Abstract
This study aimed to ferment the chicken eggshell membrane (ESM) using the lactic acid bacteria, Lactobacillus plantarum for preparation of functional and bioactive protein hydrolysates. Cultivation at an initial pH of 8.0 for 36 h resulted in maximum protein concentration (177.3 mg/g) and degree of hydrolysis (25.1%) of the hydrolysates. Fermentation resulted in the production of hydrolysates that demonstrated excellent solubility (90.7%), good foaming capacity (36.7%) and emulsification activity (94.6 m2/g). Additionally, these protein hydrolysates exhibited remarkable bioactive properties for instance reducing power (2.53), protection from DPPH radical (70.5%) and angiotensin I converting enzyme inhibition (49.3%). The fermented protein hydrolysates were also found effective against various foodborne pathogens. The protein hydrolysates obtained by fermentation of ESM can be potentially incorporated in functional foods and nutraceuticals resulting in valorization of the ESM waste.
Keywords: Eggshell membrane protein hydrolysates, Lactobacillus plantarum, Functional properties, Antioxidant, Antibacterial, ACE-inhibitory
Introduction
Food processing wastes, which are disposed of in huge amounts on a daily basis contributes to serious environmental pollution and severe health hazards. It is important to reuse and recycle these wastes for the sustainable development of the society and for preserving the environment. Also, these wastes are considered to be good sources of valuable bioactive compounds and nutraceuticals. Eggs are one of the most popularly consumed food products in the world which are gaining commercial importance due to their high nutritional value. Large amounts of by-products are generated during the processing of eggs which includes the eggshells and the eggshell membranes (ESM) that constitutes 11% of the total egg weight (Tsai et al. 2006). However, the eggshell membrane is known to be a very good source of bioactive compounds such as proteins. As the ESM contain high amounts of proteins, they can be studied for the production of functional and bioactive protein hydrolysates and peptides about which very little is known. Research has been done on protein extraction, mainly collagen from the eggshell membrane waste, but very few studies have been reported on the recovery of protein hydrolysates and bioactive peptides from it.
Protein hydrolysates and bioactive peptides have already been produced from various plant and animal sources using various enzymes. This includes bioactive protein hydrolysates and peptides from egg, meat, marine foods, rice and wheat generated using different enzymes such as Alcalase, Papain, Pepsin etc. (Zambrowicz et al. 2015; Mora et al. 2014; Harnedy and FitzGerald 2012; Cavazos and Gonzalez de Mejia 2013). However, very few studies have been reported on producing protein hydrolysates and peptides by microbial fermentation. Lactic acid fermentation is an eco-friendly and green technology, where the lactic acid bacteria (LAB) help to preserve the waste by recovering important biomolecules from them. The lactic acid bacteria are known to possess proteolytic systems made up of cell envelop proteinase and intracellular peptides. The cell envelope proteinase catalyzes the breakdown of proteins to oligopeptides which are further degraded via the intracellular peptidases to low molecular weight peptides and amino acids (Pan and Guo 2010). The fermentation method is better in comparision to the enzymatic hydrolysis method as it utilizes microorganisms that are safe and which produce protease enzymes through a low cost effective method (Singh et al. 2014).
Naturally occurring peptides in the food products are gaining more interest in the recent years in comparision to the synthetic antioxidants as they are considered to be safer (Jemil et al. 2014). These peptides are also being preferred over the antibiotics as food additives as they do not exhibit any undesirable side effects and bacterial drug resistance (Akbar and Anal 2014). They are also gaining attention for inhibiting the angiotensin converting enzyme (ACE) and for carrying out antihypertensive activities. The ACE enzyme is known to negatively regulate the blood pressure by synthesizing angiotensin II, a vasoconstrictor and also by making the vasodilator bradykinin nonfunctional (Apud et al. 2013). Many commercial ACE-inhibitors are widely used for treating hypertension, but are known to have some side effects associated with them such as cough, kidney destruction and angineurotic edema (Gu et al. 2011; Zhao et al. 2009). Hence, the protein hydrolysates and peptides are considered potential alternatives to the currently used ACE-inhibitors.
Various efforts have been made for producing and characterizing bioactive protein hydrolysates from different sources. However, the bioactivities of ESM protein hydrolysates generated by microbial fermentation remain unknown. Hence, this study was directed to generate protein hydrolysates by ESM fermentation followed by the study of their bioactivities.
Materials and methods
Materials
The natural ESM were collected by the manual peeling of the chicken eggshells provided by Charoen Pokphand Foods Ltd. (CPF), Thailand. They were rinsed with sterilized distilled water, dried, ground and stored at −18 °C until further use. ACE and 2,2-diphenyl-1-picrylhydrazyl (DPPH) was supplied by Sigma-Aldrich (St. Louis, MO, USA) and all other chemicals employed were of analytical grade. The strains of Lactobacillus plantarum (TISTR 858) and Staphylococcus aureus (TISTR 746) were obtained from Thailand Institute of Scientific and Technological Research (TISTR), Thailand. Further, bacterial strains namely, Escherichia coli, Salmonella typhimurium, and Streptococcus agalactiae were acquired from the Bioprocess Technology Laboratory at the Asian Institute of Technology, Thailand.
Fermentation and production of eggshell membrane protein hydrolysates
Lactobacillus plantarum was activated by culturing in deMan Rogosa Sharpe (MRS) agar plate at 30 °C for 18 h followed by inoculation of the obtained colonies in MRS broth medium and incubation at the same conditions. The bacteria were sub-cultured twice before inoculation of the sterilized fermentation media to reach a concentration of 107 CFU/mL. The submerged fermentation process was then initiated by adding the ESM (5 g) to the culture media (100 mL) in sterilized conical flasks (500 mL). Various parameters such as initial pH (4, 5, 6 and 8) and incubation time (6, 12, 24, 36, 48, 60 and 72 h) were varied to optimize the fermentation process of L. plantarum in presence of eggshell membranes as substrate. The cultures were incubated at 30 °C at 120 rpm. At regular intervals, the aliquots of the samples (5 mL) were removed, filtered and centrifuged (6000×g) for 20 min and supernatant was collected for analyzing the efficiency of protein hydrolysate production. The optimal condition to ferment the ESM for the efficient production of hydrolysates was then selected for further analysis of their functional and bioactive properties. Their cell-free supernatants were subjected to ammonium sulphate precipitation up to 70% saturation at 4 °C and dialyzed through cellulose-based dialysis tubing (1000 Dalton molecular weight cut-off, Spectra/Por, Spectrum Laboratories, California, USA) for 14 h against PBS (pH 7.0). Dialyzed solution was then subjected to freeze drying (Scanvac Coolsafe 55-4, Labogene, Lynge, Denmark) followed by their storage at −18 °C for their further applications.
Determination of protein concentration of the hydrolysates
Protein concentration in fermented product was determined by using the Bradford method (1976). Bovine serum albumin (BSA) was used as a standard. The protein hydrolysate supernatant (100 µL) was mixed with Coomassie Brilliant Blue dye solution (Bio-Rad, USA) and allowed to incubate at room temperature for 15 min. Their optical density determination was carried out at a wavelength of 595 nm and protein content was deduced.
Estimation of the degree of hydrolysis
The degree of hydrolysis (DH) was measured by the method of Hoyle and Merritt (1994), which was modified slightly. The protein hydrolysate solution (1 mL) was blended with an equal volume of 20 g/100 mL trichloroacetic acid (TCA), incubated at 25 °C for 30 min and centrifuged at 3000×g for 15 min. Protein concentration in supernatant was then ascertained by a modified Lowry method (1951) using a standard curve of BSA. Hydrolysis degree was then determined as:
Electrophoretic characterization
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of the fermented ESM protein hydrolysate was performed on a resolving gel (12%) and stacking gel (5%). The protein hydrolysate solution (300 µL) was diluted following the addition of 4× Laemmli sample buffer (277.8 mM Tris–HCl (pH 6.8), 44.4% (v/v) glycerol, 4.4% SDS and 0.02% bromophenol blue; 90 µL) and β-mercaptoethanol (10 µL) and boiled for 5 min. Electrophoresis was then conducted at 150 V till the bands reached the bottom of the gel. The Bio-Rad Coomassie Blue R-250 was used for staining and Bio-Rad pre-stained broad range molecular weight standards (MW 2.0-250.0 kDa) were used as molecular markers.
Amino acid analysis
Amino acid analysis was carried out following the method of Niu et al. (2013). The lyophilized protein hydrolysate samples were hydrolyzed with 6 mol/L HCl at 110 °C for 24 h. The samples were then resuspended in distilled water and injected onto a Biochrom 30 + Amino Acid Analyzer (Biochrom, Cambridge, UK) for detecting the concentration of each amino acid in the protein hydrolysate sample.
Functional properties of the protein hydrolysates
Solubility
Solubility was analyzed using a methodology described by Tsumura et al. (2005), which was slightly modified. The samples (300 mg) were dispersed in distilled water (30 mL) followed by pH adjustment to a wide pH range (3.0–10.0) using 0.1 M HCl or 0.1 M NaOH. The sample mixtures were then centrifuged at 7500×g for 15 min at 25 °C. Protein concentration in the supernatant was measured by the Bradford method (1976) using BSA as a standard and solubility was then determined using the following equation:
Foaming capacity (FC) and foam stability (FS)
The foaming properties of the fermented hydrolysate were analyzed through the method of Shahidi et al. (1995). The sample solution (V2; 20 mL) at a range of concentrations (0.5, 1, 2% w/v) was homogenized (IKA RW-20, Staufen, Germany) at 2000 rad/min for 10 min following which they were transferred to measuring cylinders with a total volume of 50 mL. Their total volume (V1) was then noted subsequently after 30 min. Foaming capacity was determined as follows:
Further, the homogenized samples were then incubated at 20 °C for 30 min and their final volume was noted (V3). Foam stability was determined as follows:
Emulsifying properties
A modified method of Pearce and Kinsella (1978) was employed for determining the emulsifying properties of the fermented hydrolysates. The lyophilized ESM protein hydrolysate was dissolved in distilled water at a range of concentrations (0.5, 1 and 2% w/v) and added to soybean oil (10 mL) followed by their homogenization at 2000 rad/min for a minute using a homogenizer (IKA RW-20, Staufen, Germany). After 0 and 10 min of homogenization, emulsion samples (50 µL) were collected and dissolved in 1 g/L SDS solution (5 mL), respectively. Their absorbance was read at 0 min (A0) and 10 min (A10) at 500 nm by a spectrophotometer (UNICAM, Alva, U.K). Emulsifying activity index (EAI) and emulsion stability were then determined as:
where ΔA = A0 − A10 and Δt = 10 min.
Evaluation of the antioxidant activities
Inhibition activity against the DPPH radical
The ability of the hydrolysates to scavenge the DPPH free radicals was measured by the slightly modified method of Bersuder et al. (1998). The fermented protein hydrolysates dissolved in distilled water at varying concentrations (1–5 mg/mL) were dispersed in an equal volume of an ethanol solution of DPPH (0.1 mM) and the following dispersions were stored at 25 °C for 60 min in the absence of light. The absorbance of the resulting mixtures was then measured at 517 nm (Asample). The control reaction comprised of distilled water substituting the sample (Acontrol). The ability of the fermented hydrolysates to inhibit DPPH was compared to that of ascorbic acid and determined as follows:
Reducing power
A slightly modified method of Chen et al. (2012) was used for determining the reducing power ability of the hydrolysates. The protein hydrolysates with varying concentrations (1–5 mg/mL) were blended with 0.2 mol/L phosphate buffer (pH 6.6) and potassium ferricyanide solution. The resulting mixture was heated at 50 °C for 30 min and then cooled down. Trichloroacetic acid (10 g/100 mL) was then incorporated and resulting solution was centrifuged at 3000×g for 10 min. Following centrifugation, the supernatant obtained was blended in distilled water and ferric chloride solution (1 g/L) and their absorbance was measured at 700 nm.
Lipid peroxidation inhibition
The ability of the fermented protein hydrolysate to inhibit lipid peroxidation was determined following the method of Naqash and Nazeer (2013) with some modifications. Fermented ESM protein hydrolysate (5 mg) was dispersed in 50 mM phosphate buffer (pH 7.0; 5 mL) and 50 mM linoleic acid solution (5 mL). Eventually, they were reconstituted in distilled water to obtain a final volume of 12.5 mL. Following their exposure to dark at 45 °C, their level of oxidation was determined by measuring their ferric thiocyanate values every day for 6 days at 500 nm by UV–Vis Spectrophotometer (UNICAM, Alva, U.K).
Evaluation of antibacterial activity
The efficiency of the fermented ESM protein hydrolysates to inhibit the growth of test pathogens such as Staphylococcus aureus, Streptococcus agalactiae, Salmonella typhimirium and Escherichia coli was assessed. Antibacterial activity was determined using the disc diffusion method similar to a method reported by Marrufo-Estrada et al. (2013) with slight modifications. Bacterial suspensions (100 µL) of different microorganisms were spread evenly over the agar plates and the solutions of protein hydrolysate were prepared by their dissolution in distilled water to achieve concentrations of 100 mg/mL. Sterile filter paper discs were then loaded with 100 µL of hydrolysate solution and placed on the surface of the agar plates, which were then kept at 37 °C for 24 h. A standard amikacin disc (30 µg) was used as a positive reference and a water-loaded disc was used as a negative control. The inhibition zone diameter (including the disc diameter of 5 mm) was then measured.
Angiotensin I converting enzyme inhibitory activity
The ability to inhibit the ACE enzyme was evaluated in vitro by a slightly modified method of Li et al. (2005) with slight modifications. The protein hydrolysate sample solutions (20 µL) of varying concentrations (1 and 2 mg/mL) were blended with the substrate (hippuryl-histidyl-leucine) (50 µL) and pre-incubated at 37 °C for 5 min. This was followed by further incubation with 10 µL ACE enzyme (0.1 U/mL) at 37 °C for 30 min. The reaction was stopped with the incorporation of 1 M HCl (100 µL) and absorbance was recorded at 492 nm by UV–Vis Spectrophotometer (UNICAM, Alva, U.K). The inhibition activity was then determined as follows:
where A, B and C are the absorbance of sample, control and the reaction blank (1 mol/L HCl added before ACE), respectively.
Statistical analysis
All tests for functional and bioactive properties were conducted with three replicates. Data was expressed as mean ± SD. SPSS software (SPSS Version 16, Chicago, IL, USA) was employed for conducting the statistical analysis where ANOVA was carried out and the significant differences among the samples were determined by the Tukey’s test having a confidence interval of 95% (p < 0.05).
Results and discussion
Effect of pH and fermentation time on the protein concentration and degree of hydrolysis of ESM protein hydrolysates
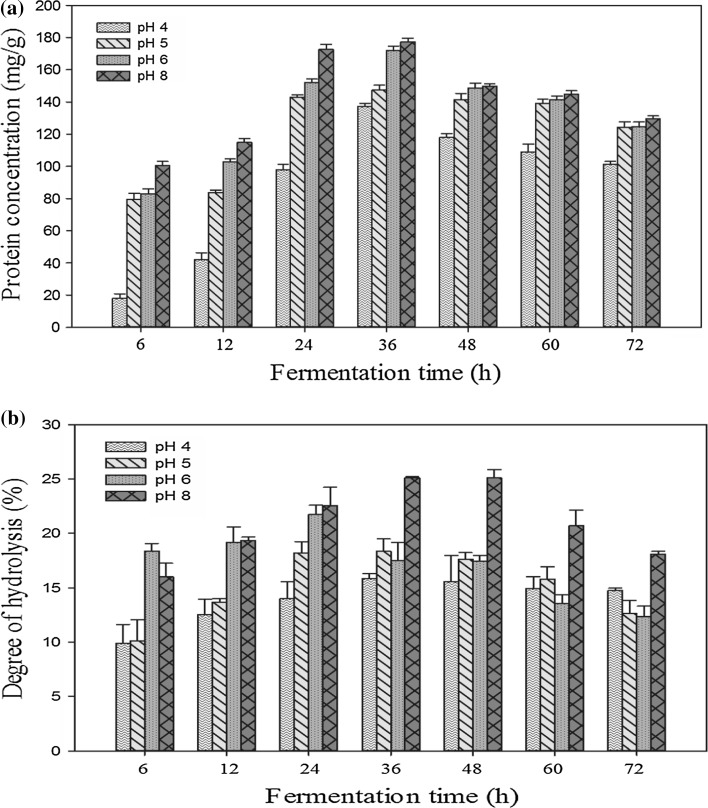
The consequences of two parameters i.e. the initial pH of the media and fermentation time were analyzed for fermenting the chicken eggshell membrane using Lactobacillus plantarum for producing functional protein hydrolysates and peptides. This was done in a similar manner to a study conducted by Fakhfakh et al. (2011). The protein concentration was found to increase significantly (p < 0.05) reaching their maximum value at 36 h, after which no significant changes were observed as shown in Fig. 1. This can be attributed to reduced microbial growth as organic acids produced during fermentation are known to inhibit their growth. These organic acids increase the amount of protons that acidifies the cytoplasm and inhibits their metabolic functions. The highest protein concentration (177.3 mg/g) was observed at pH 8.0. Pan and Guo (2010) reported the synthesis of large amounts of proteolytic enzymes by Lactobacillus helveticus during the fermentation of milk at initial pH of 7.5, which improves the protein hydrolysate production. The highest degree of hydrolysis was also observed at pH 8.0 as seen in Fig. 1. The degree of hydrolysis began to increase in the middle of the log phase (12 h) and the maximum activity was reached at the early stationery phase (36 h). In the early stationery phase, the cell growth rate decreased but microbial protease enzyme remained active. The intracellular enzymes are generally released later in the fermentation process and thus, the protein hydrolysates and peptides were formed only after cell lysis after a fermentation period of about 36 h. However, a slight reduction was observed after 36 h at all initial pH values, which may be due to lower proteolytic activity of the microbial protease after 36 h. Different pH values were evaluated for protein hydrolysate production by fermentation. Highest degree of hydrolysis was observed at pH 8.0. This can be explained by the fact that proteolytic enzymes synthesized by different microorganisms are most active at alkaline conditions (Fakhfakh et al. 2011). This is similar to a study of Pan and Guo (2010), which found highest degree of hydrolysis at pH 7 after sour milk fermentation.
Fig. 1.
Effect of initial pH value and fermentation time on the a protein concentration and b degree of hydrolysis of the eggshell membrane protein hydrolysates produced by Lactobacillus plantarum. Error bars indicate the standard errors of the mean of three independent experiments
Both time and initial pH value were found to have a significant (p < 0.05) effect on the protein concentration and degree of hydrolysis of the hydrolysates and from the results obtained, it can be suggested that fermentation of ESM using Lactobacillus plantarum is an effective approach to recover the protein hydrolysates and peptides from ESM.
Molecular weight determination of fermented protein hydrolysates
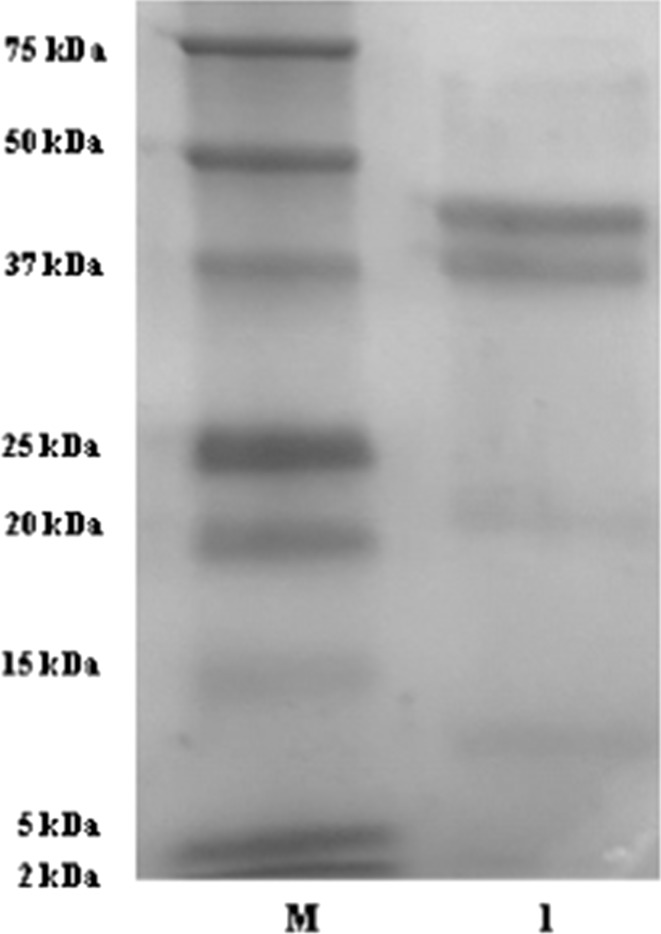
The electrophoretic profile of the fermented ESM protein hydrolysates was studied and several bands ranging from 50 to 10 kDa molecular mass were observed as seen in Fig. 2. The presence of protein bands lesser than 20 kDa indicates that notable modification occurred in the structure of the protein hydrolysates during fermentation. This includes protein unfolding followed by hydrolysis with the intracellular protease enzyme of Lactobacillus plantarum that led to the generation of free amino acids. These hydrolysates generated have higher chances of crossing the intestinal barrier and exerting biological effects due to their low molecular weight, as it allows them to easily migrate to the different tissues and organs (Chalamaiah et al. 2015). Hence, fermentation could be a potential alternative for producing protein hydrolysates and peptides. These results were found to be similar to a study of Fakhfakh et al. (2011), who carried out the fermentation of chicken feathers using Bacillus pumilus A1 strain for producing protein hydrolysates and had observed protein hydrolysates with bands corresponding to 14 kDa, respectively.
Fig. 2.
SDS-PAGE profiles of ESM protein hydrolysates obtained after fermentation with Lactobacillus plantarum for 36 h with initial pH value of culture media of 8.0 (Lane M = Molecular marker (Biorad), 1 = Fermented protein hydrolysate)
Amino acid composition
The amino acids present in the fermented protein hydrolysates were determined as shown in Table 1. They were analyzed as the functional and biological activities of the protein hydrolysates are known to rely mainly on the types of amino acids present and the content of each amino acid within their protein sequence. The amino acids—glutamic acid and aspartic acid were found to be present in highest amounts (8.4 and 5.7%) in the protein hydrolysates. Also, these hydrolysates showed high levels of hydrophobic amino acids (isoleucine, leucine, tryptophan, proline, methionine, cysteine, valine, tyrosine, alanine and phenylalanine) contributing to 21.4% and good quantities of aromatic amino acids (phenylalanine, tyrosine and tryptophan) contributing to 3.6%, respectively. These amino acids are known to contribute to the antioxidant and ACE-inhibitory activities of proteins and peptides and the presence of such amino acids might justify the high free radical scavenging and ACE inhibitory abilities obtained from the fermented protein hydrolysates. The fermented hydrolysates also demonstrated good amounts of essential amino acids (leucine, isoleucine, histidine, methionine, lysine, phenylalanine, valine, tryptophan and threonine) amounting to 15.41% making them useful in various food applications.
Table 1.
Amino acid composition (%) of the fermented protein hydrolysates
| Amino acid | Fermented hydrolysate (%) |
|---|---|
| Aspartic acid | 5.7 |
| Threonine | nd |
| Serine | 3.4 |
| Glutamic acid | 8.4 |
| Glycine | nd |
| Proline | 5.1 |
| Alanine | 2.3 |
| Cysteine | nd |
| Histidine | 2.4 |
| Arginine | 4.0 |
| Tyrosine | 1.1 |
| Valine | 3.3 |
| Methionine | 2.3 |
| Phenylalanine | 1.6 |
| Isoleucine | 2.1 |
| Leucine | 2.8 |
| Lysine | nd |
| Tryptophan | 0.9 |
| Hydroxyproline | 0.6 |
| Hydrophobic amino acids | 21.4 |
| Aromatic amino acids | 3.6 |
| Essential amino acids | 15.4 |
Hydrophobic amino acids: alanine, valine, isoleucine, leucine, tyrosine, phenylalanine, tryptophan, proline, methionine and cysteine; aromatic amino acids: phenylalanine, tyrosine and tryptophan; essential amino acids: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine
nd not detected
Functional properties of the fermented hydrolysates
Solubility
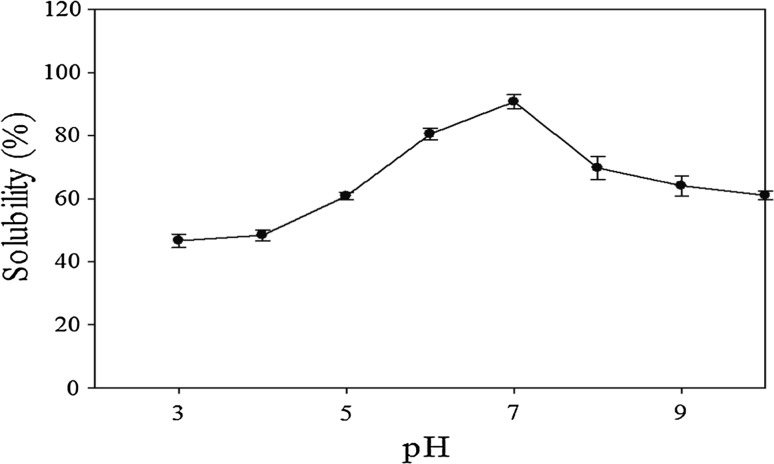
It is desired to generate protein hydrolysates with high solubility values to be used in a number of applications in the food industries, which are also noted to affect the different functional properties such as the emulsifying, foaming and the sensory properties. The solubility values of the fermented protein hydrolysates in a wide range of pH 3.0–10.0 were investigated and it was observed that the protein solubility was significantly (p < 0.05) affected by changes in their pH values. Figure 3 illustrates the solubility profiles of ESM protein hydrolysates obtained by fermentation. Very high solubility (90.7%) was seen at pH 7.0, which indicated the presence of small peptides with molecular weight less than 20 kDa and was further confirmed by SDS-PAGE analysis *(Fig. 3). Small peptides increase the polar groups of the protein hydrolysates and thus allow better interactions with water resulting in increased solubility (Jain and Anal 2016). The protein hydrolysates were found to exhibit significantly (p < 0.05) higher solubility at higher pH values (pH 6, 7, 8, 9 and 10) as compared to that at lower pH values (pH 3, 4 and 5). Chalamaiah et al. (2015) have also reported the lower solubility values of common carp roe (egg) hydrolysates at low pH values of 2, 3, 4 and 5, respectively. The low solubility values are known to be exhibited by the proteins at their isoelectric point because of electrostatic repulsions and their enhanced ionic hydration.
Fig. 3.
Solubility profile of ESM fermented protein hydrolysate at optimum conditions as a function of pH (n = 3). Error bars indicate the standard errors of the mean of three independent experiments
Foaming properties
The ability of protein hydrolysates to generate stabilized foams by reducing the interfacial tension at the interface between the air and water phases governs their functional properties, which are based on how efficient they are in migrating to the interface, forming a thick stabilizing layer and providing stability. The FC and FS of fermented hydrolysates were evaluated at different concentrations as demonstrated in Table 2. There was an insignificant (p < 0.05) increase in the foaming abilities of the fermented hydrolysates with increasing concentrations. This can be attributed to a fact that larger concentrations of proteins can result in their stronger interactions to form a multilayer film, which can lower the interfacial tension resulting in enhanced foaming abilities (Wani et al. 2015). Ability to form stable foams is further dependant on the composition of hydrophobic amino acids that were found to be present in high levels in the fermented hydrolysates. These amino acids enhance the binding at the interface leading to good foaming properties. The fermented hydrolysates demonstrated good foam stability, but decreased with time and similar trend was observed by Sila et al. (2014).
Table 2.
Foaming and emulsifying properties of fermented protein hydrolysates derived from eggshell membrane at different hydrolysate concentrations
| 0.5% | 1% | 2% | Pooled SD | |
|---|---|---|---|---|
| Foaming capacity (%) | 36.67 ± 2.89a | 43.33 ± 3.33ab | 48.33 ± 2.89a | 3.73 |
| Foam stability (%) | 23.33 ± 3.33a | 25.00 ± 2.89a | 30.00 ± 2.89a | 3.73 |
| Emulsifying activity index (m2/g) | 84.63 ± 1.81a | 94.58 ± 1.33b | 100.10 ± 1.86c | 2.06 |
| Emulsion stability (min) | 20.62 ± 1.17a | 29.68 ± 0.52b | 33.74 ± 2.43c | 1.94 |
All the values are mean ± SD of three individual determinations
Different superscripts in the same row indicate significant differences (p < 0.05)
Emulsifying properties
Emulsifying properties are important functional properties for the development of food products as it allows the interactions between the proteins and lipids. The EAI and emulsion stability of fermented ESM protein hydrolysate at different concentrations is shown in Table 2. It was observed that they exhibited significantly (p < 0.05) good EAI and emulsion stability values, respectively at different concentrations. The possible reason can be that the fermented protein hydrolysates had higher degree of hydrolysis due to which they generated smaller peptides that can migrate easily to the oil–water interface resulting in good emulsifying properties. Chalamaiah et al. (2015) also reported similar results of high emulsifying abilities with higher degree of hydrolysis. Also, at higher concentrations, their higher emulsifying abilities were seen, which may be due to their better binding to the interface due to them forming a thick film that can stabilize the droplets in a better manner. Hence, it can be concluded that the emulsifying properties of the protein hydrolysates are mainly governed by their size as well as their amount used for preparation of the emulsions.
In-vitro antioxidant properties of fermented ESM protein hydrolysates
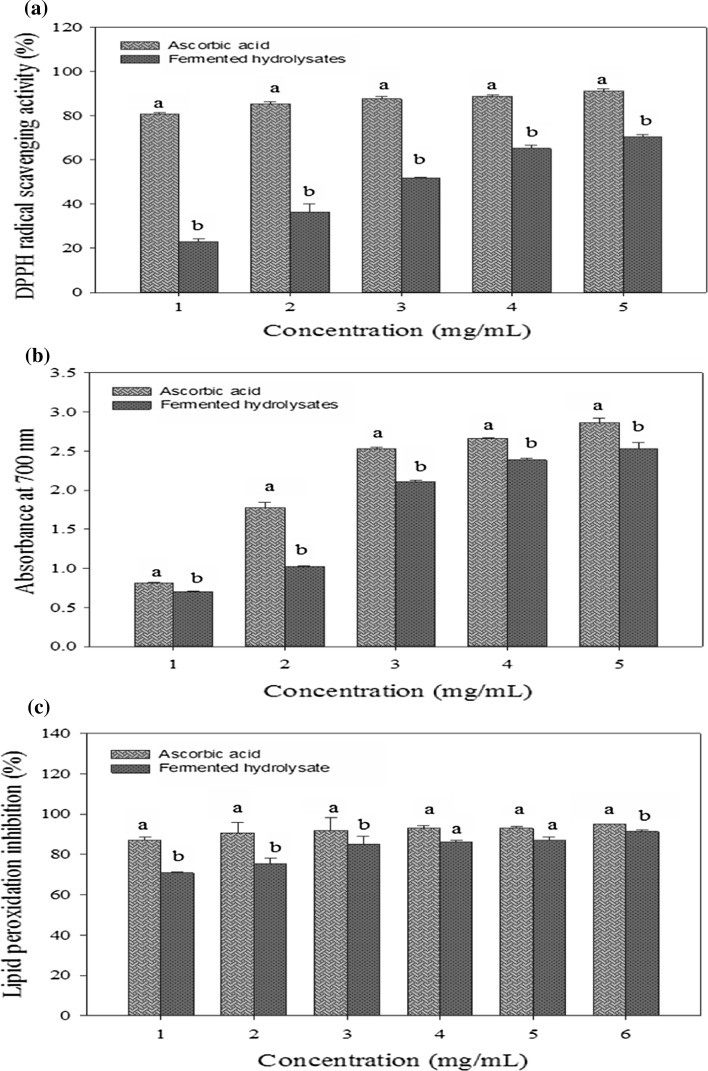
As illustrated in Fig. 4, different in vitro methods including DPPH radical scavenging activity, reducing power and lipid peroxidation inhibition assay were carried out in this study to determine the antioxidant activities of fermented protein hydrolysates and peptides. The DPPH radical scavenging assay is useful in determining the ability of a substrate to transfer electrons and hydrogen atoms, which can react with the free radicals to form stable compounds. Reducing power is the ability of a compound to donate an electron to the free radicals for converting them to stable components and the lipid peroxidation inhibition assay determines their ability of preventing the oxidation of linoleic acids in the different food systems (Fig. 4).
Fig. 4.
Antioxidant activities of the fermented ESM protein hydrolysates at different concentrations. a DPPH radical scavenging activity, b reducing power and c lipid peroxidation inhibition. Different superscript letters indicate significant differences (p < 0.05). All values represent the mean of triplicate analyses ± SD
All antioxidant activities showed a dose dependant behavior at all range of concentrations tested and increased significantly (p < 0.05) with an increase in the concentration. The fermented protein hydrolysates demonstrated radical scavenging activities and very high reducing power, which indicates their better ability to donate electrons and hydrogen atoms. However, these values were found to be less than ascorbic acid at the same concentrations. But, at concentrations of 3, 4 and 5 mg/mL, the hydrolysates demonstrated strong reducing power values, which were very close to that of ascorbic acid (2.1–2.5). The potency of inhibiting lipid peroxidation by the fermented hydrolysates was studied as well. It was predicted during the 6 day period that their degree of oxidation along with their inhibition activity was comparable to ascorbic acid as seen by their lower absorbance values at 500 nm that indicates stronger inhibition of lipid peroxidation.
The scavenging and reducing abilities of the fermented hydrolysates can be correlated with their amino acid composition and molecular size. Low molecular weight peptides (<20 kDa) present in the fermented protein hydrolysates and the presence of amino acids such as phenylalanine, alanine, valine, isoleucine, leucine, cysteine and lysine have been reported to act as good radical scavengers (Suetsuna et al. 2000). These amino acids were present in the protein hydrolysates at a concentration of 12.0%. Also, the presence of tyrosine and histidine residues contribute to good antioxidant properties, which may be related to their imidazole groups (Samaranayaka and Li-Cha 2011). From the results obtained, it can be predicted that good antioxidant activities were demonstrated by the fermented ESM protein hydrolysates. This affirms the existence of electron donating peptides, which can inhibit the free radicals through a radical chain reaction. This would make possible to use them as primary antioxidants applicable in many food, pharmaceutical and cosmetic industrial products.
Inhibition of bacterial growth by ESM protein hydrolysates
The antibacterial activity of ESM protein hydrolysates was evaluated against different food pathogens by assessing their zone of inhibitions. The fermented ESM protein hydrolysates exhibited small inhibition zone against Streptococcus agalactiae (7.6 mm) and moderate zones of inhibition against Escherichia coli (9.4 mm), Salmonella typhimurium (9.6 mm) and Staphylococcus aureus (9.6 mm). It has been reported that peptides (<20 kDa) possess improved antibacterial activity (Beaulieu et al. 2015). Also the presence of hydrophobic amino acids contributes to their antibacterial activity due to their interactions with the bacterial cytoplasmic membranes. The existence of positively charged amino acids (arginine and histidine) also affects the antibacterial activity of the hydrolysates. These amino acids help them to interact with the negatively charged phospholipids resulting in bacterial membrane destruction. In this study, fermentation produced peptides having molecular weights less than 20 kDa, which also consisted of suitable amounts of hydrophobic and positively charged amino acids contributing to their antibacterial activities.
Angiotensin-I-converting enzyme inhibitory activity
The protein hydrolysates obtained after fermentation were tested for their ACE-inhibitory activity and they were found to exhibit an ACE inhibition corresponding to 40.5% (1 mg/mL) and an ACE-inhibition corresponding to 49.3% (2 mg/mL). Their IC50 value was reported to be 2.1 ± 0.1 mg/mL respectively, which was comparable to that of protein hydrolysates obtained from Alaska pollak, sardine and sea bream (Naqash and Nazeer 2013). The existences of amino acids that are hydrophobic in nature are known to contribute to their ACE-inhibitory activity as they bind with the catalytic sites of ACE and inhibit them (Wijesekara and Kim 2010). Furthermore, the fermented protein hydrolysates also presented high levels of proline, glutamic acid, leucine and methionine, which are the frequently observed amino acids present in the ACE-inhibitory peptides (Wu et al. 2015). Hence, the results suggest the existence of peptides demonstrating ACE-inhibition within the protein sequence of the fermented hydrolysates.
The higher values of ACE-inhibition ability of the fermented protein hydrolysates can be correlated with their degree of hydrolysis and molecular weight. The protein hydrolysates with a higher degree of hydrolysis and lower molecular weight are known to have a higher potency of ACE-inhibitory activity (Li et al. 2016). Higher degree of hydrolysis contributes to the release of more active peptides, whereas a lower molecular weight allows easy binding to the ACE-catalytic site along with their inhibition.
Conclusion
The eggshell membrane was found to be a potential substrate for fermentation using Lactobacillus plantarum for the release of functional and bioactive protein hydrolysates and peptides. Effective functional, antioxidant and antihypertensive activities were observed by the fermented hydrolysates. Therefore, they can be utilized in the food industries for hindering food product oxidation during processing and storage and to synthesize functional foods and nutraceuticals. They were also found to exert significant antibacterial activity against the microorganisms tested, which makes them applicable in food preservation. Hence, fermentation of ESM is a novel and effective approach to manage the ESM waste along with their value addition.
References
- Akbar A, Anal AK. Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in ready-to-eat poultry meat. Food Control. 2014;38:88–95. doi: 10.1016/j.foodcont.2013.09.065. [DOI] [Google Scholar]
- Apud GR, Vaquero MJR, Rollan G, Stivala MG, Fernandez PA. Increase in antioxidant and antihypertensive peptides from Argentinean wines by Oenococcus oeni. Int J Food Microbiol. 2013;163(2–3):166–170. doi: 10.1016/j.ijfoodmicro.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Beaulieu L, Bondu S, Doiron K, Rioux LE, Turgeon SL. Characterization of antibacterial activity from protein hydrolysates of the microalga Saccharina longicruris and identification of peptides implied in bioactivity. J Funct Foods. 2015;17:685–697. doi: 10.1016/j.jff.2015.06.026. [DOI] [Google Scholar]
- Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine-glucose model system I: investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J Am Oil Chem Soc. 1998;75:181–187. doi: 10.1007/s11746-998-0030-y. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cavazos A, Gonzalez de Mejia E. Identification of bioactive peptides from cereal storage proteins and their potential role in prevention of chronic diseases. Compr Rev Food Sci Food Saf. 2013;12(4):364–380. doi: 10.1111/1541-4337.12017. [DOI] [PubMed] [Google Scholar]
- Chalamaiah M, Jyothirmayi T, Diwan PV, Kumar BD. Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg) J Food Sci Technol. 2015;52:5817–5825. doi: 10.1007/s13197-015-1714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chi YJ, Zhao MY, Lv L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids. 2012;43:457–466. doi: 10.1007/s00726-011-1102-0. [DOI] [PubMed] [Google Scholar]
- Fakhfakh N, Ktari N, Haddar A, Mnif IH, Dahmen I, Nasri M. Total solubilization of the chicken feathers by fermentation with a keratinolytic bacterium, Bacillus pumilus A1, and the production of protein hydrolysate with high antioxidative activity. Process Biochem. 2011;46:1731–1737. doi: 10.1016/j.procbio.2011.05.023. [DOI] [Google Scholar]
- Gu RZ, Li CY, Liu WY, Yi WX, Cai MY. Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Res Int. 2011;44:1536–1540. doi: 10.1016/j.foodres.2011.04.006. [DOI] [Google Scholar]
- Harnedy PA, FitzGerald RJ. Bioactive peptides from marine processing waste and shellfish: a review. J Funct Foods. 2012;4(1):6–24. doi: 10.1016/j.jff.2011.09.001. [DOI] [Google Scholar]
- Hoyle NT, Merritt JOHN. Quality of fish protein hydrolysates from herring (Clupea harengus) J Food Sci. 1994;59:76–79. doi: 10.1111/j.1365-2621.1994.tb06901.x. [DOI] [Google Scholar]
- Jain S, Anal AK. Optimization of extraction of functional protein hydrolysates from chicken eggshell membrane (ESM) by ultrasonic assisted extraction (UAE) and enzymatic hydrolysis. LWT Food Sci Technol. 2016;69:295–302. doi: 10.1016/j.lwt.2016.01.057. [DOI] [Google Scholar]
- Jemil I, Jridi M, Nasri R, Ktari N, Salem RBSB, Mehiri M, Nasri M. Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochem. 2014;49:963–972. doi: 10.1016/j.procbio.2014.03.004. [DOI] [Google Scholar]
- Li GH, Liu H, Shi YH, Le GW. Direct spectrophotometric measurement of angiotensin I-converting enzyme inhibitory activity for screening bioactive peptides. J Pharm Biomed Anal. 2005;37:219–224. doi: 10.1016/j.jpba.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Sadiq FA, Fu L, Zhu H, Zhong M, Sohail M. Identification of angiotensin I-converting enzyme inhibitory peptides derived from enzymatic hydrolysates of Razor Clam Sinonovacula constricta. Mar Drugs. 2016;14(6):110. doi: 10.3390/md14060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marrufo-Estrada DM, Segura-Campos MR, Chel-Guerrero LA, Betancur-Ancona DA. Defatted Jatropha curcas flour and protein isolate as materials for protein hydrolysates with biological activity. Food Chem. 2013;138:77–83. doi: 10.1016/j.foodchem.2012.09.033. [DOI] [PubMed] [Google Scholar]
- Mora L, Reig M, Toldra F. Bioactive peptides generated from meat industry by-products. Food Res Int. 2014;65(1):344–349. doi: 10.1016/j.foodres.2014.09.014. [DOI] [Google Scholar]
- Naqash SY, Nazeer RA. Antioxidant and functional properties of protein hydrolysates from pink perch (Nemipterus japonicus) muscle. J Food Sci Technol. 2013;50:972–978. doi: 10.1007/s13197-011-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu LY, Jiang ST, Pan LJ. Preparation and evaluation of antioxidant activities of peptides obtained from defatted wheat germ by fermentation. J Food Sci Technol. 2013;50(1):53–61. doi: 10.1007/s13197-011-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Guo Y. Optimization of sour milk fermentation for the production of ACE-inhibitory peptides and purification of a novel peptide from whey protein hydrolysate. Int Dairy J. 2010;20:472–479. doi: 10.1016/j.idairyj.2010.01.007. [DOI] [Google Scholar]
- Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- Samaranayaka AG, Li-Cha EC. Food-derived peptidic antioxidants: a review of their production, assessment, and potential applications. J Funct Foods. 2011;3:229–254. doi: 10.1016/j.jff.2011.05.006. [DOI] [Google Scholar]
- Shahidi F, Han XQ, Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus) Food Chem. 1995;53:285–293. doi: 10.1016/0308-8146(95)93934-J. [DOI] [Google Scholar]
- Sila A, Sayari N, Balti R, Martinez-Alvarez O, Nedjar-Arroume N, Moncef N, Bougatef A. Biochemical and antioxidant properties of peptidic fraction of carotenoproteins generated from shrimp by-products by enzymatic hydrolysis. Food Chem. 2014;148:445–452. doi: 10.1016/j.foodchem.2013.05.146. [DOI] [PubMed] [Google Scholar]
- Singh BP, Vij S, Hati S. Functional significance of bioactive peptides derived from soybean. Peptides. 2014;54:171–179. doi: 10.1016/j.peptides.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Suetsuna K, Ukeda H, Ochi H. Isolation and characterization of free radical scavenging activities of peptides derived from casein. J Nutr Biochem. 2000;1:128–131. doi: 10.1016/S0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Tsai WT, Yang JM, Lai CW, Cheng YH, Li CC, Yeh CW. Characterization and adsorption properties of eggshells and eggshell membranes. Bioresour Technol . 2006;97:488–493. doi: 10.1016/j.biortech.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Tsumura K, Saito T, Tsuge K, Ashida H, Kugimiya W, Inouye K. Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT Food Sci Technol. 2005;38:255–261. doi: 10.1016/j.lwt.2004.06.007. [DOI] [Google Scholar]
- Wani IA, Sogi DS, Gill BS. Physico-chemical and functional properties of native and hydrolyzed protein isolates from Indian black gram (Phaseolus mungo L.) cultivars. LWT Food Sci Technol. 2015;60:848–854. doi: 10.1016/j.lwt.2014.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesekara I, Kim SK. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: prospects in the pharmaceutical industry. Mar Drugs. 2010;8:1080–1093. doi: 10.3390/md8041080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Xu N, Sun X, Yu H, Zhou C. Hydrolysis and purification of ACE inhibitory peptides from the marine microalga Isochrysis galbana. J Appl Phycol. 2015;27:351–361. doi: 10.1007/s10811-014-0347-x. [DOI] [Google Scholar]
- Zambrowicz A, Pokora M, Setner B, Dąbrowska A, Szołtysik M, Babij K, Szewczuk Z, Trziszka T, Lubec G, Chrzanowska J. Multifunctional peptides derived from an egg yolk protein hydrolysate: isolation and characterization. Amino Acids. 2015;47(2):369–380. doi: 10.1007/s00726-014-1869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Li B, Dong S, Liu Z, Zhao X, Wang J, Zeng M. A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate. Peptides. 2009;30:1028–1033. doi: 10.1016/j.peptides.2009.03.002. [DOI] [PubMed] [Google Scholar]