Abstract
This study aimed to investigate the effect of tea catechin (C) and 4 of its derivatives on the Pacific white shrimp PPO inhibition and melanosis during refrigerated storage. Epigallocatechin gallate (EGCG) exhibited the highest inhibition towards PPO, followed by C. Inhibitory activity of all compounds tested was in a dose dependent manner (0.1–2.0 mM). Based on activity staining, EGCG most effectively inhibited PPO. For inhibition kinetic studies, C and epicatechin (EC) showed uncompetitive type, whereas epicatechin gallate (ECG), epigallocatechin (EGC) and EGCG exhibited mixed type inhibition. When whole shrimps were treated with EGCG solution at various concentrations (0.25–0.75%), those treated with 0.5 or 0.75% EGCG had lower melanosis scores throughout storage for 10 days at 4 °C, compared with the control and the 1.25% sodium metabisulfite treated samples (P < 0.05). Therefore, EGCG could be used as a potential inhibitor for melanosis in raw Pacific white shrimp during refrigerated storage.
Keywords: Catechin, Inhibition kinetics, Polyphenoloxidase, Melanosis, Pacific white shrimp
Introduction
Pacific white shrimp (Litopenaeus vannamei) is an economically important commercial species primarily cultured in Thailand and accounts for 90% of global aquacultured shrimp production (Nirmal and Benjakul 2009a). Despite their delicacy, shrimps are highly perishable with a limited shelf-life, mainly associated with melanosis (discoloration) and microbial deterioration (Gokoglu and Yerlikaya 2008). Melanosis is caused by the action of polyphenoloxidase (PPO), which oxidizes phenols to quinone. Quinone subsequently undergoes polymerization, giving rise to high molecular weight black pigment (Benjakul et al. 2005). To retard melanosis, icing or refrigeration has been traditionally implemented. However, during iced or refrigerated storage, melanosis still takes place since PPO still remains active under these conditions (Nirmal and Benjakul 2010). Even though blackspot or melanosis in shrimp is harmless, it is unappealing and objectionable and connotes quality loss to consumers (Gómez-Guillén et al. 2005; Simpson et al. 1988). Melanosis in shrimp drastically reduces its market value, leading to considerable financial loss (Martínez-Alvarez et al. 2005).
To overcome or alleviate melanosis in shrimp and other crustacean species, sulfiting agents and 4-hexylresorcinol have been widely used (Martínez-Álvarez et al. 2008; Montero et al. 2001). Nevertheless, the increasing consumer awareness of the health risks of chemicals and their resistance to the use of chemicals in foods, have compelled regulatory agencies to stringently limit their use in foods; thus creating an urgent need to discover novel and safer alternatives (McEvily et al. 1991). Moreover, sulfiting agents elicit allergic type reactions in some individuals (Collins-Williams 1983; Gunnison et al. 1987). Phenolics or plant extracts have been used to inhibit melanosis in shrimp. Grape seed extract (Gokoglu and Yerlikaya 2008) and enokitake extract (Jang et al. 2003) were used to prevent melanosis in deep-water rose and southern rough shrimp, respectively. Nirmal and Benjakul (2011b) reported that ethanolic green tea extract (0.5%) with prior chlorophyll removal showed the inhibitory activity towards melanosis of Pacific white shrimp during iced storage. They similarly reported that catechin in combination with ascorbic acid exhibited an inhibitory activity towards PPO of Pacific white shrimp (Nirmal and Benjakul 2009b).
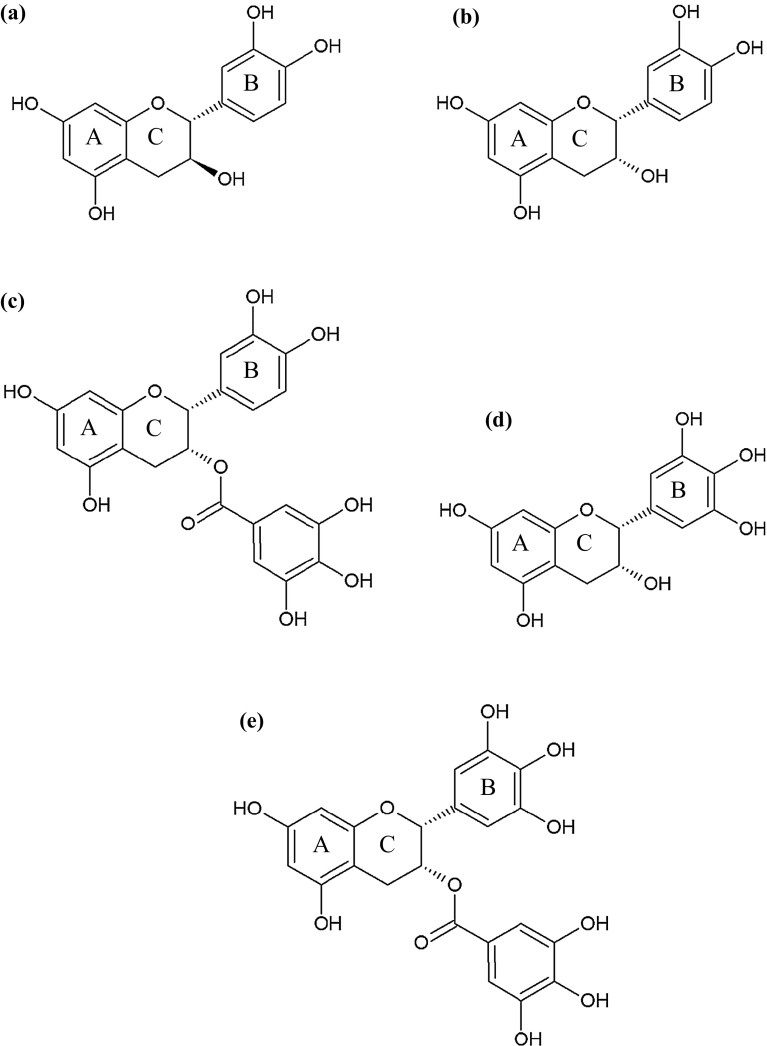
Catechins are flavanols, and are a group of low molecular weight flavan-3-ols isomers including (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), and (−)-epigallocatechin gallate (EGCG), (+)-catechin (C), etc., which are present in a variety of foods, such as tea (Wang et al. 2000). Tea catechins have been reported to possess antioxidant, antimicrobial, anticarcinogenic and anti-inflammatory properties (Wang et al. 2000). Apart from catechin, EC, EGC, ECG and EGCG (Fig. 1) were found in green tea (Yilmaz 2006). Different individual compounds might have varying inhibition efficiency towards melanosis. This might be associated with different inhibitory activities as well as kinetics towards PPO. Nevertheless, a little information regarding the use of catechin and its derivatives to inhibit PPO and melanosis of shrimps during refrigerated storage exists. The aims of this study were to extend our previous studies on the inhibitory effects of green tea, catechin and ascorbic acid on shrimp PPO, to encompass investigation of the direct inhibition of PPO from the cephalothorax of Pacific white shrimp by catechin and its isolated derivatives, epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC) and epigallocatechin gallate (EGCG), and to verify the impact of those compounds in curtailing melanosis in Pacific white shrimp during refrigerated storage.
Fig. 1.
Chemical structures of catechin and its derivatives. Catechin (a), epicatechin (b), epicatechin gallate (c), epigallocatechin (d) and epigallocatechin gallate (e)
Materials and methods
Chemicals
L-β-(3, 4 dihydroxylphenyl) alanine (L-DOPA), Brij-35 and β-mercaptoethanol (βME) were purchased from Sigma Aldrich (St. Louis, MO, USA). Sodium dodecyl sulfate (SDS), Coomassie Brilliant Blue R-250, and N,N,N´,N´-tetramethylethylenediamine (TEMED) were procured from Bio-Rad Laboratories (Hercules, CA, USA). Catechin and its derivatives including (+)-catechin (C), (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC) and (−)-epigallocatechin gallate (EGCG) were obtained from Chengdu Biopurify Phytochemicals Ltd., (Sichuan, China). The purity of catechin and its derivatives was greater than 98% as determined by HPLC. Sodium metabisulfite was purchased from Fisher Scientific (Loughborough, UK). High molecular weight protein markers were obtained from GE Healthcare UK Limited (Buckinghamshire, UK). All chemicals were of analytical grade.
Shrimp collection and preparation
Pacific white shrimp (L. vannamei) with the size amounting to 55–60 shrimps/kg were purchased from a local supplier in Songkhla, Thailand. The freshly caught shrimp, completely free of additives, were kept in ice with a shrimp/ice ratio of 1:2 (w/w) and transported to the Department of Food Technology, Prince of Songkla University, Hat Yai within 1 h after collection. Upon arrival, shrimps were washed in ice cold water (1–3 °C) and stored in ice until used (less than 1 h).
Extraction and partial purification of PPO from the cephalothoraxes of Pacific white shrimp
The cephalothoraxes of shrimps were separated, pooled and powdered by grinding in liquid nitrogen. The powder obtained was kept in polyethylene bags, stored at −20 °C and used within 1 week. PPO from the powdered cephalothoraxes was extracted according to the method of Nirmal and Benjakul (2009a).
All procedures were performed at 4 °C. The powder (50 g) was mixed with 150 mL of the extracting buffer (0.05 M sodium phosphate buffer, pH 7.2, containing 1.0 M NaCl and 0.2% Brij 35). The mixture was stirred continuously for 30 min, followed by centrifugation at 8000×g for 30 min using a refrigerated centrifuge (model CR22 N, Hitachi, Hitachi Koki Co., Ltd., Tokyo, Japan). Solid ammonium sulfate was added into the supernatant to obtain 40% saturation and allowed to stand for 30 min. The precipitate was collected by centrifugation at 12,500×g for 30 min using a refrigerated centrifuge. The pellet obtained was dissolved in a minimum volume (10 mL) of 0.05 M sodium phosphate buffer, pH 7.2 and dialyzed against 3 changes of 50 volumes of the same buffer for 18 h. The insoluble precipitate was removed by centrifugation at 3000×g for 30 min.
The ammonium sulfate fraction was subjected to ion exchange chromatography at 4 °C using DEAE-Sepharose column (1.6 × 16 cm), previously equilibrated with 0.05 M phosphate buffer (pH 7.2). The column was then washed with the same phosphate buffer until A280 was below 0.05. PPO was eluted with a linear gradient of 0–1.2 M NaCl in 0.05 M phosphate buffer (pH 7.2) at a flow rate of 0.5 mL/min. Fractions of 1.5 mL were collected and those with PPO activity were pooled. The pooled fractions were dialyzed with two changes of 50 volumes of 0.05 M phosphate buffer (pH 7.2). DEAE-Sepharose fraction had the purification fold of 78.6 with the specific activity of 173.1 units/mg and protein content of 1.74 mg/mL.
Measurement of PPO activity
PPO activity was assayed using L-DOPA as a substrate as per the method of Nirmal and Benjakul (2009a) with a slight modification. The assay system consisted of 20 µL of PPO solution, 120 µL of 15 mM L-DOPA in deionized water, 80 µL of 0.05 M phosphate buffer (pH 6.0) and 20 µL of deionized water. PPO activity was determined by monitoring the formation of dopachrome at 475 nm for 3 min at 45 °C using a FLUOstar® Omega microplate reader (BMG LABTECH GmbH, Ortenberg, Germany). One unit of activity was defined as the PPO causing an increase in the absorbance at 475 nm by 0.001/min. Enzyme and substrate blanks were prepared by excluding the substrate and enzyme, respectively, from the reaction mixture and replacing them with deionized water instead.
Study on impact of catechin and its derivatives on PPO inhibition
Effect of concentrations of catechin and its derivatives
To study the inhibitory effect of catechin and its derivatives towards PPO, DEAE-Sepharose fractions containing PPO (20 µL) was incubated with catechin or its derivatives (20 µL) to obtain the final concentrations of 0.1, 0.25, 0.5, 1.0 and 2.0 mM. The reaction mixtures were incubated for 30 min at room temperature (25–28 °C), and then 80 µL of the assay buffer (0.05 M phosphate buffer, pH 6.0) was added. To initiate the reaction, 120 µL of pre-incubated 15 mM L-DOPA (45 °C) were added. The reaction was conducted at 45 °C and the absorbance at 475 nm was measured after 3 min of reaction using a microplate reader. The control was run in the same manner, except deionized water was used instead of catechin or its derivatives. Residual activity was calculated and the inhibitory activity was expressed as the percentage inhibition as follows:
where A is PPO activity of control and B is PPO activity in the presence of catechin or its derivatives.
SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and PPO activity staining
The DEAE-Sepharose fraction was mixed with sample buffer containing 1.5 M βME at a ratio of 1:1 (v/v) without heating. The samples (40 µg protein) were loaded onto the polyacrylamide gel made of 7.5% running gel and 4% stacking gel and subjected to electrophoresis at a constant current of 15 mA per gel at room temperature (25–28 °C) using a Mini Protein III unit (Bio-Rad Laboratories, Inc., Richmond, CA, USA). After separation, one of two identical gels was immersed in a sodium phosphate buffer (pH 6.0) containing 15 mM L-DOPA for 1 h at 45 °C. The PPO activity zone appeared as a dark band. Another gel was stained with 0.125% Coomassie Brilliant Blue R-250 and destained in 25% methanol and 10% acetic acid. High molecular weight protein markers including myosin (220 kDa), α2-macroglobulin (170 kDa), β-galactosidase (116 kDa), transferrin (76 kDa) and glutamic dehydrogenase (53 kDa) were used to estimate the molecular weight of PPO.
To study the inhibitory effect of catechin and its derivatives towards PPO, DEAE-Sepharose fraction containing PPO was incubated with catechin or its derivatives (2 mM) at a ratio of 1:1 (v/v) for 30 min at room temperature (25–28 °C). The mixtures were loaded onto polyacrylamide gel, followed by activity staining as previously described. A lower activity zone indicated the higher PPO inhibition.
Study on inhibition kinetics of catechin and its derivatives towards PPO
The Michaelis–Menten constant (K m) for PPO was determined by Lineweaver–Burk plots using L-DOPA as substrate (Lineweaver and Burk 1934). For the inhibition kinetics study, DEAE-Sepharose fraction containing PPO (20 µL) was mixed with catechin or its derivatives solution (20 µL) to obtain final concentrations of 0.25, 0.5 and 1.0 mM. The mixtures were incubated for 5 min at room temperature (25–28 °C). To initiate the reaction, 200 µL of L-DOPA in 0.05 M sodium phosphate buffer (pH 6.0) were added. At each concentration of catechin or its derivatives, L-DOPA with seven different concentrations (0.5–5 mM) was used as the substrate. The reaction was incubated for 3 min at 45 °C and the absorbance at 475 nm was measured using a microplate reader.
Study on melanosis of Pacific white shrimp as influenced by EGCG treatment
Preparation of shrimp treated with EGCG
EGCG with the highest PPO inhibitory activity was used for treatment of shrimp. Whole shrimps were immersed in solution containing EGCG at various concentrations (0.25, 0.5 and 0.75%, w/v) using a shrimp/solution ratio of 1:2 (w/v) at 4 °C for 15 min. Another portion of shrimps were soaked in 1.25% sodium metabisulfite (SMS) at a ratio of 1:2 (w/v) for 1 min at 4 °C. Treated shrimps were drained on a screen for 3 min at 4 °C. Shrimps without any EGCG or metabisulfite treatment were used as the control. The samples (20 shrimps) were placed on a polystyrene tray, covered with plastic wrap and stored at 4 °C. Samples were monitored for melanosis every 2 days up to 10 days.
Melanosis assessment
Melanosis or blackening of Pacific white shrimp was evaluated through visual inspection by ten trained panelists using 10-point scoring test (Montero et al. 2001). Panelists were asked to give the melanosis score (0–10), where 0 = absent; 2 = slight (up to 20% of shrimps’ surface affected); 4 = moderate (20–40% of shrimps’ surface affected); 6 = notable (40–60% of shrimps’ surface affected); 8 = severe (60–80% of shrimps’ surface affected); 10 = extremely heavy (80–100% of shrimps’ surface affected).
Statistical analyses
All analyses were performed in triplicate and a completely randomized design (CRD) was used. The data were subjected to analysis of variance (ANOVA). Comparison of means was carried out by the Duncan’s multiple range test. Statistical analysis was performed using a SPSS package (SPSS 11.0 for windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Inhibitory effect of catechin and its derivatives on PPO from Pacific white shrimp
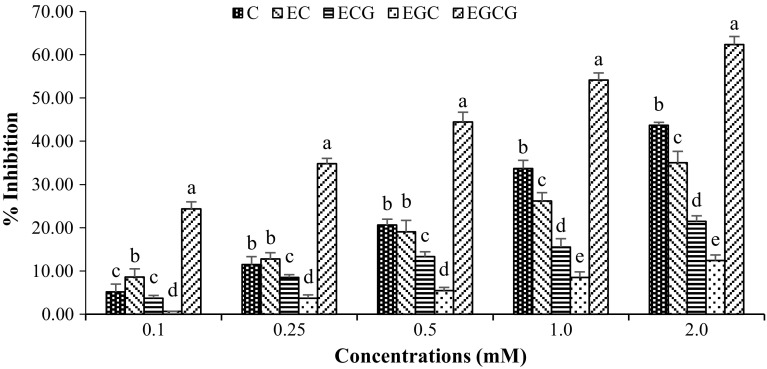
PPO inhibitory activity of catechin and its derivatives expressed as percent inhibition is represented in Fig. 2. All compounds showed PPO inhibitory activity in a dose-dependent manner. At the same concentration used, EGCG generally exhibited the highest inhibitory activity towards PPO, while EGC showed the lowest activity (P < 0.05). It has been reported that some phenolic compounds inhibit PPO activity by interacting with the active sites of the enzymes or via reduction of quinone formed (Janovitz-Klapp et al. 1990; Nirmal and Benjakul 2012). Ferulic acid with different concentrations (0.1–2.0%, w/v) showed inhibitory activity towards Pacific white shrimp PPO in a dose dependent manner (Nirmal and Benjakul 2009a). Kim et al. (2000) reported that aromatic carboxylic acids of cinnamic acid and its analogues, p-coumaric, ferulic, and sinapic acids were all potent PPO inhibitors. Benzoic acid and cinnamic acid derivatives also acted as PPO inhibitors due to their structural similarities to its phenolic substrates (Krueger 1955). Catechin is composed of two benzene rings (A and B rings) and a dihydropyran heterocyclic ring (C ring) with a hydroxyl group on carbon 3 (Fig. 1a). EC has an o-dihydroxyl group in the B ring at carbons 3′ and 4′, while EGC has a trihydroxyl group in the B ring at carbons 3′, 4′, and 5′. ECG differs from EC with a gallate moiety esterified at carbon 3 of the C ring, while EGCG has both trihydroxyl group in the B ring at carbons 3′, 4′, and 5′, and a gallate moiety esterified at carbon 3 of the C ring (Graham 1992). Tea has the highest level of catechin and derivatives among all the food sources (Wang et al. 2000). Phenolic compounds are known to have reducing power and metal chelating capacity (Heim et al. 2002). Apart from serving as PPO inhibitor, catechin and its derivatives could inhibit PPO, possibly due to the combined effects of metal chelation, reduction of quinone, etc. (Nirmal and Benjakul 2012). In the present study, EGCG showed the highest PPO inhibitory activity, which was much higher than ECG. Based on structure (Fig. 1), it is suggested that the hydroxyl group at 5′ position of B ring plays an essential role in PPO inhibition. With the same structure of B ring between EGC and EGCG, gallate moiety more likely contributed to enhanced PPO inhibitory activity of EGCG.
Fig. 2.
Effect of catechin and its derivatives on the inhibition of polyphenoloxidase from the cephalothorax of Pacific white shrimp. Bars represent the standard deviation (n = 3). Different letters on the bars indicate significant differences (P < 0.05). C catechin, EC epicatechin, ECG epicatechin gallate, EGC epigallocatechin, EGCG epigallocatechin gallate
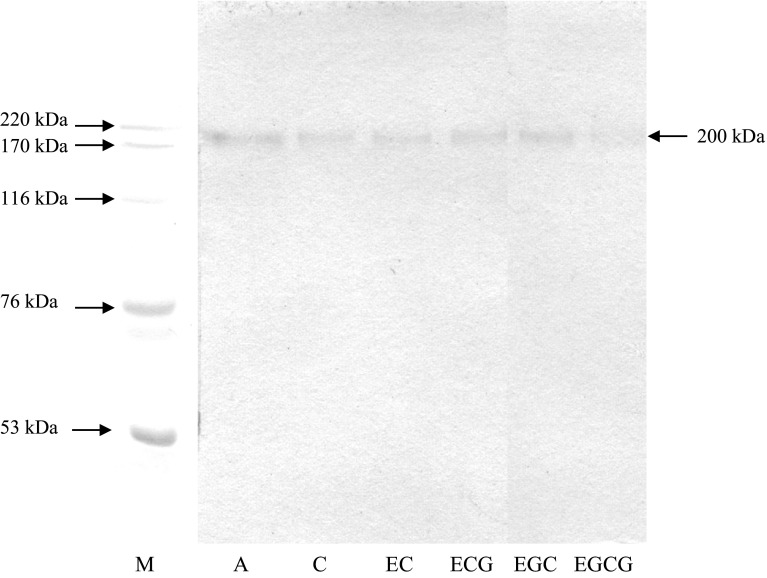
Activity staining of DEAE-Sepharose fraction containing PPO from cephalothorax of Pacific white shrimp in the absence and the presence of catechin or its derivatives at a concentration of 2 mM is shown in Fig. 3. PPO was able to induce the oxidation of DOPA to DOPA-quinone and other intermediate products, which subsequently underwent polymerization to melanin. This was evidenced by the highest activity band intensity for the control (without catechin and derivatives). PPO activity band appeared with MW of 200 kDa. PPO from carapace of Pacific white shrimp had MW of 210 kDa (Manheem et al. 2012). The active PPO from deep water pink shrimp (Parapenaeus longirostris) had MW of 212 kDa (Zamorano et al. 2009). Benjakul et al. (2005) reported that the PPO from the kuruma prawn cephalothoraxes had MW of 160 kDa. PPO from viscera and carapace extracts of cephalothorax of Norway lobster (Nephrops norvegicus) had MW about 200–220 kDa as determined by activity staining using l-tyrosine and 4-tert-butyl-catechol as substrates (Giménez et al. 2010). Among all compounds tested, C and EGCG showed high inhibitory effect towards PPO from Pacific white shrimp as indicated by the lower activity band intensity than the others (Fig. 3). On the other hand, the higher activity band intensity was observed when EGC or ECG was incorporated.
Fig. 3.
Activity staining of polyphenoloxidase of DEAE-Sepharose fraction from cephalothorax of Pacific white shrimp in the absence and presence of catechin or its derivatives at a concentration of 2 mM. M molecular weight marker, A DEAE-Sepharose fraction, C catechin, EC epicatechin, ECG epicatechin gallate, EGC epigallocatechin, EGCG epigallocatechin gallate
Inhibition kinetics of catechin and its derivatives towards PPO from Pacific white shrimp
The Michaelis constant (K m) for the oxidation of L-DOPA by PPO in DEAE-Sepharose fraction was 1.16 mM. Similar K m (1.6 mM) of PPO from pink shrimp (Panaeus duorarum) using L-DOPA as substrate was reported by Simpson et al. (1988). Simpson et al. (1988) reported that K m for oxidation of L-DOPA by PPO from white shrimp (Panaeus setiferus) was 2.8 mM but K m for oxidation of L-DOPA by PPO from the same shrimp was reported by Chen et al. (1991) as 3.48 mM. A K m value of 0.26 mM was found for oxidation of L-DOPA by PPO from kuruma prawn (Penaeus japonicus) (Benjakul et al. 2006). The small K m values reflect the high affinity of enzymes for their substrates. Conversely, higher K m value indicates the lower catalytic efficiency of the enzyme towards substrate (Liu et al. 2006).
Inhibition kinetics of catechin and its derivatives towards PPO from cephalothorax of Pacific white shrimp were elucidated by Lineweaver–Burk plots as shown in Fig. 4. Catechin and its derivatives at different concentrations affected both K m and V max values of PPO. The inhibitory action of C and EC towards PPO was uncompetitive as evidenced by the parallel lines on a Lineweaver–Burk plot with all concentrations used. The result suggested that C and EC most likely bound to the enzyme-substrate complex. Arslan et al. (2004) reported that p-amino benzene sulfonamide and sulfosalicylic acid showed uncompetitive inhibition towards mulberry PPO when catechol was used as a substrate. Agartine showed uncompetitive inhibition with mushroom (Agaricus bisporus) PPO when L-DOPA was used as a substrate (Espín et al. 1998). The binding of the uncompetitive inhibitor distorts the active site of the enzyme, thereby rendering it catalytically inactive without affecting substrate affinity. This type of inhibition causes a decrease in both V max and the K m value (Voet and Voet 2011).
Fig. 4.
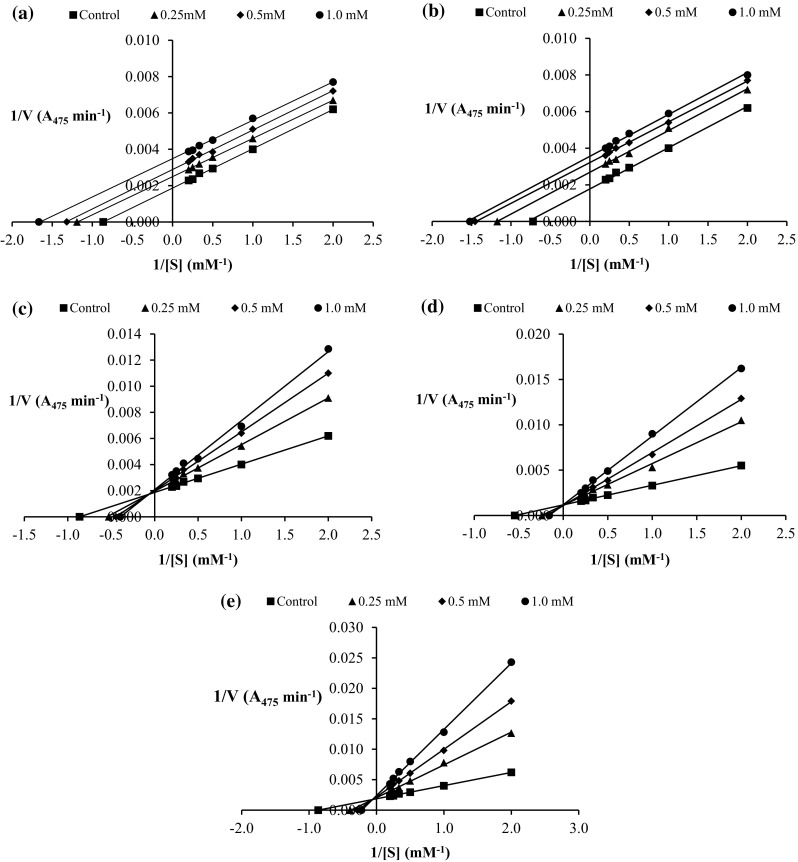
Lineaweaver–Burk plots of polyphenoloxidase from cephalothorax of Pacific white shrimp in the absence and presence of catechin (a), epicatechin (b), epicatechin gallate (c), epigallocatechin (d) and epigallocatechin gallate (e) at different concentrations. L-DOPA at levels of 0.5–5 mM were used as substrate
ECG, EGC and EGCG showed mixed type inhibition towards PPO from Pacific white shrimp. K m increased while V max decreased with increasing concentrations of all aforementioned compounds. The results indicated that those compounds could bind both enzyme and enzyme-substrate complex, but with different affinities (Voet and Voet 2011). Kojic acid showed a mixed type inhibition towards PPO from white shrimp, grass prawn and lobster using L-DOPA and l-tyrosine as substrates (Chen et al. 1991). Dodecyl gallate was found as a mixed-type inhibitor for mushroom tyrosinase, when L-DOPA was used as a substrate (Kubo et al. 2000). Nirmal and Benjakul (2011a) reported that mimosine exhibited mixed type reversible inhibition on PPO from Pacific white shrimp. Based on the structure, o-dihydroxyl group in the B ring of C and EC possibly played an important role in uncompetitive inhibition kinetics. Considering the similar structure to C and EC, hydroxyl group at 5′ position of the B ring might be associated with the mixed type inhibition kinetics of EGC. Gallate moiety of ECG and EGCG could also contribute to the mixed type kinetics. The result suggested that catechin and its derivatives showed different types of inhibition on PPO from the cephalothorax of Pacific white shrimp. This might be governed by varying structures and functional groups on A, B and C rings of different compounds.
Effect of EGCG on melanosis of Pacific white shrimp during refrigerated storage
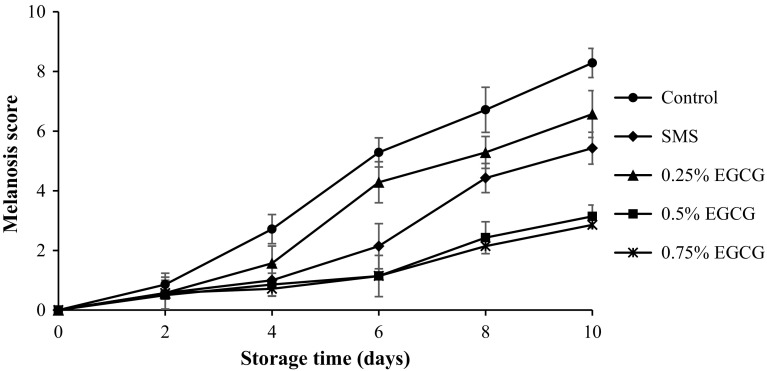
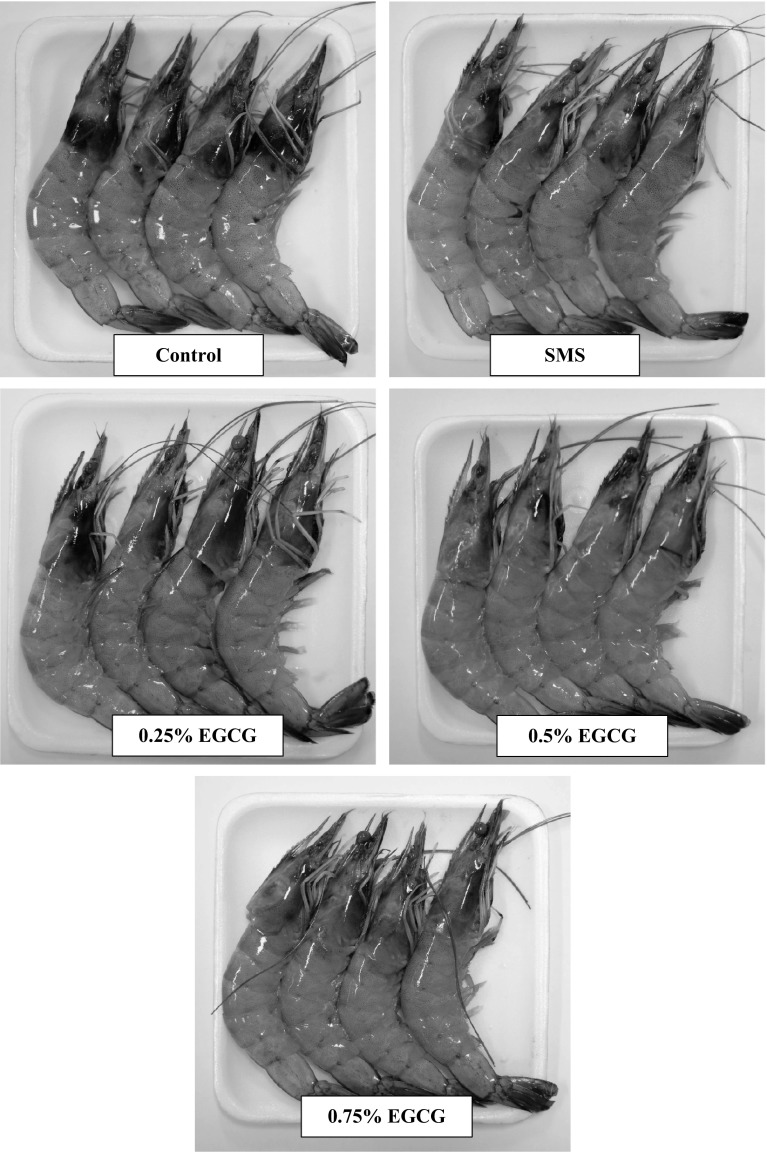
Melanosis scores for Pacific white shrimp with and without treatments of SMS, 0.25, 0.5 or 0.75% EGCG during the refrigerated storage are illustrated in Fig. 5. At day 0, all samples had no melanosis (score = 0). During 2 days of storage, all samples showed no difference in melanosis scores (P > 0.05). When the storage time increased, melanosis score in the control (without treatment) increased (P < 0.05). However, no difference in melanosis was noticeable in samples treated with SMS, 0.5 or 0.75% EGCG within the first 4 days of storage (P > 0.05). Generally, SMS treated shrimps had lower melanosis score than 0.25% EGCG treated shrimp (P < 0.05). Treatment of shrimps with 0.5 and 0.75% EGCG showed higher effectiveness in lowering melanosis, when compared with SMS treatment. During the first 4 days of storage, SMS at 1.25% used in this study was effective in melanosis prevention. However, SMS might not be stable during the extended storage, in which sulfur dioxide formed could be liberated (Taylor et al. 1986). Additionally, the mechanism of melanosis inhibition by SMS and EGCG might be different. SMS inhibits melanosis by reacting with intermediate quinone, forming sulfoquinone or it can act as a competitive inhibitor (Ferrer et al. 1989). During 8–10 days of storage, the lowest melanosis score was observed in the sample treated with 0.5 or 0.75% EGCG, followed by those treated with SMS and 0.25% EGCG, respectively. Shrimp treated with 0.5 or 0.75% EGCG showed the lowest blackspot (melanosis) as compared to the others at the end of storage (day 10) (Fig. 5), while severe melanosis was found in the control. The retardation of melanosis formation in EGCG treated shrimp was related with high PPO inhibitory activity of EGCG (Figs. 2, 3). Phenolic compounds and plant extracts have been shown to inhibit PPO and reduce melanosis in crustaceans. Nirmal and Benjakul (2009a) reported that Pacific white shrimps treated with 2% ferulic acid had the lower melanosis score after 10 days of iced storage. Shrimp (Parapenaeus Iongirostris) treated with 1.5% grape seed extract and stored at 4 °C had the best melanosis score (score 6) as compared to the other treatments (Gokoglu and Yerlikaya 2008). Pacific white shrimp treated with 0.5% ethanolic green tea extract with prior chlorophyll removal possessed the lower melanosis, compared with the control, and showed similar score to those treated with SMS (Nirmal and Benjakul 2011b). The result suggested that 0.5 or 0.75% EGCG showed the higher efficiency in retardation of melanosis in Pacific white shrimp, compared to SMS and EGCG at lower concentration (0.25%) (Fig. 6).
Fig. 5.
Melanosis score of Pacific white shrimp without and with treatment of EGCG at different concentrations during 10 days of refrigerated storage. Bars represent the standard deviation (n = 3). SMS sodium metabisulfite, EGCG epigallocatechin gallate
Fig. 6.
Photographs of Pacific white shrimp without and with the treatment of EGCG at different concentrations after 10 days of refrigerated storage. SMS sodium metabisulfite, EGCG epigallocatechin gallate
Conclusion
Catechin or its derivatives showed inhibitory activity towards PPO from cephalothorax of Pacific white shrimp in a dose dependent manner. Different inhibition kinetics were observed among different compounds. C and EC exhibited uncompetitive inhibition, while ECG, EGC and EGCG showed the mixed type inhibition towards PPO. EGCG was more effective in PPO inhibition than other compounds. Pacific white shrimp treated with 0.5 or 0.75% EGCG had the lower melanosis, compared with the control and those treated with SMS throughout the refrigerated storage. Thus, prevention of melanosis in Pacific white shrimp during refrigerated storage could be achieved by using EGCG at an appropriate concentration.
Acknowledgements
This research was supported by Prince of Songkla University and the Postdoctoral Fellowship from Prince of Songkla University, Thailand to Dr. Thanasak Sae-leaw. National Research Council of Thailand and the Thailand Research Fund (TRF) Distinguished Research Professor Grant are also acknowledged for the financial support.
References
- Arslan O, Erzengin M, Sinan S, Ozensoy O. Purification of mulberry (Morus alba L.) polyphenol oxidase by affinity chromatography and investigation of its kinetic and electrophoretic properties. Food Chem. 2004;88:479–484. doi: 10.1016/j.foodchem.2004.04.005. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Tanaka M. Properties of phenoloxidase isolated from the cephalothorax of kuruma prawn (Penaeus japonicus) J Food Biochem. 2005;29:470–485. doi: 10.1111/j.1745-4514.2005.00042.x. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Tanaka M. Inhibitory effect of cysteine and glutathione on phenoloxidase from kuruma prawn (Penaeus japonicus) Food Chem. 2006;98:158–163. doi: 10.1016/j.foodchem.2005.05.056. [DOI] [Google Scholar]
- Chen JS, C-i Wei, Rolle RS, Otwell WS, Balaban MO, Marshall MR. Inhibitory effect of kojic acid on some plant and crustacean polyphenol oxidases. J Agric Food Chem. 1991;39:1396–1401. doi: 10.1021/jf00008a008. [DOI] [Google Scholar]
- Collins-Williams C. Intolerance to additives. Ann Allergy. 1983;51:315–316. [PubMed] [Google Scholar]
- Espín JC, Jolivet S, Wichers HJ. Inhibition of mushroom polyphenol oxidase by agaritine. J Agric Food Chem. 1998;46:2976–2980. doi: 10.1021/jf9802732. [DOI] [Google Scholar]
- Ferrer OJ, Koburger JA, Otwell WS, Gleeson RA, Simpson BK, Marshall MR. Phenoloxidase from the cuticle of Florida spiny lobster (Panulirus argus): mode of activation and characterization. J Food Sci. 1989;54:63–67. doi: 10.1111/j.1365-2621.1989.tb08568.x. [DOI] [Google Scholar]
- Giménez B, Martínez-Alvarez Ó, Montero P, Gómez-Guillén MC. Characterization of phenoloxidase activity of carapace and viscera from cephalothorax of Norway lobster (Nephrops norvegicus) LWT Food Sci Technol. 2010;43:1240–1245. doi: 10.1016/j.lwt.2010.02.017. [DOI] [Google Scholar]
- Gokoglu N, Yerlikaya P. Inhibition effects of grape seed extracts on melanosis formation in shrimp (Parapenaeus longirostris) Int J Food Sci Technol. 2008;43:1004–1008. doi: 10.1111/j.1365-2621.2007.01553.x. [DOI] [Google Scholar]
- Gómez-Guillén MC, Martínez-Alvarez Ó, Llamas A, Montero P. Melanosis inhibition and SO2 residual levels in shrimps (Parapenaeus longirostris) after different sulfite-based treatments. J Sci Food Agric. 2005;85:1143–1148. doi: 10.1002/jsfa.1990. [DOI] [Google Scholar]
- Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-F. [DOI] [PubMed] [Google Scholar]
- Gunnison AF, Jacobsen DW, Schwartz HJ. Sulfite hypersensitivity. A critical review. Crit Rev Toxicol. 1987;17:185–214. doi: 10.3109/10408448709071208. [DOI] [PubMed] [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Jang MS, Sanada A, Ushio H, Tanaka M, Ohshima T. Inhibitory effect of enokitake extract on melanosis of shrimp. Fish Sci. 2003;69:379–384. doi: 10.1046/j.1444-2906.2003.00632.x. [DOI] [Google Scholar]
- Janovitz-Klapp AH, Richard FC, Goupy PM, Nicolas JJ. Inhibition studies on apple polyphenol oxidase. J Agric Food Chem. 1990;38:926–931. doi: 10.1021/jf00094a002. [DOI] [Google Scholar]
- Kim J, Marshall MR, Wei C. Polyphenoloxidase. In: Haard NF, Simpson BK, editors. Seafood enzyme: utilization and influence on post harvest seafood quality. New York: Marcel Dekker; 2000. pp. 271–315. [Google Scholar]
- Krueger RC. The inhibition of tyrosinase. Arch Biochem Biophys. 1955;57:52–60. doi: 10.1016/0003-9861(55)90175-2. [DOI] [PubMed] [Google Scholar]
- Kubo I, Kinst-Hori I, Kubo Y, Yamagiwa Y, Kamikawa T, Haraguchi H. Molecular design of antibrowning agents. J Agric Food Chem. 2000;48:1393–1399. doi: 10.1021/jf990926u. [DOI] [PubMed] [Google Scholar]
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–666. doi: 10.1021/ja01318a036. [DOI] [Google Scholar]
- Liu G, et al. Purification and characterization of phenoloxidase from crab Charybdis japonica. Fish Shellfish Immunol. 2006;20:47–57. doi: 10.1016/j.fsi.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Manheem K, Benjakul S, Kijroongrojana K, Visessanguan W. The effect of heating conditions on polyphenol oxidase, proteases and melanosis in pre-cooked Pacific white shrimp during refrigerated storage. Food Chem. 2012;131:1370–1375. doi: 10.1016/j.foodchem.2011.10.001. [DOI] [Google Scholar]
- Martínez-Alvarez O, Montero P, Gómez-Guillén MC. Controlled atmosphere as coadjuvant to chilled storage for prevention of melanosis in shrimps (Parapenaeus longirostris) Eur Food Res Technol. 2005;220:125–130. doi: 10.1007/s00217-004-1015-1. [DOI] [Google Scholar]
- Martínez-Álvarez Ó, Gómez-Guillén MC, Montero P. Chemical and microbial quality indexes of Norwegian lobsters (Nephrops norvegicus) dusted with sulphites. Int J Food Sci Technol. 2008;43:1099–1110. doi: 10.1111/j.1365-2621.2007.01576.x. [DOI] [Google Scholar]
- McEvily AJ, Iyengar R, Otwell S. Sulfite alternative prevents shrimp melanosis. Food Technol. 1991;9:80–86. [Google Scholar]
- Montero P, Lopez-Caballero ME, Perez-Mateos M. The effect of inhibitors and high pressure treatment to prevent melanosis and microbial growth on chilled prawns (Penaeus japonicus) J Food Sci. 2001;66:1201–1206. doi: 10.1111/j.1365-2621.2001.tb16105.x. [DOI] [Google Scholar]
- Nirmal NP, Benjakul S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009;116:323–331. doi: 10.1016/j.foodchem.2009.02.054. [DOI] [PubMed] [Google Scholar]
- Nirmal NP, Benjakul S. Melanosis and quality changes of pacific white shrimp (Litopenaeus vannamei) treated with catechin during iced storage. J Agric Food Chem. 2009;57:3578–3586. doi: 10.1021/jf900051e. [DOI] [PubMed] [Google Scholar]
- Nirmal NP, Benjakul S. Effect of catechin and ferulic acid on melanosis and quality of Pacific white shrimp subjected to prior freeze–thawing during refrigerated storage. Food Control. 2010;21:1263–1271. doi: 10.1016/j.foodcont.2010.02.015. [DOI] [Google Scholar]
- Nirmal NP, Benjakul S. Inhibitory effect of mimosine on polyphenoloxidase from cephalothoraxes of Pacific white shrimp (Litopenaeus vannamei) J Agric Food Chem. 2011;59:10256–10260. doi: 10.1021/jf201603k. [DOI] [PubMed] [Google Scholar]
- Nirmal NP, Benjakul S. Use of tea extracts for inhibition of polyphenoloxidase and retardation of quality loss of Pacific white shrimp during iced storage. LWT Food Sci Technol. 2011;44:924–932. doi: 10.1016/j.lwt.2010.12.007. [DOI] [Google Scholar]
- Nirmal NP, Benjakul S. Inhibition kinetics of catechin and ferulic acid on polyphenoloxidase from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) Food Chem. 2012;131:569–573. doi: 10.1016/j.foodchem.2011.09.025. [DOI] [Google Scholar]
- Simpson BK, Marshall MR, Otwell WS. Phenoloxidases from pink and white shrimp: kinetic and other properties. J Food Biochem. 1988;12:205–218. doi: 10.1111/j.1745-4514.1988.tb00373.x. [DOI] [Google Scholar]
- Taylor SL, Higley NA, Bush RK. Sulfites in foods: uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity, and hypersensitivity. In: Chichester CO, Mrak EM, Schweigert BS, editors. Advances in food research. New York: Academic Press; 1986. pp. 1–76. [DOI] [PubMed] [Google Scholar]
- Voet D, Voet JG. Rates of enzymatic reactions. In: Voet D, Voet JG, editors. Biochemistry. New Jersey: Wiley; 2011. pp. 482–505. [Google Scholar]
- Wang H, Provan GJ, Helliwell K. Tea flavonoids: their functions, utilisation and analysis. Trends Food Sci Technol. 2000;11:152–160. doi: 10.1016/S0924-2244(00)00061-3. [DOI] [Google Scholar]
- Yilmaz Y. Novel uses of catechins in foods. Trends Food Sci Technol. 2006;17:64–71. doi: 10.1016/j.tifs.2005.10.005. [DOI] [Google Scholar]
- Zamorano J-P, Martínez-Álvarez O, Montero P, Gómez-Guillén MC. Characterisation and tissue distribution of polyphenol oxidase of deepwater pink shrimp (Parapenaeus longirostris) Food Chem. 2009;112:104–111. doi: 10.1016/j.foodchem.2008.05.061. [DOI] [Google Scholar]