Abstract
Phenolic composition and antioxidant capacity of different fruit part including peel, pulp, juice, whole fruit and seed from five lemon cultivars (Feiminailao, Cuningmeng Limeng, Pangdelusaningmeng, Beijingningmeng) were investigated. Caffeic acid (9.31–741.4 μg/g FW) and chlorogenic acid (2.7–527.5 μg/g FW) were the dominant phenolic acid in fruit tested, Pangdelusaningmeng (PD) and Limeng peels with the highest contents, respectively. Hesperidin was the predominant flavanone (10.27–3315 μg/g FW), Cuningmeng (CN) peels with the highest level. PD peels had rich rutin, CN seeds had rich eriocitrin. Nobiletin was the main polymethoxylated flavonoids identified, PD with the highest level. CN peels contained rich tangeretin. Overall, peels and whole fruit had significantly higher level of phenolics than other fruit parts, and seeds were good source of flavonoids. PD and CN not only contained higher level of phenolic, but also presented higher antioxidant capacity than other cultivars tested, and are of great value for human nutrition.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2544-5) contains supplementary material, which is available to authorized users.
Keywords: Lemon (Citrus limon Burm.), Flavonoid, Phenolic acid, PMFs, Antioxidant capacity
Introduction
Lemon (Citrus limon Burm.) is the third major Citrus species after orange and mandarin in the world (Miran et al. 2016), and it is widely used as fresh, beverages, cook material and preservative for food (González-Molina et al. 2010; Ngugi et al. 2016). Owe to its tart flavor, lemon is popularly used in beverages, ice creams, desserts, salad dressings, and many meat and vegetable dishes. Citrus limon contains many important natural nutritional components, such as phenolic compounds, dietary fiber, essential oils and carotenoids (Del Río et al. 2004). There is now evidence to show that lemon fruit have strong antioxidant, antimicrobial and anti-inflammatory properties, and intake of lemon is associated with a decrease risk of cardiovascular diseases and certain forms of cancer. Therefore, lemon fruit are more and more becoming popular health-promoting fruit (Benavente-Garcia and Castillo 2008).
Polyphenol is one of the most important group of phytochemical antioxidants in lemon fruit. Up to now, many previous studies have identified the individual phenolics in lemon fruit and evaluated their several bioactivities. The eriocitrin (eriodictyol 7-rutinoside) from lemon fruit was isolated and its antioxidant capacity was evaluated (Miyake et al. 1997). The coumarins from lemon fruit was identified and the inhibition for tumor promotion and superoxide and nitric oxide generation was characterized (Miyake et al. 1999). New limonoid glycosides was isolated and their structures from lemon peels was established (Matsubara et al. 1990). At the same time, some researchers have also investigated the role of induced cells apoptosis (Ogata et al. 2000), hepatoprotective capacity (Bhavsar et al. 2007), antimicrobial properties (Lopes Campêlo et al. 2011; Agarwal et al. 2012) and antinociceptive effects of C. limon fruit extract (Campêlo et al. 2011). Even these abovementioned works have concerned about lemon, however, these studies mainly focused on identifying individual phenolic compounds and evaluating one specific bioactivity. Now, a few studies have sought to determine the phenolic compound and antioxidant capacity of C. limon cultivar, and most these studies only referred to one cultivar or the bioactivity of one compound. As one of the main food material, only juice of lemon fruit was used in our daily life, other inedible parts such as peels, seeds and pulps matrix were wasted, these high amounts of wastes and by-products that constitute an important source of bioactive compounds with potential for animal feed, manufactured foods, and health care (González-Molina et al. 2010). However, the comprehensive information about lemon nutrition is still scarce. To the best of our knowledge, the phenolic distributed in different fruit parts of C. limon and their antioxidant capacities is so far unknown.
The present study aimed to determine the content and composition of phenolic compounds in five different fruit part including peels, seeds, juice and whole fruit from five lemon cultivars and to evaluate their antioxidant capacities. The results obtained will provide information for guide consumer and future utilization of C. limon germplasm.
Materials and methods
Chemicals
Gallic acid, chlorogenic acid, caffeic acid, ferulic acid, eriocitrin, naringin, hesperidin, naringenin, hesperetin, rutin, diosmin, eriodictyol, sinensetin, nobiletin and tangeretin were obtained from Sigma (St. Louis, MO, USA). 2,2-Diphenyl-1-picrylhydrazyl radicals (DPPH), 2,4,6-tris (2-pyridyl)-S-triazine (TPTZ), dimethyl sulfoxide (DMSO), acetic acid and acetonitrile were purchased from Fluka (St. Louis, MO, USA). All other reagents were of analytical grade and were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Fruit materials
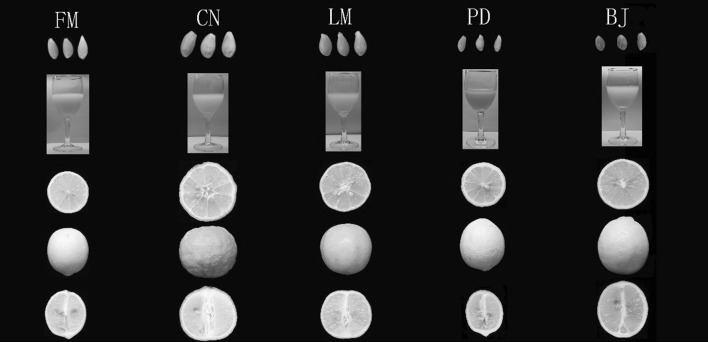
Five lemon (C. limon Burm.) cultivars were grown at the National Citrus Germplasm Repository, Citrus Research Institute of Chinese Academy of Agricultural Sciences, Chongqing, China (Table 1). Fruits were harvested at the commercial stage according to the appearance on base of color, size and shape, and were transported to the laboratory on the day of harvest (Fig. 1; Table 2). Fruits were divided into peel, pulp and seeds, and a part of pulp was squeezed into juice. Peel, pulp, whole fruit and seeds of each genotype were grounded into powder by using a freezer-mill (6750) apparatus (Glen Creston, Middlesex, UK). The powders and juice were stored at −80 °C until analysis.
Table 1.
Lemon cultivars used in the present study
| Repository number | Scientific name | Cultivars | Abbreviation | SSC (%) | TA (mmol/L H+) |
|---|---|---|---|---|---|
| LM0082 | Citrus limon (L.) Burm.f. | Feiminailao | FM | 9.07 | 22.19 |
| LM0027 | Citrus jambhiri Lush. | Cuningmeng | CN | 8.00 | 24.17 |
| LM0030 | Citrus limonia Osbeck | Limeng | LM | 9.50 | 21.73 |
| LM0044 | Citrus limon (L.) Burm.f. | Pangdelusaningmeng | PD | 9.10 | 23.63 |
| LM0126 | Citrus limon (L.) Burm.f. | Beijingningmeng | BJ | 9.20 | 28.93 |
Fig. 1.
Lemon cultivars used in the present study. The abbreviations represent cultivars in the Table 1
Table 2.
Total phenolic (μg/g FW) and total flavonoid (μg/g FW) contents of different fruit part of five lemon cultivars
| Cultivars | Peel | Pulp | Juice | |||
|---|---|---|---|---|---|---|
| Total phenolic | Total flavonoid | Total phenolic | Total flavonoid | Total phenolic | Total flavonoid | |
| FM | 3.99 ± 0.06 | 7.12 ± 0.29 | 2.70 ± 0.09 | 4.41 ± 0.12 | 0.52 ± 0.00 | 0.41 ± 0.00 |
| CN | 4.63 ± 0.11 | 7.58 ± 0.39 | 2.67 ± 0.07 | 4.24 ± 0.19 | 0.35 ± 0.01 | 0.26 ± 0.01 |
| LM | 3.73 ± 0.10 | 7.18 ± 0.26 | 2.43 ± 0.11 | 3.86 ± 0.03 | 0.29 ± 0.01 | 0.28 ± 0.01 |
| PD | 4.71 ± 0.06 | 8.30 ± 0.33 | 3.46 ± 0.07 | 5.38 ± 0.23 | 0.47 ± 0.01 | 0.44 ± 0.01 |
| BJ | 3.17 ± 0.06 | 5.12 ± 0.29 | 2.82 ± 0.10 | 4.16 ± 0.22 | 0.38 ± 0.01 | 0.33 ± 0.00 |
| Cultivars | Whole fruit | Seeds | ||
|---|---|---|---|---|
| Total phenolic | Total flavonoid | Total phenolic | Total flavonoid | |
| FM | 1.88 ± 0.10 | 9.27 ± 0.19 | 3.36 ± 0.04 | 22.16 ± 0.12 |
| CN | 3.04 ± 0.08 | 4.67 ± 0.17 | 3.11 ± 0.12 | 24.97 ± 0.12 |
| LM | 1.63 ± 0.04 | 3.16 ± 0.14 | 2.56 ± 0.04 | 25.33 ± 0.12 |
| PD | 2.87 ± 0.04 | 5.96 ± 0.37 | 2.28 ± 0.05 | 18.61 ± 0.09 |
| BJ | 2.32 ± 0.03 | 4.58 ± 0.24 | 2.12 ± 0.03 | 22.63 ± 0.17 |
Data are expressed as mean ± standard error of triplicate samples
Total phenolic and total flavonoid were expressed as gallic acid equivalents (GAE) and rutin equivalents (RE), respectively
Determination of total soluble solids and titratable acidity
Total soluble solids (TSS) and titratable acidity (TA) were determined using juice samples collected from eight fruit for one replicate, and triplicates were used. TSS values of each fruit were measured with a hand-held refractometer (Model: B32T Brix Meter, Guangzhou Ruiqi Trade Co. Ltd, Guangdong, China). 10 mL of juice was titrated with 0.2 M NaOH until reaching a pH of 8.2, and the values were expressed as mmol L−1 H+.
Extraction of phenolic compounds
Extraction of phenolic compounds was determined according to the previous method (Ramful et al. 2011). Briefly, methanol (80%, 12 mL) and dimethyl sulphoxide (1:1, v/v) were added to 1 g of fruit powder. After shaken for 12 h, the homogenized was centrifuged at 3000g for 10 min at 4 °C. The residue was washed twice with methanol (80%, 24 mL). The supernatants from three extractions were collected and diluted to 50 mL with methanol. The solutions were then stored at −20 °C for determination of phenolic compounds and antioxidant capacity.
Determination of total phenolic and total flavonoid content
Total phenolic content was determined by the Folin–Ciocalteu (FC) colorimetric method described previously (Xu et al. 2008). Briefly, the above extract (1 µL) was diluted with three milliliters, and then 0.5 mL of Folin–Ciocalteu reagent was added. After 3 min, 2 mL of 20% Na2CO3 was added and mixed thoroughly. After incubating for 60 min at room temperature, the absorbance value was read at 650 nm using a Microplate spectrophotometer (Benchmark Plus, Biorad, Hercules, CA, USA). Total phenolic content was expressed as mg/g FW of gallic acid equivalent (GAE). All tests were run in triplicate.
The total flavonoid content was determined according to the method described previously (Wang et al. 2008). 2.5 g of sample was added to a Soxhlet extractor extracted and refluxed with methanol for more than 12 h at 85 °C. The extract was dried in a rotary vacuum evaporator at less than 40 °C and then dissolved with methanol. 0.3 mL of 5% NaNO2 was added to a 1 mL extract in a volumetric flask, and the mixture was kept for 6 min at room temperature. 0.3 mL of 10% Al(NO3)3 was added to the mixture and incubated for 6 min again, then 4 mL of 1 N NaOH was added. After incubating for 15 min at room temperature, the absorbance was measured at 510 nm using the above spectrophotometer. Total flavonoid content was expressed as mg/g DW of rutin equivalents (RE). All tests were run in triplicate.
Determination of phenolic compounds
The phenolic compounds were determined as described by our previous report (Zhang et al. 2012). After filtration on Millipore membrane (0.22 µm), the filtrate (10 µL) was injected into the HPLC system. Chromatographic separation was carried on using a reverse phase column (ZORABX SB-C18, 250 mm × 4.6 mm internal diameter). The mobile phase was composed of (A) 0.1% formic acid (aqueous) and (B) methanol. Gradient elution was performed as follows: from 0 to 20 min, 37–50% B; from 20 to 35 min, 50–80% B; from 35 to 40 min, 80–100% B; from 40 to 50 min, 100% B; from 50 to 60 min, 37–50% B. The column temperature was maintained at 25 °C and the flow rate was 0.7 mL/min.
Eriocitrin, naringin, hesperidin, naringenin, hesperetin, rutin, diosmin, eriodictyol, and gallic acid were detected at wavelengths of 283 nm, sinensetin, nobiletin and tangeretin detected at 330 nm, chlorogenic acid, caffeic acid and ferulic acid detected at 320 nm (Fig. S1). All compounds were identified by comparing their retention time and the spectral characteristics of peaks with those of standards and quantified based on the peak area of the sample and the corresponding standard.
Antioxidant capacity assays
The antioxidant capacity assay was determined by DPPH, ABTS and FRAP methods. The DPPH was performed according to the method descripted previously (Barreca et al. 2011). For each sample (50 μL), 63 μM of DPPH was added, and the a final volume was adjusted to 4.0 mL with methanol. After 25 min, the absorbance was detected at wavelength of 517 nm. The inhibition percentage of radical scavenging capacity was the DPPH value.
ABTS values were measured according to the previous method (Barreca et al. 2011). 5 mL aqueous ABTS solution (7 mM) was added to 88 µL of 140 mM of a potassium per sulfate solution. The mixture was kept in dark at 29 °C for 14 h before being used. The decrease of absorbance was measured in 6 min at 734 nm.
The FRAP assay was conducted by the previous method described (Jang et al. 2010). 1.8 mL of FRAP reagent was added to 20 μL of fruit extract and mixed with 1.8 mL deionised water. After 30 min, the absorbance was detected at wavelength of 593 nm. Aqueous solutions of 0–5 mM ferrous sulphate heptahydrate were used for calibration and reducing power was expressed as mM. All absorbance values were determined by using the above spectrophotometer. All tests were run in triplicate. DPPH, FRAP and ABTS were expressed as μM rutin equivalents (TE)/g FW.
Statistical analysis
All data are expressed as the mean ± standard error of three replicates. Statistical analysis was performed using SPSS v19.0 software (SPSS Inc., Chicago, IL, USA). Significant differences among the samples were calculated using one-way ANOVA followed by Duncan’s multiple-range test at the 5% level (p ≤ 0.05).
Results and discussion
Titratable acidity and soluble solids content
The SSC and TA of the lemon juices are shown in Table 1. The SSC of mature fruits ranged 8.00–9.50%. CN was characterized by the highest SSC value followed by BJ and PD, and they had Brix greater than 9%. The lowest SSC value was found in CN, with a Brix of 8.00%. The TA of all fruits tested ranged 17.96–28.93 mmol/L H+. The juice of PD was the most acidic whilst the juice of BJ was the least acidic. TA values of FM, CN, BJ and PD were more than 22 mmol/L H+, LM was characterized by low TA (<20 mmol/L H+).
Total phenolic and total flavonoid contents
The total phenolic contents exhibited obvious variations among the different cultivars and fruit part tested, ranging from 3.17 to 4.63 mg/g GAE FW in peel, from 2.43 to 3.46 mg/g FW in pulp, from 0.29 to 0.52 mg/g FW in juice, from 1.63 mg/g FW in whole fruit, and from 2.12 to 3.36 mg/g FW in seeds (Table 2). Peels presented the highest total phenolic contents, followed by whole fruit and seeds, while juice presented the lowest. As for peel and pulp, PD had significantly higher total phenolic than other cultivars tested, while FM juice and seeds had higher total phenolic than other cultivars. For whole fruit, CN had significant higher total phenolic than other cultivars.
Similarly, the total flavonoid contents differed significantly (p < 0.05) among the fruit part and cultivars. The total flavonoid contents varied from 5.12 to 8.30 mg/g RE FW in peel, from 3.86 to 5.38 mg/g FW in pulp, from 0.26 to 0.44 mg/g FW in juice, from 3.16 to 9.27 mg/g FW in whole fruit, and from 18.61 to 25.33 mg/g FW in seeds (Table 2). Seeds showed the highest level of total flavonoid content, followed by peels and whole fruit, while juice presented the lowest. Total flavonoid content in seeds was 54–96 times than that in juice. As for tissues, PD peel, pulp and juice had higher total flavonoid than other cultivars tested. FM whole fruit had significantly total flavonoid than other cultivars tested, while LM seeds contained higher total flavonoid than other cultivars tested, and except for PD, seeds of other cultivars were over 20 mg/g FW.
Individual phenolic compound contents
A total of 14 phenolic compounds, including four phenolic acids, seven flavanones and three polymethoxylated flavones (PMFs), were identified from peel, pulp, juice, whole fruit and seed (Fig. S1; Table 3).
Table 3.
Phenolic composition and concentration (μg/g FW) in different fruit part of five lemon cultivars
| Cultivars | Phenolics acid | Flavanones | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid | Chlorogenic | Caffeic | Ferulic acid | Eriocitrin | Hesperidin | Rutin | Diosmin | Eriodictyol | Naringenin | Hesperetin | |
| Peel | |||||||||||
| FM | 90.69 ± 4.2 | 222.7 ± 17.04 | 538.8 ± 58.78 | nd | 7.73 ± 1.97 | 1465 ± 29.43 | 31.55 ± 1.55 | nd | 8.04 ± 2.54 | nd | 30.82 ± 2.15 |
| CN | 23.81 ± 1.8 | 219.6 ± 11.87 | 388.4 ± 73.42 | 1.19 ± 0.31 | 49.61 ± 18.82 | 3315 ± 262.3 | 4.92 ± 2.38 | 14.66 ± 3.45 | 3.87 ± 1.35 | 12.35 ± 1.93 | 58.11 ± 6.43 |
| LM | 9.61 ± 2.46 | 527.5 ± 170.3 | 367.7 ± 123.4 | 2.75 ± 0.47 | 40.77 ± 19.25 | 1563 ± 415.9 | 21.55 ± 7.39 | 6.91 ± 1.62 | 6.60 ± 2.46 | 3.52 ± 0.00 | 5.79 ± 2.85 |
| PD | 5.33 ± 0.94 | 474.2 ± 52.04 | 741.6 ± 79.22 | 2.28 ± 1.00 | 18.19 ± 3.57 | 2145 ± 177.0 | 60.59 ± 8.30 | 9.33 ± 4.96 | 79.27 ± 10.70 | 4.82 ± 1.00 | 13.69 ± 0.80 |
| BJ | 1.63 ± 0.29 | 138.7 ± 19.34 | 293.7 ± 33.78 | 1.17 ± 0.29 | 30.72 ± 14.5 | 2966 ± 354.8 | 13.76 ± 0.00 | 1.80 ± 0.36 | 1.57 ± 0.25 | nd | 88.12 ± 7.15 |
| Pulp | |||||||||||
| FM | nd | 38.37 ± 2.74 | 233.5 ± 13.74 | nd | nd | 622.8 ± 72.4 | 14.87 ± 4.47 | nd | nd | nd | nd |
| CN | 28.42 ± 4.2 | 9.28 ± 2.32 | 58.94 ± 12.99 | nd | nd | 769.4 ± 139.7 | nd | nd | nd | nd | nd |
| LM | 0.95 ± 0.00 | 29.47 ± 1.84 | 44.67 ± 1.52 | nd | nd | 525.3 ± 18.25 | nd | nd | nd | nd | nd |
| PD | 1.06 ± 0.09 | 83.92 ± 22.49 | 210.3 ± 48.28 | nd | nd | 734.1 ± 168.9 | 11.72 ± 5.48 | nd | nd | nd | nd |
| BJ | nd | 10.43 ± 1.29 | 64.75 ± 5.72 | nd | nd | 1419 ± 61.26 | 1.70 ± 0.65 | 2.49 ± 0.95 | nd | nd | nd |
| Juice | |||||||||||
| FM | 1.39 ± 0.08 | 17.06 ± 0.39 | 119.5 ± 2.85 | nd | nd | 157.4 ± 1.11 | 3.82 ± 0.53 | nd | nd | nd | 4.71 ± 0.01 |
| CN | 7.62 ± 0.36 | 2.70 ± 0.45 | 20.51 ± 1.35 | nd | nd | 131.1 ± 1.18 | nd | nd | nd | nd | 1.73 ± 0.14 |
| LM | 0.38 ± 0.12 | 9.40 ± 1.10 | 17.83 ± 1.95 | nd | nd | 105.5 ± 6.79 | nd | nd | nd | nd | 1.05 ± 0.00 |
| PD | 0.46 ± 0.02 | 22.08 ± 0.36 | 128.4 ± 3.39 | nd | nd | 143.2 ± 4.77 | 0.92 ± 0.27 | nd | 1.75 ± 0.11 | nd | 1.18 ± 0.05 |
| BJ | 4.59 ± 0.85 | 8.13 ± 0.13 | 34.16 ± 0.57 | nd | nd | 210.3 ± 7.89 | 1.98 ± 0.07 | 2.21 ± 0.10 | 0.28 ± 0.06 | nd | 0.83 ± 0.06 |
| Whole fruit | |||||||||||
| FM | 9.05 ± 1.72 | 174.6 ± 107.9 | 299.8 ± 26.53 | nd | 0.59 ± 0.00 | 971.55 ± 61.3 | 12.33 ± 0.43 | nd | 2.47 ± 0.86 | nd | 8.25 ± 0.76 |
| CN | 4.38 ± 1.16 | 72.66 ± 19.92 | 134.5 ± 10.84 | 0.61 ± 0.04 | 19.63 ± 9.10 | 2338 ± 169.33 | 2.29 ± 1.62 | nd | nd | nd | 10.10 ± 2.01 |
| LM | 1.64 ± 0.24 | 17167 ± 27.77 | 127.3 ± 6.44 | nd | nd | 889.8 ± 24.14 | 15.22 ± 0.70 | nd | nd | nd | 2.50 ± 0.15 |
| PD | 2.96 ± 1.12 | 184.2 ± 39.12 | 389.2 ± 5.00 | nd | 1.44 ± 0.78 | 9260 ± 136.4 | 30.57 ± 2.81 | 7.66 ± 0.15 | 26.50 ± 1.37 | nd | 1.45 ± 0.31 |
| BJ | 7.05 ± 1.29 | 75.03 ± 11.88 | 205.5 ± 27.24 | nd | 6.09 ± 1.64 | 2269 ± 244.5 | 3.22 ± 0.24 | 6.50 ± 4.55 | 1.00 ± 0.38 | nd | 24.49 ± 5.38 |
| Seeds | |||||||||||
| FM | 9.32 ± 3.52 | 52.51 ± 21.56 | 32.43 ± 23.79 | 0.67 ± 0.02 | 81.92 ± 24.46 | 22.57 ± 6.94 | nd | nd | nd | nd | nd |
| CN | 3.50 ± 2.19 | 48.52 ± 7.48 | 116.8 ± 2.60 | nd | 150.9 ± 33.43 | 29.96 ± 3.11 | nd | 23.38 ± 4.20 | 0.96 ± 0.44 | nd | nd |
| LM | 11.95 ± 2.9 | 10.11 ± 7.11 | 17.90 ± 2.71 | 0.63 ± 0.00 | 6.66 ± 2.55 | 49.86 ± 7.00 | 7.21 ± 5.00 | 5.16 ± 1.21 | nd | 1.73 ± 0.39 | nd |
| PD | 9.73 ± 1.26 | 124.4 ± 29.10 | 26.34 ± 1.73 | 0.73 ± 0.00 | nd | 22.64 ± 3.28 | 2.01 ± 0.45 | nd | nd | 1.32 ± 0.11 | nd |
| BJ | 8.21 ± 1.44 | 11.94 ± 3.91 | 9.31 ± 6.33 | nd | 8.30 ± 3.02 | 10.27 ± 7.16 | nd | 1.29 ± 0.00 | nd | nd | nd |
| Cultivars | Polymethoxylated flavones | ||
|---|---|---|---|
| Sinensetin | Nobiletin | Tangeretin | |
| Peel | |||
| FM | 19.27 ± 1.32 | 54.30 ± 2.98 | 1.23 ± 0.1 |
| CN | 7.55 ± 1.25 | 174.5 ± 20.33 | 62.08 ± 7.75 |
| LM | 5.45 ± 2.46 | 45.96 ± 13.11 | 12.37 ± 4.12 |
| PD | 12.91 ± 1.10 | 107.3 ± 10.13 | 1.29 ± 0.24 |
| BJ | 10.30 ± 0.90 | 7.45 ± 2.43 | 25.91 ± 7.58 |
| Pulp | |||
| FM | nd | 5.51 ± 0.00 | nd |
| CN | nd | nd | nd |
| LM | nd | nd | 2.04 ± 0.00 |
| PD | nd | nd | nd |
| BJ | nd | nd | nd |
| Juice | |||
| FM | nd | 17.13 ± 2.18 | nd |
| CN | nd | 3.19 ± 0.58 | 1.90 ± 0.07 |
| LM | nd | nd | nd |
| PD | nd | 1.12 ± 0.04 | nd |
| BJ | nd | nd | nd |
| Whole fruit | |||
| FM | 6.83 ± 0.49 | nd | nd |
| CN | 1.18 ± 0.31 | 72.44 ± 4.56 | 14.68 ± 6.42 |
| LM | nd | 12.18 ± 0.78 | 1.49 ± 0.25 |
| PD | 3.39 ± 0.28 | 59.77 ± 9.48 | nd |
| BJ | 2.62 ± 1.17 | 0.80 ± 0.21 | 4.68 ± 1.12 |
| Seeds | |||
| FM | nd | nd | nd |
| CN | nd | nd | nd |
| LM | nd | nd | nd |
| PD | nd | nd | nd |
| BJ | nd | nd | 4.49 ± 0.00 |
Data are expressed as mean ± standard error of triplicate samples
nd not detectable
Among phenolic acids identified from the tested cultivars, including gallic acid, chlorogenic acid, caffeic acid and ferulic acid, caffeic acid was the most dominant phenolic acid, followed by chlorogenic acid. The contents of caffeic acid varied from 293.7 to 741.6 μg/g FW in peels, 44.67–233.5 μg/g FW in pulps, 20.51–128.4 μg/g FW in juice, 127.3–299.8 in whole fruit and 9.31–32.43 μg/g FW in seeds, respectively. The caffeic acid in different parts was largely as: peels > whole fruit > pulps > juice > seeds. FM contained the highest level of caffeic acid, compared with other cultivars tested, CN seeds were rich in caffeic acid. Chlorogenic acid was the second rich phenolic acids in lemon tested, it varied from 138.7 to 527.5 μg/g FW in peels, 9.28–83.92 μg/g FW in pulps, 2.70–22.08 μg/g FW in juice, 72.66–184.2 in whole fruit and 10.11–124.4 μg/g FW in seeds, respectively. The chlorogenic acid in different fruit parts was largely as: peels > whole fruit > seeds > pulps > juice. LM peels and whole fruit contained the highest level of chlorogenic acid, PD pulps, juice and seed contained the highest level of chlorogenic acid. Gallic acid ranged from nd to 90.69 μg/g FW in all tested fruit parts. Compared with other fruit parts, peels contained higher gallic acid. FM peels presented the highest level of gallic acid, ferulic acid was only detected in peels, whole fruit and seeds of cultivars tested. Bocco et al. (1998) identified five phenolic acids from sweet orange and sour orange, and found that ferulic acid was the most abundant phenolic acid analyzed in orange. The order of concentration in orange is ferulic > sinapinic > p-coumaric > caffeic acids. Similar with orange, ferulic acid is also the most abundant phenolic acid in the peels and pulps of the wild mandarins tested (Zhang et al. 2014; Xi et al. 2014b). Gallic acid was the major phenolic acid in grapefruit different parts, followed by chlorogenic acid, caffeic acid and ferulic acid (Xi et al. 2015). Caffeic acid and chlorogenic acid were the dominant phenolic acid in lemon, followed by gallic acid and ferulic acid, lemon peel and whole fruit contained the richest ferulic acid.
Flavanones were the major flavonoid of the lemon tested, and hesperidin was the predominant flavanone, followed by hesperetin and eriocitrin. The hesperidin contents varied from 1563 to 3315 μg/g FW in peels, from 525.3 to 1419 μg/g FW in pulps, from 105.5 to 210.3 μg/g FW in juice, from 889.8 to 2269 μg/g FW in whole fruit, and from 10.27 to 49.86 μg/g FW in seeds, respectively. The hesperidin in different parts was largely as: peels > whole fruit > pulps > juice > seeds. BJ contained the highest level of hesperidin. Rutin contents varied from 4.92 to 60.59 μg/g FW in peels, from nd to 11.72 μg/g FW in pulp, from nd to 3.82 μg/g FW in juice, from 2.29 to 30.57 μg/g FW in whole fruit, and from nd to 7.21 μg/g FW in seeds, respectively. The rutin in different parts was largely as: peels > whole fruit > pulps > juice > seeds. PD peel and whole fruit contained the highest level of rutin. Hesperetin content varied from 5.79 to 88.12 μg/g FW in peels, from 0.83 to 4.71 μg/g FW in juice, and from 1.45 to 24.49 μg/g FW in whole fruit, respectively. Hesperetin was not detected in pulps and seeds. BJ peel and whole fruit presented significantly higher hesperetin than other cultivars (p < 0.05). The hesperetin in different parts was largely as: peels > whole fruit > juice. Eriocitrin content varied from 7.73 to 49.61 μg/g FW in peels, from nd to 19.63 μg/g FW in whole fruit, and from nd to 150.9 μg/g FW in seeds, respectively. The eriocitrin in different parts is largely as: peels > seed > whole fruit. No eriocitrin were detected in pulps and juice. CN seed presented the highest eriocitrin. Naringenin was only detected in peels and seeds, it ranged from nd to 12.35 μg/g FW, and CN contained the highest level of naringenin. Rich eriodictyol (79.27 μg/g FW) was detected in PD peels. Flavanones are the typical polyphenols of Citrus species. Our previous result showed that wild mandarin was characterized by highest level of hesperidin (Zhang et al. 2014), while sweet orange, tangelo, lemon and lime were characterized by rich hesperidin, didymin and narirutin, naringenin is rich in sour orange (Ooghe et al. 1994). Generally, pummelo and grapefruit have a distinct flavanone profile, naringin was the predominant flavanone in pummelo cultivars, whereas naringin and neohesperidin were the principal flavanones in grapefruit (Xi et al. 2014a, 2015). The present results showed that hesperidin was the predominant flavanone, followed by hesperetin and eriocitrin, which revealed lemon present unique flavonoids profile in Citrus species.
Among the three PMFs identified, nobiletin was the most abundant PMFs, they were almost detected in all lemon cultivars tested and fruit parts except for seeds. The content of nobiletin ranged from 7.45 to 107.3 μg/g FW in peels, from nd to 5.51 μg/g FW in pulp, from nd to 17.13 μg/g FW in juice, from nd to 72.44 μg/g FW in whole fruit, and no nobiletin was detected in seeds. The nobiletin in different parts was largely as: peels > whole fruit > juice > pulps. PD peel and whole fruit have the highest level of nobiletin. The tangeretin content varied from 1.23 to 62.08 μg/g FW in peels, from nd to 2.04 μg/g FW in pulp, from nd to 1.90 μg/g FW in juice, from nd to 14.68 μg/g FW in whole fruit, and nd to 4.49 μg/g FW in seeds, respectively. The peels contained the highest level of tangeretin, followed by whole fruit. PD presented the highest level of tangeretin. Sinensetion was only detected in peels and whole fruit tested, it ranged from 5.45 to 19.27 μg/g FW in peels and nd to 6.83 μg/g FW in whole fruit, FM was the richest in sinensetion. Previous study showed that tangeretin was the dominant PMFs in C. grandis cv. Foyou, while nobiletin and tangeretin were the dominant PMFs in C. paradise cv. Huyou (Sun et al. 2013), which was similar with pummelo and grapefruit (Xi et al. 2014a, 2015). Nobiletin was the most abundant PMFs in lemon, it ranged from nd to 107.3 μg/g FW, which was significantly higher than the contents reported in wild mandarin and grapefruit (Xi et al. 2015; Zhang et al. 2014), except for peels, lemon whole fruit, pulp and juice were also good source of sinensetin, nobiletin and tangeretin.
Antioxidant capacity
DPPH assay is routine method for assessment of free radical scavenging potential of an antioxidant molecule or extract from plant, and it is considered as one of the standard and easy colorimetric methods for the evaluation of antioxidant properties (Mishra et al. 2012). The DPPH values of the lemon varied from 1.08 to 8.20% in peel, from 4.00 to 7.29% in pulp, from 0.22 to 0.59% in juice, from 3.10 to 7.96% in whole fruit and from 0.50 to 4.01% in seed (Table 3). The DPPH values in different tissues was largely as follows: peels > pulps > whole fruit > seeds > juice. Except for seed, PD peel, pulp, whole fruit and juice had significantly higher DPPH values than those in other cultivars, FM seed had the highest DPPH value among cultivars tested.
ABTS is based on the capacity of a sample to scavenge the ABTS radical cation (ABTS·+) as compared to a standard antioxidant (Trolox), the method is also commonly used to study the antioxidant capacity of plants (Touriño et al. 2005). The ABTS values of the lemons tested varied from 8.65 to 14.40 mM in peel, from 0.94 to 3.85 mM in pulp, from 0.42 to 0.71 mM in juice, from 8.79 to 13.09 mM in juice vesicle and from 7.74 to 11.97 mM (Table 3). The highest ABTS value was found in CN peel, whereas the lowest ABTS value was found in CN juice. As a whole, peels, whole fruit and seeds of all cultivars tested presented significantly higher ABTS values than pulps and juice. CN peels and whole fruit had the higher ABTS values than other cultivars peels. PD pulp had the highest ABTS value, while FM juice and seed had the highest ABTS values.
The FRAP assay is a simple and reproducible method which can be applied to the study of the antioxidant capacity of antioxidants in food extracts (Pulido et al. 2000). The FRAP values of the lemons tested varied from 1.62 to 6.60 mM in peel, from 0.37 to 1.85 mM in pulp, from 0.07 to 0.71 mM in juice, from 1.15 to 3.65 mM in whole fruit and from 2.30 to 3.40 mM in seed (Table 4). FD peel had the highest FRAP values, while LM juice had the lowest FRAP value. For all tissues, peels of tested lemon had higher FRAP values than other tissues, followed by seeds and whole fruit, while juice had the lowest FRAP value. The peel, pulp and whole fruit of PD had significantly higher FRAP values than other cultivars tested, FM seed had higher FRAP values than other cultivars tested.
Table 4.
Antioxidant capacities of different fruit part of five lemon cultivars
| Cultivars | Peel | Pulp | Juice | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | |
| FM | 7.45 ± 0.24 | 8.65 ± 0.09 | 5.25 ± 0.00 | 5.11 ± 0.32 | 1.83 ± 0.17 | 0.71 ± 0.05 | 0.59 ± 0.02 | 0.71 ± 0.00 | 0.65 ± 0.03 |
| CN | 3.58 ± 0.65 | 14.40 ± 0.96 | 2.71 ± 0.12 | 4.00 ± 0.18 | 2.13 ± 0.58 | 0.45 ± 0.07 | 0.32 ± 0.01 | 0.42 ± 0.03 | 0.11 ± 0.01 |
| LM | 4.55 ± 0.13 | 11.12 ± 0.53 | 3.05 ± 0.07 | 4.22 ± 0.09 | 0.94 ± 0.10 | 0.37 ± 0.04 | 0.22 ± 0.01 | 0.44 ± 0.04 | 0.07 ± 0.01 |
| PD | 8.20 ± 0.33 | 12.04 ± 0.12 | 6.60 ± 0.16 | 7.29 ± 0.22 | 3.85 ± 0.14 | 1.85 ± 0.18 | 0.44 ± 0.01 | 0.63 ± 0.01 | 0.60 ± 0.01 |
| BJ | 1.08 ± 0.04 | 10.49 ± 0.44 | 1.62 ± 0.09 | 4.14 ± 0.16 | 1.91 ± 0.07 | 0.39 ± 0.04 | 0.45 ± 0.01 | 0.57 ± 0.03 | 0.29 ± 0.00 |
| Cultivars | Whole fruit | Seeds | ||||
|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | |
| FM | 3.66 ± 0.33 | 8.98 ± 0.90 | 1.15 ± 0.05 | 4.01 ± 0.97 | 11.97 ± 0.30 | 3.40 ± 0.07 |
| CN | 3.78 ± 0.27 | 13.09 ± 0.19 | 1.33 ± 0.08 | 2.30 ± 0.04 | 9.24 ± 0.19 | 2.80 ± 0.04 |
| LM | 3.10 ± 0.33 | 8.79 ± 0.27 | 1.08 ± 0.23 | 0.50 ± 0.05 | 9.47 ± 0.50 | 2.30 ± 0.11 |
| PD | 7.96 ± 0.27 | 12.43 ± 0.32 | 3.65 ± 0.24 | 2.35 ± 0.01 | 7.74 ± 0.19 | 2.48 ± 0.05 |
| BJ | 3.76 ± 0.44 | 11.40 ± 0.63 | 1.18 ± 0.10 | 1.47 ± 0.04 | 8.98 ± 0.55 | 2.34 ± 0.05 |
Data are expressed as mean ± standard error of triplicate samples
DPPH, FRAP and ABTS were expressed as μM rutin equivalents (TE)/g FW
Even DPPH, ABTS and FRAP assay measured the antioxidant activities of plant extract based on different mechanisms, the result for the same sample is almost consistent with each other. Lemon peels showed the highest antioxidant activity, followed by whole fruit, juice was the lowest, which is consistent with level of chlorogenic, eriocitrin and total flavonoid, similar with the ranking of the flavonoid content and antioxidant in peel, pulp and juice of 42 species and cultivars of Citrus genus observed by Nogata et al. (2006), showing that flavonoid may play important role in lemon antioxidant determination. In the present study, pulps presented higher DPPH activity than seeds, while had lower ABTS and FRAP activity than seeds, which may be due to the composition of individual phenolic, and proved that lemon seeds are good antioxidant source. Our previous study found that grapefruit seeds had lower antioxidant activity than flavedo, segment membrane, juice vesicle, albedo and seed, which is mainly associated with the level of naringin (Xi et al. 2015). Therefore, antioxidant activity of different fruit parts or citrus mainly depended on the composition and content level of individual phenolic, and even their complicated interaction. As for cultivars, PD and CN not only contained higher total phenolic and total flavonoid, but also exhibited higher antioxidant activities than other cultivars. At the same time, significantly higher phenolics, including chlorogenic, caffeic, hesperidin, and nobiletin, were detected in CN and PD than those in other cultivars tested, which was consistent with the antioxidant activities, indicating that these compounds may play important role in the total antioxidant activities.
Sun et al. (2013) found that physiological drop of Eureka lemon had FRAP and DPPH activity than those of Foyou and Huyou. However, lemon juice had the lowest FRAP activity than other 14 citrus cultivars (Xu et al. 2008), which is consistent with the present study. Agarwal et al. (2012) found that thought Emblica officinalis were found to have the maximum activities determined by phosphomolybdenum assay, but lemon peel also show good antioxidant activity (Agarwal et al. 2012). Misharina and Samusenko (2008) found that mixtures essential oils from lemon, pink grapefruit, coriander, and clove buds strongly inhibited oxidation of hexanal, the stability of components of lemon and coriander essential oils in mixtures increased compared to individual essential oils. Higher DPPH-scavenging activity was found in essential oils of lemon than peppermint (Yang et al. 2010). Based on these, lemon or its different fruit parts have irreplaceable antioxidant activity, and should be utilization comprehensively.
Conclusion
Caffeic acid and chlorogenic acid were the most dominant phenolic acid in lemon tested, varying 9.31–741.6 and 2.70–527.5 μg/g FW, FM and LM peels with the highest contents, respectively. FM peels presented the highest level of gallic acid, ferulic acid was only detected in peels. Hesperidin was the predominant flavanone, varying 10.27–3315 μg/g FW, CN contained the highest level of hesperidin. PD peel and whole fruit contained rich rutin. BJ peel and whole fruit presented significant higher hesperetin than other cultivars. CN seed presented rich eriocitrin. Naringenin was only detected in peels and seeds. Nobiletin was the most abundant PMFs, they were almost detected in all lemon cultivars and fruit parts tested except for seeds, PD peel and whole fruit have the highest level of nobiletin. FM had the richest level of sinensetin. Taken together, PD and CN not only had significantly higher polyphenol content, but also exhibited higher antioxidant capacities than other cultivars tested. The order of polyphenols and antioxidant capacities for different fruit part was largely as: peels > whole fruit > pulp > seed > juice, and seeds are good source of flavonoids. Our findings provide useful information for consumer and utilization of lemon.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Natural Science Foundation of Chongqing City (cstc2013jcyjA80012), China Agriculture Research System (No. CARS-27), and National Risk Assessment Program for Agricultural Products Quality and Safety (GJFP2016004, GJFP2016015).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Agarwal M, Kumar A, Gupta R, Upadhyaya S. Extraction of polyphenol, flavonoid from Emblica officinalis, Citrus limon, Cucumis sativus and evaluation of their antioxidant activity. Orient J Chem. 2012;28:993–998. doi: 10.13005/ojc/280248. [DOI] [Google Scholar]
- Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G. Distribution of C- and O-glycosyl flavonoids, (3-hydroxy-3-methylglutaryl) glycosyl flavanones and furocoumarins in Citrus aurantium L. juice. Food Chem. 2011;124:576–582. doi: 10.1016/j.foodchem.2010.06.076. [DOI] [Google Scholar]
- Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- Bhavsar SK, Joshi P, Shah MB, Santani D. Investigation into hepatoprotective activity of Citrus limon. Pharm Biol. 2007;45:303–311. doi: 10.1080/13880200701214995. [DOI] [Google Scholar]
- Bocco A, Cuvelier ME, Richard H, Berset C. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J Agric Food Chem. 1998;46:2123–2129. doi: 10.1021/jf9709562. [DOI] [Google Scholar]
- Campêlo LML, de Almeida AAC, de Freitas RLM, Cerqueira GS, de Sousa GF, Saldanha GB, Feitosa CM, de Freitas RM. Antioxidant and antinociceptive effects of Citrus limon essential oil in mice. Biomed Res Int. 2011 doi: 10.1155/2011/678673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Río JA, Fuster MD, Gómez P, Porras I, García-Lidón A, Ortuño A. Citrus limon—a source of flavonoids of pharmaceutical interest. Food Chem. 2004;84:457–461. doi: 10.1016/S0308-8146(03)00272-3. [DOI] [Google Scholar]
- González-Molina E, Domínguez-Perles R, Moreno D, García-Viguera C. Natural bioactive compounds of Citrus limon for food and health. J Pharm Biomed. 2010;51:327–345. doi: 10.1016/j.jpba.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Jang HD, Chang KS, Chang TC, Hsu CL. Antioxidant potentials of buntan pumelo (Citrus grandis Osbeck) and its ethanolic and acetified fermentation products. Food Chem. 2010;118:554–558. doi: 10.1016/j.foodchem.2009.05.020. [DOI] [Google Scholar]
- Lopes Campêlo LM, Moura Gonçalves FC, Feitosa CM, de Freitas RM. Antioxidant activity of Citrus limon essential oil in mouse hippocampus. Pharm Biol. 2011;49:709–715. doi: 10.3109/13880209.2010.541924. [DOI] [PubMed] [Google Scholar]
- Matsubara Y, Sawabe A, Iizuka Y. Structures of new limonoid glycosides in lemon (Citrus limon Burm. f.) peelings. Agric Biol Chem. 1990;54:1143–1148. [Google Scholar]
- Miran W, Nawaz M, Jang J, Lee DS. Sustainable electricity generation by biodegradation of low-cost lemon peel biomass in a dual chamber microbial fuel cell. Int Biodeterior Biodegrad. 2016;106:75–79. doi: 10.1016/j.ibiod.2015.10.009. [DOI] [Google Scholar]
- Misharina TA, Samusenko AL. Antioxidant properties of essential oils from lemon, grapefruit, coriander, clove, and their mixtures. Appl Biochem Microbiol. 2008;44:438–442. doi: 10.1134/S0003683808040182. [DOI] [PubMed] [Google Scholar]
- Mishra K, Ojha H, Chaudhury NK. Estimation of antiradical properties of antioxidants using DPPH assay: a critical review and results. Food Chem. 2012;130:1036–1043. doi: 10.1016/j.foodchem.2011.07.127. [DOI] [Google Scholar]
- Miyake Y, Yamamoto K, Morimitsu Y, Osawa T. Isolation of C-glucosylflavone from lemon peel and antioxidative activity of flavonoid compounds in lemon fruit. J Agric Food Chem. 1997;45:4619–4623. doi: 10.1021/jf970498x. [DOI] [Google Scholar]
- Miyake Y, Murakami A, Sugiyama Y, Isobe M, Koshimizu K, Ohigashi H. Identification of coumarins from lemon fruit (Citrus limon) as inhibitors of in vitro tumor promotion and superoxide and nitric oxide generation. J Agric Food Chem. 1999;47:3151–3157. doi: 10.1021/jf980999y. [DOI] [PubMed] [Google Scholar]
- Ngugi CC, Oyoo-Okoth E, Muchiri M. Effects of dietary levels of essential oil (EO) extract from bitter lemon (Citrus limon) fruit peels on growth, biochemical, haemato-immunological parameters and disease resistance in Juvenile Labeo victorianus fingerlings challenged with Aeromonas hydrophila. Aquac Res. 2016;47:1–13. doi: 10.1111/are.12471. [DOI] [Google Scholar]
- Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. Flavonoid composition of fruit tissues of citrus species. Biosci Biotechnol Biochem. 2006;70:178–192. doi: 10.1271/bbb.70.178. [DOI] [PubMed] [Google Scholar]
- Ogata S, Miyake Y, Yamamoto K, Okumura K, Taguchi H. Apoptosis induced by the flavonoid from lemon fruit (Citrus limon Burm. f.) and its metabolites in HL-60 cells. Biosci Biotech Biochem. 2000;64:1075–1078. doi: 10.1271/bbb.64.1075. [DOI] [PubMed] [Google Scholar]
- Ooghe WC, Ooghe SJ, ClM Detavernier, Huyghebaert A. Characterization of orange juice (Citrus sinensis) by flavanone glycosides. J Agric Food Chem. 1994;42:2183–2190. doi: 10.1021/jf00046a020. [DOI] [Google Scholar]
- Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Ramful D, Tarnus E, Aruoma OI, Bourdon E, Bahorun T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res Int. 2011;44:2088–2099. doi: 10.1016/j.foodres.2011.03.056. [DOI] [Google Scholar]
- Sun Y, Qiao L, Shen Y, Jiang P, Chen J, Ye X. Phytochemical profile and antioxidant activity of physiological drop of citrus fruits. J Food Sci. 2013;78:C37–C42. doi: 10.1111/j.1750-3841.2012.03002.x. [DOI] [PubMed] [Google Scholar]
- Touriño S, Selga A, Jiménez A, Juliá L, Lozano C, Lizárraga D, Cascante M, Torres JL. Procyanidin fractions from pine (Pinus pinaster) bark: radical scavenging power in solution, antioxidant activity in emulsion, and antiproliferative effect in melanoma cells. J Agric Food Chem. 2005;53:4728–4735. doi: 10.1021/jf050262q. [DOI] [PubMed] [Google Scholar]
- Wang YC, Chuang YC, Hsu HW. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008;106:277–284. doi: 10.1016/j.foodchem.2007.05.086. [DOI] [Google Scholar]
- Xi W, Fang B, Zhao Q, Jiao B, Zhou Z. Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chem. 2014;161:230–238. doi: 10.1016/j.foodchem.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Xi W, Zhang Y, Sun Y, Shen Y, Ye X, Zhou Z. Phenolic composition of Chinese wild mandarin (Citrus reticulata Balnco.) pulps and their antioxidant properties. Ind Crop Prod. 2014;52:466–474. doi: 10.1016/j.indcrop.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Xi W, Zhang G, Jiang D, Zhou Z. Phenolic compositions and antioxidant activities of grapefruit (Citrus paradisi Macfadyen) varieties cultivated in China. Int J Food Sci Nutr. 2015;66:858–866. doi: 10.3109/09637486.2015.1095864. [DOI] [PubMed] [Google Scholar]
- Xu G, Liu D, Chen J, Ye X, Ma Y, Shi J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008;106:545–551. doi: 10.1016/j.foodchem.2007.06.046. [DOI] [Google Scholar]
- Yang SA, Jeon SK, Lee EJ, Shim CH, Lee IS. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat Prod Res. 2010;24:140–151. doi: 10.1080/14786410802496598. [DOI] [PubMed] [Google Scholar]
- Zhang YM, Zhou ZQ, Sun YJ, Shen Y, Zhong LZ, Qiao LP, YX Q. Simultaneous determination of 18 flavonoids in citrus fruits by high-performance liquid chromatography (in Chinese) Sci Agric Sin. 2012;45:3558–3565. [Google Scholar]
- Zhang Y, Sun Y, Xi W, Shen Y, Qiao L, Zhong L, Ye X, Zhou Z. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 2014;145:674–680. doi: 10.1016/j.foodchem.2013.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.