Abstract
The aim of this study was to investigate the role of tetrahydrocurcumin (THC) against various skin health parameters using in vitro human foreskin fibroblast and melanoma cell lines (i.e. HFF-1 and B16-F10). The study was assessed using cell viability by MTT assay, identification of extracellular matrix component in HFF-1 cell line (i.e. collagen, elastin and hyaluronic acid), melanin synthesis in B16-F10 cells, cell viability against UVB-induced stress in HFF-1 cells, and in vitro wound healing by the scratch assay. THC was found to be safe and nontoxic up to the concentration of 10 µg/mL with improved level of collagen (37.90%), elastin (90.1%), and hyaluronic acid (74.19%) at 1 µg/mL. Besides, melanin was significantly inhibition by 78.5% at the lowest THC concentration of 0.1 µg/mL. UVB-protection rate was significantly improved by 61.2% and improved cell viability by THC in HFF-1 cells, which indicated protection from photoaging. In addition, THC showed significant wound healing activity (78.51%) and greater migration of fibroblast in HFF-1 cells at different time period. It can be concluded from the study that THC can protect the skin matrix with improved extracellular component synthesis and would healing via collagen synthesis in the skin, which improved the skin elasticity and tightness. Overall, it might be suggested that THC can be used as a safe skin whitening agent, wounds management, cosmetic applications, and treating various skin-related disorders.
Keywords: B16-F10, Extracellular matrix, HFF-1, Hyaluronic acid, Scratch assay, Tetrahydrocurcumin
Introduction
Natural products isolated from plants and microbes used as traditional medicines, which are believed to be inexpensive and beneficial source for exploration of novel pharmacophores. More than 30% of the worldwide drugs, cosmetics, and agrochemicals are mostly based on the natural products (Helmstädter and Staiger 2014). Phytochemicals continued to serve for designing a novel herbal and pharmaceutical formulations against various diseases. In general, both the active principles and molecule targets are well defined, which helps to understand the mechanism of action and acceptance of the interested molecule.
Turmeric (Curcuma longa) is commonly defined as a perennial dietary spice in Indian cuisines, also used as coloring agent in foods and textiles along with wide variety of ailments. Curcumin, the active constituent of turmeric had remarkable medicinal properties and used to treat a wide range of disorders without any side effects even at high doses (Gupta et al. 2012). Tetrahydrocurcumin (THC) is the polyphenolic compounds, with almost similar physiological and pharmacological properties of curcumin, but THC exhibits strongest antioxidant property among the naturally occurring curcuminoids (Lai et al. 2011). THC is one of the major metabolite of curcumin and non-toxic in nature. THC possesses clinically more advantageous properties than the curcumin. However, THC has different molecular targets, signaling pathways, and cellular responses as compared with the curcumin (Aggarwal et al. 2015). THC has been reported with significant results in preventing cancer, inflammation, atherosclerotic lesions treatment, hepatotoxicity and nephrotoxicity (Naito et al. 2002).
Nowadays, modern cosmetology is focused to incorporate valuable crude plant constituents compared with the synthetic chemicals in the manufacturing of cosmetic products, due to the wide plethora of active ingredients. Skin care products supposed to have the significant antioxidant and anti-inflammatory activity (Gao et al. 2008). Because, ultraviolet radiation on exposure to skin generates reactive oxygen species by reacting with DNA, proteins and fatty acids. This could liberate the pro-inflammatory mediators causing irritation of the epidermis. Besides, UV radiation damage skin regulatory mechanisms, photoaging effect like wrinkles, hyperpigmentation, and loss of skin firmness. Phytoconstituents and raw plant materials are used in the cosmetic products to reduce the negative effects of UV radiations and also provide protecting and skin whitening properties with almost less toxicity and side effects (Chanchal and Swarnlata 2008). In cosmetics, THC and other curcuminoids exhibits a broad spectrum of therapeutic activities such as anti-inflammatory, antioxidant, antiaging, and many more. Traditional use of herbs and spices for cosmetic purposes has been highly recommended as skin colorant due to their impressive effects without producing any side effects (Arct et al. 2014). However, THC with various biological activities does not have toxicity even at higher doses and can be used against tumors of colon, skin, pancreas, breast, scleroderma, psoriasis, and other skin diseases. It protect skin by minimizing the inflammation, free radical quenching, impaired wound-healing, improved collagen deposition, improved fibroblast and vascular density (Wu et al. 2014). THC has beneficial role in angiogenesis and improves accumulation of extracellular matrix (ECM) components through remodeling of the wound repair.
To our knowledge, there are no such in depth scientific reports available explaining the effects of THC against wide range of skin rejuvenating parameters as a nutraceutical. The aim of this study was to evaluate the effect of THC on skin health parameters using human foreskin fibroblast and mouse melanoma cell lines (i.e. HFF-1 and B16-F10).
Materials and methods
Reagents
Tetrahydrocurcumin (THC) with 95% purity was purchased from Novel Nutrients Pvt. Ltd., India. L-Ascorbic acid as a positive control was purchased from Alfa-Aesar, while Kojic acid was purchased from Sigma, USA. Epidermal growth factor (EGF) was procured from Gibco, ThermoFisher, USA. All the other chemicals used in this experiment were analytical grade procured from local vendors. ELISA kits for the estimation of elastin and hyaluronic acid were procured from CUSABIO and CusAb Co. Pvt. Ltd, USA.
Cell culture and treatment with THC
HFF-1 (human foreskin fibroblast) cells were procured from American Type Culture Collection (ATCC), USA, originated from normal human skin fibroblast cells. B16-F10 cells were procured from National Centre for Cell Science (NCCS), Pune. HFF-1 and B16-F10 cell lines were maintained in the growth medium DMEM supplemented with 15% FBS, with added antibiotics penicillin (100 U/mL) and streptomycin (100 μg/mL). The growth condition of cell lines were 37 °C, 5% CO2, and 95% humidity. Melanocyte stimulating hormone (α-MSH) was dissolved in sterile PBS buffer to obtain stock solution for estimation of melanin synthesis. L-ascorbic acid (positive control for ECM, UVB protection, and wound healing assay) was dissolved in DMSO to obtain a stock solution of 20 mM, which was further diluted in DMEM to obtain the concentrations range from 10 to 1000 µM. Kojic acid (positive control for melanin study) was dissolved in DMEM to obtain a stock solution of 20 mM, which was further diluted in DMEM to obtain the concentrations range from 1 to 10 mM. The cells were then treated with THC (0.1, 0.5, and 1.0 µg THC mL−1) for the estimation of various skin health parameters and was compared with respective to the positive control.
Determination of non-cytotoxic concentrations
The cell proliferation in cell lines such as B16-F10 and HFF-1 were performed by MTT assay. The cells were counted and plated in 96 well plates at the density corresponding to 5 × 103–10 × 103 cells/well/180 µL of cell growth medium. The cells were incubated overnight under specific growth conditions and allowed for recovery and exponential growth, which were subjected to serum stripping or starvation. The cells were subsequently exposed to the THC at various concentrations (0.1, 0.5, and 1.0 µg/mL) and ascorbic acid (10 µM), which were incubated for 24–72 h in CO2 incubator at 37 °C, 5% CO2 and 95% humidity. Further, serum free MTT media (20 µL of 5 mg/mL) was added to each well followed by incubation for 3 h at 37 °C. The supernatant was aspirated and 150 µL of DMSO was added to each well to dissolve the formazan crystals. Thereafter, the absorbance of each well was recorded at 540 nm using Synergy HT micro plate reader, BioTek, USA. The concentrations that exhibit percentage cytotoxicity of <30% was considered as non-cytotoxic (Plumb 2004).
Estimation of extracellular matrix component synthesis
Synthesis of extracellular matrix component (i.e. collagen, elastin and hyaluronic acid) in HFF-1 cell line were estimated for determining the potential of THC to improve skin strength, hydration level and overall suppleness. HFF-1 cells were counted using hemocytometer and plated in 48 well plate at the density corresponding to 10 × 103 cells/well in DMEM supplemented with 15% FBS. The plates were then incubated overnight under specified growth conditions followed by cells to serum stripping. Further, the cells were treated with different group viz. vehicle control (DMSO-0.05%), positive control (ascorbic acid, at 10 µM concentration), and the test item treatment, THC at concentrations 0.1, 0.5, and 1.0 µg/mL). Further, after 72 h of incubation with the test items and positive control, the supernatants from all the cell plates were taken out and collected in a pre-labeled centrifuge tubes for the estimation of elastin and hyaluronic acid levels. However, the corresponding cell layers were processed for estimation of collagen level using Direct Sirius red dye binding assay. Elastin and hyaluronic acid were estimated using ELISA kits from Cusabio Biotech Co. Ltd, Human Elastin ELN Elisa kit 96T and Human Hyaluronic Acid, Elisa kit 96T, respectively (Hahn et al. 2006) with three independent assay.
Estimation of melanin synthesis
B16-F10 cells were used for melanin synthesis estimation, which were counted using hemocytometer and plated in 90 mm culture dish at the density corresponding to 2 × 106 per 6 mL in culture plates. Further, the cells were incubated overnight under specified growth conditions and allowed for cell recovery and exponential growth. After incubation, the cells were treated with α-Melanocyte-stimulating hormone (α-MSH) for a time point ranging from 4 to 24 h for stimulation of intracellular melanin synthesis. Further, the cells were incubated with α-MSH, and then treated with THC at concentrations 0.1, 0.5, and 1.0 µg/mL for a time period from 48 to 96 h. After incubation, intracellular melanin was extracted in NaOH and the absorbance was recorded at 405 nm. The level of melanin was extrapolated using standard curve obtained from purified melanin (Zhang et al. 1992). The percentage of melanin with respect to the untreated cells (baseline group) was calculated with three independent assay using the formula
where, X represents melanin level in cells corresponding to positive control and test item groups, and R represents melanin level in cells corresponding to baseline (control cells) group.
Cell viability HFF-1 cells against UVB-induced stress
UVB-induced stress was evaluated in HFF-1 cells along with its viability in the presence of THC. The cells were counted using hemocytometer and plated in 96 well plate at the density corresponding to 5 × 103–10 × 103 cells/well in DMEM supplemented with 15% FBS cells/plates, which were incubated overnight under growth conditions to allow cell recovery and exponential growth. The cells were treated with non-cytotoxic concentrations of THC for 2–24 h. After treatment with THC, the cells were subjected to lethal dose of UVB-irradiation (200 mJ/cm2) that can lead to approx. 50% cytotoxicity (302 nm, CL-1000 M, UVP, USA) (Wen et al. 2011). The percent cell viability was assessed with three independent assay using this formula
where X represents the absorbance of cells corresponding to positive control and test groups, and R represents the absorbance of cells corresponding to baseline (control cells) group.
In vitro wound healing assay
HFF-1 cell were counted using hemocytometer and plated in 12 well plates at the densities 0.08 × 106/well/mL of cell growth medium. The plates were incubated overnight under growth conditions and allowed for cell recovery and exponential growth. After overnight incubation, the cells were subjected to the serum starvation in DMEM for 24 h. Mechanical scratch representing wound was created in the near confluent monolayer of cells by gently scraping with sterile 200 µL micropipette tip. The cells were then rinsed with serum free DMEM and treated with THC (1 µg/mL). The scratched area was then monitored for a time period ranging from 0 to 48 h for closure of wound area. The photomicrography was performed at the selected time point’s for the quantitative assessment of migrated cells and its area of wound closure using digital camera (Nikon, Tokyo, Japan), which was connected to the inverted microscope (Nikon, TMS-F, Japan). Further, fibroblast cells migration distance in each wells were monitored using MetaMorph software, version: 6.1. (Carl Zeiss, Germany). It represented fibroblast distance covered and subsequent scratch closure. All the observations were calculated and compared with vehicle control and all the experiments were performed in triplicates (Fronza et al. 2009).
Statistical analysis
Each experiments were carried out in three independent assay and was represented as mean values with standard deviation. Student’s t-test was used to compare two groups to judge the statistical significance. For multiple group comparison, one-way analysis of variance (ANOVA) was used followed by post-hoc analysis by Tukey’s test using statistical software SigmaPlot Version 11.0. Statistically significant values were set at the level of p ≤ 0.05.
Results
Cytotoxic effect of THC on cell lines
The two cell lines i.e. HFF-1 and B16-F10 were treated with THC at various concentrations for the estimation of percentage of the viability. The results in term of %viability of L-ascorbic acid at concentration of 10 µM was in the range of 89–95% in the tested cell lines. However, the cell viability percentage in THC at all the tested concentrations were from 77 to 93%. THC was found to be safe and nontoxic up to 10 µg/mL concentration (data not shown). The results of MTT assay against cell lines in presence of THC (at concentrations of 0.1, 0.5, and 1 µg/mL) treatment. This suggested the safe dose range of THC against tested cell lines, and cell viability greater than 70% was considered as safe and this dose range was selected for further studies. According to the results of the MTT assay, THC was found safe up to the tested concentrations and showed dose dependent effects.
Estimation of extracellular matrix component synthesis
The effect of THC was evaluated in order to find out the action with respect to skin strength, hydration level and overall suppleness. Extracellular matrix (ECM) components synthesis such as collagen, elastin and hyaluronic acid were evaluated in HFF-1 cell line. The results of ECM components viz. collagen, elastin and hyaluronic acid are presented in Figs. 1, 2, and 3, respectively.
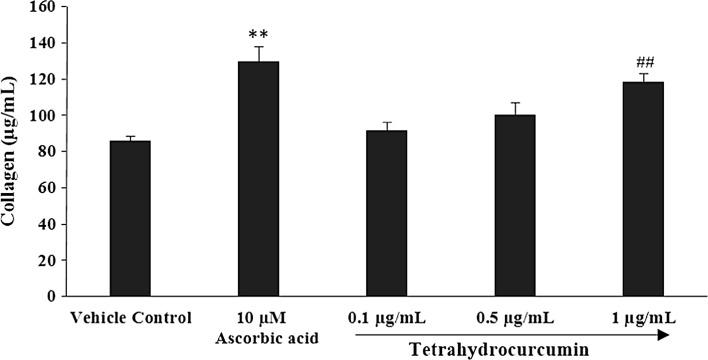
Fig. 1.
Concentration-dependent effects of THC on human foreskin fibroblast (HFF-1) cell line for extracellular matrix component, collagen. **p ≤ 0.01 versus vehicle control and ## p ≤ 0.01 versus ascorbic acid (using Student’s t-test with three independent assay)
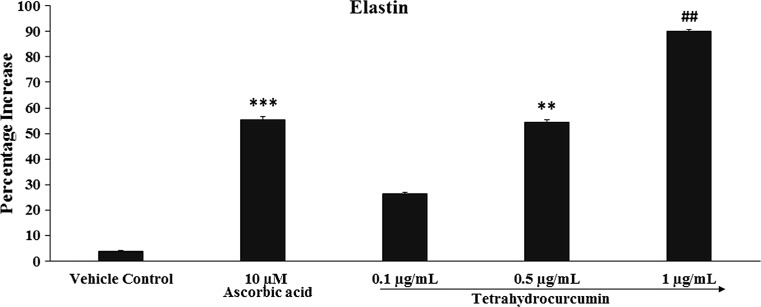
Fig. 2.
Concentration-dependent effects of THC on human dermal fibroblast cells (HFF-1) cell lines for extracellular matrix component, elastin. ***p ≤ 0.001 and **p ≤ 0.01 versus vehicle control and ## p ≤ 0.01 versus positive control (ascorbic acid) group using one way ANOVA (using Tukey’s test with three independent assay)
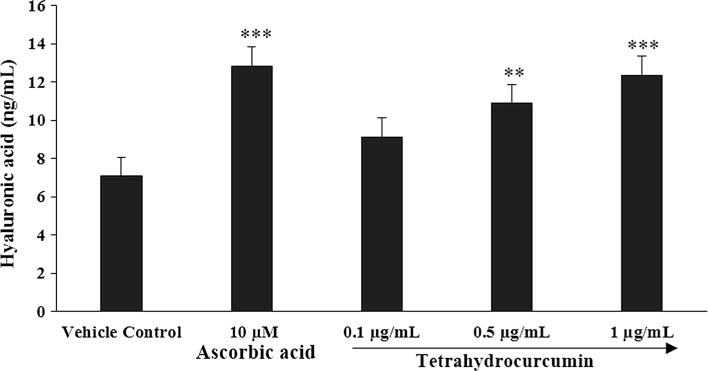
Fig. 3.
Synthesis of extracellular matrix component, hyaluronic acid by THC against UVB-induced stress in human foreskin fibroblasts (HFF-1) cell lines. ***p ≤ 0.001 and **p ≤ 0.01 all statistical comparison with respect to vehicle control using one-way ANOVA (using Tukey’s test with three independent assay)
Analysis of collagen
The analysis showed significant increase in the content of collagen and dose dependent effect of THC at the concentrations 0.1, 0.5, and 1.0 µg/mL in HFF-1 cell line. Collagen results after treatment with THC are presented in Fig. 1. Ascorbic acid (10 µM), positive control showed significant increased collagen content by 51.14% (p ≤ 0.01), while THC at concentrations of 0.1, 0.5, and 1 µg/mL reported with increase percentage of collagen by 6.67, 16.80, and 37.90% respectively, as compared with the ascorbic acid. However, THC at 1 µg/mL showed significant (p ≤ 0.01) increase in the collagen level as compared with ascorbic acid.
Analysis of elastin
Similarly, the results suggest significant increase in the elastin content in a dose-dependent manner at three concentrations of THC in HFF-1 cell line. The results of elastin synthesis after treatment with THC is presented in Fig. 2. Ascorbic acid (10 µM), positive control showed significant (p ≤ 0.001) increase in the elastin content by 55.4%, while THC at concentration of 0.1, 0.5, and 1 µg/mL reported with significant (p ≤ 0.01) increased percentage of elastin by 26.5, 54.5, and 90.1% respectively, as compared with the vehicle control. Besides, THC at 1 µg/mL concentration showed significant (p ≤ 0.01) increase elastin content by 62.64% as compared with the positive control (ascorbic acid). The values of elastin in vehicle control and ascorbic acid were 6.06 and 9.07 pg/mL, while elastin values at THC concentrations of 0.1, 0.5, and 1 µg/mL were 7.38, 9.01, and 11.09 pg/mL respectively.
Analysis of hyaluronic acid
Hyaluronic acid synthesis analysis showed a significant increase in the hyaluronic acid concentration with the dose dependent manner when exposed to various THC concentrations of 0.1, 0.5, and 1 µg/mL in HFF-1 cell line. The results of hyaluronic acid synthesis after the treatment with THC are presented in Fig. 3. Ascorbic acid (10 µM), positive control showed significant (p ≤ 0.001) increase in the hyaluronic acid content by 80.96%, while THC at the concentration of 0.1, 0.5, and 1 µg/mL reported with significant increased percentage of hyaluronic acid by 28.78%, 53.60% (p ≤ 0.01), and 74.19% (p ≤ 0.001) respectively, as compared with the vehicle control. The values of hyaluronic acid in vehicle control and ascorbic acid were 7.09 and 12.83 ng/mL, while hyaluronic acid values at tested THC concentrations of 0.1, 0.5, and 1 µg/mL were 9.13, 10.89, and 12.35 ng/mL, respectively. This showed that THC has the significant capacity to increase the extra skin cellular component, i.e. hyaluronic acid.
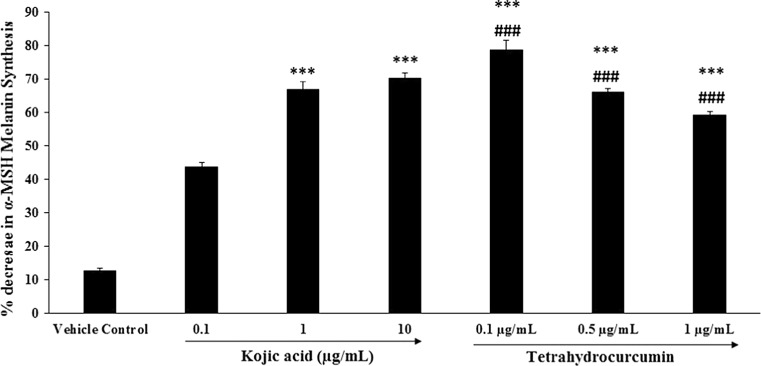
Estimation of melanin synthesis inhibition
To investigate the effects of THC on melanogenesis, mouse melanoma (B16-F10) cell line was cultured in DMEM supplemented media containing various concentrations of THC for 48–96 h. An acid insoluble fraction was prepared from the cells and the amount of melanin was quantitatively measured by the method of Hosoi et al. (1985). Kojic acid, which is used in many cosmetic products for skin whitening has been used in this study for comparing the skin whitening effect of THC. The cellular content of melanin was reduced by the addition of THC to the medium, and the results of inhibition was in dose-dependent manner (Fig. 4). α-MSH melanin synthesis was significantly (p ≤ 0.001) inhibited at the kojic acid concentrations of 0.1, 1, and 10 µg/mL by 43.7, 66.8, and 70.3%, while THC showed maximum decrease in α-MSH melanin synthesis at lowest concentration (0.1 µg/mL) by 78.5% (p ≤ 0.001). Besides, at higher concentration i.e. at 0.5 and 1.0 µg/mL showed significant (p ≤ 0.001) percentage decrease in α-MSH melanin synthesis by 65.90 and 59.10% respectively. Thus, it can be concluded from the results that THC seems to be efficient in inhibiting melanin production in the B16-F10 melanoma cell line.
Fig. 4.
Inhibitory effect of THC on melanogenesis (skin whitening potential) in mouse melanoma (B16-F10) cell line. ***p ≤ 0.001 versus vehicle control and ### p ≤ 0.001 versus positive control (ascorbic acid) using one-way ANOVA (using Tukey’s test with three independent assay)
Effects of THC on UVB-induced photoaging
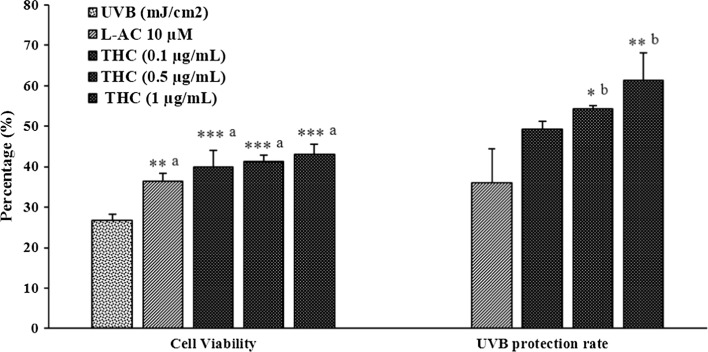
The effect of THC was measured in UVB-induced stress in HFF-1 cells and the cell viability was identified using hemocytometer. The cells were subjected to lethal dose of UVB-irradiation (200 mJ/cm2) and percentage cell viability and UVB-protection rate was presented in Fig. 5. The control cells on exposure to UVB showed high degree of cell death with only 26.73% of cell viability and considered to have no UVB-protection. Besides, ascorbic acid (10 µM) showed 36.34% cell viability and 36% UVB-protection rate. On the other hand, THC was reported with dose dependent effect at THC concentrations of 0.1, 0.5, and 1.0 µg/mL with cell viability 39.86, 41.22, and 43.09% respectively. However, the percentage UVB-protection rate at THC concentrations of 0.1, 0.5, and 1.0 µg/mL was reported as 49.2, 54.2, and 61.2% respectively.
Fig. 5.
Anti-wrinkling potential and cytoprotective effect of THC against UVB-induced stress in human foreskin fibroblasts (HFF-1) cell lines. L-AC L-ascorbic acid, THC tetrahydrocurcumin. ***p ≤ 0.001, **p ≤ 0.01 and *p ≤ 0.05; a and b represents statistical comparison with vehicle control and ascorbic acid group respectively using one way ANOVA (using Tukey’s test with three independent assay)
In vitro wound-healing assay for cell migration
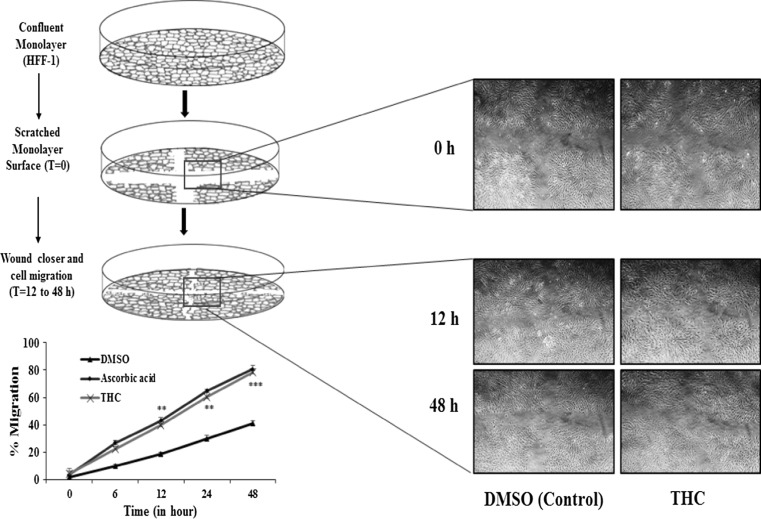
In vitro scratch assay was performed in order to measure the rate of HFF-1 cell migration in the presence of THC and the represented images are presented in Fig. 6. From the experimental observations, it was found that the percent migration of HFF-1 fibroblasts cells was 41.11% in response to the treatment with DMSO (vehicle control); 80.90% in response to the treatment with ascorbic acid (positive control); and 78.51% in response to the treatment with the THC at the end of 48 h observation period. Confluent monolayer was scratched and cell migrations was recorded at different time points. Represented photographs showed significant migration of cells at time point 12 h onwards. Further, significant wound closure and cell migration were monitored at t = 12–48 h in the presence of THC as compared with vehicle control and ascorbic acid.
Fig. 6.
Representative pictures show the migration of HFF-1 cells after induction of a scratch. All the pictures were taken immediately after the scratch was induced (i.e. at 0 h), after 12 h (12 h), and 48 h (48 h). The fibroblast on the pictures were cultured in conditioned media. Pictures are taken at 50 times magnification. THC tetrahydrocurcumin, DMSO dimethyl sulfoxide (vehicle control). ***p ≤ 0.001 and **p ≤ 0.01 represents statistical comparison with vehicle control using Student’s t-test with three independent assay
Discussion
ECM, which is present in all the tissues and organs provides an important physical scaffolding for cellular mater along with initiating the various biochemical and biomechanical parameters for controlling the tissue differentiation, morphogenesis, and homeostasis. The importance of the ECM with respect to collagen, elastin, and hyaluronic acid has been related with a wide range of skin related diseases, which might be from genetic reason or other abnormalities. Collagen is considered as the most abundant fibrous protein present in interstitial extracellular matrix component, while 30% of the total protein mass is constituted in multicellular animal. Collagen represents as the main structural element of the ECM, which support chemotaxis and migration, provide tensile strength, and regulate the cell adhesion. It is responsible for direct tissue development, mechanical strength and texture (Rozario and DeSimone 2010). Besides, collagen is the major proteins in tendons, ligaments, cartilage, bone, and skin. Skin appearance is largely regulated with the quality and quantity of collagen. In this experiment, it was assumed that THC might inhibit the collagenase synthesis (such as matrixmetalloproteinase, MMP-1) and promote the collagen production, which could also reduce and neutralize the free radicals to help in anti-ageing activity. This suggested the significant role of THC in cosmetics to improve the collagen content, which is one of the important skin parameter in ageing.
Elastin is another important ECM major component along with collagen. It is observed that the elastin fibers maintain stretch conditions and provide recoil to the tissues. Collagen fibrils and elastin stretch fibers have strong association and form tight junction. Elastin fibers are covered by the glycoprotein microfibrils that mostly have fibrillins, which are important for the integrity of the elastin fiber (Frantz et al. 2010). Hence, elastin is important which regulates and activates the dermal metabolism, while increased level of elastin would be beneficial for skin health. Experimental data suggest that THC has the capacity to significantly increase the elastin synthesis. This showed that THC has significant capacity to increase the extracellular component and microenvironment that is very critical and important for cell growth, survival, differentiation and morphogenesis.
Hyaluronic acid (HA) is considered as a key molecule that plays an important role in skin moisture regulation, which has a unique capacity in retaining the water. Out of total body HA, 50% accounting for skin as an abundant molecule. The major biological functions of HA includes lubrication of joints, hydration, tissue repair, activation of inflammatory cells, framework to blood vessels, fibroblast migration, angiogenesis. In dermis of skin, it regulates osmotic pressure, water balance, ion flow, enhancing the extracellular domain of cell surfaces, and help to stabilize the skin structures. HA has been now used in the treatment and prevention of skin disorders in term of clinical aspect (Weindl et al. 2004). Study results suggest significant increase in the HA value after treatment with THC, which might be a novel finding that can offer new horizons for the therapeutic use of THC against many skin disorders.
Melanin is synthesized in the basal layer of the epidermis, cells are known as melanocytes, which produced melanin that contains packets known as melanosomes. Melanogenesis process has been initiated once the skin nuclei cells become damaged from ultraviolet radiation (UVA and UVB) emitted by the sun. Melanin absorbs the radiations and is responsible for skin darkening. In order to control the skin darkening, several natural and synthetic skin whitening components, which have been added in the cosmetics such as azelaic acid, aloesin, piperlonguminine, eugenol, and curcuminoids, and many more (Wattanakrai et al. 2007). However, THC is a novel approach for the treatment for skin-related disorders. Many scientific evidences indicated the role of THC for the treatment of inflammatory dermatoses, skin infections, chronic pain, wound closure, along with cosmetic ailments like dyspigmentation. THC also reported to have strong antioxidant activity, which suggests its use in skin related disorders. Our experimental results showed maximum decrease in percentage α-MSH melanin synthesis at lowest concentration and showed better effect with respect to kojic acid. Study results of THC suggested that melanin inhibition might be due to the tyrosinase inhibition effect. α-MSH was used in the study, which generally binds to the melanocortin 1 receptor (MC1R) and activates the adenylate cyclase, which in turn catalyzes the ATP to cAMP conversion that increases the intracellular cAMP levels (Busca and Ballotti 2000). Our experimental results showed that the THC at lowest concentration inhibited the melanogenesis induced by α-MSH-mediated intracellular cAMP up-regulation. Hence, THC could be a good alternative as compared with the synthetic compounds and would be a safe and effective skin whitening agent in the fields of cosmetic research and development.
UVB-protection results indicated that THC would be a potential topical agent to protect against UV-induced skin damage or aging-related disorders. Therefore, THC might be a promising agent to protect against UVB-induced photo damage. It might be expected that UVB will induce stress resulting in free radicals generation that could further promote the downstream signal transduction in human skin fibroblasts. It will lead to direct or indirect DNA damage and induces inflammatory responses (Ho et al. 2005). However, it was also reported that UV stimulates the inhibitory role in receptor activation (Smad protein) followed by phosphorylation, which leads to reducing the procollagen and elastin expression. It might be suggested that THC protects the skin and cell viability from UVB-irradiations by inhibiting the reactive oxygen species and phosphorylated mechanism. Hence, THC attenuated the UVB-induced photo damage and inflammation by modulating the inflammatory expression and can be used as potent antiphotoaging agent.
In vitro scratch assay is particularly suitable and well-developed method for the estimation of cell migration, study the cell-matrix and cell-cell interactions during wound healing and for monitoring the intracellular event during migration (Liang et al. 2007). Cell migration and proliferation are important in wound repair process and collagen deposition is needed to repair tissue injury. Fibroblasts, connective tissue cells are responsible for collagen deposition. Collagen is required to repair the defect and it will provide strength, integrity and structure. Fibroblast activity is related to replication and migration in proliferation phase, and fibroblast cells will begin to synthesize collagen. Curcumin was reported to have important role in wound healing process (Akbik et al. 2014). However, the role of curcumin in fibroblast proliferation and wound contraction exhibited by antioxidant mechanism. THC at various tested concentrations revealed significant improve in the fibroblast migration and showed significant results, which implicated its significant wound repair activity.
The limitation of the study falls in the evaluation of THC wound healing mechanism or pathway i.e. either by inhibiting the collagenase synthesis by MMP-1 or it might work by suppression of NF-κB activation pathway. These mechanistic studies would be helpful for better understanding the molecular aspect of THC for the improvement and protective role in skin health. Further research is needed in order to elucidate the mechanistic perspective of THC in fibroblast and melanoma cell line.
Conclusion
Overall, the current study established the potential application of THC in improving various skin parameters using human foreskin fibroblast and mouse melanoma cell lines (i.e. HFF-1 and B16-F10). However, sufficient number of phytoconstituents have been reported, but dose dependent and non-cytotoxic action of THC, with improved extra cellular matrix components like collagen, elastin, and hyaluronic acid, will provide a great insight of THC as a novel phytoconstituents in cosmetic industries. The use of THC in sunscreen could also be an effective approach for reducing the harmful effect of UVB-irradiation and might help in skin whitening. Further development of novel delivery systems using THC will be a good approach for development of novel cosmetic for skin. This research would be helpful for the formulation of stable herbal cosmetic formulations without any negative effects, high efficiency, and longer duration of action. The current findings inferred that THC could be useful compound for skin whitening, anti-ageing, and skin rejuvenator in the skin care cosmetic industry.
Acknowledgements
The authors extend their sincere thanks and grateful to Dabur Research Foundation, India for providing the facilities and support that enabled the successful completion of the work. Further, the authors report no conflicts of interest in this work. However, no external funding is associated with this study.
Compliance with ethical standards
Conflict of interest
The authors state no conflict of interest.
References
- Aggarwal BB, Deb L, Prasad S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules. 2015;20:185–205. doi: 10.3390/molecules20010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R. Curcumin as a wound healing agent. Life Sci. 2014;116:1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Arct J, Ratz-Łyko A, Mieloch M, Witulska M. Evaluation of skin colouring properties of curcuma longa extract. Indian J Pharm Sci. 2014;76:374–378. [PMC free article] [PubMed] [Google Scholar]
- Busca R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- Chanchal D, Swarnlata S. Novel approaches in herbal cosmetics. J Cosmet Dermatol. 2008;7:89–95. doi: 10.1111/j.1473-2165.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronza M, Heinzmann B, Hamburger M, Laufer S, Merfort I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J Ethnopharmacol. 2009;126:463–467. doi: 10.1016/j.jep.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Gao XH, Zhang L, Wei H, Chen HD. Efficacy and safety of innovative cosmeceuticals. Clin Dermatol. 2008;26:367–374. doi: 10.1016/j.clindermatol.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MS, Kobler JB, Starcher BC, Zeitels SM, Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix. I: elastic fibers and hyaluronic acid. Ann Otol Rhinol Laryngol. 2006;115:156–164. doi: 10.1177/000348940611500213. [DOI] [PubMed] [Google Scholar]
- Helmstädter A, Staiger C. Traditional use of medicinal agents: a valid source of evidence. Drug Discov Today. 2014;19:4–7. doi: 10.1016/j.drudis.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Ho JN, Lee YH, Lee YD, Jun WJ, Kim HK, Hong BS, Shin DH, Cho HY. Inhibitory effect of Aucubin isolated from Eucommia ulmoides against UVB induced matrix metalloproteinase-1 production in human skin fibroblasts. Biosci Biotechnol Biochem. 2005;69:2227–2231. doi: 10.1271/bbb.69.2227. [DOI] [PubMed] [Google Scholar]
- Hosoi J, Abe E, Suda T, Kuroki T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985;45:1474–1478. [PubMed] [Google Scholar]
- Lai CS, Wu JC, Yu SF, Badmaev V, Nagabhushanam K, Ho CT, Pan MH. Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Mol Nutr Food Res. 2011;55:1819–1828. doi: 10.1002/mnfr.201100290. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Naito M, Wu X, Normura H, Kodama M, Kato Y, Osaswa T. The protective effect of tetrahydrocurcumin on oxidative stress in cholesterol-fed rabbits. J Atheroscler Thromb. 2002;9:243–250. doi: 10.5551/jat.9.243. [DOI] [PubMed] [Google Scholar]
- Plumb JA. Cell sensitivity assays: the MTT assay. Methods Mol Med. 2004;88:165–169. doi: 10.1385/1-59259-406-9:165. [DOI] [PubMed] [Google Scholar]
- Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanakrai P, Suwanachote S, Kulkollakarn S, Rajatanavin N. The study of human skin irritation of a novel herbal skin care product and ingredients by human single closed patch testing. J Med Assoc Thail. 2007;90:1116–1122. [PubMed] [Google Scholar]
- Weindl G, Schaller M, Schäfer-Korting M, Korting HC. Hyaluronic acid in the treatment and prevention of skin diseases: molecular biological, pharmaceutical and clinical aspects. Skin Pharmacol Physiol. 2004;17:207–213. doi: 10.1159/000080213. [DOI] [PubMed] [Google Scholar]
- Wen KC, Shih IC, Hu JC, Liao ST, Su TW, Chiang HM. Inhibitory effects of Terminalia catappa on UVB-Induced photodamage in fibroblast cell line. Evid Based Complement Alternat Med. 2011;2011:904532. doi: 10.1155/2011/904532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Tsai ML, Lai CS, Wang YJ, Ho CT, Pan MH. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food Funct. 2014;5:12–27. doi: 10.1039/C3FO60370A. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yoshida T, Kuroiwa Y. Stimulation of melanin synthesis of B16-F10 mouse melanoma cells by bufalin. Life Sci. 1992;51:17–24. doi: 10.1016/0024-3205(92)90213-9. [DOI] [PubMed] [Google Scholar]