Abstract
In this research, maltodextrin (0, 1 and 2% w/w) and resistant starch (0, 1 and 2% w/w) were used in the formulation of low-fat ice cream (4% fat) and their effects on the physicochemical and sensory properties were investigated. The optimum levels of maltodextrin and resistant starch were determined by response surface methodology. Increment of maltodextrin and resistant starch increased acidity, viscosity, melting rate, time of dripping and overrun but decreased melting rate of ice cream. Results showed that the incorporation of maltodextrin and resistant starch at 0 and 2% w/w respectively, resulted into ice cream with suitable viscosity, melting rate, first dripping time, overrun and acidity.
Keywords: Maltodextrin, Resistant starch, Low-fat ice cream, Optimization
Introduction
Ice cream is a complex product that has an emulsification fat, colloidal protein and a solution of lactose and salt (Goff and Hartel 2013; Flores and Goff 1999). The greatest concern about ice cream relates to its fat content that has a significant effect on the ice cream properties.
Nowadays, due to the negative effects of fat, trend for consuming low-fat products in the diet, such as low-fat ice cream has increased. Low-fat ice cream has a fat content lower than 5% by weight (Goff and Hartel 2013). Fat is widely used in foods and in many ways, such as texture, shape, appearance, smell, flavor and mouth feel, is participating. According to the role of fat in the ice cream, reducing of its amount in the ice cream formulations, lead to several problems in the structure and texture of the produced ice cream such as coarseness and roughness, iciness, crushing, reduces the size and bad flavor. To resolve these problems, using of fat substitutes is essential in the low-fat ice cream production. Nowadays, generally the fat substitute material can be classified as substitutes based on carbohydrates, proteins, low-calorie fats, emulsifiers and combined products (Marshall and Arbuckle 1996; Guzeler et al. 2011; Goff and Hartel 2013).
Fat substitutes based on carbohydrates such as processed starch and dietary fibers as fat emulator lead to soft texture in food by linking up with water. Resistant starch is a relatively new classification of carbohydrates is considered in the diet as a fat substitute based on carbohydrates. Resistant starch is defined as a component of starch that not digests in the small intestine and may be fermented by the intestinal flora. Resistant starch may be naturally exists in foods or formed due to processing conditions in foods containing starch (Fuentes-Zaragoza et al. 2010, 2011; Surapat and Rugthavon 2003). It is reported that the resistant starch provides a good appearance, desirable and mouth feel than normal fibers in foods (Surapat and Rugthavon 2003).
Maltodextrin is produced through starch fragmentation by enzyme or acid. Maltodextrin gel can be easily combined with solid and liquid fats to form a stable emulsion gel. Since maltodextrin has a taste similar to the fat taste, lead to easily break down in the food into its component parts in the mouth and mainly use in margarine, mayonnaise, pastries, salads and dairy products (Guzeler et al. 2011). Finally, the main purpose of this study was to evaluate the effect of fat substitutes (maltodextrin and resistant starch) on the physicochemical, rheological, and sensory properties of low-fat ice cream and optimizing the process conditions for the same by response surface methodology.
Materials and methods
Preparation of ice cream
In all samples, the ice cream mixture, was contained 6 g salep, 160 g sugar and 1 g vanilla (per a liter), and depending on the type of attendance, various percentages of maltodextrin (0, 1 and 2% w/w) and Hi-Maize resistant starch (0, 1 and 2% w/w) were added to the ice cream formulation (Table 1). Also, 1.5% fat milk with cream (25% fat) was mixed and its fat content was reduced to 4%. The required dry material was weighted and homogenized (10,000 rpm at 5 min) with 4% fat milk by homogenizer (T25 digital, ULTRA-TURRAX, Heidolph, Germany) and finally was pasteurized (72 °C for 20 min). After this step, the resulting mixture passed the maturity step for hydration of the mixture components (aging) at a temperature of 5 °C for 24 h. The resulted mixture after this step was prepared by the ice cream maker for 20 min and finally was purred in 30 g plastic containers and was kept up at −20 °C for hardening. The experiments in this regard were done two times.
Table 1.
Experimental runs to study the effect of RS and maltodextrin on the properties of ice cream samples
| Run | Block | RS (b1) | Maltodextrin (b2) |
|---|---|---|---|
| 1 | Block 1 | 1 | 1 |
| 2 | Block 1 | 0 | 2 |
| 3 | Block 1 | 0 | 0 |
| 4 | Block 1 | 1 | 1 |
| 5 | Block 1 | 0 | 1 |
| 6 | Block 1 | 2 | 2 |
| 7 | Block 1 | 1 | 1 |
| 8 | Block 1 | 1 | 1 |
| 9 | Block 1 | 1 | 0 |
| 10 | Block 1 | 1 | 1 |
| 11 | Block 1 | 2 | 0 |
| 12 | Block 1 | 1 | 2 |
| 13 | Block 1 | 2 | 1 |
| 14 | Block 1 | 1 | 1 |
Measuring the pH and acidity
The pH value of samples before freezing was measured using the pH meter (Methrom model 827, Switzerland). In order to measure the acidity before freezing, 10 ml of sample was picked up and titrated with 0.1 normal NaOH in the presence of phenolphthalein until pink color was appeared (Akalin and Erisir 2008). The amount of acidity was calculated based on Dornik (°D) as follows:
| 1 |
Increasing volume coefficient (overrun) measurement
For overrun measurement, the containers with a defined volume were used. After freezing of the product in ice cream maker, sampling was made from the produced mixture. The sample was weighed and overrun was calculated through the following equation (Rezaei et al. 2012).
| 2 |
Melting rate
A piece of ice cream with weight of 30 ± 1 g was placed on a sieve with pore diameter of 2 mm and incubated at 25 ± 1 °C in oven. After 60 min the molten liquid weight, based on the percentage of the initial weight was measured as the melting rate. The first dripping time also was recorded during melting (Sun-Waterhouse et al. 2013).
Viscosity and rheological properties
The ice cream mixture viscosity was measured at a 5 °C by Brookfield viscometer Model DVII (Akin et al. 2007). After preliminary tests, spindle number 5 was chosen as the most appropriate spindle. The viscosity was measured in rpm = 100. To determine the rheological behavior of the produced ice cream, raw data obtained from the viscometer, was converted to shear speed and shear stress using a mathematical relationship described by Mitschka (1982) and flow behavior diagram of the samples was compared on this basis (Constenla and Lozano 2005; Rezaei et al. 2012; Briggs and Steffe 1997). The flow behavior of samples were compared with different rheological models including Power law, Casson and Herschel–Bulkley. These relationships are shown in the following equations:
| 3 |
| 4 |
| 5 |
where τ, γ, k, n, are shear stress (Pa), shear rate (1/s), consistency coefficient (cp) and flow behavior index (dimensionless) respectively. Also in Eq. 4, k2c and k20c = τ0c are Casson viscosity (Pa s) and Casson yield stress (Pa), respectively.
Sensory evaluation
The sensory properties of the products were evaluated by use of 10 trained panelists. After initial training of the panelists to acquaint the desired properties, the samples were evaluated in term of texture, flavor, color and total acceptability (Garmakhany et al. 2008). In this test, the 5 points hedonic scale (excellent sample 5 score, good 4, Average 3, bad 2 and too bad 1) was used.
Statistical design
After the initial Pre-attendance and obtaining the proper domain of independent variables, the central composite design in the form of response surface methodology (RSM), was designed with Design Expert software (6.0.2). The applied central composite design was included 8 factorial points, 6 axis points and 6 repetitions at the central point. All attendances were done in two replications. The experimental design or coding levels of the independent variable and the results of data attendance are shown in Table 1.
In the response surface method for evaluating parameters, different levels of resistant starch and maltodextrin were used. Response functions (Y) on the measured parameters were studied by using of linear model (Eq. 6) and quadratic polynomial model (Eq. 7).
| 6 |
| 7 |
where Y is the predicted response; x1 and x2 is the independent variables related to resistant starch and maltodextrin, respectively; b0 is a model constant; b1 and b2 is the linear effects; b11 and b22 is the Quadratic effects; and b12 is the interaction effect.
Results and discussion
Statistical design
Linear model of the melting rate parameter and quadratic polynomial model of overrun, first dripping time and viscosity in fitting data according to the other proposed models were significantly different (p < 0.01). As can be seen from Table 2, for each parameters the appropriate model was selected according to different conditions, including the significant test F (p < 0.01), non significant lack of fit amount (p > 0.01), correlation coefficient (R2) and modified R2 values. According to the Table 2 and the R2 values, it could be seen the suitable data fitting by the selected models. The R2 value of overrun changes, melting rate, first dripping time and viscosity have respectively been 0.90, 0.95, 0.90 and 0.87 (Table 2). The very low variation coefficient is also another reason for the fact that the models were the best fitted data. Effective parameters were placed in the model according to the analysis of variance and the selected ANOVA table.
Table 2.
Regression coefficients of predicted linear and quadratic polynomial models for physicochemical properties of ice cream containing RS and maltodextrin
| Source | df | Overrun | Melting rate | First dripping time | Apparent viscosity (cp) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | Sum of squares | p value | Coefficient | Sum of squares | p value | Coefficient | Sum of squares | p value | Coefficient | Sum of squares | p value | ||
| Modela | 5 (2) | +37.09 | 291.91 | 0.0009 | +25.84 | 271.03 | <0.0001 | ||||||
| Linear | |||||||||||||
| b1 | 1 | +2.31 | 32.02 | 0.0251 | −2.08 | 26.04 | 0.0010 | ||||||
| b2 | 1 | +5.52 | 183.15 | 0.0002 | −6.39 | 244.99 | <0.0001 | ||||||
| Quadratic | |||||||||||||
| b11 | 1 | +3.13 | 27.79 | 0.0336 | – | – | – | ||||||
| b22 | 1 | +1.93 | 10.52 | 0.1537 | – | – | – | ||||||
| Interaction | |||||||||||||
| b12 | 1 | −1.81 | 13.14 | 0.1162 | – | – | – | ||||||
| Residualb | 8 (11) | 33.89 | 14.53 | 22,154.00 | 40,187.49 | ||||||||
| Lack of fitc | 3 (6) | 7.69 | 0.7048 | 11.65 | 0.1016 | 13,548.00 | 0.1626 | 38,788.16 | 0.0005 | ||||
| Pure error | 5 | 26.20 | 2.88 | 8606.00 | 1399.33 | ||||||||
| Total | 13 | 325.80 | 285.56 | 2.200E+005 | 3.108E+005 | ||||||||
| R2 | 0.90 | 0.95 | 0.90 | 0.87 | |||||||||
| Adj-R2 | 0.83 | 0.94 | 0.84 | 0.80 | |||||||||
| Pred-R2 | 0.67 | 0.91 | 0.32 | 0.20 | |||||||||
| CV | 5.24 | 4.45 | 5.43 | 7.31 | |||||||||
b1 Resistant starch (RS), b2 Maltodextrin
a df of model for Overrun, first dripping and apparent viscosity is 5 and for melting rate is 2
b df of residual for Overrun, first dripping and apparent viscosity is 8 and for melting rate is 11
c df of lack of fit for Overrun, first dripping and apparent viscosity is 3 and for melting rate is 6
Acidity and pH
The results obtained from pH and acidity measurement showed that the resistant starch and maltodextrin didn’t have any significant effect on pH and acidity value (p > 0.05). Minimum and maximum pH value was related to the control sample and the sample containing 2% resistant starch and 2% maltodextrin that had respectively 6.7 and 6.9 values. Also, the minimum and maximum acidity value were related to the sample containing 2% resistant starch and 1% maltodextrin and the control sample, respectively and the obtained values were respectively been 14.5 and 22.6 °D.
Overrun
Ice cream and related products were known as frozen and aerated foams. The amount of the air in ice cream is very important; because of its effect on the quality and profitability of ice cream. As a result, in order to achieve optimal overrun, it was necessary to consider both economic and consumer acceptance according to national standards. The amount of overrun depends on various factors, including the type of mixture components such as fat content, solid materials, sweeteners and the presence of stabilizer materials (Bahram-Parvar and Mazaheri Tehrani 2011).
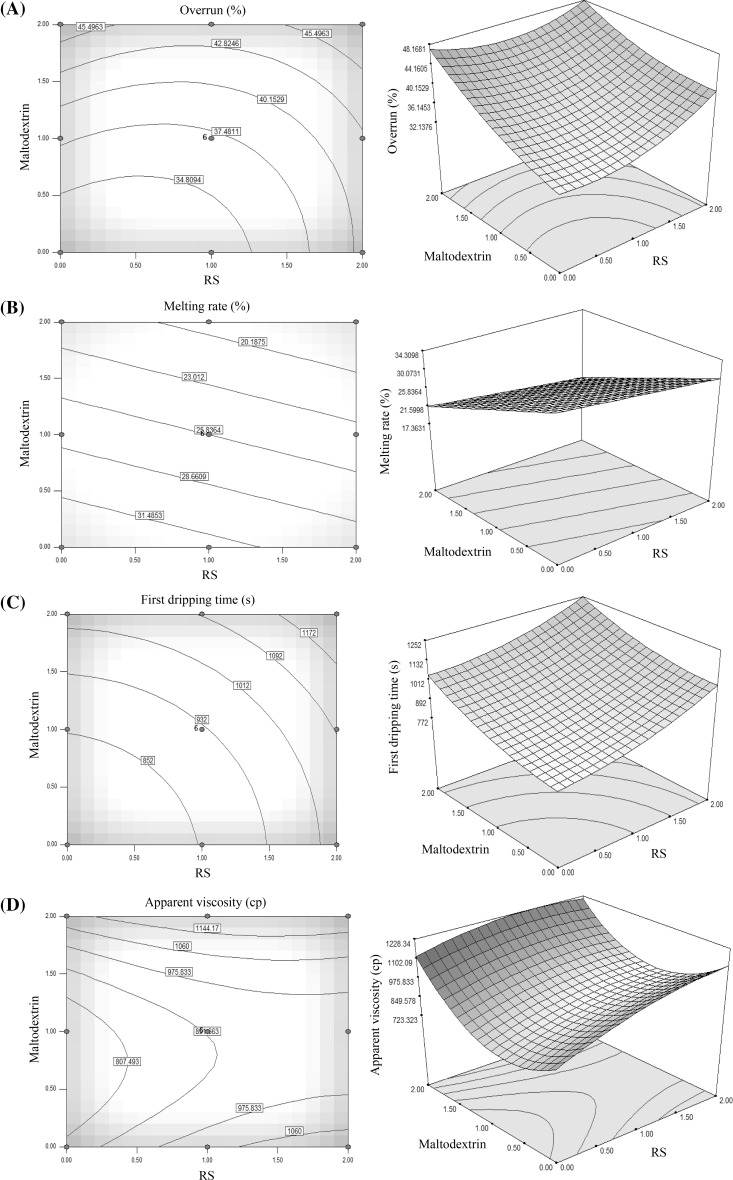
In Fig. 1a, the combined effects of resistant starch (RS) and maltodextrin on the overrun amount of ice cream samples are shown. As can be seen by the increasing RS and maltodextrin concentrations, the amount of overrun was increased. The amount of overrun increase in the RS and maltodextrin concentrations higher than 1.5% was more and about 45% that was comparable with the overrun amount in maltodextrin with concentrations lower than 5% and resistant starch with concentrations lower than 1.5%, which was about 34% (Fig. 1). According to Table 2 and comparison of the results of ice cream samples containing maltodextrin and resistant starch, linear and quadratic effects were significant (p < 0.05).
Fig. 1.
Response surface showing (contour and 3D surface) the effect of RS and maltodextrin concentrations on overrun (a), melting rate (b), first dripping time (c) and apparent viscosity (d)
Similar results also indicate that the use of maltodextrin (Guzeler et al. 2011) and modified starch (Surapat and Rugthavon 2003) as a fat substitute in the ice cream formulation lead to increase overrun. On the other hand, researchers such as Marshall et al. (2003), Rezaei et al. (2015) and Goff and Hartel (2013) expressed that by increasing viscosity during mixing and freezing process, air is properly distributed into the texture and so lead to increase sample overrun.
Melting rate
While the ice cream prepared in the form of a cone or wood, melting rate creates the most importance feels for the consumer. Slow melting, maintained a good shape and slower texture decay was an important qualitative parameter that described the ice cream (Bahram-Parvar and Mazaheri Tehrani 2011).
Table 2 shows the melting rate of the ice cream samples at 25 °C. As can be seen from Table 2 the melting rate of ice cream samples decreased with increase in the amount of RS and maltodextrin. The minimum and maximum amount of melting rate was related to the control sample and the sample containing the mixture of 2% RS and 2% maltodextrin, respectively. These results can be related to the ability of RS and maltodextrin in trapping water and forming gel network structure (Akalin et al. 2008; Rezaei et al. 2015).
Different factors affected the intensity of the ice cream melting rate including, the amount of air entering to the ice cream (overrun), ice crystal structures and fat globules network during freezing. Another reason for this phenomenon was the increasing of the fat substitute that may increase serum phase viscosity. Therefore, more time for water was necessary to spread in condensed serum phase (Sofjan and Hartel 2004).
Maltodextrin has a high water holding capacity, while samples containing RS have a higher viscosity and so their melting rate was higher than the samples containing more amounts of maltodextrin (Fig. 1b) (Guzeler et al. 2011; Surapat and Rugthavon 2003; Sajilata et al. 2005). However, by increasing RS and maltodextrin concentrations, first dripping time was increased. The use of RS and maltodextrin may change the thermal properties such as thermal conductivity and melting rate of ice cream (Table 2; Fig. 1c) (Goff and Hartel 2013; Bahram-Parvar and Mazaheri Tehrani 2011; Guzeler et al. 2011).
Apparent viscosity
Viscosity is defined as the resistance to stream and how much its amount is higher, the more force is needed for freezing and aeration of products (Marshall and Arbuckle 1996). Viscosity is one of the most important rheological properties of the ice cream mixture and the part of non-frozen ice cream that was impressed by the mixture composition, type and quality of component material processed (Goff and Hartel 2013).
According to the analysis of variance results (ANOVA), RS and maltodextrin showed a significant effect on the viscosity of the ice cream mixture (p ≤ 0.01); But the interaction of these two compounds was not significant (Table 2). The amount of viscosity in the control sample was 748 cP and the addition of RS and maltodextrin led to a significant increase in the ice cream mixture viscosity (p ≤ 0.01), so that the amount of viscosity at the highest levels of RS and maltodextrin, was 1264 cP, that compared to the control sample showed a significant increase (p ≤ 0.01). Also, the quadratic effect of maltodextrin on the viscosity of the ice cream mixture was significant (p ≤ 0.01). Dietary fibers, such as RS and maltodextrin can increase the functional properties of foods such as water-holding capacity, which may had led to increase in viscosity (Fuentes-Zaragoza et al. 2010; Elleuch et al. 2010; Soukoulis et al. 2009).
As can be seen from Fig. 1d, the variation of maltodextrin concentration had higher effect on the sample viscosity compared to RS and this may be related to the higher water absorption and strong gel network formation ability of maltodextrin compared to RS (Rezaei et al. 2015; Guzeler et al. 2011; Sajilata et al. 2005).
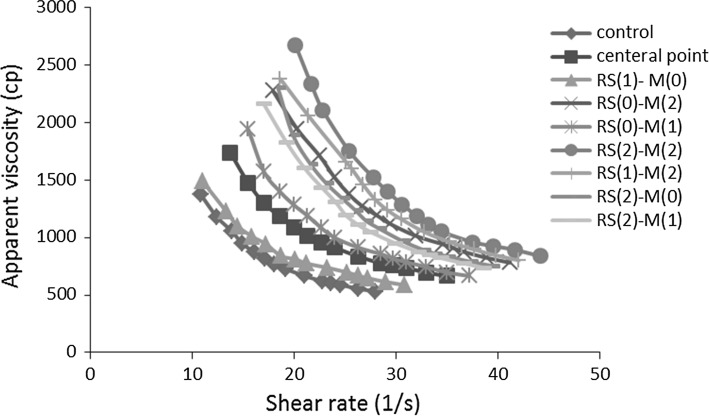
In general, increasing the viscosity of the samples containing fat substitutes can be related to the reaction of RS and maltodextrin with the liquid part of the mixture and the high water absorption capacity of these compounds (Rezaei et al. 2015). As a result, these compounds by increasing the water holding capacity led to decrease the stream acceptation and increase the sample resistance to streaming or appearance viscosity (Fig. 3).
Fig. 3.
Apparent viscosity as functions of shear rate for ice cream systems with different concentrations of RS and maltodextrin (w/w)
Rheological properties
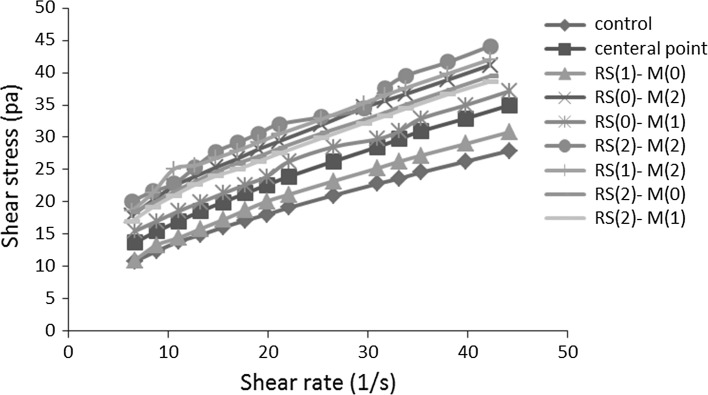
The soft texture and mouth feel describe the ice cream acceptability during the consumption (Dogan and Kayacier 2007). Figure 2 shows the relationship between shear stress and shear rate in samples containing RS and maltodextrin. Non-linear relationship of shear stress and shear rate showed the non-Newtonian behavior of flow. Figure 3 represents the effect of shear rate on the viscosity and showed that by increasing shear rate, the apparent viscosity of samples changed and this effect is higher in the samples containing high amounts of maltodextrin.
Fig. 2.
Shear stress as functions of shear rate for ice cream systems with different concentrations of RS and maltodextrin (w/w)
Table 3 shows the results of the data fitting of the rheological behavior of ice cream with different rheological models. As can be seen, the control samples (without RS and Maltodextrin) have flow behavior index lower than 1 and confirmed that Herschel–Bulkley model can describe this sample better than the other samples, in fact by increasing the amounts of these two compounds, pseudoplastic don’t change.
Table 3.
The values obtained of Power law, Herschel–Bulkley and Casson model parameter for ice cream containing RS and maltodextrin
| Run | RS (%) | M (%) | Power Law model | Herschel–Bulkley model | Casson model | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | n | R 2 | χ2 | RMSE | k | τ 0 | n | R 2 | χ2 | RMSE | k 2c | τ 0c | R 2 | χ2 | RMSE | |||
| 1 | 1 | 1 | 4.94 | 0.51 | 0.998 | 0.024 | 0.013 | 4.86 | 0.15 | 0.52 | 0.999 | 0.021 | 0.012 | 0.59 | 4.33 | 0.989 | 0.034 | 0.018 |
| 2 | 0 | 2 | 4.93 | 0.52 | 0.997 | 0.025 | 0.015 | 4.87 | 0.15 | 0.51 | 0.999 | 0.022 | 0.010 | 0.55 | 4.13 | 0.989 | 0.030 | 0.019 |
| 3 | 0 | 0 | 4.01 | 0.50 | 0.997 | 0.025 | 0.015 | 3.96 | 0.10 | 0.51 | 0.995 | 0.024 | 0.011 | 0.52 | 3.50 | 0.988 | 0.028 | 0.020 |
| 4 | 1 | 1 | 4.94 | 0.51 | 0.995 | 0.027 | 0.018 | 4.76 | 0.14 | 0.52 | 0.999 | 0.022 | 0.012 | 0.56 | 4.22 | 0.989 | 0.037 | 0.025 |
| 5 | 0 | 1 | 4.84 | 0.55 | 0.995 | 0.027 | 0.018 | 4.76 | 0.15 | 0.53 | 0.999 | 0.028 | 0.017 | 0.59 | 4.32 | 0.990 | 0.025 | 0.018 |
| 6 | 2 | 2 | 4.22 | 0.49 | 0.997 | 0.027 | 0.022 | 4.16 | 0.11 | 0.50 | 0.999 | 0.023 | 0.018 | 0.52 | 3.71 | 0.987 | 0.028 | 0.023 |
| 7 | 1 | 1 | 7.77 | 0.44 | 0.998 | 0.031 | 0.018 | 7.70 | 0.11 | 0.44 | 0.999 | 0.021 | 0.016 | 0.61 | 6.70 | 0.979 | 0.032 | 0.026 |
| 8 | 1 | 1 | 5.69 | 0.48 | 0.998 | 0.025 | 0.019 | 5.57 | 0.21 | 0.49 | 0.998 | 0.027 | 0.013 | 0.59 | 5.02 | 0.986 | 0.026 | 0.024 |
| 9 | 1 | 0 | 4.89 | 0.54 | 0.997 | 0.021 | 0.012 | 4.66 | 0.15 | 0.53 | 0.999 | 0.025 | 0.012 | 0.59 | 4.43 | 0.989 | 0.028 | 0.019 |
| 10 | 1 | 1 | 8.54 | 0.43 | 0.995 | 0.029 | 0.014 | 8.43 | 0.17 | 0.43 | 0.996 | 0.022 | 0.015 | 0.62 | 7.34 | 0.976 | 0.029 | 0.024 |
| 11 | 2 | 0 | 8.93 | 0.41 | 0.998 | 0.030 | 0.015 | 8.88 | 0.07 | 0.41 | 0.998 | 0.031 | 0.017 | 0.59 | 7.64 | 0.972 | 0.035 | 0.025 |
| 12 | 1 | 2 | 7.98 | 0.42 | 0.998 | 0.031 | 0.016 | 7.87 | 0.16 | 0.42 | 0.999 | 0.034 | 0.013 | 0.58 | 6.84 | 0.976 | 0.032 | 0.024 |
| 13 | 2 | 1 | 7.40 | 0.44 | 0.998 | 0.034 | 0.017 | 7.32 | 0.13 | 0.44 | 0.999 | 0.032 | 0.015 | 0.58 | 6.38 | 0.979 | 0.034 | 0.025 |
| 14 | 1 | 1 | 4.99 | 0.52 | 0.997 | 0.020 | 0.014 | 4.96 | 0.17 | 0.53 | 0.998 | 0.020 | 0.015 | 0.60 | 4.41 | 0.989 | 0.027 | 0.022 |
Ice cream mixtures showed the Non-Newtonian and pseudoplastic behavior, which means that, there was a non-linear relationship between shear stress and shear rate. And a decrease in apparent viscosity with increase in shear speed was observed. Pseudoplastic or shear thinning behavior was related to the increasing of molecular components of the system (Farhoosh and Riazi 2007). The n and k values are important in the rheological properties of liquid foods and these quantities were determined the food streams (Bahram-Parvar and Mazaheri Tehrani 2011). As can be seen by the increasing of the samples viscosity the consistency coefficient was increased.
In general, the Herschel–Bulkley model with the R2 ranged between 0.995 and 0.999 for all attendances and other statistical parameters such as chi-square (χ2) and root mean square error (RMSE) lower than the other studied model, can predict the flow behavior better than other models. As can be seen in Table 3, the control sample has the highest correlation coefficient (R2 = 0.999). Many studies had done on the rheological properties of ice cream, ice cream mixture and effective factors on these properties (Rezaei et al. 2012; Soukoulis et al. 2009; Adapa et al. 2000). For now, it was shown that increase in concentration and decrease in temperature, increases pseudoplastic behavior (Bahram-Parvar and Mazaheri Tehrani 2011). Goff and Davidson (1994) reported that the flow behavior index (n) of the ice cream mixture is close to 0.7. However, the values that were obtained by other researchers are between 0.37 and 0.98.
Sensory evaluation
Sensory evaluation results showed that the panelists detect a significant difference in color, texture, flavor and total acceptability of the ice cream samples (Table 4). In fact, the control sample compared to the sample which had the highest RS and maltodextrin created a significant difference (p < 0.05). An increase in RS amount in the ice cream led to decrease in its overall acceptance. This may be due to flour and starchy taste that RS had imparted to the ice cream, while this taste was not contributed by maltodextrin. Sensory evaluation scores revealed that ice cream containing 1% maltodextrin and 1% RS had best majority of sensory score (Table 4).
Table 4.
Sensory evaluation scores of ice cream made using RS and maltodextrin
| RS (%) | M (%) | Colour | Texture | Flavour | Total |
|---|---|---|---|---|---|
| 0 | 0 | 4.4 ± 0.89ab | 2.6 ± 0.54a | 3.8 ± 0.83bc | 2.8 ± 0.83a |
| 1 | 1 | 4.4 ± 0.54ab | 3.6 ± 0.89b | 4.4 ± 0.54c | 4.2 ± 0.44 cd |
| 1 | 0 | 4.2 ± 0.83ab | 3.8 ± 0.44b | 4.2 ± 0.83bc | 3.6 ± 0.89abc |
| 0 | 2 | 4.6 ± 0.54bab | 3.6 ± 0.54b | 4.0 ± 0.00bc | 3.8 ± 0.44bcd |
| 0 | 1 | 4.4 ± 0.54ab | 3.6 ± 0.54b | 4.0 ± 0.00bc | 3.4 ± 0.54abc |
| 2 | 2 | 4.2 ± 0.44ab | 4.0 ± 0.70b | 2.6 ± 0.54a | 3.4 ± 0.54abc |
| 1 | 2 | 3.8 ± 0.44ab | 4.0 ± 0.70b | 3.4 ± 0.54ab | 4.6 ± 0.54d |
| 2 | 0 | 3.6 ± 0.54a | 4.0 ± 0.70b | 3.0 ± 0.70a | 3.0 ± 0.70ab |
| 2 | 1 | 3.8 ± 0.44ab | 3.8 ± 0.45b | 2.8 ± 0.44a | 3.4 ± 0.89abc |
Mean with the same letter in each column are not significantly different at 95% level
Optimization
The results of the optimization of viscosity, melting rate, first dripping time, overrun and acidity showed that the best levels of the independent variables of the RS and maltodextrin were 0 and 2 respectively. Also, at optimal levels, the amount of viscosity, melting rate, first dripping time, overrun and acidity were obtained 1116.02 centipoise, 21.53%, 1040 s, 47.17% and 20.31 °D respectively that was selected with the desirability of 80%.
Conclusion
Nutritionists increase emphasis on the use of low-fat food and increase of consumers’ knowledge according to the adverse effects of fat on the body health, causing the consumers worldwide trend toward low-fat food products. The results of this research showed that the use of maltodextrin and resistant starch in the ice cream sample led to increase the viscosity, overrun and first dripping time of the final products while the melting rate of ice cream reduced. According to the results, due to the high water holding capacity of maltodextrin compared to the resistant starch, the obtained values from this research were more observed. On the other hand, by study the rheological properties and fitting the experimental data with mentioned models, Herschel–Bulkley model was the best model for the prediction flow behavior of ice cream samples. As a general result, maltodextrin and resistant starch showed better physicochemical properties. The amount of obtained viscosity, melting rate, first dripping time, overrun and acidity were 1116.02 centipoise, 21.53%, 1040 s, 47.17% and 20.31 °D respectively, in the optimum ice cream formulation. Therefore, these fat substitutes based on carbohydrates, can be proposed to ice cream manufacturers, especially in the production of reduced fat mixtures.
References
- Adapa S, Dingeldein H, Schmidt KA, Herald TJ. Rheological properties of ice cream mixes and frozen ice creams containing fat and fat replacers. J Dairy Sci. 2000;83:2224–2229. doi: 10.3168/jds.S0022-0302(00)75106-X. [DOI] [PubMed] [Google Scholar]
- Akalin AS, Erisir D. Effects of inulin and oligofructose on the rheological characteristics and probiotic culture survival in low fat probiotic ice cream. J Food Sci. 2008;73(4):184–188. doi: 10.1111/j.1750-3841.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- Akalin AS, Karagözlü C, Ünal G. Rheological properties of reduced-fat and low fat ice cream containing whey protein isolate and inulin. Eur Food Res Technol. 2008;227(3):889–895. doi: 10.1007/s00217-007-0800-z. [DOI] [Google Scholar]
- Akin MB, Akin MS, Kirmaci Z. Effects of inulin and sugar levels on the viability of yogurt and probiotic bacteria and the physical and sensory characteristics in probiotic ice-cream. J Food Chem. 2007;104:93–99. doi: 10.1016/j.foodchem.2006.11.030. [DOI] [Google Scholar]
- Bahram-Parvar M, Mazaheri Tehrani M. Application and functions of stabilizers in ice cream. Food Rev Int. 2011;27(4):389–407. doi: 10.1080/87559129.2011.563399. [DOI] [Google Scholar]
- Briggs JL, Steffe JF. Using Brookfield data and the Mitschka method to evaluate power law foods. J Texture Stud. 1997;28:517–522. doi: 10.1111/j.1745-4603.1997.tb00134.x. [DOI] [Google Scholar]
- Constenla DT, Lozano JE. Effect of pretreatments and processing conditions on the chemical, physical, microbiological and sensory characteristics of garlic paste. J Food Process Eng. 2005;28:313–329. doi: 10.1111/j.1745-4530.2005.00407.x. [DOI] [Google Scholar]
- Dogan M, Kayacier A. The effect of ageing at low temperature on the rheological properties of Kahramanmaras-type ice cream mix. Int J Food Prop. 2007;10(1):19–24. doi: 10.1080/10942910600610729. [DOI] [Google Scholar]
- Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C, Attia H. Dietary fiber and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. J Food Chem. 2010;124(2):411–421. doi: 10.1016/j.foodchem.2010.06.077. [DOI] [Google Scholar]
- Farhoosh R, Riazi A. A compositional study on two types of salep in Iran and their rheological properties as a function of concentration and temperature. Food Hydrocoll. 2007;21:660–666. doi: 10.1016/j.foodhyd.2006.07.021. [DOI] [Google Scholar]
- Flores AA, Goff HD. Ice crystal size distribution in dynamically frozen model solutions and ice cream as affected by stabilizers. J Dairy Sci. 1999;82:1399. doi: 10.3168/jds.S0022-0302(99)75366-X. [DOI] [Google Scholar]
- Fuentes-Zaragoza E, Riquelme-Navarrete MJ, Sánchez-Zapata E, Pérez-Álvarez JA. Resistant starch as functional ingredient: a review. Food Res Int. 2010;43:931–942. doi: 10.1016/j.foodres.2010.02.004. [DOI] [Google Scholar]
- Fuentes-Zaragoza E, Sanchez-Zapata E, Sendra E, Sayas E, Lope JF, Perez-Alvarez A. Resistant starch as prebiotic: a review. Starch J. 2011;63:406–415. doi: 10.1002/star.201000099. [DOI] [Google Scholar]
- Garmakhany AD, Mirzaei HO, Nejad MK, Maghsudlo Y. Study of oil uptake and some quality attributes of potato chips affected by hydrocolloids. Eur J Lipid Sci Technol. 2008;110(11):1045–1049. doi: 10.1002/ejlt.200700255. [DOI] [Google Scholar]
- Goff HD, Davidson VJ. Controlling the viscosity of ice cream mixes at pasteurization temperatures. Mod Dairy. 1994;73:12–14. [Google Scholar]
- Goff HD, Hartel RW. Ice cream. 7. Berlin: Springer; 2013. pp. 75–443. [Google Scholar]
- Guzeler N, Kagar A, Say D. Effect of milk powder, maltodextrin and polydextrose use on physical and sensory properties of low calorie ice cream during storage. Akademik Gıda. 2011;9(2):6–12. [Google Scholar]
- Marshall RT, Arbuckle WS. Ice cream. 5. New York: Chapman and Hall; 1996. p. 349. [Google Scholar]
- Marshall RT, Goff HD, Hartel RW. Ice Cream. 6. New York: Kluwer Academic; 2003. pp. 80–84. [Google Scholar]
- Mitschka P. Simple conversion of Brookfield R.V.T. readings into viscosity functions. Rheol Acta. 1982;21:207–209. doi: 10.1007/BF01736420. [DOI] [Google Scholar]
- Rezaei R, Khomeiri M, Aalami M, Kashaninejad M. Effect of inulin on the physicochemical properties, flow behavior and probiotic survival of frozen yogurt. J Food Sci Technol. 2014;51(10):2809–2814. doi: 10.1007/s13197-012-0751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei R, Khomeiri M, Kashaninejad M, Mazaheri-Tehrani M, Aalami M. Effect of resistant starch and aging conditions on the physicochemical properties of frozen soy yogurt. J Food Sci Technol. 2015;52(12):8164–8171. doi: 10.1007/s13197-015-1895-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajilata MG, Sinhal RS, Kulkarni R (2005) Resistant-starch: a review. Food Engineering and Technology Department, Institute of Chemical Technology, Matunga, Mumbai–400-019
- Sofjan RP, Hartel RW. Effect of overrun on s tructural and physical characteristics of ice cream. Int Dairy J. 2004;14(3):255–262. doi: 10.1016/j.idairyj.2003.08.005. [DOI] [Google Scholar]
- Soukoulis C, Lebesi D, Tzia C. Enrichment of ice cream with dietary fiber: effects on rheological properties, ice crystallization and glass transition phenomena. Food Chem. 2009;115:665–671. doi: 10.1016/j.foodchem.2008.12.070. [DOI] [Google Scholar]
- Sun-Waterhouse D, Edmonds L, Wadhwa SS, Wibisono R. Producing ice cream using a substantial amount of juice from kiwifruit with green, gold or red flesh. Food Res Int. 2013;50:647–656. doi: 10.1016/j.foodres.2011.05.030. [DOI] [Google Scholar]
- Surapat S, Rugthavon P. Use of modified starch as fat replacer in reduced fat coconut milk ice cream. Kasetsart J. 2003;37:484–492. [Google Scholar]