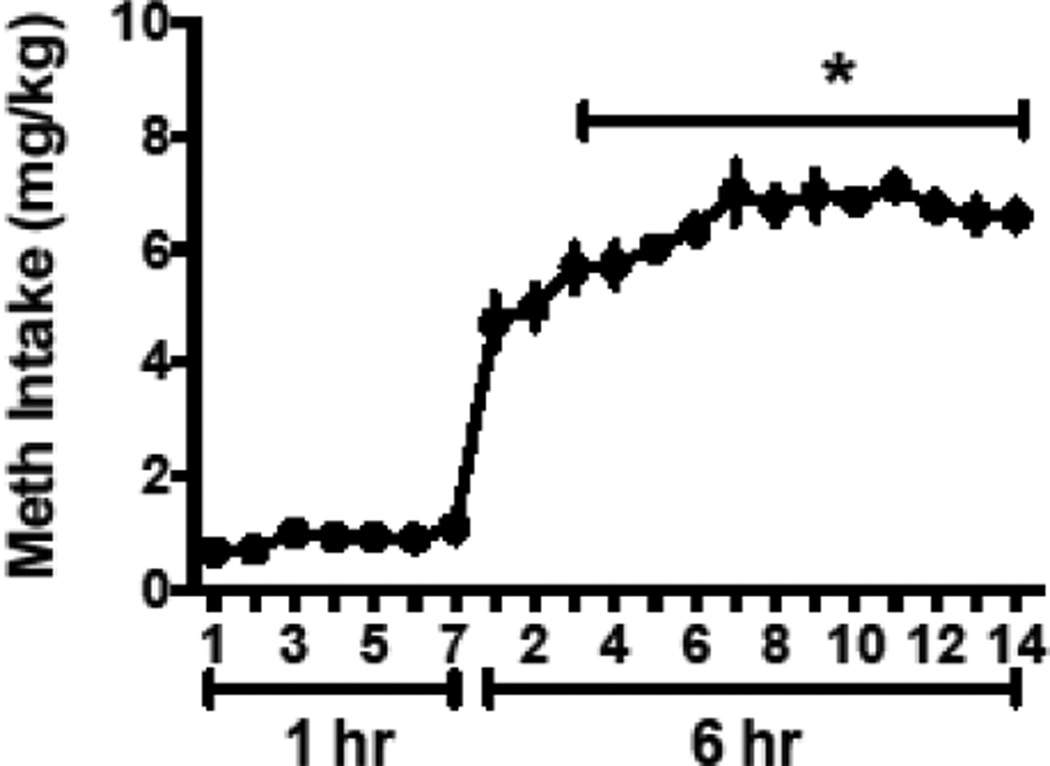
Meth self-administration escalated over time, indexed as an increase in drug intake over time (Figure 1). Intake in mg/kg increased during the 6 hour sessions on days 3–14 compared to long access day 1 [F(13, 65)=9.78, p<0.0001, Holm-Sidek multiple comparison, p<0.05]. Responding on the inactive lever did not change over the course of the 21 days [F(13, 65)=1.06, p=0.41].
Figure 1.
Meth intake (mg/kg) increased over the long access (6 hr) period.
*Significant difference from day one of long access
Meth self-administration decreases AMPA/NMDA ratio in the mPFC
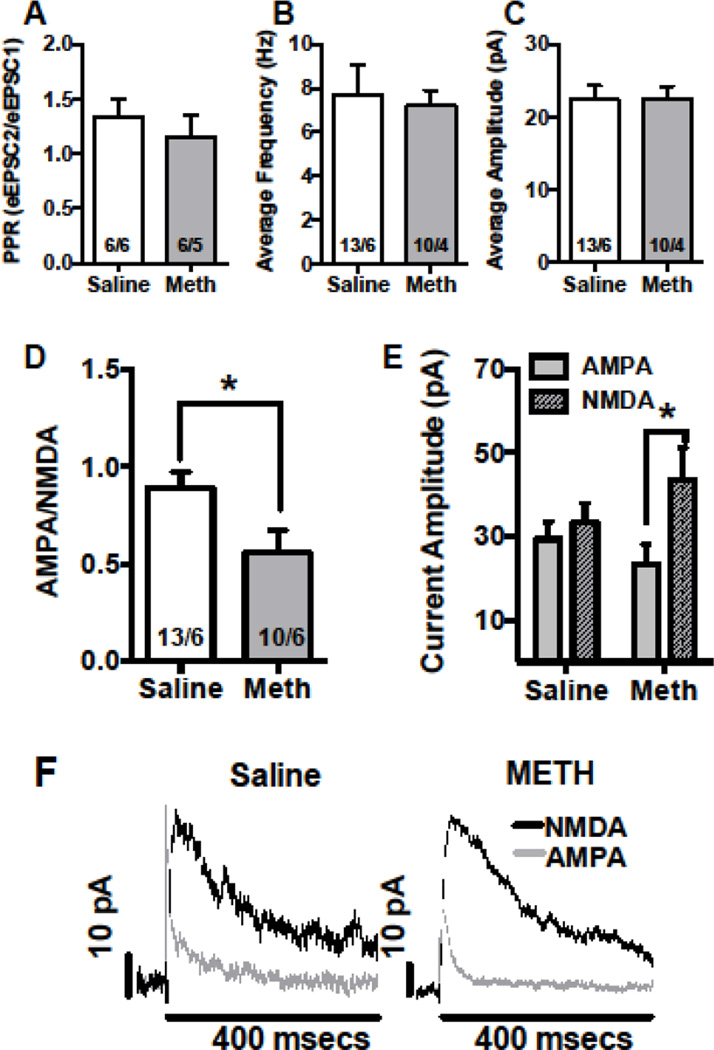
Using whole cell recordings in the mPFC, we assessed the following standard markers of pre- and post-synaptic function: paired pulse responses (PPR), AMPA/NMDA ratio, and frequency and amplitude of spontaneous (s) EPSCs. PPRs were used to provide an indication of neurotransmitter release probability (Bonci and Williams 1997; Debanne et al. 1996; Regehr 2012). The PPR ratio was calculated by dividing the amplitude of the second postsynaptic response by the first. PPR decreased when the probability of release increased (Zucker and Regehr 2002). In the mPFC, there were no changes in the PPR [Fig 2A, mean ± SEM: saline 1.33 ± 0.169, meth 1.14±0.209]. We also recorded sEPSCs (a change in frequency and amplitude of spontaneous events that suggests a change in pre- and post-synaptic transmission, respectively). For sEPSCs in the mPFC, there were no changes in frequency [Fig 2B, mean ± SEM: saline 7.73 ±1.30, meth 7.20±0.71] or amplitude [Fig 2C, mean ± SEM: saline 22.38 ± 1.89, meth 22.55±1.61].
Figure 2.
In the mPFC, meth self-administration altered post-synaptic physiology. In the mPFC, long access meth did not have an effect on whole cell recordings of PPR (A) and frequency (B) or on amplitude (C) of sEPSCs. Meth reduced AMPA/NMDA ratio (D, *significant difference from saline), and the decrease was driven by an increase in NMDA current (E, *significant difference between subtype). Representative traces of AMPA/NMDA currents are depicted (F).
The number of cells/animal is indicated in the bar graphs, which represent the mean; the errors bars represent the SEM.
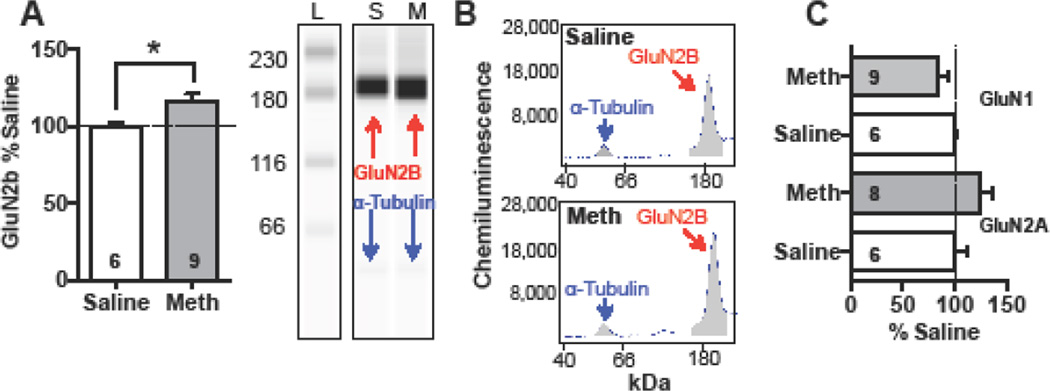
Synaptic strength is, determined by the ratio of the amplitude of AMPA to NMDA currents and is regularly used for investigating the effects of various psychostimulants on glutamate transmission (Gao and Wolf 2007). AMPA/NMDA ratio was significantly decreased in meth rats relative to saline [Fig 2D, t(21)=2.408, p<0.05, mean ± SEM: saline 0.89 ± 0.082, meth 0.55±0.117]. In a separate analysis, using a 2-way ANOVA with group (meth and saline) and receptor type (AMPA and NMDA) as the variables, we showed that the decrease in ratio was driven by a significant increase in NMDA receptor currents in the mPFC of meth rats (Fig 2E, group × receptor interaction, F(1,21)=7.28, p<0.02]. Specifically, NMDA currents in meth rats were elevated relative to AMPA currents (Sidak’s multiple comparisons test, p<0.05). Consistently, our protein analysis revealed that meth significantly increased the surface expression of the GluN2B subunits in the mPFC [Fig 3A, t(13)=2.5, p<0.05], yet other NMDA receptor subunits, GluN2A [t(12)=1.5, p=0.16] and GluN1 [t(13)=1.4, p=0.2] remained unchanged (Fig 3C).
Figure 3.
In the mPFC, meth self-administration increased surface expression of NMDA receptor subunit GluN2B relative to yoked-saline controls (A, *significantly different from saline). Representative band for GluN2B with the loading control alpha-tubulin are depicted (B). L denotes a molecular weight ladder, while S and M represent saline and meth treated rats. On the right are representative electropherograms, with the area under the curve shown in grey. Meth did not change surface expression of GluN1 or GluN2A subtypes (C).
Meth self-administration increases glutamate release probability in the NAc
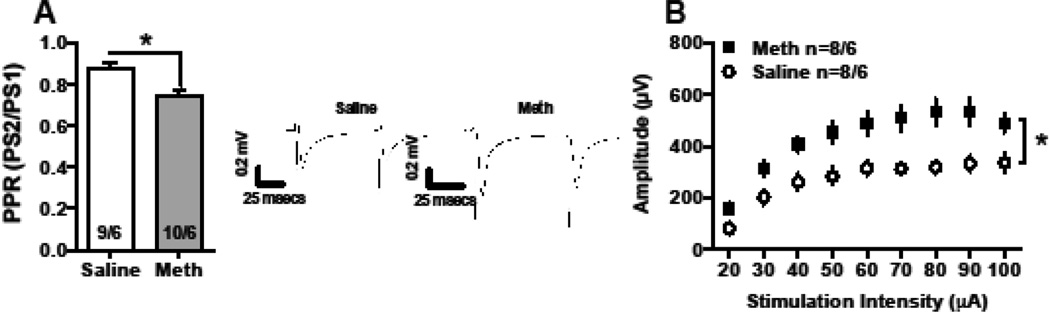
We assessed synaptic function using whole cell and field recordings in the NAc. Population spike recordings in the NAc of meth rats showed a significant depression in PPR compared to controls (measured at 50msec ISI, Fig 4A, t(17)=3.56 p<0.0024; mean ± SEM: saline 0.88 ± 0.03, meth 0.75 ± 0.02). Synaptic output was indexed as an increase in the I/O function. Specifically, there was a main effect of group, with meth rats increased relative to controls [Fig 4B, F(1, 122)=15.9, p<0.001], as well as a main effect on stimulus intensity [F(8,122=15.89, p<0.001]. However, the interaction was not significant; there were no differences in the mean stimulus intensity (mean ± SEM: saline 29.78 ± 1.68, meth 31.4 ± 1.73).
Figure 4.
In the NAc, meth self-administration altered presynaptic physiology determined by field recordings. In the NAc, long access meth decreased PPR (50 msec ISI) relative to yoked-saline controls (A). Representative traces are depicted. The input/output function was significantly increased following meth, relative to saline controls (B).
The number of cells/animal is indicated in the bar graphs, which represent the mean; the errors bars represent the SEM.
*Significant difference from controls
Meth-induced depression of PPR was consistent with data obtained from whole-cell voltage clamp recordings [Fig 5A, t(14)=2.57, p<0.05; mean ± SEM: saline 1.12 ± 0.173, meth 0.69±0.078]. The frequency of sEPSCs increased in meth rats compared to yoked saline controls [Fig 5B, unpaired t with Welch’s correction, t(11)=2.56, p<0.05; mean ± SEM: saline 3.766 ± 0.25, meth 5.54 ± 0.65]; neither did the amplitude of sEPSCs (Fig 5C, mean ± SEM: saline 18.13 ± 1.06, meth 20.37 ± 0.94) and AMPA/NMDA ratio (not shown, mean ± SEM: saline 0.92 ± 0.132, meth 0.93 ± 0.076).
Figure 5.
In the NAc, meth self-administration altered presynaptic NAc physiology. In the NAc, long access meth decreased PPR on whole cell recordings (A). Representative traces for significant findings are depicted below. Also, meth increased the frequency (B) of sEPSCs but not the amplitude (C). Representative traces of spontaneous events from three cells of each group are depicted.
The number of cells/animal is indicated in the bar graphs, which represent the mean; the errors bars represent the SEM.
*Significant difference from saline.
Discussion
Here we show that a translationally relevant model of meth self-administration altered the pre- and postsynaptic plasticity in the NAc and mPFC, respectively. In the mPFC, meth changed synaptic strength, indexed as a decrease in the AMPA/NMDA ratio, and that plasticity reflected an increase in NMDA signaling. Further, meth increased NMDA GluN2B surface expression in the mPFC. In the NAc, meth caused a decrease in PPR, shifted the I/O function, and increased the frequency of sEPSCs with no changes in amplitude, suggesting a predominantly presynaptic change in NAc. The changes in cortical and accumbens glutamatergic neurotransmission demonstrated alterations in distinct regions that drive addictive behavior.
Effects of meth self-administration in mPFC
Following meth self-administration, we found a significant decrease in the AMPA/NMDA ratio in mPFC, driven by an increase in NMDA currents. The change in synaptic strength was accompanied by a significant increase in the surface expression of GluN2B subunits, suggesting that meth alters postsynaptic mechanisms at a cortical level. Those findings may actually reflect an increase in synaptic integration in the meth animals. Support for this notion comes from reports showing that GluN2B containing synapses have slower decay times when cortico-cortical inputs to layer 5 pyramidal neurons in the frontal cortex are stimulated (Kumar and Huguenard 2003), which, in turn, facilitates summation of the synaptic inputs (Cull-Candy and Leszkiewicz 2004). This enhanced integration allows more efficient synaptic function by bringing the neurons close to spike-firing threshold (Kumar and Huguenard 2003). We tested the post hoc hypothesis that the increased GluN2B expression resulted in slower sEPSC decay kinetics by analyzing the decay time of our existing data. The hypothesis was unsupported, however, because there were no differences in the decay rate of sEPSCs in meth relative to saline rats (data not shown). However, our experiments focused on measurements of synaptic strength, and our sEPSCs recordings were performed at −70 mV. The analysis of decay time from that data set was limited to the average trace generated from the digital subtraction of the Idual − IAMPA at +40mV. Therefore, conclusions about NMDA current decay based on those data are marginal, at best, and need to be further investigated with pharmacological isolation of the NMDA-mediated evoked response.
Our data contrast with the results reported by Gonzalez and colleagues (2016), which showed that in mice there is an increase in paired-pulse facilitation in deep-layer pyramidal mPFC neurons following withdrawal from repetitive non-contingent meth administration (7 days, 1 mg/kg). The most obvious explanation for this discrepancy is the methodological differences between studies, in particular, between species (mice and rats) and the behavioral contingency used to acquire the drug. Meth self-administration produces distinct neurochemical adaptions from those produced by non-contingent meth (Lominac et al. 2012). For example, glutamate levels increased in rats that self-administered meth, relative to those that received saline on day one of withdrawal in response to a meth prime injection. This increase was potentiated after 3 weeks of abstinence. Importantly, these findings demonstrate the importance of non-pharmacological factors in meth-induced neurochemical adaptations in the NAc.
Effects of meth self-administration in NAc
In the NAc we found that meth self-administration elicited an increase in the probability of glutamate release, and also found meth self-administration increased I/O function, which is consistent with increased glutamate release probability. However, increased I/O function may also reflect increased excitability of MSNs or post-synaptic receptor population changes. Independent of the mechanisms underlying the increase in I/O function, the difference in population level response was not due to differences in the average AMPA and NMDA currents between saline and meth animals.
We report an increase in frequency of sEPSCs, with no changes in amplitude, suggesting alterations in glutamatergic presynaptic sites. Furthermore, whole-cell paired-pulse recordings show decreases in the PPR, suggesting an increase in glutamate release. This increase in glutamate release may be the result of an increase in the number of synaptic vesicles in the readily releasable pool (Stevens and Tsujimoto 1995). Meth increases striatal vesicular glutamate transporter 1 protein expression and vesicular glutamate uptake (Mark et al. 2007). Alternatively, we have shown that meth self-administration decreased the number and/or function of inhibitory presynaptic release regulating group 2 metabotropic glutamate receptors (mGluR2/3) in the NAc core following 14 days of abstinence from meth self-administration (Schwendt et al. 2012); moreover, down regulation of mGluR2/3 disinhibited presynaptic neurotransmitter release.
We did not observe changes in AMPA/NMDA ratio or amplitude of sEPSCs to indicate altered postsynaptic function. While puzzling, this lack of postsynaptic plasticity in the NAc is consistent with reports showing no changes in AMPA/NMDA ratio or amplitude in the shell of mice following repeated amphetamine injections (Jedynak et al. 2016). Recently, Wolf and colleagues (Scheyer et al. 2016) demonstrated that long access meth self-administration (10 days, 6 hr daily) followed by > 40 days of withdrawal increased CP-AMPAR (GluA2 lacking AMPAR) in the NAc core in a manner similar with cocaine (Loweth et al. 2014). In contrast with cocaine, these increases in CP-AMPAR occurred as early as 7 days following withdrawal in meth animals’ however, for cocaine the up regulation typically does not occur until 25 days of abstinence. Importantly, CP-AMPAR have been linked to drug craving (Loweth et al. 2014); thus Wolf and colleagues (2016) suggested that the rapid increase in CP-AMPAR following meth may contribute to the highly addictive nature of the drug. In the current report, we did not assess levels of CP-AMPAR in the NAc of our meth animals. Our assessment of AMPA/NMDA ratio did not indicate that we would find changes in that parameter, even though our study and the one conducted by Wolf and colleagues assessed animals at similar time points during withdrawal/abstinence. We used a different meth self-administration protocol; our animals went through 7 days of one-hour meth self-administration before 6-hour access. Thus, during the first 7 days of self-administration, our rats administered substantially lower amounts of the drug than during the 6-hour sessions. This protocol may result in a phenomenon known as meth preconditioning (Cadet et al. 2009; Johnson-Davis et al. 2003). Although untested in our model in conjunction with glutamate receptor function, it is possible that a similar effect may have occurred in our sample. Thus, the possibility of meth preconditioning warrants future study.
Effects of meth self-administration in the cortico-accumbens circuit
A strength of our experimental design stems from recording in the mPFC and NAc of the same rats. However, our methodology severs the circuit, so we can only speculate about events occurring within the cortical-striatal pathway. This design allow the assumption that changes in glutamatergic synaptic transmission observed in those two regions occur simultaneously, even though we cannot determine whether the onset of the changes occurs concurrently. Another limitation of this approach is that we can only infer that the spontaneous events detected and analyzed in our NAc sEPSC recordings are from inputs from the mPFC because they more likely reflect multiple inputs into NAc core. Despite these limitations, it is possible that the meth-induced selective increase in GluN2B containing NMDA receptors and the increase in NMDA receptor current amplitude in the mPFC contributed to the decreased PPR and increased frequency of sEPSCs in the NAc.
Our results can be interpreted two ways based on a recent review by Kourrich and colleagues (Kourrich et al. 2015). First, the synaptic changes induced by meth (i.e., up regulation of GluN2B, increased NMDA current) do not necessarily translate into changes in neuronal activity (i.e., firing frequency). Second, the permissive function hypothesis (Kourrich et al., 2015) suggests that cocaine-induced transient increases in the NAc activity are linked to delayed increases in NAc synaptic strength. Applying this hypothesis to our mPFC data, we speculate that the meth induced changes in intrinsic activity (Parsegian et al. 2011) may precede our reported differences in synaptic plasticity. Furthermore, the increase in cortical intrinsic excitability may mediate the increase in glutamate release in the NAc.
In summary, we report meth self-administration alters synaptic plasticity in the corticostriatal circuit. These findings add to a growing body of literature focused on identifying the underlying mechanisms of meth addiction. Although it remains to be determined how these physiological processes relate to documented cognitive sequelae in addicts, these results show that meth elicits different long-term synaptic changes in the reward circuit, suggesting altered synaptic function, which is one of the fundamental mechanisms affected by drugs of addiction.
Acknowledgments
We would like to thank Shannon Ghee and Carole Berini for technical assistance and the Writing Center at the Medical University of South Carolina for editing the final version of the manuscript.
Funding
This project was funded by NIH/NIDA grant R01DA033049 to CMR and Hjarnfonden Brain Foundation of Sweden to DM.
Footnotes
Author Contributions
The experiments were designed by DM, JPB, AL, and CMR. Field recordings were conducted by DM. Whole cell recordings were conducted by JPB. Western blots were performed by KCL. Electrophysiology analyses were conducted by JPB, DM, and AL with the statistical analysis conducted by CMR. DM and JPB wrote the first draft of the paper. AL and CMR prepared the final version of the manuscript and figures. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science (New York, N.Y.) 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Beccano-Kelly DA, Kuhlmann N, Tatarnikov I, Volta M, Munsie LN, Chou P, Cao LP, Han H, Tapia L, Farrer MJ, Milnerwood AJ. Synaptic function is modulated by LRRK2 and glutamate release is increased in cortical neurons of G2019S LRRK2 knock-in mice. Frontiers in Cellular Neuroscience. 2014;8:301. doi: 10.3389/fncel.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. Journal of Neuroscience. 1997;17:796–803. doi: 10.1523/JNEUROSCI.17-02-00796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, McCoy MT, Cai NS, Krasnova IN, Ladenheim B, Beauvais G, Wilson N, Wood W, Becker KG, Hodges AB. Methamphetamine preconditioning alters midbrain transcriptional responses to methamphetamine-induced injury in the rat striatum. PloS one. 2009;4:e7812. doi: 10.1371/journal.pone.0007812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Science's STKE. 2004;255:16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. J. Physiol. (Lond.) Vol. 491. Wiley-Blackwell; 1996. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release; pp. 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Wolf ME. Dopamine alters AMPA receptor synaptic expression and subunit composition in dopamine neurons of the ventral tegmental area cultured with prefrontal cortex neurons. The Journal of Neuroscience. 2007;27:14275–14285. doi: 10.1523/JNEUROSCI.2925-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews. Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González B, Rivero-Echeto C, Muñiz JA, Cadet JL, García-Rill E, Urbano FJ, Bisagno V. Methamphetamine blunts Ca2+ currents and excitatory synaptic transmission through D1/5 receptor-mediated mechanisms in the mouse medial prefrontal cortex. Addiction Biology. 2015;21:589–602. doi: 10.1111/adb.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves SM, Clark MJ, Traynor JR, Hu XT, Napier TC. Nucleus accumbens shell excitability is decreased by methamphetamine self-administration and increased by 5-HT2C receptor inverse agonism and agonism. Neuropharmacology. 2015;89:113–121. doi: 10.1016/j.neuropharm.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jedynak J, Hearing M, Ingebretson A, Ebner SR, Kelly M, Fischer RA, Kourrich S, Thomas MJ. Cocaine and amphetamine induce overlapping but distinct patterns of AMPAR plasticity in nucleus accumbens medium spiny neurons. Neuropsychopharmacology. 2016;41:464–476. doi: 10.1038/npp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Davis KL, Fleckenstein AE, Wilkins DG. The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. European Journal of Pharmacology. 2003;482:151–154. doi: 10.1016/j.ejphar.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Calu DJ, Bonci A. Intrinsic plasticity: an emerging player in addiction. Nature reviews. Neuroscience. 2015;16:173–184. doi: 10.1038/nrn3877. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Huguenard JR. Pathway-specific differences in subunit composition of synaptic NMDA receptors on pyramidal neurons in neocortex. The Journal of Neuroscience. 2003;23:10074–10083. doi: 10.1523/JNEUROSCI.23-31-10074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology. 2011;61:1060–1069. doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, Kennedy PJ, Liu QR, Cimbro R, Hope BT, Nestler EJ, Shaham Y. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. The Journal of Neuroscience. 2015;35:8232–8244. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology. 2012;37:707–722. doi: 10.1038/npp.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, Li X, Ford KA, Le T, Olive MF, Szumlinski KK, Tseng KY, Wolf ME. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nature Neuroscience. 2014;17:73–80. doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Luscher C. Pathological circuit function underlying addiction and anxiety disorders. Nature Neuroscience. 2014;17:1635–1643. doi: 10.1038/nn.3849. [DOI] [PubMed] [Google Scholar]
- Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J. Neurosci. 2007;27:6823–6831. doi: 10.1523/JNEUROSCI.0013-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. The American Journal on Addictions. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- O'Neill RA, Bhamidipati A, Bi X, Deb-Basu D, Cahill L, Ferrante J, Gentalen E, Glazer M, Gossett J, Hacker K, Kirby C, Knittle J, Loder R, Mastroieni C, Maclaren M, Mills T, Nguyen U, Parker N, Rice A, Roach D, Suich D, Voehringer D, Voss K, Yang J, Yang T, Vander Horn PB. Isoelectric focusing technology quantifies protein signaling in 25 cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16153–16158. doi: 10.1073/pnas.0607973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Jr, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biological Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Scofield MD, Ghee SM, Heinsbroek JA, Reichel CM. Perirhinal Cortex mGlu5 Receptor Activation Reduces Relapse to Methamphetamine Seeking by Restoring Novelty Salience. Neuropsychopharmacology. 2015;41:1477–1485. doi: 10.1038/npp.2015.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG. Short-term presynaptic plasticity. Cold Spring Harbor perspectives in biology. 2012;4(7):a005702. doi: 10.1101/cshperspect.a005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology. 2012;223:371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, Dolubizno H, Stefanik MT, Murray CH, Sakas C, Wolf ME. AMPA receptor plasticity in accumbens aore aontributes to a incubation of methamphetamine craving. Biological Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.04.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Reichel CM, See RE. Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. PLoS ONE. 2012;7(3):e34299–e34310. doi: 10.1371/journal.pone.0034299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Trantham-Davidson H, Schwendt M, Leong KC, Peters J, See RE, Reichel CM. Failure to recognize novelty after extended methamphetamine self-administration results from loss of long-term depression in the perirhinal cortex. Neuropsychopharmacology. 2015;40:2526–2535. doi: 10.1038/npp.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47:242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Tsujimoto T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:846–849. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annual Review of Physiology. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]