Abstract
This study examined the antibacterial activity of Thymus vulgaris on multiple antibiotic resistant (MAR) Vibrio parahaemolyticus and Vibrio fluvialis isolated from shrimps. The ethanol extract of T. vulgaris antibacterial properties was assessed using the agar diffusion method. Survival of test organisms in shrimp meat using different concentrations of T. vulgaris was done using standard method. The quantitative and qualitative phytochemical tests of T. vulgaris extract were determined. The ethanol extract had antimicrobial activities on the test organisms showing 20.00 ± 0.0 and 23.00 ± 0.0 mm zone of inhibition on V. parahaemolyticus and V. fluvialis respectively. T. vulgaris completely decreased microbial load of V. parahaemolyticus and V. fluvialis in 150 and 60 min, respectively. The phytochemical screening for the ethanol extract reported phenol, alkaloids, tannin, saponin, anthraquinone flavonoid and cardiac glycoside as 51.76, 26.60, 6.76, 54.33, 30.35 89.65 and 18.23 mg/100 g, respectively. This study reveals the antibacterial property of T. vulgaris on the MAR Vibrio species.
Keywords: Thyme (Thymus vulgaris), Vibrio parahaemolyticus, Vibrio fluvialis, Multiple antibiotics resistance (MAR)
Introduction
There is a serious apprehension on the use and abuse of antibiotics by humans and fish farmers. This abuse led to the natural emergence of bacteria resistance, which can be transmitted from animals to man (Dunstan et al. 2013). Moreover, pathogenic bacteria have shown resistance to one or many of the common antibiotics. However, due to the increasing resistance of pathogens to the synthetic antibiotics, scientists have been creating awareness to resuscitate the use of those plants, with confirmed antimicrobial properties (Gull et al. 2012).
Interestingly, there are facts on antimicrobial properties of various plants used as spices that are good therapeutic agents against some microbial infections (Gull et al. 2012). Although studies done previously have confirmed that some spices like thyme, garlic, onion, sage, cinnamon, cloves, rosemary and some other spices inhibit the growth of both Gram negative and positive food borne pathogens, molds and yeast (Issabeagloo et al. 2012; Ismail et al. 2012). These spices also have unique flavour and aroma from compounds known as phyto-chemicals as reported by Melvin et al. (2009). The phyto-chemicals have many classes which include the flavonoids, isoflavones and anthocyanins found in the spices (Melvin et al. 2009). They also have antimicrobial property which serves as photoprotectants and responses to environmental changes (Melvin et al. 2009).
Thymus vulgaris (common garden thyme), belongs to Lamiaceae family which is rich in aromatic compounds and also a known genera by their pharmaceutical properties (Figueiredo et al. 2008). This plant has been used from olden times as antiseptic, antimicrobial drugs, food preservatives, food flavour and in cosmetics (Figueiredo et al. 2008). The processed plant is known as thyme and traditionally used for the preparation for flavoring of meat. Some studies have reported antibacterial activity of T. vulgaris (Ismail et al. 2012). Snoussi et al. (2008) also reported that plants like thyme and clove can be used for seafood preparation to protect against contamination by Vibrio species strains. Therefore there is a need to discover antibacterial compounds with innovative mechanisms of action for emerging antibiotic resistance Vibrio species.
This present study was designed to determine the antibacterial activity of ethanol extract of Thymus vulgaris on MAR Vibrio species isolated from shrimps.
Materials and methods
Collection of fresh T. vulgaris samples
The fresh leaves of T. vulgaris were collected from a garden along Benin airport, Edo State between 5°36′27.6″E and 6°18′50.7″N. The plant was authenticated at Botany Department, University of Ibadan-herbarium number UIH-22499. The fresh leaves were air dried for 2 weeks and blended into powder using a sterile blender prior to use.
Standardisation of Vibrio species
The test organisms Vibrio parahaemolyticus and Vibrio fluvialis were isolated from shrimps in Lagos coastal waters, Vibrio species were phenotypically and molecularly identified as earlier described by Oramadike and Ogunbanwo (2014) and antibiotic susceptibility of the organisms were done and the organisms confirmed to be multiple antibiotic resistant (MAR) strains (Oramadike and Ogunbanwo 2015). These test organisms were maintained in tryptone soy broth (TSB) supplemented with glycerol at −80 °C prior to use.
Preparation of T. vulgaris ethanol extract
The method of Olayemi and Opaleye (1999) and Aboaba et al. (2011) was adopted for the extraction of the spices. This was carried out using 20 g of T. vulgaris. This was dispensed into two sterile beakers, each containing 80 mL of 95% ethanol. They were soaked and left to stand in the refrigerator for 72 h, after which the solution was sterilized by filtration. The solvent was removed using a rotary evaporator (Fisatom 802) with vacuum control (New Technique, Model NT 613, Piracicaba, SP, Brazil). T. vulgaris residue was reconstituted with 5% dimethylsulfoxide (DMSO). Sensitivity of the test organisms to the ethanol extract of the spice was indicated by a clear zone of inhibition of 3.0 mm around the wells. The diameter of the zone of inhibition was measured to the nearest millimeter using a transparent ruler. This was taken as an index of the degree of sensitivity of the test organisms to the extract. Varying concentrations of the extract using modified concentrations by Akintobi et al. (2013) were prepared and the above procedure was repeated. Minimum inhibitory concentrations were determined for the extract.
Determination of minimum inhibitory concentration (MIC)
MIC was determined using the agar diffusion method (Rojas et al. 2003). Wells were made into previously seeded Mueller–Hinton agar plates containing 2.0 × 108 cfu/mL (0.5 McFarland’s standard) of each of the test organism and were filled with 0.2 mL of the four different concentrations of the residue (1.3, 1.6, 3.1 and 3.9 mg/mL) using DMSO as control. They were allowed to stand for 1 h for proper diffusion and then incubated at 37 °C for 24 h. All tests were performed in triplicate. The least concentration of T. vulgaris extract that show inhibitory effects on the test organisms was taken as the minimum inhibitory concentration.
Determination of phytochemical compounds
The presence of bioactive agents, such as the flavonoids, alkaloids, steroids, terpenoid, anthraquinone, tannins, saponins, cardiac glycosides, phenols and phlobatanins was detected. The phyto-constituents were assayed by using standard methods described by Harbone (2008). The phytochemical analysis was carried out in the Department of Biochemistry, University of Lagos, Lagos State.
Flavonoids test
Five millilitres of diluted ammonia solution was added to aqueous filtrate of the test sample followed by the addition of 1 mL concentrated H2SO4. A yellow colouration indicates the presence of flavonoids (Harbone 2008).
Test for phenol
To 2 mL of the extract, a few drops of ferric chloride solution were added. Bluish green or red colour indicates the presence of phenol (Harbone 2008).
Phlobotannins test
Deposition of red precipitate when an aqueous extract of the test sample was boiled with 1% hydrochloric acid indicated the presence of phlobotannins (Harbone 2008).
Tannins test
Five grams of the extract and 5 mL of honey were stirred separately with 100 mL distilled water and filtered. One millilitre ferric chloride reagent was added to the filtrate. A blue–black or blue–green precipitate was an indication of the presence of tannins (Harbone 2008).
Test for steroid (Libermann–Burchard test)
To 2 mL of the extract, a few drops of chloroform, 3–4 drops of acetic anhydride and one drop of concentrate sulphuric acid were added. Appearance of purple colour, which changes to blue or green colour, shows the presence of steroid (Harbone 2008).
Saponins test
Five grams of the extract and 5 mL of honey were shaken separately with distilled water in a test tube. Frothing which persists on warming was taken as preliminary evidence of the presence of the saponins (Harbone 2008).
Alkaloid test
Five grams of the T. vulgaris extract and 5 mL honey were stirred with 5 mL of 1% aqueous hydrochloric acid on a steam bath at 60 °C for 5 min. The sample was filtered with a three layered muslin cloth. One millilitre of the filtrate was treated with few drops of Draggendoff’s reagent. Blue–black turbidity serves as preliminary evidence of alkanoids (Harbone 2008).
Cardiac glycosides (Keller–Killiani Test)
Five grams of the extract and 5 mL of honey were dissolved separately in 2 mL glacial acetic acid containing a drop of ferric chloride solution. This was underplayed with 1 ml concentrated H2SO4. A brown ring at the interface indicates a deoxy-sugar characteristic of cardenolides. A violet ring may appear below the brown ring, while in the acetic acid layer, a green ring may form which just gradually spreads throughout the layer (Harbone 2008).
Test for anthraquinone (Borntrager’s test)
A few drops of magnesium acetate solution were added to 5 g of the extract. Pink colour formation shows the presence of anthroquinone (Harbone 2008).
Test for terpenoid (Noller’s Test)
The extracts were warmed with a piece of tin and a few drops of thionyl chloride. Violet or purple colouration indicates the presence of terpenoid (Harbone 2008).
Survival of MAR V. parahaemolyticus and V. fluvialis in shrimp meat treated with different concentrations of T. vulgaris
Bacterial strain and preparation of inoculum
V. parahaemolyticus and V. fluvialis were sub cultured at 37 °C for 18 h. Cells from each of the pure isolates were harvested from 18 h. Tryptone soy broth culture supplemented with 3% NaCl w/v, washed in phosphate buffer (0.05 M, pH 7.0) and resuspended in normal saline with turbidity adjusted to 0.5 McFarland’s turbidity level (about 2.0 × 108 cfu/mL). The bacterial cultures were further diluted in saline peptone solution (0.1% bacteriological peptone; 0.85% sodium chloride solution) and used to obtain a final population 2.0 × 106 cfu/mL by inoculating the shrimp meat (Reis et al. 2011).
Preparation of shrimp meat with T. vulgaris
The shrimp meat was prepared according to Rabiey et al. (2014). Eighty grams of diced shrimp meat was sterilized for 15 min at 121 °C. Twenty grams each was treated with three different amounts of the extracts (1.3, 1.6 and 3.1 g) T. vulgaris. The same quantity of shrimp meat without the spice was used as control.
Sterile tubes containing sterile shrimp meat were inoculated with 0.1 mL of TSB with 3% sodium chloride containing 2.0 × 106 cfu/mL of V. parahaemolyticus/V. fluvialis. The treatments were as follows: T. vulgaris extract (1.3, 1.6, and 3.1 g) added to 20 g of sterilized shrimp meat inoculated with 0.1 mL (2.0 × 106 cfu/mL) of V. parahaemolyticus/V. fluvialis and 20 g of shrimp meat with 0.1 mL (2.0 × 106 cfu/mL) of isolates only as control. The bacterial population was monitored at 30 min interval for 150 min. These assays were performed in triplicate. One gram was added to 9 mL of sterile diluents (phosphate buffer) for serial dilution and 1 mL of appropriate dilution was plated out in TSA media using pour plate method, with incubation at 37 °C for 18 h.
Sensory evaluation of shrimp cooked with different concentrations of T. vulgaris
Twenty grams of shrimp samples each were mixed with different amounts (1.3, 1.6 and 3.1 g) of T. vulgaris. The samples were wrapped with aluminum foil and cooked in a steaming pot until the core temperature reached 70 °C (measured with a hand-held thermometer, model CTH 6200, Temp range −5 °C to 250 °C) for 15 min (Masniyom et al. 2005). The sensory evaluation was performed by ten trained panelists. The assessment was conducted for aroma, physical appearance, taste, after taste and mouth feel of shrimp samples using a nine-point hedonic scale: (1) dislike extremely; (2), dislike very much; (3) dislike moderately; (4) dislike slightly; (5) neither like nor dislike; (6) like slightly; (7) like moderately; (8) like very much and (9) like extremely (Mailgaad et al. 1999).
Statistical analyses
The descriptive statistics was done using the prism version 5.03 statistical software programmes (Graph pad software, San Diego, CA. USA).
Results
Antibacterial activity of T. vulgaris extract on MAR Vibrio species from shrimps
Inhibitory zones of T. vulgaris on tested Vibrio species
The results of the inhibitory zones of T. vulgaris are presented in Table 1. The results showed that extract of T. vulgaris produced the highest zone of inhibition on V. fluvialis 23.00 ± 0.0 mm while the extract produced 20.00 ± 0.0 mm on V. parahaemolyticus.
Table 1.
Zone of inhibition (mm) of ethanol extract of T. vulgaris on tested organisms
| Test organisms | Ethanol extract (mm) |
|---|---|
| V. parahaemolyticus | 20.00 ± 0.0 |
| V. fluvialis | 23.00 ± 0.0 |
Inhibitory zones above 3 mm is positive
Mean value represents = mean ± SD
Minimum inhibitory concentration of T. vulgaris ethanol extract on test organisms
The results showed that T. vulgaris ethanol extract at 1.3 mg/mL was the least concentration that had a mean value of 7.75 ± 0.2 mm against MAR V. parahaemolyticus while the extract of the same concentration had a mean value of 8.70 ± 0.2 mm against MAR V. fluvialis as shown in Table 2. The other concentrations of T. vulgaris, 1.6, 3.1 and 3.9 mg/mL showed inhibitory zones of 9.50 ± 0.1, 17.15 ± 0.2 and 20.00 ± 0.0 mm mean values, respectively against MAR V. parahaemolyticus. However, 1.6, 3.1 and 3.9 mg/mL of T. vulgaris ethanol extract reported mean values of 11.15 ± 0.1, 19.80 ± 0.2 and 23.00 ± 0.0 mm, respectively against MAR V. fluvialis. The control with 5% DMSO had no effect on the test isolates.
Table 2.
Minimum inhibitory concentration of T. vulgaris ethanol extract on test organisms
| Test organisms | Concentrations of T. vulgaris extract (mg/mL) | Mean values from inhibitory zones (mm) | Control |
|---|---|---|---|
| V. parahaemolyticus | 1.3 | 7.75 ± 0.2 | No effect |
| 1.6 | 9.50 ± 0.1 | No effect | |
| 3.1 | 17.15 ± 0.2 | No effect | |
| 3.9 | 20.00 ± 0.0 | No effect | |
| V. fluvialis | 1.3 | 8.70 ± 0.2 | No effect |
| 1.6 | 11.15 ± 0.1 | No effect | |
| 3.1 | 19.80 ± 0.2 | No effect | |
| 3.9 | 23.00 ± 0.0 | No effect |
Qualitative phytochemical compounds of T. vulgaris extract
The ethanol extract of T. vulgaris contained flavonoids, phenol, tannins, saponins, alkaloids, cardiac-glycosides and anthraquinone except phlobatanins, steroids and terpernoid (Table 3).
Table 3.
Quantitative and qualitative phytochemical compounds present in ethanol extract of T. vulgaris
| Bioactive constituents (mg/100 g) | |
|---|---|
| Alkaloid | 26.60 |
| phlobatanin | Absent |
| Steriod | Absent |
| Terpernoid | Absent |
| Flavonoid | 89.65 |
| Phenol | 51.76 |
| Tannin | 6.76 |
| Saponin | 54.53 |
| Cardiac glycoside | 18.23 |
| Antraquinone | 30.35 |
Quantitative bioactive constituents of T. vulgaris extract
The bioactive constituents of T. vulgaris ethanol extract of T. vulgaris had flavonoid 89.65 mg/100 g, cardiac glycoside 18.23 mg/100 g, phenol, tannin, saponin and anthraquinone alkaloids were 51.76, 6.76, 54.33, 30.35 and 26.60 mg/100 g, respectively (Table 3).
Survival of multiple antibiotics resistance (MAR) Vibrio species in shrimp meat using different concentrations of T. vulgaris
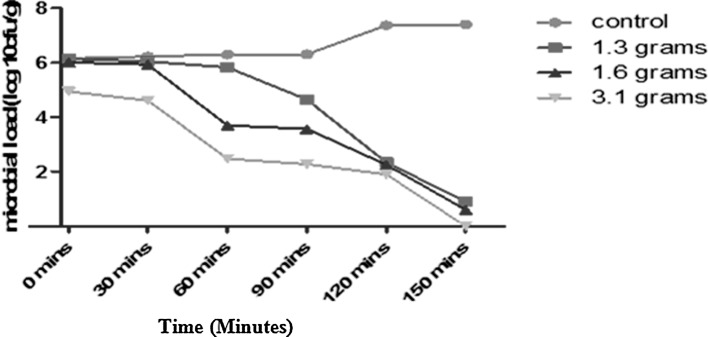
The antibacterial effects of T. vulgaris at 1.3, 1.6, and 3.1 g concentrations on V. parahaemolyticus and V. fluvialis are shown in Fig. 1. Results indicated that after 150 min of storage, the population of V. parahaemolyticus in the control was 6.14 log cfu/mL microbial counts and a final count 7.4 log cfu/mL. In 1.3 g treatment, initial microbial population of the V. parahaemolyticus at 0 min was 6.13 log cfu/mL, while the final population of the organism at 150 min showed 0.9 log cfu/mL. At 1.6 g concentration, the initial microbial load was 6.0 log cfu/mL and a final microbial load was 0.6 log cfu/mL after 150 min. However, 3.1 g concentration led to an initial microbial load of 4.9 log cfu/mL and a final count of 1.9 log cfu/mL after 120 min. No growth at 150 min was observed.
Fig. 1.
Survival of V. parahaemolyticus in shrimp meat treated with different concentrations of T. vulgaris
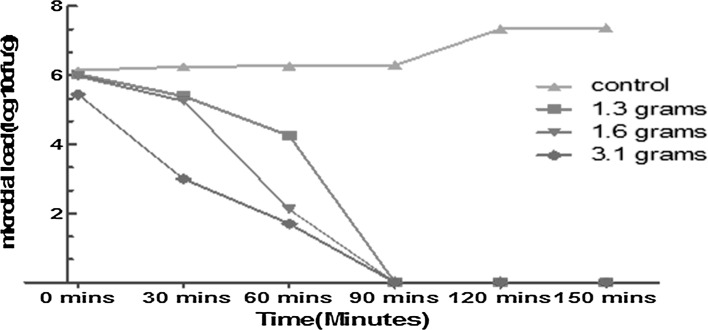
Moreover, microbial load of untreated shrimp meat inoculated with V. fluvialis showed an initial microbial population of 6.1 log cfu/mL and a final count of 7.4 log cfu/mL after 150 min. In 1.3 g treatment initial population of V. fluvialis was 6.03 log cfu/mL and final population of 4.3 log cfu/mL after 60 min. The initial population of 5.9 log cfu/mL for 1.6 g and final microbial population of 2.11 log cfu/mL after 60 min was observed. The concentration (3.1 g) for T. vulgaris treatment revealed an initial population 5.4 log cfu/mL and a final count of 1.6 log cfu/mL in 60 min. All concentrations (1.3, 1.6 and 3.1 g) treatments showed no growth of V. fluvialis after 90 min of monitoring as shown in Fig. 2.
Fig. 2.
Survival of V. fluvialis in shrimp meat treated with different concentrations of T. vulgaris
Sensory evaluation of shrimp meat cooked with different concentrations of T. vulgaris
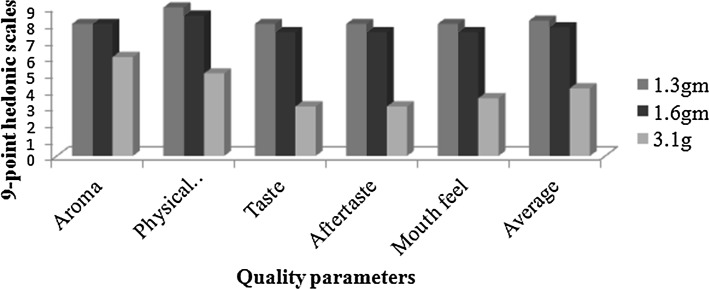
The average sensory scores for aroma, physical appearance, taste, after taste and mouth feel of the cooked shrimps mixed with different concentrations (1.3, 1.6 and 3.1 g) of T. vulgaris are given in Fig. 3.
Fig. 3.
Sensory evaluation of shrimp prepared with different concentrations of T. vulgaris
The result of the cooked shrimp meat with 1.3 g concentration of T. vulgaris revealed an average score of 8.2. The 1.6 g concentration of T. vulgaris cooked with the shrimp meat had average scores of 7.8 while those with 3.1 g concentration showed an average score of 4.1.
Discussion
This study was conducted to increase the awareness on the beneficial rule of spices on inhibitory properties against food borne microorganisms. Sensitivity of the Vibrio species to T. vulgaris extracts have shown that the spice extract can be used as a complement to the array of antimicrobial compounds available. According to Aboaba et al. (2011) the inhibitory zones shown by some of the spices can be comparable to chemotherapeutic drugs. The MIC values can also be used as a guide for the treatment of most infections (Aboaba et al. 2011). Plant based antimicrobials have huge therapeutic potentials as they may serve the purpose with lesser side effects that are often associated with synthetic antimicrobials (Poole 2001). Also the issue of resistance to these extracts may not arise as is found with antibiotics (Kareem et al. 2010).
A large number of constitutive plant components have been reported to have exhibited antimicrobial activity. T. vulgaris (thyme) extracts are known to have some antimicrobial activities and are used in different forms as flavour enhancers, food preparations and in herbal medicine (Dorman and Deans 2000). Though the modes of action of the extracts are not known, their antimicrobial agents include eugenol, thymol, terpenes, flavones, glycosides of phenolic monoterpenoids and aliphatic alcohols among other elements (Usman et al. 2015). These substances alone or in combination with other agents were reported to have broad spectrum of antimicrobial activity for both bacteria and fungi (Dorman and Deans 2000).
The inhibitory effect of T. vulgaris extracts in this study revealed a high antibacterial potential of T. vulgaris on the MAR Vibrio species. This could be suggesting the presence of high active plant secondary metabolites in the plant extract. Reports have shown that essential oils having eugenol, carvacrol and thymol (phenolic compounds) had maximum antibacterial performances and T. vulgaris have been identified to contain thymol (Ismail et al. 2012). This is not surprising as the antimicrobial nature of many edible plant extracts have been demonstrated (Benbelaïd et al. 2013; Radulovic et al. 2013). Nzeako et al. (2006) had earlier reported on antimicrobial activity of T. vulgaris against Pseudomonas aeruginosa, Salmonella spp and Staphylococcus aureus at various dilutions. Nowak et al. (2013) reported that the application of thyme oil even at low concentrations led to inhibition of the growth of these microbes in refrigerated products. Several published reports had described the antibacterial effects of different spices (Gull et al. 2012).
The active components of these extracts usually hinder the growth and metabolism of microorganisms and these are quantified by determining the MIC of the extract. These values are used as guide for the treatment of most infections (Aboaba et al. 2011). This study implies that T. vulgaris could be used as spice and also exhibit antibacterial effect on contaminated seafoods. This supports the extensive use of these plants (spices) for treatment of ailments by traditional African medical practitioners. The sensory result of cooked shrimp meat treated with T. vulgaris in this study demonstrated high acceptance of this cooking spice at 1.3/20 g of shrimp meat.
The quantitative and qualitative phytochemical tests carried out on the ethanol extract of T. vulgaris indicated that T. vulgaris constitutes some antimicrobial properties. However, it was observed that not all the constituents screened were present in the ethanol extract of T. vulgaris. Usman et al. (2015) stated that ethanol is an organic solvent and it dissolves organic substances effectively to release active compounds. This is similar to the findings of this study as organic compounds were released with ethanol extraction. These compounds are known to be biologically dynamic and therefore support the antimicrobial activity. This study has confirmed that T. vulgaris possess antimicrobial properties that can inhibit MAR Vibrio species. The sensitivity of microorganisms to thyme oil has been reported by Girova et al. (2010). More so, the susceptibility of thymol, which is the most important constituent of thyme oil, has been examined by Snoussi et al. (2008), Rivas et al. (2010), Schirmer and Langsrud (2010) and Szczepaniak et al. (2011).
The present study suggests that the plant extracts could be a very good option as alternative remedy for control of Vibrio species contamination.
Acknowledgements
We acknowledge the Nigerian Institute for Oceanography and Marine Research (NIOMR) for sponsoring this research. Special thanks to Dr. G.R. Akande, Executive Director NIOMR, for his strong support.
Contributor Information
Chigozie Eunice Oramadike, Phone: 234-8033551690, Email: chigooramadike@gmail.com, Email: chigooramadike2007@yahoo.com.
Samuel Temitope Ogunbanwo, Email: topzybanwo@yahoo.com.
References
- Aboaba OO, Eze AR, Anabuike CL. Antimicrobial activities of some Nigeria spices on some pathogens. Agric Biol J N Am. 2011;2(8):1187–1193. doi: 10.5251/abjna.2011.2.8.1187.1193. [DOI] [Google Scholar]
- Akintobi OA, Onoh CC, Ogele JO, Idowu AA, Ojo OV, Okonko IO. Antimicrobial activity of Zingiber officinale extract against some selected pathogenic bacteria. Nat Sci. 2013;11(1):7–15. [Google Scholar]
- Benbelaïd F, Khadir A, Abdoune MA, Bendahou M. Phytochemical screening and in vitro antimicrobial activity of Thymus lanceolatus Desf. from Algeria. Asian Pac J Trop Dis . 2013;3(6):454–459. doi: 10.1016/S2222-1808(13)60100-0. [DOI] [Google Scholar]
- Dorman HGD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Dunstan RA, Heinz E, Wijeyewickrema LC, Pike RN, Purcell AW, Evans TJ, Praszkier J, Robins-Browne RM, Strugnell RA, Korotkov KV, Lithgow T. Assembly of the type II secretion system such as found in Vibrio cholera depends on the Novel. PLoS Pathog. 2013;9(1):303–309. doi: 10.1371/journal.ppat.1003117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo AC, Barroso JG, Pedro LG, Salgueiro L, Miguel MG, Faleiro ML. Portuguese Thymbra and Thymus species volatiles: chemical composition and biological activities. Curr Pharm Des. 2008;14(29):3120–3140. doi: 10.2174/138161208786404218. [DOI] [PubMed] [Google Scholar]
- Girova T, Gochev V, Jirovetz L, Buchbauer G, Schmidt E, Stoyanova A. Antimicrobial activity of essential oils from spices against psychrotrophic food spoilage microorganism. Biotechnol Biotechnol Equip. 2010;24:547–552. doi: 10.1080/13102818.2010.10817895. [DOI] [Google Scholar]
- Gull I, Saeed M, Shaukat H, Aslam SM, Samra ZQ, Athar AM. Inhibitory effect of Allium sativum and Zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Ann Clin Microbiol Antimicrob. 2012;11:8. doi: 10.1186/1476-0711-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbone DB. Phytochemical methods, a guide to modern techniques of plant analysis. 3. New Delhi: Springer; 2008. [Google Scholar]
- Ismail MM, Essam TM, Mohamed AF, Mourad FE. Screening for the antimicrobial activities of alcoholic and aqueous extracts of some common spices in Egypt. Int J Microbiol Res. 2012;3(3):200–207. [Google Scholar]
- Issabeagloo E, Kermanizadeh P, Taghizadieh M, Forughi R. Antimicrobial effects of rosemary (Rosmarinus officinalis L.) essential oils against Staphylococcus spp. Afr J Microbiol Res. 2012;6(23):5039–5042. [Google Scholar]
- Kareem KT, Kareem SO, Adeyemo OJ, Egberongbe RK. In vitro antimicrobial properties of Bridelia ferruginea on some clinical isolates . Agric Biol J N Am. 2010;1(3):416–420. doi: 10.5251/abjna.2010.1.3.416.420. [DOI] [Google Scholar]
- Mailgaad M, Civille GV, Carr BT. Sensory evaluation techniques. Boca Raton: CRS; 1999. [Google Scholar]
- Masniyom P, Benjakul S, Visessanguan W. Combination effect of phosphate and modified atmosphere on quality and shelf-life extension of refrigerated seabass slices. LWT Food Sci Technol. 2005;38:745–756. doi: 10.1016/j.lwt.2004.09.006. [DOI] [Google Scholar]
- Melvin MJ, Jayachitra J, Vijayapriya M. Antimicrobial activity of some common spices against certain human pathogens. J Med Plant Res. 2009;3(11):1134–1136. [Google Scholar]
- Nowak A, Kalemba D, Piotrowska M, Czyżowska A. Effects of thyme (Thymus vulgaris L.) and rosemary (Rosmarinus officinalis L.) essential oils on growth of Brochothrix thermosphacta. Afr J Microbiol Res. 2013;7(26):3396–3404. doi: 10.5897/AJMR12.1618. [DOI] [PubMed] [Google Scholar]
- Nzeako BC, Al-Kharousi ZSN, Al-Mahrooqui Z. Antimicrobial activities of clove and thyme extracts. Sult Qaboos Univ Med J. 2006;6(1):34–39. [PMC free article] [PubMed] [Google Scholar]
- Olayemi AB, Opaleye FI. Antibiotic resistance among coliform bacteria isolated from hospital and urban waste waters. World J Microbiol Biotechnol. 1999;6:285–288. doi: 10.1007/BF01201298. [DOI] [PubMed] [Google Scholar]
- Oramadike C, Ogunbanwo ST. Incidence of Vibrio species in seafood samples collected from Lagos Lagoon, Nigeria. Am J Food Sci Nutr. 2014;1:76–82. [Google Scholar]
- Oramadike C, Ogunbanwo ST. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from seafoods in Lagos Lagoon Nigeria. Cogent Food Agric. 2015;1:1041349. [Google Scholar]
- Poole K. Overcoming antimicrobial resistance by targeting resistance mechanisms. J Pharm Pharmacol. 2001;53:283–284. doi: 10.1211/0022357011775514. [DOI] [PubMed] [Google Scholar]
- Rabiey S, Hosseini H, Rezaei M. Use Carum copticum essential oil for controlling the Listeria monocytogenes growth in fish model system. Braz J Microbiol. 2014;45(1):89–96. doi: 10.1590/S1517-83822014000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic NS, Blagojevic PD, Stojanovic-Radic ZZ, Stojanovic NM. Antimicrobial plant metabolites: structural diversity and mechanism of action. Curr Med Chem. 2013;20(7):932–952. [PubMed] [Google Scholar]
- Reis FBD, Souza VMD, Thomaz MRS, Fernandes LP, Oliveira WPD, Martinis ECP. Use of carnobacterium maltaromaticum cultures and hydroalcoholic extract of Lippia sidoides Cham. against Listeria monocytogenes in fish model systems. Int J Food Microbiol. 2011;146:228–234. doi: 10.1016/j.ijfoodmicro.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Rivas L, McDonnel MJ, Burgess CM, O’Brien M, Navarro-Villa A, Fanning S, Duffy G. Inhibition of verocytotoxigenic Escherichia coli in model broth and rumen systems by carvacrol and thymol. Int J Food Microbiol. 2010;139:70–78. doi: 10.1016/j.ijfoodmicro.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Rojas R, Bustamante B, Bauer J, Fernández I, Albán J, Lock O. Antimicrobial activity of selected peruvian medicinal plants. J Ethnopharmacol. 2003;88(2–3):199–204. doi: 10.1016/S0378-8741(03)00212-5. [DOI] [PubMed] [Google Scholar]
- Schirmer BC, Langsrud S. Evaluation of natural antimicrobials on typical meat spoilage bacteria and in vacuum-packed pork meat. J Food Sci. 2010;75:98–102. doi: 10.1111/j.1750-3841.2009.01485.x. [DOI] [PubMed] [Google Scholar]
- Snoussi M, Hajlaoui H, Noumi E, Usai D, Sechi L, Zanetti S, Bakhrouf A. In vitro anti Vibrio spp. activity and chemical composition of some Tunisian aromatic plants. World J Microbiol Biotechnol. 2008;24:3071–3076. doi: 10.1007/s11274-008-9811-6. [DOI] [Google Scholar]
- Szczepaniak S, Polanska M, Van Assche A, Moloney R, Willems KA. The synergism of natural compounds in the pursuit of safe and healthier ford. J Ind Microbiol Biotechnol. 2011;38:215–220. doi: 10.1007/s10295-010-0822-6. [DOI] [PubMed] [Google Scholar]
- Usman UZ, Usman HM, Mainasara AS. Role of African spices against Escherichia coli isolated from potable water sample in Sokoto, Nigeria. Adv Med Plant Res. 2015;3(2):62–68. [Google Scholar]