Abstract
The effect of kefiran on cold-set gelation of whey protein isolate (WPI) at 25 °C was studied using rheological measurements and environmental scanning electron microscopy (ESEM). The gelation of samples was induced by the addition of glucono-δ-lactone to the dispersions. WPI concentration was maintained at 8% (w/v) and the concentration of kefiran varied from 0 to 0.08% (w/v). According to rheological measurements, the addition of kefiran into WPI dispersions resulted in a significant increase in the gel strength, the yield stress, and the shear stress values at the flowing point. The gelling point and gelation pH of samples decreased significantly with an increase in kefiran concentration. ESEM micrographs showed that the presence of kefiran played an important role in the microstructure formation of gels. The microstructure of kefiran-WPI mixed gels was more compact and dense, compared to the WPI gel. Depletion interactions between kefiran and whey protein aggregates can be regarded as the chief factor which was responsible for these effects. The present work demonstrated that rheological and microstructural properties of acid-induced whey protein gels were improved by the addition of kefiran.
Keywords: Whey protein, Kefiran, Gel, Rheology, Microscopy
Introduction
Polysaccharides and proteins are naturally present as ingredients in many food systems. These macromolecules are widely used in the food industry because of their unique characteristics of improving texture, stability, and nutritional properties of food products. The rheological and structural properties of protein–polysaccharide mixtures depend on the macromolecular interactions between these biopolymers. The interactions between proteins and polysaccharides can be either attractive or repulsive depending on the concentration and molecular attributes (such as size and charge density) of these macromolecules and the medium conditions (Le et al. 2016; Spotti et al. 2017).
Whey proteins are widely used in food products as nutritional and functional ingredients due to emulsification, thickening, gelation, and foaming properties (Patel 2015). One of the most important functional properties of whey proteins is their ability to form gels (Fox et al. 2017). Cold-set gelation is an alternative to heat-induced gelation. The benefits of using cold gelation are gelation at lower protein concentration and low temperature. Thus, it is possible to use thermolabile ingredients in the gel matrix (Wijaya et al. 2017). Cold-set gelation is a two-step process: (i) heating a whey protein solution above the thermal denaturation temperature, at neutral pH, low ionic strength, and a protein concentration lower than the minimum required for thermal gelation; (ii) induction of gelation by salt addition (Mleko et al. 2002) or by decreasing the pH (Wijaya et al. 2017). Ingredients that are often used to modify the structural properties of protein systems are polysaccharides. Polysaccharides are usually added into whey protein solution before the second step to modify gelation behaviour of whey protein (Zhang and Vardhanabhuti 2014).
Kefiran is a novel microbial exopolysaccharide (EPS) extracted from kefir grains. It is a water-soluble non-adsorbing heteropolysaccharide containing galactose and glucose (Ghasemlou et al. 2012). Kefiran is GRAS (generally recognized as safe) and found to improve viscosity and viscoelastic properties of acid milk gels (Dimitreli et al. 2016; Rimada and Abraham 2006). It can form gels alone at low temperature (Zavala et al. 2015) and edible films with good mechanical and barrier properties (Ghasemlou et al. 2011; Zolfi et al. 2014). Also, in comparison with other polysaccharides such as galactomanans, kefiran has some important benefits such as antibacterial, antifungal, and antitumor properties (Güzel-Seydim et al. 2016).
The effects of different polysaccharides on the properties of cold-set whey protein gels has been studied (De Jong et al. 2009; Huan et al. 2016; Wijaya et al. 2017). But there is no study related to whey protein/kefiran gels. Thus, the objective of this study was to evaluate the effect of kefiran on cold-set whey protein gels formed by adding glucono-δ-lactone (GDL). To achieve this aim, both small-amplitude oscillatory rheology and environmental scanning electron microscopy (ESEM) were used.
Materials and methods
Materials
Whey protein isolate (WPI) was provided by Self Ominutrition (Sweden). The main ingredients of WPI were 84% (w/w) protein, 3% (w/w) fat, and 4% (w/w) lactose. GDL was purchased from VWR International (Chemicals and Laboratory Scientific Supplies, England). Sodium azide was supplied from Merck (Darmstadt, Germany). The kefir grains were kept in skimmed milk at room temperature and the medium was replaced daily for new culture to maintain the grains’ viability. The grains were considered active after seven subsequent days. Deionized water was used for all tests.
Isolation and purification of kefiran
Kefiran was extracted from kefiran grains by the method of Zolfi et al. (2014). In brief, a weighed amount of kefir grains was added to hot water (1:10, w/w) and stirred strongly for 1 h. The mixture was centrifuged (Sigma 3–16 k Frankfurt, Germany) at 10,000g for 15 min at 20 °C. The resulting mixture was blended with an equal level of chilled ethanol to precipitate the kefiran and maintained at −20 °C for 24 h. The pellets were collected through centrifugation at 10,000g for 20 min at 4 °C. The precipitates were re-suspended in hot distilled water and the precipitation process was then repeated twice. The resulting solution was concentrated, yielding a crude polysaccharide. The samples were tested for the absence of other proteins and sugars using high-performance liquid chromatography (HPLC) and the phenol–sulfuric acid method (Dubois et al. 1956).
Preparation of solutions
Protein stock solutions were prepared (9%, w/v) by dissolution of the WPI powder in deionized distilled water (pH 6.8) with magnetic stirring for 2 h at room temperature (25 °C). Then, the solution was placed in a temperature controlled water bath and heat treated at 68 °C for 2 h. The thermally denatured protein solution was then rapidly cooled to room temperature with tap water. Then, it was maintained overnight at 4 °C to promote full hydration.
Kefiran aqueous solutions were prepared (0.2, 0.4, and 0.8%, w/v) in deionized distilled water using constant stirring for 30 min at room temperature. All the solutions were maintained overnight at 4 °C to promote complete polysaccharide hydration prior to mixing with the protein solution to form bi-polymeric gels.
Preparation of the mixed solutions and gels
The mixed solutions were prepared by blending protein and kefiran solutions until they were completely homogeneous (stirring at 25 °C) to get samples with a fixed 8% (w/v) WPI content and varied kefiran content (%, w/v): 0 (no kefiran addition), 0.02, 0.04, and 0.08. Gelation of the mixed systems was induced by the addition of 0.8% (w/v) glucono-δ-lactone to the dispersions.
Monitoring of pH
The decrease in the patterns of pH throughout acidification of samples with or without kefiran was monitored by a pH meter (Metrohm, 827 pH Lab, Switzerland) equipped with an electrode. The measurements were done following a two-point calibration at the incubation temperature (25 °C).
Dynamic rheological measurements
Both the formation of the gels and the measurement of rheological properties were carried out using Physica MCR 301 rheometer (Anton Paar GmbH, Graz, Austria). Low-amplitude dynamic oscillation with a concentric cylinder measurement system (CC27) was chosen. The temperature was set to 25 °C by a peltier system equipped with fluid circulator with a precision of 0.01. Following the addition of GDL, 20 mL of solution (25 °C) was transferred to the rheometer cup (25 °C, controlled by Peltier). A solvent trap was used to prevent evaporating the samples. Rheological data were collected using Rheoplus software version 3.21 (Anton-Paar). Time-sweep measurements were performed throughout the gelation process at an angular frequency of 6.28 rad/s and with an applied strain of 1% that was within the linear viscoelastic range (LVE). Measurements were taken every 15 s for 65 min. Strain sweep tests were performed at strain range (γ) of 0.1–100% at constant angular frequency of 6.28 rad/s to measure the critical strain (γc), the yield stress (τy); the modulus at flow point (Gf); and the flow point (τf). Frequency sweep tests were carried out at the angular frequency (ω) range of 0.628–62.8 rad/s. The strain of the frequency sweep tests was chosen to be 1% (LVE range) to avoid network destruction.
Environmental scanning electron microscopy
The microstructure of gels was observed by environmental scanning electron microscopy (ESEM, XL 30, Philips, USA). Gel samples (5 mm cubes) were mounted on a double-sided tape attached to the specimen stubs. The samples were observed by the gaseous secondary electron detector (GSED) at acceleration voltage of 20 kV. The samples were analysed at a magnification of 1000×. The brightness and contrast were controlled during acquiring of pictures. Thus, the values of these parameters were maintained steady for every magnification level throughout the procedure of image attainment.
Statistical analysis
The analysis on samples was carried out in triplicate. An analysis of variance (ANOVA) was conducted using SPSS software (SPSS, 16). The comparison of treatment means was performed with the Duncan test at P < 0.05.
Results and discussion
Rheological properties
Time sweep test
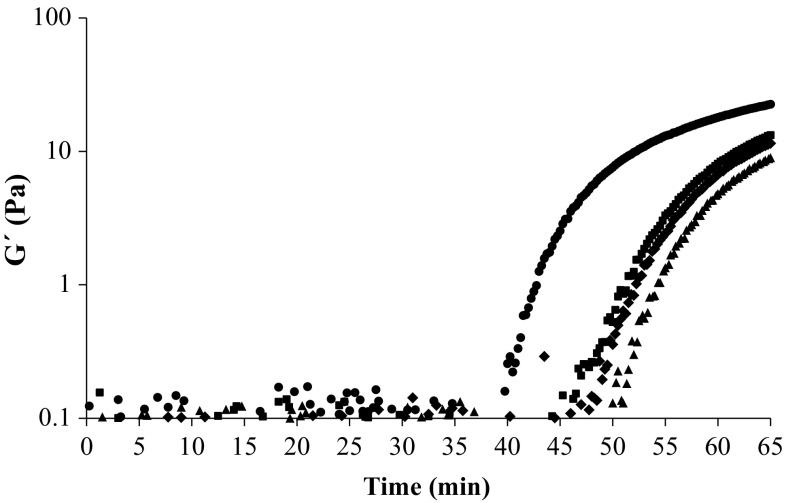
The effects of the addition of kefiran on the gelation process of whey proteins, as investigated with time sweep tests, are shown in Fig. 1. The loss modulus (G′′) was not detected. Therefore, only the storage modulus is presented here. In the first stage of the acidification a primary ‘lag period’ in the development of G′ was observed. This was related to the attractive interactions among the protein aggregates that were not efficient enough to overcome the repulsive interactions among them. As a result, it prevented the formation of the three-dimensional network (O’Kennedy et al. 2006). When the temperature was maintained at 25 °C, the ring structure of GDL started to linearize. It caused the pH to decrease because of the increase in acidity. By decreasing the pH of samples until about isoelectric point of whey proteins (pH ~5.1), the repulsive interactions among protein aggregates also decrease. So, these aggregates interact with one another. Thus, an abrupt raise in the value of G′ is observed. The increase in G′ is a sign that the gel is being formed. The time when G′ exceeds 1 Pa is considered as the gelling point (Girard and Schaffer-Lequart 2007; Mende et al. 2013).
Fig. 1.
Storage modulus G′ as a function of time for whey protein gels (8%, w/v) including different concentrations of kefiran (%, w/v): 0 (filled triangle), 0.02 (filled diamond), 0.04 (filled square) and 0.08 (filled circle) as determined at angular frequency, 6.28 rad/s; strain,1%; temperature, 25 °C
The key gelation parameters in time sweep tests are presented in Table 1. Comparing different samples shows that increasing kefiran causes the elastic modulus of gels to increase. Upon addition of kefiran the gel strength becomes higher as G′65 increases significantly from 9 to 23 Pa. These effects have also been reported by Rimada and Abraham (2006), who found that the addition of kefiran up to 500 mg/L to heat treated milk mixtures, led to an increase in the elastic modulus of final gels. The addition of kefiran up to 0.04% (w/v) to dispersions resulted in a little change in the gelation time (tg) and the gelation pH (pHg). However, addition of 0.08% kefiran to the system resulted in a significant reduction in the gelation time. Correspondingly, the gelation pH for this sample was significantly higher than other samples (Table 1). These results confirm the role of kefiran in improving the rheological properties of WPI gel. In WPI/kefiran mixed system, since kefiran is a non-gelling polysaccharide under the conditions applied, an increase in the gel strength is because of changes in the gelation behaviour of whey proteins. Since kefiran is uncharged (Ghasemlou et al. 2011) it behaves as a non-adsorbing polysaccharide and induces attractive interactions among protein aggregates. Addition of greater amounts of kefiran to the system results in earlier aggregation since more polysaccharides occupy more space and the protein aggregates are forced to interact with one another by a process like depletion flocculation at shorter time and higher pH (Everett and McLeod 2005). The strong protein–protein interactions induced by these depletion interactions are also responsible for the higher stiffness of gels containing kefiran (Tobin et al. 2012). The effect of galactomannans and konjac (glucomanan) on WPI heat-set gels has been investigated by some authors (Tavares and Da Silva 2003; Tobin et al. 2012). In agreement with the present work on kefiran they found that adding these polysaccharides to whey protein solution causes the gel strength of heat-set gels to increase.
Table 1.
Gelation parameters of WPI gels (8%, w/v) including different concentrations
| Parameters | Kefiran concentration (%, w/v) | |||
|---|---|---|---|---|
| 0 | 0.02 | 0.04 | 0.08 | |
| G′65 (Pa) | 9.00 ± 0.45a | 11.80 ± 0.50b | 13.00 ± 0.75b | 23.00 ± 0.40c |
| tg (min) | 54.0 ± 0.2a | 52.0 ± 0.1b | 52.0 ± 0.1b | 43.0 ± 0.1c |
| pHg | 5.11 ± 0.02a | 5.14 ± 0.02a | 5.14 ± 0.02a | 5.20 ± 0.02b |
Values represent the mean ± standard deviation of three independent experiments
Means within the same row with different superscript letters are significantly different (P < 0.05)
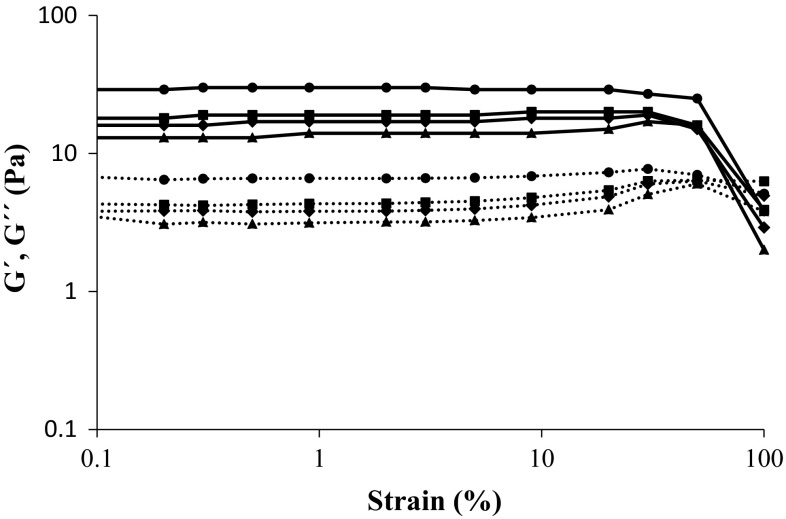
Strain sweep test
Strain sweep tests were performed on the final gels to find out the limit of the linear viscoelastic domains and the other viscoelastic properties of gels. As shown in Fig. 2, two distinguished regions for all gels are observed: a linear viscoelastic region in which G′ and G′′ are almost constant and deformation in the gel structure is reversible (small deformation); and a nonlinear region in which G′ and G′′ are variable (large deformations). The structural character of the sample in the LVE region is determined by comparing the values of G′ and G′′; if G′ > G′′, there is a gel character (solid character), but if G′′ > G′ then a liquid character exist (Nejatian et al. 2013). For the WPI-only gel and gels containing kefiran G′ was greater than G′′ in the whole range of applied strain in the LVE region and all samples showed tan δ values of less than 1. The rheological parameters obtained from the strain sweep tests are summarized in Table 2.
Fig. 2.
Strain sweep at 6.28 rad/s angular frequency modulus G ′(filled line) and G′′ (dashed line) for whey protein gels including different concentrations of kefiran (%, w/v): 0 (filled triangle), 0.02 (filled diamond), 0.04 (filled square) and 0.08 (filled circle) as determined at 25 °C
Table 2.
Variation of γc, τy, τf and Gf of WPI gels (8%, w/v) including different concentrations of kefiran as determined by strain sweep tests
| Parameters | Kefiran concentration (%, w/v) | |||
|---|---|---|---|---|
| 0 | 0.02 | 0.04 | 0.08 | |
| γc (%) | 50.43 ± 1.13a | 50.45 ± 1.11a | 50.28 ± 1.23a | 50.49 ± 1.12a |
| τy (Pa) | 7.11 ± 0.25a | 8.22 ± 0.20b | 8.41 ± 0.21b | 9.91 ± 0.11c |
| τf (Pa) | 4.18 ± 0.11a | 5.59 ± 0.33b | 6.12 ± 0.25b | 7.21 ± 0.12c |
| Gf (Pa) | 5.05 ± 0.13a | 5.99 ± 0.24b | 5.58 ± 0.18b | 7.72 ± 0.10c |
Values represent the mean ± standard deviation of three independent experiments
Means within the same row with different superscript letters are significantly different (P < 0.05)
In general, all parameter values increased upon addition of kefiran. By increasing the strain, stress increased to a certain point where it started to decrease. This point was defined as the yield stress (τy) and indicated the resistance of a gel to the mechanical forces. Yield stress is also a measure of the structural strength of a gel and is considered as an important parameter in many technical applications and food processing (Hatami et al. 2014). For mixed gels, the yield stress is higher significantly (P < 0.05) than the WPI-only gel (Table 2). It indicated that the addition of kefiran significantly (P < 0.05) increased the mechanical resistance of gels. A mixed gel containing 0.08% (w/v) kefiran showed a yield stress value ~1.4 times higher than those without kefiran.
The increased τf value of WPI gels in the presence of kefiran indicates that samples with greater amounts of kefiran need larger mechanical forces to exhibit a visible flow (Table 2). Increased Gf (a measure of structural strength or shape maintenance ability) is accompanied by increased τf (a measure of resistance to mechanical force) (Table 2). These results indicated the role of kefiran on the depletion interactions which led the protein aggregates to become closer to one another and the gel to become stronger.
In a strain sweep test G′ remains constant until the strain reaches a critical point at which G′ begins to decrease sharply (Fig. 2) and the gel structure begins to break. This point was defined as the critical strain (γc). Thus, critical strain (γc) indicated the deformability of a gel (Hesarinejad et al. 2014). Strong gels were reported to stay within the LVE region up to much greater strain values than weak gels (Tan et al. 2015). No significant differences (P < 0.05) in γc values of gels with and without kefiran were observed (Table 2). Therefore, the addition of kefiran to the system did not cause any significant change in the deformability of gels (P < 0.05).
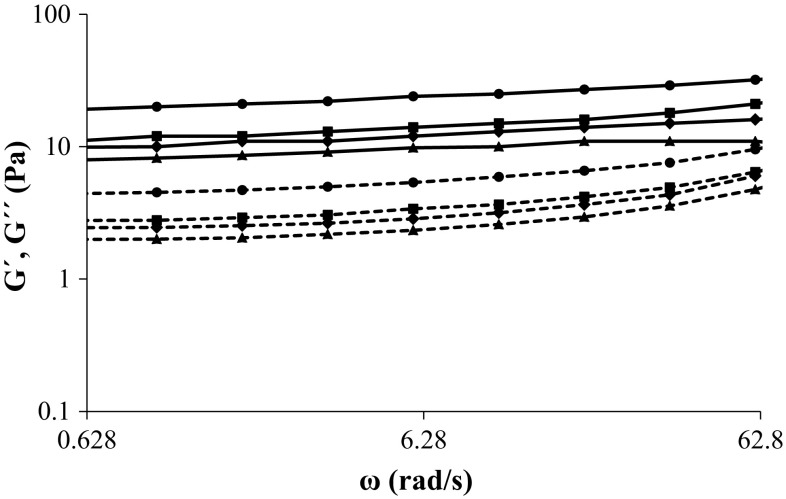
Frequency sweep test
Dynamic rheological tests give information about the type of gel structure. Thus, the frequency sweep tests were carried out from 0.628 to 62.8 rad/s to provide a better understanding of the effect of kefiran on the gel network. The frequency dependence of G′ and G′′ for all gels is illustrated in Fig. 3. The mechanical spectra of all gels showed that all samples are elastic gels with G′ > G′′. The graph clearly shows that G′ and G′′ values for the WPI gel were lower than the mixed gels.
Fig. 3.
The effect of angular frequency on modulus G′ (filled line) and G′′ (dashed line) of whey protein gels including different concentrations of kefiran (%, w/v): 0 (filled triangle), 0.02 (filled diamond), 0.04 (filled square) and 0.08 (filled circle) as determined at 25 °C
To determine the degree of the frequency dependence of the storage modulus, the power-law model (G′ = a.ωb) was fitted to the experimental data obtained from frequency sweep test to determine structure strength (a) and type of structure (b) as used earlier (Hatami et al. 2014; Nejatian et al. 2013). The power-law parameters are presented in Table 3. Here, the coefficient (a) is a measure of the strength of interactions between rheological units. The exponent (b), which is the slope of logarithmic plot of G′ versus ω, indicates the degree of interactions or the amount of rheological units associated with one another in the three-dimensional structure (Hatami et al. 2012). Therefore, a value and b value are correlated to the strength and the nature of the gel, respectively. b ≈ 1 for a viscous gel, low b values are characteristic of elastic gels, and b = 0 for covalent gels (Nejatian et al. 2013). A significant difference in the calculated b values was observed between the gel samples. The values of b are found to increase with kefiran content. This indicates the importance of the non-covalent interactions at higher polysaccharide concentration (Rocha et al. 2009). These results were similar to gels prepared from flaxseed gum-WPI (Kuhn et al. 2011) and mixed whey protein hydrolysates with locust bean gum (Rocha et al. 2009). WPI-only gel had the lowest b value and finally the least sensitivity to frequency variation (Table 3). According to the b values of the samples, all gels were elastic, or physical gels. For mixed gels, adding kefiran increased the a value of the system, which indicates that the incorporation of kefiran to the mixture causes a stronger elastic structure than the WPI-only gel. The a value of sample with 0.08% (w/v) kefiran was about 2.5 times more than the a value of sample without kefiran.
Table 3.
Power-law parameters for the elastic modulus for WPI gels (8%, w/v) including different concentrations of kefiran as determined by frequency sweep test
| Parameters | Kefiran concentration (%, w/v) | |||
|---|---|---|---|---|
| 0 | 0.02 | 0.04 | 0.08 | |
| a (Pa.s) | 9.759 ± 0.29a | 12.463 ± 1.11b | 14.544 ± 1.21b | 24.047 ± 1.10c |
| b | 0.090 ± 0.003a | 0.108 ± 0.003b | 0.115 ± 0.004b | 0.126 ± 0.002c |
Values represent the mean ± standard deviation of three independent experiments
Means within the same row with different superscript letters are significantly different (P < 0.05)
Microstructural properties
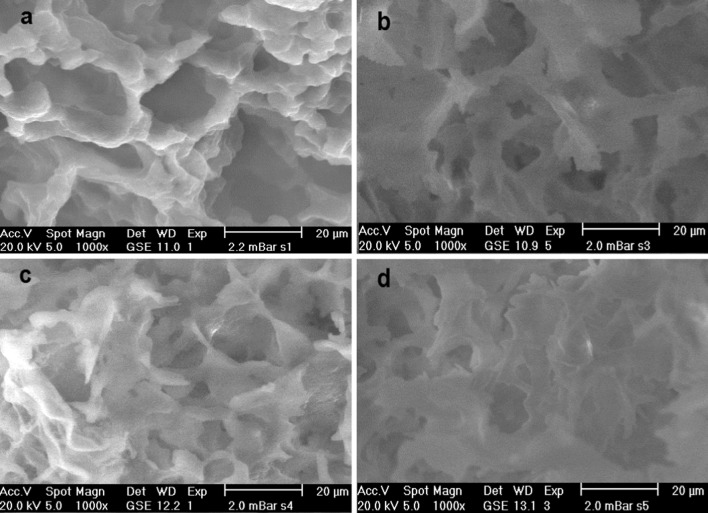
Microscopic observations have been performed by a newly developed apparatus, the ESEM, in order to examine the microstructure of gels. This technique of microscopy was selected because it allows obtaining high-quality images without sample preparation (Barrera et al. 2013). The micrographs of mixed gels (Fig. 4b, c, d) are different from gel without kefiran (Fig. 4a). The presence of kefiran in the system results in a local increase in protein concentration. Addition of greater amounts of kefiran to the system causes the protein aggregates to get closer to each other, which results in a greater interaction between them. Since whey proteins are globular proteins containing sulfhydryl groups that form the gel network by disulfide bonds, a higher local concentration of molecules suitable for interaction will result in more compact gel network with low porosity (as shown in Fig. 4). These results are in accordance with data obtained from rheological tests that kefiran causes the gel strength to increase. These effects have been referred for some mixed protein gels containing tara gum (Sittikijyothin et al. 2007) or locust bean gum (Tavares and Da Silva 2003).
Fig. 4.
ESEM micrographs of whey protein gels including different concentrations of kefiran (%, w/v): 0 (a), 0.02 (b), 0.04 (c) and 0.08 (d) as determined at 25 °C
Conclusion
This study showed that the rheological properties and the microstructure of WPI gel are significantly influenced by adding kefiran. By adding kefiran to whey protein solution, the gelling point and gelation pH decreased. Moreover, the presence of kefiran in mixture resulted in the mixed gels with more compact microstructures (smaller voids) to form. This is due to the depletion interaction phenomenon induced by kefiran. Findings of the present work can provide a better perspective to relative food industry sectors while particularly developing new food products.
Acknowledgements
The authors wish to extend their appreciation to research council of University of Tehran for providing the financial support of this study. Special thanks also goes to the University of Shahid Beheshti and Institute for Colour Science and Technology for supporting this study.
References
- Barrera GN, Calderón-Domínguez G, Chanona-Pérez J, Gutiérrez-López GF, León AE, Ribotta PD. Evaluation of the mechanical damage on wheat starch granules by SEM, ESEM, AFM and texture image analysis. Carbohydr Polym. 2013;98:1449–1457. doi: 10.1016/j.carbpol.2013.07.056. [DOI] [PubMed] [Google Scholar]
- De Jong S, Klok HJ, Van de Velde F. The mechanism behind microstructure formation in mixed whey protein–polysaccharide cold-set gels. Food Hydrocoll. 2009;23:755–764. doi: 10.1016/j.foodhyd.2008.03.017. [DOI] [Google Scholar]
- Dimitreli G, Exarhopoulos S, Goulas A, Antoniou KD, Raphaelides SN. Effect of kefiran and milk proteins addition on the rheological behavior of glucono-delta-lactone induced milk gels. J Food Res. 2016;5:121–128. doi: 10.5539/jfr.v5n1p121. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Everett DW, McLeod RE. Interactions of polysaccharide stabilisers with casein aggregates in stirred skim-milk yoghurt. Int Dairy J. 2005;15:1175–1183. doi: 10.1016/j.idairyj.2004.12.004. [DOI] [Google Scholar]
- Fox PF, Guinee TP, Cogan TM, McSweeney PL. Fundamentals of cheese science. Berlin: Springer; 2017. Whey and whey products; pp. 755–769. [Google Scholar]
- Ghasemlou M, Khodaiyan F, Oromiehie A, Yarmand MS. Development and characterisation of a new biodegradable edible film made from kefiran, an exopolysaccharide obtained from kefir grains. Food Chem. 2011;127:1496–1502. doi: 10.1016/j.foodchem.2011.02.003. [DOI] [Google Scholar]
- Ghasemlou M, Khodaiyan F, Jahanbin K, Gharibzahedi SMT, Taheri S. Structural investigation and response surface optimisation for improvement of kefiran production yield from a low-cost culture medium. Food Chem. 2012;133:383–389. doi: 10.1016/j.foodchem.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Girard M, Schaffer-Lequart C. Gelation of skim milk containing anionic exopolysaccharides and recovery of texture after shearing. Food Hydrocoll. 2007;21:1031–1040. doi: 10.1016/j.foodhyd.2006.07.012. [DOI] [Google Scholar]
- Güzel-Seydim ZB, Cagdas MDE, Seydim AC. Effect of kefir on Fusobacterium nucleatum potentially causing intestinal cancer. Funct Foods Health Dis. 2016;6:469–477. [Google Scholar]
- Hatami M, Nejatian M, Mohammadifar MA. Effect of co-solute and gelation temperature on milk protein and gum tragacanth interaction in acidified gels. Int J Biol Macromol. 2012;50:1109–1115. doi: 10.1016/j.ijbiomac.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Hatami M, Nejatian M, Mohammadifar MA, Pourmand H. Milk protein–gum tragacanth mixed gels: effect of heat-treatment sequence. Carbohydr Polym. 2014;101:1068–1073. doi: 10.1016/j.carbpol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Hesarinejad MA, Koocheki A, Razavi SMA. Dynamic rheological properties of Lepidium perfoliatum seed gum: effect of concentration, temperature and heating/cooling rate. Food Hydrocoll. 2014;35:583–589. doi: 10.1016/j.foodhyd.2013.07.017. [DOI] [Google Scholar]
- Huan Y, Zhang S, Vardhanabhuti B. Effect of CMC molecular weight on acid-Induced gelation of heated WPI-CMC soluble complex. J Food Sci. 2016;81:502–507. doi: 10.1111/1750-3841.13209. [DOI] [PubMed] [Google Scholar]
- Kuhn KR, Cavallieri ÂLF, Da Cunha RL. Cold-set whey protein–flaxseed gum gels induced by mono or divalent salt addition. Food Hydrocoll. 2011;25:1302–1310. doi: 10.1016/j.foodhyd.2010.12.005. [DOI] [Google Scholar]
- Le XT, Rioux L-E, Turgeon SL. Formation and functional properties of protein-polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv Colloid Interface Sci. 2016 doi: 10.1016/j.cis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Mende S, Peter M, Bartels K, Rohm H, Jaros D. Addition of purified exopolysaccharide isolates from S. thermophilus to milk and their impact on the rheology of acid gels. Food Hydrocoll. 2013;32:178–185. doi: 10.1016/j.foodhyd.2012.12.011. [DOI] [Google Scholar]
- Mleko S, Glibowski P, Gustaw W, Janas P. Calcium ions induced gelation of double-heated whey protein isolate. J Food Sci Technol. 2002;39:563–565. [Google Scholar]
- Nejatian M, Hatami M, Mohammadifar MA. Effect of gum tragacanth exuded by three Iranian Astragalus on mixed milk protein system during acid gelation. Int J Biol Macromol. 2013;53:168–176. doi: 10.1016/j.ijbiomac.2012.11.001. [DOI] [PubMed] [Google Scholar]
- O’Kennedy BT, Mounsey JS, Murphy F, Duggan E, Kelly PM. Factors affecting the acid gelation of sodium caseinate. Int Dairy J. 2006;16:1132–1141. doi: 10.1016/j.idairyj.2005.11.003. [DOI] [Google Scholar]
- Patel S. Emerging trends in nutraceutical applications of whey protein and its derivatives. J Food Sci Technol. 2015;52:6847–6858. doi: 10.1007/s13197-015-1894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimada PS, Abraham AG. Kefiran improves rheological properties of glucono-δ-lactone induced skim milk gels. Int Dairy J. 2006;16:33–39. doi: 10.1016/j.idairyj.2005.02.002. [DOI] [Google Scholar]
- Rocha C, Teixeira J, Hilliou L, Sampaio P, Gonçalves M. Rheological and structural characterization of gels from whey protein hydrolysates/locust bean gum mixed systems. Food Hydrocoll. 2009;23:1734–1745. doi: 10.1016/j.foodhyd.2009.02.005. [DOI] [Google Scholar]
- Sittikijyothin W, Sampaio P, Gonçalves M. Heat-induced gelation of β-lactoglobulin at varying pH: effect of tara gum on the rheological and structural properties of the gels. Food Hydrocoll. 2007;21:1046–1055. doi: 10.1016/j.foodhyd.2006.07.019. [DOI] [Google Scholar]
- Spotti MJ, Tarhan Ö, Schaffter S, Corvalan C, Campanella OH. Whey protein gelation induced by enzymatic hydrolysis and heat treatment: comparison of creep and recovery behaviour. Food Hydrocoll. 2017;63:696–704. doi: 10.1016/j.foodhyd.2016.10.014. [DOI] [Google Scholar]
- Tan T-C, Foo W-T, Liong M-T, Easa AM. Comparative assessment of dynamic oscillatory measurements on network development and mechanical spectra of gelatine or gellan in maize starch–egg white composite gels. Food Hydrocoll. 2015;45:93–101. doi: 10.1016/j.foodhyd.2014.11.003. [DOI] [Google Scholar]
- Tavares C, Da Silva JL. Rheology of galactomannan–whey protein mixed systems. Int Dairy J. 2003;13:699–706. doi: 10.1016/S0958-6946(03)00095-5. [DOI] [Google Scholar]
- Tobin JT, Fitzsimons SM, Chaurin V, Kelly AL, Fenelon MA. Thermodynamic incompatibility between denatured whey protein and konjac glucomannan. Food Hydrocoll. 2012;27:201–207. doi: 10.1016/j.foodhyd.2011.07.004. [DOI] [Google Scholar]
- Wijaya W, Van der Meeren P, Patel AR. Cold-set gelation of whey protein isolate and low-methoxyl pectin at low pH. Food Hydrocoll. 2017;65:35–45. doi: 10.1016/j.foodhyd.2016.10.037. [DOI] [Google Scholar]
- Zavala L, Roberti P, Piermaria JA, Abraham AG. Gelling ability of kefiran in the presence of sucrose and fructose and physicochemical characterization of the resulting cryogels. J Food Sci Technol. 2015;52:5039–5047. doi: 10.1007/s13197-014-1577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Vardhanabhuti B. Acid-induced gelation properties of heated whey protein–pectin soluble complex (Part II): effect of charge density of pectin. Food Hydrocoll. 2014;39:95–103. doi: 10.1016/j.foodhyd.2013.12.020. [DOI] [Google Scholar]
- Zolfi M, Khodaiyan F, Mousavi M, Hashemi M. Characterization of the new biodegradable WPI/clay nanocomposite films based on kefiran exopolysaccharide. J Food Sci Technol. 2014;52:3485–3493. doi: 10.1007/s13197-014-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]