Abstract
Black tea manufacture usually involves the processes of withering, cutting, fermentation and drying. The aim of present study was to evaluate the effect of the relationship between the quality and withering with different light sources (ultraviolet, yellow, blue, purple, orange, red, cyan, green and white) an quality attribute of tea. The results indicated that the yellow, orange and red light withering significantly improved the aroma and taste, imparting the tea a sweet flavor and a fresh and mellow taste. Tea treated with yellow light was scored highest the sensory scores and showed the highest content in catechins, theaflavins, amino acids and aroma components, followed by the orange and red light treatments. The black tea withered with ultraviolet light showed a strong astringency, probably resulting from low contents of theaflavins, amino acids and soluble sugar. The green light irradiation remarkably damaged the aroma and taste of the tea, leading to a strong greenish flavor and an astringent taste, probably owing to the lowest contents of chemical compositions. No significant cumulative effect was found in the hybrid light withering treatments. Therefore, monochromatic yellow, orange and red lights were suggested for withering the black tea to improve its overall quality.
Keywords: Black tea, Withering, Light, Sensory quality, Chemical components
Introduction
Research shows that there are at least three kinds of photoreceptors in plants to perceive and detect optical signals (Franklin et al. 2005). Two of them are pigment protein dimers and can be excited after absorbing different light wavelengths. Most excitation energy was transformed into photochemical energy for reactions in physiological metabolism in plants, such as electron transport, phosphorylation and changes of molecular configuration. Meanwhile, the growth and quality of plants also varied with different lights (Ahmad and Cashmore 1993; Christie 1998; Lin et al. 1997). Talbott et al. found a 173% increase of malic acid in stomatal guard cells of broad bean after 30 min irradiation of blue light and a 215% increase of sugar content with 2 h blue light treatment (Talbott and Zeiger 1993). Additionally, light also has a close relationship with plant secondary metabolites. Wang et al. (1989) used light film mulching on ginseng and found that purple and yellow film mulching could significantly increase the content of ginsenoside. Several reports also showed that ultraviolet light and blue light could promote the synthesis of flavonoids while red light had the opposite function (Zhang et al. 2004; Zhao et al. 1999).
Tea, a widely consumed global beverage, has been reported to be beneficial to health in terms of reducing the risk of cardiovascular diseases and some forms of cancer, antibacteria, antiviral activity and so on (Friedman 2007). Basically, tea is classified into six types: green tea, black tea, oolong tea, white tea, yellow tea and dark tea (Wu et al. 2014). Improving the quality of these teas to meet the increasing requirements of consumers has always been the goal of tea processing enterprises. In the processing of oolong and black tea, withering fresh leaves under sunlight is popular in China since this procedure was believed to decrease the bitter taste and improve the aroma quality (Wan et al. 2015). Increasing lines of evidence showed that light irradiation on plucked leaves during withering of oolong tea could change the chemical components. For example, Fan et al. (2012) found that, after withering oolong tea with irradiation of infrared lamp, the contents of Epigallocatechingallate (EGCG) and Epicatechingallate (ECG) decreased while Epicatechin (EC), catechin (C), amino acids, theaflavin and water extract prominently increased. Compared with oolong tea, the withering of black tea takes more time (≥8 h at 25–35 °C) and loses more water (about 15–20%). During this process, the activity of hydrolase rises remarkably so that the proteins and polysaccharides slowly hydrolyze, which contributes to the tea quality. Sunlight was often used in withering black tea, but the function and mechanism by which the sunlight modifies the chemical components and qualities remain unknown. In the present study, different lights (ultraviolet, yellow, blue, purple, orange, red, cyan, green and white) were exploited to wither plucked leaves, then the withered leaves were subjected to cutting, fermentation and drying, and finally the relationship between the tea quality and light illumination was investigated by evaluating the sensory quality and chemical compositions of the made tea.
Materials and methods
Materials
Catechin, theaflavins and theanine were purchased from Sigma-Aldrich Co., USA. Acetonitrile and methanol of chromatographically pure grade were purchased from Fisher Chem. Alert Guide (New Jersey, USA). Diethyl ether of chromatographically pure grade was purchased from Duksan Co., Korea. The lamps of each monochromater were gifts from China Jiliang University (Hangzhou, China). Other reagents were all purchased from Shanghai Pharmaceutical Group Co., LTD (Shanghai, China) and were of analytical grade.
Methods
The design of experimental equipment
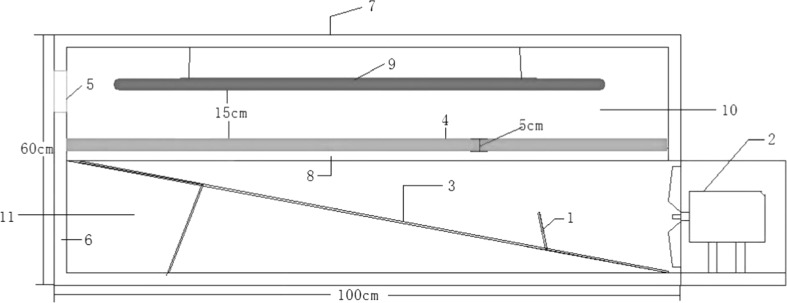
The withering equipment (Fig. 1) was designed as a cubic wooden framework (1.0 × 0.5 × 0.6 m3), with a wire net dividing the structure into an upper and a lower layer. On the right side of the lower layer, a fan was fixed to blow the fresh air in, and a foam board of the same width was installed to form an inclined plane, on which a 10-cm-high wind deflector was fixed about 30 cm away from the fan. Each surface of the equipment was covered by a foam board with an air outlet, with a 225-cm2 (15 × 15 cm2) hole on the left side of the upper layer. For each withering equipment, two lamp tubes were arranged in parallel and suspended from the ceiling.
Fig. 1.
Structure of light withering equipment. 1 wind deflector, 2 fan, 3 inclined plane, 4 plucked tea shoots, 5 air outlet, 6 wooden framework covered with foam board, 7 foam board cover, 8 wire net, 9 lamp tubes, 10 the upper layer, 11 the lower layer
Experiment of withering with different lights
Young tea shoots for processing (most with two leaves and a bud, and a few with one leaf and a bud) were harvested from the clone Fudingdabai (Camellia sinensis L.) in July, 2012 from the Experimental Farm at Huazhong Agricultural University. The freshly plucked tea leaves were loaded in the withering equipment at a thickness of 5 cm and a distance of 15 cm between lamp tubes and tea leaves. Then the tea shoots were withered separately at room temperature of 26 °C and relative humidity of 70% for 9 h under one type of the following light sources (yellow (585–590 nm, 1120–1430 lx), blue (410–430 nm, 190–270 lx), purple (390–410 nm, 200–260 lx), orange (590–600 nm, 1038–2000 lx), red (630–640 nm, 725–1015 lx), cyan (515–525 nm, 240–300 lx), green(555–565 nm, 2610–3680 lx), white (380–710 nm, 480–620 lx), ultraviolet light (260 nm, 540–710 lx)), and stirred every 1.5 h, with the ambient air of the leaves regulated by fan (on for 4 h, then off for 1 h and then on again). Meanwhile, dark treatment (no light in the withering equipment) was also performed and white light treatment was taken as control. The withered leaves were subjected to twisting and cutting machine in a continuous five-cut system. The machine-rolled leaves were fermented in an artificial climate box (RXZ-328A, China) at 30 °C and 85% relative humidity for 5.5 h with air flow, followed by treatment in a far infrared drying oven first at 135 °C for 30 min, then at 105 °C for about 1.5 h to obtain black tea containing about 5% moisture.
Experiment of withering with hybrid lights
The interaction between different lights was investigated by using 4 hybrid lights (red + yellow light, yellow + ultraviolet light, red + ultraviolet light and red + yellow + ultraviolet light) for the withering experiment. For each equipment, two or three lamp tubes with different lights were arranged in parallel and suspended from the ceiling. Meanwhile, three individual lights (red, yellow and ultraviolet light) were taken as control. Other procedures were the same as those described in Sect. Experiment of withering with different lights.
Sensory quality analysis
Black tea samples (10 g) were infused with 150 ml of boiled water for 5 min, and evaluated for sensory quality by three professional tea tasters from the Department of Tea Science at Huazhong Agricultural University, using the black tea quality grading system widely adopted in China (Liang et al. 2005). Evaluation was based on a total score of 100 marks, with 10% for the appearance of the dry tea, 30% for the aroma, 15% for the infusion color, 35% for the taste and 10% for the infused leaves.
Main composition analysis
Total tea polyphenols content was analyzed by using colorimetric method with Folin-Ciocalteu reagent according to the reference of ISO 14502-1:2005 (ISO 2005a).
Soluble sugar content was analyzed by using anthrone-sulfuric acid method as previously reported (Zhong 1989).
Catechins component was analyzed by High Performance Liquid Chromatography (HPLC) according to the reference of ISO 14502-2:2005 (ISO 2005b). The separation was performed on an ODS reversed-phase column (4.6 mm i.d. × 250 mm, 4.5 μm, TC-C18, Agela Technologies) in an Agilent LC1200 system (Agilent, USA) with a mobile phase of 9% acetonitrile containing 0.2% ethylene diamine tetraacetic acid (EDTA) and 2% acetic acid, at a flow-rate of 1.0 ml/min.
The free amino acids content was determined by using L-8800 automatic amino acid analyzer (Hitachi, Japan) according to the reference of the national standard of the PRC (GB/T5009.124-2003) (GB 2003). An ion exchange column (2622sc.ph, 4.6 × 60 mm) was chosen for the analysis with the temperatures of the reaction column and the separation column set at 57 and 135 °C, the column pressures at 10.0 MPa and 1.07 MPa, and the flow velocities at 0.40 and 0.35, respectively.
Aroma component was determined by using the simultaneous distillation and extraction (SDE) method to obtain aroma oils, and the Gas Chromatography-Mass Spectrometer (GC–MS) (Trace GM - PolarisQ MS) was used for separation and identification. Helium (He) was used as a carrier gas with a flow rate of 1.0 mL/min. The capillary column (DB-5MS, 30 m × 0.25 mm × 0.22 mm) was temperature programmed to 40 °C and hold for 2 min, then up to 110 °C at 2.0 °C/min and hold for 2 min, then up to 160 °C at 5.0 °C/min and hold for 1 min, and finally up to 220 °C at 5.0 °C/min and hold for 5 min. The GC-MS analysis was performed by electron impact (EI) ionization with electron energy of 70 eV, and the scan was from 50 to 650 amu. The aroma components were determined according to the spectral library and the literatures. The relative content of aroma components was calculated by using the ratio of peak area of the internal standard.
Theaflavin content was analyzed by quantifying the theaflavin extract using HPLC as described above in “3) Tea catechins component” (ISO 2005b).
Statistical analysis
All experimental data were analyzed by Excel and SPSS. The significance of differences between experimental groups was analyzed by variance analysis (ANOVA). For the significant values, the means were separated by the least significant difference (LSD) test at P ≤ 0.05 (* significant) and 0.01(** highly significant).
Results
Effect of light treatments on sensory quality
As shown in Table 1, the different light treatments during withering caused a remarkable influence on aroma, taste and appearance of black tea, but little on liquor color and infused leaf. Sweet aroma was detected in the yellow, red, orange and ultraviolet light treatments, resulting in a high score (≥26.3 ± 1.5), but no obvious sweet aroma was found in the cyan, dark and white light treatments. Interestingly, strong greenish flavor was sensed in the green light treatment, leading to the lowest sensory score (23.2 ± 0.8). The difference in taste is also listed in Table 1. The yellow, orange and red light treatments significantly improved the black tea taste, imparting it a fresh and mellow taste, which contributed to a high score (≥31.5 ± 0.5). However, the taste of black tea treated with dark and white light was just mellow, lacking fresh taste. Apart from the greenish aroma, the green light treatment also damaged the taste quality with green and astringency, which caused the lowest sensory score (27.3 ± 1.2). Overall, the yellow light treatment showed the best sensory quality, followed by the orange and red light treatments.
Table 1.
Sensory quality score of the black tea treated with different light sources (mean ± SD)
| Samples | Appearance (10) | Aroma (35) | Liquor color (15) | Taste (35) | Infused leaf (10) | TQS (100) |
|---|---|---|---|---|---|---|
| Dark | 8.1 ± 0.1cde | 24.3 ± 1.2bc | 12.5 ± 0.5a | 28.7 ± 0.6de | 8.5 ± 0.1a | 82.1 ± 0.4de |
| Ultraviolet light | 8.8 ± 0.3ab | 26.3 ± 1.5a | 12.7 ± 0.6a | 29.7 ± 0.6cd | 8.5 ± 0.2a | 85.9 ± 1.3bc |
| Yellow light | 8.9 ± 0.3a | 26.7 ± 1.3a | 13.2 ± 0.3a | 32.0 ± 0.5a | 8.5 ± 0.1a | 89.3 ± 1.9a |
| Blue light | 8.6 ± 0.4abc | 25.7 ± 0.6ab | 13.5 ± 0.5a | 30.5 ± 0.5abc | 8.5 ± 0.2a | 86.8 ± 1.1abc |
| Purple light | 8.5 ± 0.3abc | 25.5 ± 0.5ab | 13.5 ± 0.5a | 30.0 ± 1.0bcd | 8.5 ± 0.1a | 86.0 ± 1.3bc |
| White light | 8.0 ± 0.2de | 24.0 ± 1.3bc | 12.6 ± 0.6a | 28.7 ± 0.6de | 8.5 ± 0.1a | 81.7 ± 0.6e |
| Orange light | 8.9 ± 0.2a | 26.7 ± 1.0a | 13.0 ± 1.0a | 31.5 ± 0.5ab | 8.5 ± 0.2a | 88.6 ± 2.3ab |
| Red light | 8.9 ± 0.4ac | 26.7 ± 0.3a | 12.7 ± 0.6a | 31.5 ± 1.5ab | 8.5 ± 0.2a | 88.3 ± 1.7ab |
| Cyan light | 8.3 ± 0.3bd | 25.0 ± 1.0abc | 12.7 ± 0.3a | 30.0 ± 1.0bcd | 8.5 ± 0.3a | 84.5 ± 2.0cd |
| Green light | 7.8 ± 0.3e | 23.2 ± 0.8c | 12.7 ± 0.3a | 27.3 ± 1.2e | 8.5 ± 0.3a | 79.4 ± 1.4e |
Mean values with the same lower case letters in the same column indicate no significant difference at p = 0.05
SD standard deviation, TQS total quality score; each value was the average of three different samples
Effect of light treatments on soluble sugar, polyphenol and catechins
The effect of different withering lights on soluble sugar, polyphenol and catechins of black tea was presented in Table 2. Except for polyphenol, the light treatments varied significantly in their effects on the contents of soluble sugar (P < 0.05) and total tea catechins (P < 0.01). In the content of soluble sugar, the red and yellow light treatments, when compared with the white light treatment, showed a significant increase of 7.38 and 7.16% (P < 0.05), respectively, while the other treatments except for the dark treatment all had a slight but not significant increase.
Table 2.
Effects of light sources on catechins, polyphenols and soluble sugar content of tea (mean ± SD)
| Treatment | GC (mg/g) | EGC (mg/g) | C (mg/g) | EGCG (mg/g) | EC (mg/g) | ECG (mg/g) | Total tea catechins (mg/g) | Polyphenols (mg/g) | Soluble sugar (%) |
|---|---|---|---|---|---|---|---|---|---|
| Dark | 5.43 ± 0.19bc | 3.74 ± 0.07bcd | 0.47 ± 0.001 | 1.57 ± 0.01bcd | 0.57 ± 0.12bc | 1.25 ± 0.02de | 13.03 ± 0.36 cd | 83.71 ± 3.42 | 4.41 ± 0.04c |
| Ultraviolet light | 6.50 ± 0.69ab | 4.34 ± 0.42ab | 0.50 ± 0.02 | 1.99 ± 0.15ab | 0.88 ± 0.06ab | 1.60 ± 0.17ab | 15.82 ± 1.55ab | 87.73 ± 1.64 | 4.45 ± 0.25bc |
| Yellow light | 7.45 ± 0.78a | 3.51 ± 0.16 cd | 0.47 ± 0.01 | 2.26 ± 0.20a | 0.73 ± 0.06abc | 1.77 ± 0.16a | 16.18 ± 1.51a | 87.18 ± 2.62 | 4.79 ± 0.15a |
| Blue light | 6.97 ± 0.69ab | 3.84 ± 0.37bc | 0.51 ± 0.07 | 1.77 ± 0.06bcd | 0.77 ± 0.07abc | 1.59 ± 0.04ab | 15.45 ± 1.38abc | 88.92 ± 2.04 | 4.52 ± 0.20abc |
| Purple light | 5.93 ± 0.56abc | 3.42 ± 0.21cd | 0.54 ± 0.04 | 1.62 ± 0.08bcd | 0.67 ± 0.05abc | 1.44 ± 0.10bcd | 13.62 ± 1.23bcd | 85.87 ± 2.22 | 4.64 ± 0.16abc |
| White light | 5.48 ± 0.53bc | 3.12 ± 0.09d | 0.59 ± 0.06 | 1.37 ± 0.07d | 0.55 ± 0.04c | 1.20 ± 0.11e | 12.31 ± 1.17d | 84.72 ± 0.44 | 4.47 ± 0.26bc |
| Orange light | 7.40 ± 0.71a | 3.41 ± 0.21cd | 0.52 ± 0.06 | 1.69 ± 0.11bcd | 0.90 ± 0.03a | 1.39 ± 0.13bcde | 15.30 ± 1.19abc | 84.21 ± 1.17 | 4.62 ± 0.09abc |
| Red light | 5.49 ± 0.21bc | 3.67 ± 0.23cd | 0.52 ± 0.07 | 1.79 ± 0.05bc | 0.47 ± 0.00c | 1.53 ± 0.07bc | 13.47 ± 0.11bcd | 85.63 ± 4.78 | 4.80 ± 0.03a |
| Cyan light | 5.50 ± 0.57bc | 3.64 ± 0.35cd | 0.53 ± 0.03 | 1.54 ± 0.04cd | 0.50 ± 0.05c | 1.38 ± 0.12bcde | 13.09 ± 1.26 cd | 84.62 ± 2.44 | 4.73 ± 0.10ab |
| Green light | 4.67 ± 0.05c | 4.72 ± 0.14a | 0.53 ± 0.03 | 1.44 ± 0.03cd | 0.49 ± 0.03c | 1.34 ± 0.05cde | 13.18 ± 0.09 cd | 84.41 ± 3.62 | 4.52 ± 0.02abc |
| Significance | * | ** | Ns | ** | * | ** | ** | Ns | * |
SD standard deviation
*, ** indicate p < 0.05, and 0.01, respectively; Ns means not significant; each value was the average of three different samples; mean values with the same lowercase letters in the same column indicate no significant difference at p = 0.01 or 0.05
The effect of lights on the content of total catechins was in the order of yellow light > ultraviolet light > blue light > orange light > purple light > green light > red light > cyan light > dark > white light, with no significant difference among the ultraviolet, blue and orange light treatments or among the green, red, cyan, dark and white light treatments. Table 2 also listed the effects of different withering lights on main categories of catechin, with the highest amounts of gallocatechin (GC), EGCG and ECG found in the yellow light, and the highest EC in the orange light. It was also found that the different lights could not remarkably affect C, but green light could significantly increase epigallocatechin (EGC) content with the highest amount of 4.72 ± 0.14 mg/g.
Effect of light treatments on theaflavins
Theaflavins are important components of black tea taste. As shown in Table 3, light treatments had significant influence on theaflavins (P < 0.01), with yellow > orange = cyan > blue = red > purple > green > dark > ultraviolet > white lights. No obvious difference (P > 0.01) was observed among yellow, orange, cyan, blue and red light treatments, and between dark and ultraviolet light treatments. The predominant categories of theaflavin are Theaflavin (TF1), Theaflavin-3-gallate (TF2A), Theaflavin-3′-gallate (TF2B) and Theaflavin-3, 3′-digallate (TF3), which showed the same tendency as total theaflavins. The results indicated that yellow, orange and red light treatments could improve the taste quality of black tea.
Table 3.
Effects of light sources on contents of theaflavins in black tea (mg/g)(mean ± SD)
| Treatment | TF1 | TF2A | TF3 | TF2B | Total content |
|---|---|---|---|---|---|
| Dark | 0.22 ± 0.03ab | 0.41 ± 0.01cd | 0.20 ± 0.003d | 0.10 ± 0.002c | 0.92 ± 0.01cd |
| Ultraviolet light | 0.20 ± 0.01bc | 0.39 ± 0.02d | 0.19 ± 0.04d | 0.09 ± 0.005c | 0.87 ± 0.07d |
| Yellow light | 0.24 ± 0.01a | 0.49 ± 0.02ab | 0.34 ± 0.01a | 0.12 ± 0.005ab | 1.20 ± 0.04a |
| Blue light | 0.24 ± 0.01a | 0.48 ± 0.01ab | 0.28 ± 0.02c | 0.12 ± 0.007ab | 1.13 ± 0.02ab |
| Purple light | 0.21 ± 0.02ab | 0.45 ± 0.03bc | 0.30 ± 0.01abc | 0.12 ± 0.01b | 1.08 ± 0.06b |
| White light | 0.17 ± 0.002c | 0.32 ± 0.004e | 0.12 ± 0.001e | 0.08 ± 0.001d | 0.67 ± 0.01e |
| Orange light | 0.23 ± 0.02a | 0.51 ± 0.04a | 0.33 ± 0.02ab | 0.13 ± 0.01a | 1.16 ± 0.02ab |
| Red light | 0.23 ± 0.003a | 0.48 ± 0.03ab | 0.29 ± 0.05bc | 0.12 ± 0.01ab | 1.13 ± 0.08ab |
| Cyan light | 0.23 ± 0.02a | 0.49 ± 0.04ab | 0.32 ± 0.03abc | 0.13 ± 0.01ab | 1.16 ± 0.10ab |
| Green light | 0.23 ± 0.01a | 0.45 ± 0.003bc | 0.21 ± 0.001d | 0.12 ± 0.01b | 0.99 ± 0.01c |
| Significance | ** | ** | ** | ** | ** |
SD standard deviation
*, ** indicate p < 0.05, and 0.01, respectively; each value was the average of three different samples; mean values with the same lowercase letters in the same column indicate no significant difference at p = 0.01 or 0.05
Effect of light treatments on amino acids
The highest content of amino acids was found in the yellow light (9.92 mg/g), followed by purple (9.81 mg/g), cyan (9.47 mg/g), orange (9.47 mg/g), red (9.37 mg/g), ultraviolet (9.26 mg/g), blue (9.34 mg/g), white (9.11 mg/g), dark (9.08 mg/g) and green light (8.77 mg/g). No significant difference was observed between yellow and purple light treatments, nor among the cyan, orange and red light treatments or among the red, blue and ultraviolet light treatments. On the contrary, the green light treatment could significantly decrease the content of amino acids. According to Table 4, light treatments could produce obvious effect on most amino acids except for glycine, methionine and ornithine. Furthermore, the influence varied with lights. For instance, the yellow light had the highest content of theanine, glutamic acid, leucine, isoleucine, lysine and proline, while ultraviolet light showed the highest content of alanine and γ-aminobutyric acid. However, the green light exhibited the lowest content of all the amino acids. The results suggested that, compared with white light and dark treatment, yellow, purple, cyan, orange, red, blue and ultraviolet light could increase the content of amino acids, but not the green light.
Table 4.
Effects of light sources on amino acid composition (mg/g) of tea (mean ± SD)
| Amino acid | Dark | Ultraviolet light | Yellow light | Blue light | Purple light |
|---|---|---|---|---|---|
| Aspartic acid | 0.56 ± 0.010Dd | 0.56 ± 0.006Dd | 0.59 ± 0.002BCbc | 0.60 ± 0.014Bb | 0.63 ± 0.013Aa |
| Serine | 0.35 ± 0.001Cc | 0.36 ± 0.006BCbc | 0.36 ± 0.00Cc | 0.36 ± 0.004BCbc | 0.38 ± 0.001Aa |
| Glutamic acid | 0.89 ± 0.007Ha | 1.08 ± 0.007Cc | 1.14 ± 0.004Aa | 1.08 ± 0.002Cc | 1.10 ± 0.003Bb |
| Glycine | 0.01 ± 0.001 | 0.02 ± 0.006 | 0.01 ± 0.00 | 0.02 ± 0.004 | 0.01 ± 0.001 |
| Alanine | 0.23 ± 0.001Gg | 0.32 ± 0.00Aa | 0.29 ± 0.002Bb | 0.28 ± 0.001Cc | 0.28 ± 0.003Cc |
| Cystine | 0.12 ± 0.002Dd | 0.08 ± 0.002Dd | 0.10 ± 0.007Cc | 0.08 ± 0.005Dd | 0.10 ± 0.003Cc |
| Yaline | 0.39 ± 0.003Ee | 0.28 ± 0.003Ee | 0.30 ± 0.003Cc | 0.29 ± 0.002Dd | 0.32 ± 0.003Bb |
| Methionine | 0.02 ± 0.002 | 0.01 ± 0.00 | 0.02 ± 0.006 | 0.02 ± 0.004 | 0.02 ± 0.001 |
| Isoleucine | 0.12 ± 0.002Dd | 0.14 ± 0.003c | 0.16 ± 0.004ABa | 0.15 ± 0.002BCbc | 0.16 ± 0.003Aa |
| Leucine | 0.14 ± 0.002Dd | 0.15 ± 0.002Cc | 0.16 ± 0.002Aa | 0.16 ± 0.003BCbc | 0.16 ± 0.002ABa |
| Tyrosine | 0.48 ± 0.005Cd | 0.52 ± 0.002ABb | 0.54 ± 0.002Aab | 0.53 ± 0.012Aab | 0.54 ± 0.004Aab |
| Phenylalanine | 0.31 ± 0.002Ee | 0.32 ± 0.005Ee | 0.37 ± 0.010ABCab | 0.36 ± 0.005CDc | 0.38 ± 0.001Aa |
| γ-aminobutyricAcid | 0.11 ± 0.004Ee | 0.17 ± 0.002Aa | 0.16 ± 0.003Bb | 0.15 ± 0.001Cc | 0.15 ± 0.001Cc |
| Ornithine | 0.01 ± 0.003 | 0.01 ± 0.002 | 0.01 ± 0.002 | 0.01 ± 0.002 | 0.01 ± 0.002 |
| Lysine | 0.21 ± 0.002Ee | 0.28 ± 0.001ABa | 0.28 ± 0.005Aa | 0.28 ± 0.006ABab | 0.28 ± 0.004ABa |
| Proline | 1.08 ± 0.098ABa | 1.06 ± 0.001ABCDEab | 1.09 ± 0.081ABCa | 0.95 ± 0.008BCDEbc | 1.14 ± 0.065Aa |
| Histidine | 0.04 ± 0.001 | 0.05 ± 0.002 | 0.05 ± 0.003 | 0.06 ± 0.002 | 0.05 ± 0.002 |
| Tryptophan | 0.06 ± 0.004 | 0.05 ± 0.002 | 0.06 ± 0.003 | 0.05 ± 0.00 | 0.06 ± 0.003 |
| Arginine | 0.31 ± 0.00Aa | 0.28 ± 0.003Cd | 0.30 ± 0.003Aab | 0.29A ± 0.003Bbc | 0.30 ± 0.004Aab |
| Theanine | 3.67 ± 0.002Cc | 3.52 ± 0.023Ff | 3.93 ± 0.004Aa | 3.62 ± 0.006Cc | 3.74 ± 0.008Bb |
| Total content | 9.11 ± 0.152Ee | 9.26 ± 0.082DEd | 9.92 ± 0.150Aa | 9.34 ± 0.087Dd | 9.81 ± 0.127ABa |
| Amino acid | White light | Orange light | Red light | Cyan light | Green light | Significance |
|---|---|---|---|---|---|---|
| Aspartic acid | 0.54 ± 0.013De | 0.60 ± 0.003Bb | 0.58 ± 0.005Cc | 0.58 ± 0.003BCc | 0.59 ± 0.002BCbc | ** |
| Serine | 0.34 ± 0.003Dd | 0.37 ± 0.002Bb | 0.36 ± 0.004Cc | 0.37 ± 0.001Bb | 0.36 ± 0.003Cc | ** |
| Glutamic acid | 1.08 ± 0.00Cc | 1.06 ± 0.008Dd | 1.04 ± 0.003Ff | 1.05 ± 0.003Ff | 1.00 ± 0.006Gg | ** |
| Glycine | 0.01 ± 0.003 | 0.01 ± 0.002 | 0.01 ± 0.004 | 0.01 ± 0.001 | 0.01 ± 0.003 | Ns |
| Alanine | 0.28 ± 0.004Cc | 0.26 ± 0.004Ee | 0.28 ± 0.001Cc | 0.27 ± 0.002Dd | 0.24 ± 0.003Ff | ** |
| Cystine | 0.12 ± 0.006ABab | 0.10 ± 0.003Cc | 0.11 ± 0.011ABCbc | 0.13 ± 0.008Aa | 0.12 ± 0.006ab | ** |
| Yaline | 0.30 ± 0.002Cc | 0.32 ± 0.004Bb | 0.32 ± 0.003Bb | 0.33 ± 0.002Bb | 0.32 ± 0.003Bb | ** |
| Methionine | 0.01 ± 0.002 | 0.02 ± 0.005 | 0.01 ± 0.001 | 0.02 ± 0.006 | 0.02 ± 0.001 | Ns |
| Isoleucine | 0.15 ± 0.003BCbc | 0.16 ± 0.001ABa | 0.15 ± 0.001BCbc | 0.16 ± 0.001ABa | 0.16 ± 0.003ABab | ** |
| Leucine | 0.16 ± 0.002BCbc | 0.16 ± 0.003Aa | 0.15 ± 0.003BCbc | 0.16 ± 0.001ABa | 0.15 ± 0.001BCbc | ** |
| Tyrosine | 0.52 ± 0.010ABb | 0.54 ± 0.009Aab | 0.53 ± 0.009Aab | 0.54 ± 0.005Aa | 0.50 ± 0.01Bc | ** |
| Phenylalanine | 0.34 ± 0.009Dd | 0.37 ± 0.002ABCab | 0.36 ± 0.003BCDbc | 0.38 ± 0.006Aa | 0.35 ± 0.003Dcd | ** |
| γ-aminobutyricAcid | 0.15 ± 0.002Cc | 0.14 ± 0.002Dd | 0.15 ± 0.002Cc | 0.16 ± 0.004BCb | 0.14 ± 0.003Dd | ** |
| Ornithine | 0.01 ± 0.001 | 0.01 ± 0.003 | 0.01 ± 0.002 | 0.01 ± 0 | 0.01 ± 0.001 | Ns |
| Lysine | 0.26 ± 0.003CDcd | 0.27 ± 0.002BCbc | 0.27 ± 0.001BCbc | 0.26 ± 0.005CDcd | 0.26 ± 0.005Dd | ** |
| Proline | 0.90 ± 0.070DEc | 1.10 ± 0.019ABa | 1.07 ± 0.006ABCDab | 0.8 ± 0.0189Ec | 0.92 ± 0.092CDEc | ** |
| Histidine | 0.06 ± 0.006 | 0.05 ± 0.002 | 0.06 ± 0.006 | 0.06 ± 0.001 | 0.04 ± 0 | Ns |
| Tryptophan | 0.06 ± 0.00 | 0.06 ± 0.006 | 0.06 ± 0.003 | 0.06 ± 0.002 | 0.06 ± 0.002 | Ns |
| Arginine | 0.28 ± 0.005Cd | 0.30 ± 0.001Aa | 0.28 ± 0.003BCcd | 0.29A ± 0.003Bbc | 0.14 ± 0.002De | ** |
| Theanine | 3.54 ± 0.008EFe | 3.57 ± 0.004Dd | 3.56 ± 0.008DEd | 3.74 ± 0.003Bb | 3.28 ± 0.005Gg | ** |
| Total content | 9.11 ± 0.152Ee | 9.47 ± 0.083CDc | 9.37 ± 0.073Dcd | 9.470 ± 0.246CDc | 8.77 ± 0.154Ff | ** |
SD standard deviation
*, ** indicate p < 0.05, and 0.01, respectively; Ns means not significant; each value was the average of three different samples; mean values with the same lowercase letters or capital letters in the same row indicate no significant difference at p = 0.05 or 0.01
Effect of light treatments on aroma components
A total of 186 aroma substances were identified in aroma extracts from black tea and the main components were presented in Table 5. Hydrocarbons, alcohols, amides, acidic and esters, aldehydes and ketones, aromatics and heterocyclic compounds were the predominant aroma components in the tested black teas as showed in Table 6. The effect of the treatments on the total aroma content was in the order of yellow (24.50 ± 0.79) > ultraviolet (24.03 ± 0.66) > blue (24.29 ± 0.16) > red (23.80 ± 1.40) > dark (22.78 ± 0.25) > purple (22.70 ± 0.27) > orange (21.87 ± 0.51) > green (21.47 ± 0.88) > white (18.81 ± 0.09) > cyan (17.47 ± 0.48). According to Table 6, light treatments varied in their effects on aroma components. Firstly, the light treatments except cyan light all caused a significant increase of aldehydes and ketones content than the CK (white light), among which orange light had the highest content (10.53 ± 0.16) and yellow, red, blue light and dark treatments showed no significant differences with orange light. Secondly, red lights (7.50 ± 0.01), ultraviolet (7.29 ± 0.18), yellow (7.05 ± 0.25), blue (7.05 ± 0.10) all had a significant rise of the alcohols content than the control (5.76 ± 0.09) with no significant differences among each other. Thirdly, blue light had a significant increase of acidic and esters content (3.11 ± 0.05), followed with yellow (2.87 ± 0.08), purple (2.80 ± 0.25), ultraviolet (2.78 ± 0.02) light. Also, hydrocarbons, aromatics and heterocyclic compounds both had the same increase tendency. Blue light (1.96 ± 0.02) had the highest hydrocarbons content while red light (2.23 ± 0.13) had the highest aromatics and heterocyclic compounds. However, red (0.27 ± 0.09), yellow (0.24 ± 0.00), orange light (0.19 ± 0.00) showed a decrease of amides content than the control (0.30 ± 0.07). The results also revealed that the yellow, red and orange light treatments, which had a high aroma sensory score, also showed a high content of key aroma compounds, including hexanal, trans-3-Propyalacrolein, phenylacetaldehyde, geraniol, nerol, cedrol and so on. Among them, nerol [(Z)-isomer] and its isomer geraniol [(E)-isomer], which produce a sweet smell, were important indicators of high-grade black tea. The orange and yellow light treatments had the highest contents of nerol (0.13) and geraniol (1.23), respectively, which were 2.6 and 1.23 times that of cyan light treatment (the lowest, 0.05, 0.61, respectively). Additionally, the red light treatment with a high aroma score also had a high content of nerol (0.12) and geraniol (1.21). Conversely, the green light treatment had some compounds that are almost not present in other treatments, such as 1-octen-3-ol, 2-sec-butyl-cyclopentane, cis-11-eicosenamide and phenylethyl alcohol, which may cause bad smells to black tea, leading to the lowest aroma score.
Table 5.
Effects of light sources on major aroma compounds of black tea
| Time (min) | Aroma components | Relative content of aroma components with different light treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dark | Ultraviolet light | Yellow light | Blue light | Purple light | White light | Orange light | Red light | Cyan light | Green light | ||
| 3.28 | Cyclohexanol, 4-methyl-, tran- | 0.11 | 0.12 | 0.15 | 0.20 | 0.07 | 0.09 | 0.17 | 0.10 | 0.11 | 0.27 |
| 3.46 | 1-Octen-3-ol | – | – | – | – | – | 0.37 | – | 0.01 | 0.38 | |
| 3.58 | 1-Penten-3-ol | 0.48 | 0.47 | 0.43 | 0.57 | 0.02 | – | – | 0.29 | 0.30 | – |
| 4.54 | 2-Pentenal, (E)- | 0.05 | 0.07 | 0.07 | 0.05 | 0.09 | 0.07 | 0.05 | |||
| 4.82 | Oxirane, 2-(1,1-dimethyl ethyl)-3-methyl- | 0.18 | 0.20 | 0.15 | 0.18 | 0.38 | 0.23 | 0.12 | 0.43 | 0.17 | 0.12 |
| 4.88 | Oxirane, 2-(1,1-dimethylethyl)-3-ethyl-, cis- | 0.28 | 0.18 | 0.30 | 0.21 | 0.23 | 0.31 | – | – | 0.14 | |
| 5.07 | 2-Penten-1-ol, (Z)- | 0.12 | – | – | – | 0.04 | 0.07 | 0.10 | – | – | – |
| 5.36 | Hexanal | 1.22 | 0.88 | 0.96 | 1.05 | 1.51 | 0.65 | 1.22 | 0.90 | 1.01 | 0.69 |
| 6.71 | Furfural | 0.10 | 0.06 | 0.11 | – | – | – | 0.09 | 0.05 | 0.04 | 0.11 |
| 6.81 | 3-Methylpenta-1, 3-diene-5-ol, (E)- | 0.04 | 0.04 | 0.04 | – | 0.08 | 0.05 | – | – | – | – |
| 7.02 | 2-Hexenal, (E)- | 1.54 | 1.47 | 1.58 | 1.43 | 1.75 | 0.91 | 1.78 | 1.14 | 0.56 | 1.29 |
| 7.43 | 2-Octenal, (E)- | 0.06 | 0.12 | – | – | 0.02 | 0.02 | – | – | ||
| 7.86 | Cyclohexanol | 0.07 | 0.13 | 0.16 | 0.10 | 0.06 | 0.11 | 0.09 | 0.01 | 0.08 | 0.06 |
| 7.90 | Cyclobutane, 1,2-diethyl- | – | – | 0.05 | 0.04 | – | – | 0.06 | – | 0.02 | – |
| 7.94 | 1-Octene, 7-methyl- | 0.05 | 0.04 | – | – | – | 0.04 | – | – | – | 0.03 |
| 9.01 | trans-2-Cyclohexene-1,4-diol | 0.06 | 0.07 | 0.11 | 0.11 | 0.02 | 0.04 | 0.19 | 0.11 | 0.09 | 0.16 |
| 9.42 | 2,4-Hexadienal, (E,E)- | 0.07 | 0.05 | 0.06 | 0.04 | 0.03 | 0.04 | 0.07 | 0.02 | 0.03 | 0.06 |
| 11.35 | 6-(Hydroxy-phenyl-methyl)-2, 2-dimethyl-cyclohexanone | 0.04 | – | 0.04 | – | – | – | 0.07 | – | – | 0.02 |
| 11.45 | Benzaldehyde | 0.43 | 0.35 | 0.40 | 0.31 | 0.36 | 0.24 | 0.48 | 0.28 | 0.18 | 0.33 |
| 12.03 | Heptyl trifluoroacetate | 0.07 | 0.05 | 0.06 | 0.07 | – | 0.06 | 0.08 | 0.02 | 0.05 | |
| 12.43 | 1-Octen-3-ol | 0.08 | 0.06 | 0.07 | 0.08 | – | 0.06 | 0.10 | 0.02 | 0.02 | 0.05 |
| 12.64 | Bicyclo(3.1.1)heptane-2, 3-diol, 2,6,6-trimethyl- | 0.02 | 0.02 | 0.03 | 0.03 | 0.08 | 0.01 | 0.03 | 0.02 | 0.01 | 0.01 |
| 12.73 | 5-Hepten-2-one, 6-methyl- | – | – | 0.02 | – | – | 0.01 | 0.04 | – | – | – |
| 12.84 | Furan, 2-pentyl- | 0.49 | 0.39 | 0.42 | 0.50 | 0.66 | 0.41 | 0.36 | 0.51 | 0.34 | 0.55 |
| 13.43 | Octanal | 0.05 | 0.07 | 0.04 | 0.07 | 0.04 | 0.06 | 0.02 | 0.02 | 0.03 | |
| 13.67 | 2,4-Heptadienal, (E,E)- | 0.85 | 0.60 | 0.79 | 0.68 | 0.50 | 0.49 | 0.94 | 0.34 | 0.31 | 0.73 |
| 14.32 | Benzaldehyde, 3-benzyloxy-2-fluoro-4-methoxy- | 0.04 | 0.03 | 0.04 | – | – | – | – | – | 0.12 | |
| 14.91 | Benzene, [(methoxymethoxy)methyl]- | – | – | – | – | 0.03 | – | – | 0.03 | 0.09 | – |
| 14.94 | Benzene, [(2-propenyloxy)methyl]- | 0.11 | 0.10 | 0.08 | 0.12 | 0.08 | 0.09 | 0.29 | 0.15 | ||
| 15.14 | Benzene acetaldehyde | 1.58 | 1.75 | 1.91 | 1.50 | 1.45 | 1.09 | 2.07 | 1.16 | 0.79 | 1.56 |
| 15.53 | Icosapent | 0.02 | 0.02 | 0.02 | 0.02 | – | – | – | 0.01 | – | – |
| 15.97 | 1,2-Cyclooctanediol, trans- | 0.17 | 0.11 | 0.14 | 0.14 | 0.14 | 0.11 | 0.17 | 0.14 | 0.09 | 0.12 |
| 16.63 | à-Methyl-à-[4-methyl-3-pentenyl] oxiranemethanol | 1.41 | 1.57 | 1.80 | 1.83 | 1.33 | 1.37 | 1.70 | 0.88 | 0.84 | 1.49 |
| 17.66 | 3, 5-Octadien-2-one | 0.06 | – | 0.05 | – | 0.06 | 0.05 | 0.06 | – | – | 0.06 |
| 18.02 | 1, 6-Octadien-3-ol, 3, 7-dimethyl- | 0.54 | 0.60 | 0.69 | 0.72 | 0.40 | 0.55 | 0.72 | 0.36 | 0.27 | 0.57 |
| 18.25 | Nonanal | 0.40 | 0.32 | 0.46 | 0.43 | 0.30 | 0.35 | 0.46 | 0.34 | 0.27 | 0.29 |
| 18.69 | Phenylethyl Alcohol | 0.32 | 0.42 | 0.32 | 0.39 | 0.15 | 0.26 | 0.33 | 0.26 | 0.22 | 0.44 |
| 20.30 | trans-3-Nonen-2-one | – | – | 0.01 | – | – | – | 0.02 | – | – | 0.01 |
| 20.66 | (R,S)-5-Ethyl-6-methyl-3E-hepten-2-one | 0.10 | 0.10 | 0.11 | 0.11 | 0.11 | 0.08 | 0.13 | 0.07 | 0.07 | 0.09 |
| 21.04 | 2,6-Nonadienal, (E,Z)- | 0.01 | 0.02 | 0.03 | 0.03 | 0.04 | |||||
| 21.43 | 2-Nonenal, (E)- | 0.08 | 0.07 | 0.10 | 0.09 | 0.07 | 0.08 | 0.10 | 0.07 | 0.08 | 0.07 |
| 21.97 | 2H-Pyran-3-ol, 6-ethenyltetrahydro-2, 2, 6-trimethyl- | 0.62 | 0.85 | 0.71 | 0.72 | 0.36 | 0.58 | 0.67 | 0.73 | 0.59 | 0.77 |
| 22.11 | Cyclohexanol, 5-methyl-2-(1-methylethyl)-, [1R-(1à,2á,5à)]- | – | – | – | 0.02 | – | 0.01 | – | – | – | 0.03 |
| 22.49 | Naphthalene | 0.13 | 0.14 | 0.16 | 0.10 | 0.10 | 0.07 | 0.12 | 0.07 | 0.06 | 0.07 |
| 23.22 | 3-Cyclohexene-1-methanol, à, à, 4-trimethyl-, (S)- | 0.08 | 0.11 | 0.10 | 0.09 | 0.04 | 0.08 | 0.09 | 0.04 | 0.05 | 0.09 |
| 23.39 | Methyl salicylate | 0.19 | 0.21 | 0.25 | 0.25 | 0.21 | 0.19 | 0.22 | 0.24 | 0.15 | 0.16 |
| 23.72 | 1,3-Cyclohexadiene-1-carboxaldehyde, 2, 6, 6-trimethyl- | 0.04 | 0.05 | 0.05 | 0.05 | 0.04 | 0.04 | 0.05 | – | – | 0.04 |
| 23.88 | cis-Z-à-Bisabolene epoxide | – | – | 0.02 | – | 0.03 | – | – | 0.04 | 0.03 | – |
| 24.27 | Decanal | 0.11 | 0.10 | 0.13 | 0.16 | 0.08 | 0.11 | 0.13 | 0.13 | 0.10 | 0.12 |
| 24.51 | Icosapent | – | – | – | – | – | – | 0.01 | – | – | – |
| 24.53 | 1-Heptatriacotanol | – | 0.01 | – | – | – | – | – | – | – | – |
| 24.71 | 2,4-Nonadienal, (E,E)- | 0.04 | 0.06 | 0.04 | 0.03 | 0.04 | 0.04 | 0.04 | 0.03 | ||
| 25.00 | 1-Cyclohexene-1-carboxaldehyde, 2,6,6-trimethyl- | 0.19 | 0.14 | 0.20 | 0.19 | 0.17 | 0.15 | 0.20 | 0.14 | 0.13 | 0.13 |
| 25.20 | Cholestan-3-ol, 2-methylene-, (3á,5à)- | 0.10 | 0.10 | 0.14 | 0.02 | 0.03 | 0.10 | 0.10 | 0.05 | 0.03 | |
| 25.49 | Bicyclo[2.2.1]hept-2-ene, 1,7,7-trimethyl- | 0.10 | 0.12 | 0.11 | 0.11 | 0.10 | 0.08 | 0.11 | 0.11 | 0.09 | 0.10 |
| 25.65 | 2,6-Octadien-1-ol, 3,7-dimethyl-, (Z)- | 0.12 | 0.09 | 0.13 | 0.12 | 0.10 | 0.12 | 0.13 | 0.12 | 0.05 | 0.10 |
| 27.39 | 2,6-Octadien-1-ol, 3,7-dimethyl-, (E)- | 1.01 | 1.26 | 1.23 | 1.21 | 1.16 | 1.05 | 1.23 | 1.21 | 0.61 | 1.07 |
| 27.71 | 8-Hexadecenal, 14-methyl-, (Z)- | 0.40 | 0.45 | 0.45 | 0.43 | 0.43 | 0.38 | 0.46 | 0.62 | 0.46 | 0.43 |
| 27.93 | 7-Methyl-Z-tetradecen-1-ol acetate | 0.03 | 0.01 | 0.01 | 0.01 | – | 0.02 | 0.04 | 0.03 | 0.05 | – |
| 28.78 | Cyclohexene, 4-methyl-1-(1-methylethenyl)- | 0.03 | 0.03 | 0.03 | 0.03 | – | 0.02 | 0.03 | – | 0.02 | 0.04 |
| 29.23 | Naphthalene, 1-methyl- | 0.16 | 0.17 | 0.20 | 0.22 | 0.15 | 0.14 | 0.15 | 0.16 | 0.12 | 0.13 |
| 30.57 | Undecanal | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | |
| 30.84 | Alloaromadendrene oxide-(1) | 0.06 | 0.05 | 0.08 | 0.06 | 0.06 | 0.05 | 0.06 | 0.05 | 0.05 | 0.05 |
| 31.06 | 2,4-Decadienal, (E,E)- | 0.44 | 0.47 | 0.51 | 0.50 | 0.48 | 0.41 | 0.45 | 0.65 | 0.42 | 0.44 |
| 33.16 | Naphthalene, 1,2-dihydro-1,5,8-trimethyl- | 0.05 | 0.04 | 0.05 | 0.04 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 | 0.03 |
| 33.43 | Naphthalene,1,2,3,4-tetrahydro-1,5,7-trimethyl- | – | – | – | 0.02 | – | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 |
| 34.25 | 8-Hexadecenal, 14-methyl-, (Z)- | 0.21 | 0.24 | 0.23 | 0.22 | 0.22 | 0.20 | 0.21 | 0.32 | 0.20 | 0.19 |
| 34.73 | Benzene, (2,4-cyclopentadien-1-ylidenemethyl)- | 0.06 | 0.08 | 0.10 | 0.07 | 0.06 | 0.04 | 0.06 | 0.06 | 0.04 | 0.05 |
| 35.38 | 2-Buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-, (E)- | 0.16 | 0.20 | 0.18 | 0.20 | 0.20 | 0.15 | 0.14 | 0.25 | 0.18 | 0.16 |
| 35.71 | Hexanoic acid, hexyl ester | – | – | 0.04 | 0.04 | 0.06 | 0.02 | 0.03 | 0.04 | 0.03 | 0.02 |
| 35.90 | Hexanoic acid, 2-hexenyl ester, (E)- | 0.03 | 0.02 | 0.03 | – | 0.02 | 0.01 | 0.02 | 0.02 | – | |
| 36.02 | Naphthalene, 1,5-dimethyl- | 0.07 | 0.07 | 0.07 | 0.07 | – | 0.05 | 0.06 | 0.08 | 0.07 | – |
| 36.77 | Naphthalene, 1,3-dimethyl- | 0.10 | 0.12 | 0.12 | 0.10 | 0.10 | 0.10 | 0.09 | 0.13 | 0.09 | 0.09 |
| 37.08 | 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)but-3-en-2-one | 0.02 | 0.03 | 0.03 | 0.02 | 0.01 | 0.02 | 0.02 | 0.06 | 0.02 | 0.04 |
| 37.52 | 3-Buten-2-one, 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-, (E)- | 0.10 | 0.11 | 0.12 | 0.12 | 0.11 | 0.09 | 0.10 | 0.13 | 0.11 | 0.10 |
| 37.70 | 4-(2,4,4-Trimethyl-cyclohexa-1,5-dienyl)-but-3-en-2-one | 0.05 | 0.06 | 0.05 | 0.06 | 0.06 | 0.04 | 0.05 | 0.07 | 0.07 | 0.06 |
| 38.16 | 9,10-Secocholesta-5,7,10(19)-triene-3,25,26-triol, (3á,5Z,7E)- | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | – | 0.02 | 0.02 | – | – |
| 38.31 | 4-(2,6,6-Trimethyl-cyclohex-1-enyl)-butan-2-ol | 0.08 | 0.10 | 0.11 | 0.11 | 0.06 | 0.09 | 0.09 | 0.15 | 0.10 | 0.13 |
| 38.50 | 1,4,7,-Cycloundecatriene, 1,5,9,9-tetramethyl-, Z,Z,Z- | 0.01 | – | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 38.69 | 5,9-Undecadien-2-one, 6,10-dimethyl-, (E)- | 0.26 | 0.26 | 0.29 | 0.27 | 0.25 | 0.25 | 0.24 | 0.34 | 0.25 | 0.25 |
| 39.21 | E,E,Z-1,3,12-Nonadecatriene-5,14-diol | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | – | – | – | – |
| 39.44 | Acenaphthene | 0.07 | 0.08 | 0.09 | 0.06 | 0.08 | 0.06 | 0.05 | 0.08 | 0.06 | 0.06 |
| 39.55 | 1-Tetradecanol | – | – | – | – | – | – | – | 0.02 | – | – |
| 39.65 | 1,1′-Biphenyl, 2-methyl- | 0.05 | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 | 0.03 | 0.04 | 0.03 | 0.03 |
| 39.90 | 3-Buten-2-one, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-, (E)- | 1.11 | 1.26 | 1.26 | 1.15 | 1.16 | 1.04 | 1.03 | 1.50 | 1.04 | 1.09 |
| 40.47 | Pentadecane | 0.11 | 0.13 | 0.14 | 0.12 | 0.16 | 0.10 | 0.10 | 0.16 | 0.09 | 0.13 |
| 40.62 | Dibenzofuran | 0.11 | 0.13 | 0.11 | 0.08 | 0.10 | 0.07 | 0.07 | 0.12 | 0.08 | 0.09 |
| 40.75 | à-Farnesene | 0.13 | 0.36 | 0.31 | 0.29 | 0.42 | 0.17 | 0.30 | 0.43 | 0.30 | 0.32 |
| 40.91 | Phenol, 2,4-bis(1,1-dimethylethyl)- | 0.23 | 0.25 | 0.29 | 0.22 | 0.29 | 0.25 | 0.28 | 0.34 | 0.15 | 0.13 |
| 41.18 | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)- | 0.09 | 0.13 | 0.13 | 0.12 | 0.11 | 0.09 | 0.09 | 0.25 | 0.17 | 0.08 |
| 41.26 | 1,8(2H,5H)-Naphthalenedione, hexahydro-8a-methyl-, cis- | 0.22 | 0.20 | 0.21 | 0.17 | 0.17 | 0.15 | 0.12 | 0.34 | 0.22 | 0.18 |
| 41.44 | 1-Heptatriacotanol | 0.07 | 0.05 | 0.03 | 0.12 | 0.07 | 0.13 | 0.09 | 0.16 | 0.20 | 0.07 |
| 42.02 | Toluene-4-sulfonic acid, 2,7-dioxatricyclo[4.3.1.0(3,8)]dec-10-yl ester | 0.02 | – | – | – | 0.05 | 0.03 | 0.02 | – | – | 0.01 |
| 42.52 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl- | 1.16 | 1.73 | 1.56 | 1.37 | 1.61 | 1.12 | 0.98 | 2.16 | 1.32 | 1.29 |
| 42.69 | 3-Hexen-1-ol, benzoate, (Z)- | 0.08 | 0.17 | 0.10 | 0.13 | 0.10 | 0.08 | 0.10 | 0.26 | 0.15 | 0.16 |
| 42.76 | Fluorene | 0.12 | 0.27 | 0.13 | 0.09 | 0.10 | 0.09 | 0.06 | 0.15 | 0.07 | 0.09 |
| 42.90 | Benzoic acid, hexyl ester | – | – | – | 0.02 | – | – | 0.02 | 0.04 | 0.03 | |
| 43.14 | 2-Butenal, 2-methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 0.06 | 0.07 | 0.06 | 0.07 | 0.07 | 0.04 | 0.12 | 0.07 | 0.08 | |
| 43.52 | Cedrol | 0.37 | 0.46 | 0.56 | 0.45 | 0.43 | 0.43 | 0.33 | 0.59 | 0.40 | 0.42 |
| 43.79 | 12-Oxabicyclo[9.1.0]dodeca-3,7-diene, 1,5,5,8-tetramethyl-, [1R-(1R*,3E,7E,11R*)]- | 0.03 | 0.04 | 0.04 | 0.04 | 0.03 | – | 0.03 | 0.06 | – | 0.04 |
| 44.39 | Cubenol | 0.10 | 0.14 | 0.12 | 0.10 | 0.11 | 0.09 | 0.07 | 0.18 | – | 0.11 |
| 44.83 | tau.-Muurolol | 0.09 | 0.13 | 0.12 | 0.10 | 0.13 | 0.09 | 0.08 | 0.21 | 0.15 | 0.13 |
| 44.95 | 1-Naphthalenol, 1,2,3,4,4a,7,8,8a-octahydro-1,6-dimethyl-4-(1-methylethyl)-, [1R-(1à,4á,4aá,8aá)]- | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 | – | 0.02 | 0.06 | – | 0.04 |
| 45.20 | à-Cadinol | 0.07 | 0.12 | 0.06 | 0.06 | 0.07 | 0.09 | 0.05 | 0.12 | 0.07 | 0.08 |
| 46.50 | Tetratetracontane | 0.07 | 0.09 | 0.08 | 0.09 | 0.09 | 0.13 | 0.06 | 0.06 | – | – |
| 46.66 | Pentadecane, 2,6,10,14-tetramethyl- | – | – | – | – | – | 0.03 | 0.01 | 0.02 | – | 0.01 |
| 46.75 | 1,1′-Biphenyl, 2,2′,5,5′-tetramethyl- | 0.03 | 0.05 | 0.05 | 0.03 | 0.02 | – | 0.02 | 0.04 | 0.02 | 0.02 |
| 48.22 | Benzyl Benzoate | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | – | – | – | – | – |
| 48.37 | Phenanthrene | 0.12 | 0.16 | 0.14 | 0.08 | 0.12 | 0.06 | 0.06 | 0.19 | 0.09 | 0.11 |
| 48.63 | 4,6,6-Trimethyl-2-(3-methylbuta-1,3-dienyl)-3-oxatricyclo[5.1.0.0(2,4)]octane | – | – | – | 0.06 | 0.04 | – | 0.02 | 0.10 | – | 0.05 |
| 49.06 | Heneicosane | – | – | 0.02 | 0.02 | 0.02 | 0.04 | 0.01 | 0.02 | – | 0.01 |
| 49.99 | 2(1H)-Benzocyclooctenone, decahydro-10a-methyl-, trans- | 0.01 | 0.01 | 0.02 | 0.01 | – | – | – | 0.03 | 0.02 | 0.02 |
| 50.13 | 2-Pentadecanone, 6,10,14-trimethyl- | 0.34 | 0.39 | 0.29 | 0.29 | 0.31 | 0.26 | 0.16 | 0.43 | 0.26 | 0.24 |
| 50.67 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 0.19 | 0.22 | 0.19 | 0.19 | 0.22 | 0.17 | 0.13 | 0.32 | 0.20 | 0.19 |
| 51.79 | 5,9,13-Pentadecatrien-2-one, 6,10,14-trimethyl-, (E,E)- | 0.07 | 0.10 | 0.08 | 0.08 | 0.11 | 0.08 | 0.06 | 0.14 | 0.09 | 0.08 |
| 51.92 | Hexadecanoic acid, methyl ester | 0.21 | 0.21 | 0.20 | 0.15 | 0.21 | 0.12 | 0.09 | 0.18 | 0.11 | 0.11 |
| 52.38 | Isophytol | 0.02 | 0.03 | 0.02 | 0.02 | 0.01 | – | 0.02 | 0.02 | 0.01 | |
| 52.70 | n-Hexadecanoic acid | 0.13 | – | – | 0.13 | 0.27 | 0.13 | – | 0.42 | 0.33 | 0.27 |
| 54.30 | Kaur-16-ene | – | 0.04 | – | – | 0.03 | 0.04 | – | 0.01 | – | – |
| 54.69 | Heptasiloxane, hexadecamethyl- | 0.06 | 0.08 | 0.03 | 0.05 | – | 0.04 | – | 0.10 | 0.07 | 0.03 |
| 55.30 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | – | 0.02 | 0.02 | 0.02 | – | – | 0.01 | 0.02 | – | – |
| 55.30 | E,E,Z-1,3,12-Nonadecatriene-5,14-diol | – | – | – | – | 0.02 | 0.01 | – | 0.02 | – | 0.01 |
| 55.42 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | 0.03 | 0.05 | 0.04 | 0.04 | 0.05 | 0.03 | 0.02 | 0.07 | 0.05 | 0.04 |
| 55.77 | Phytol | 0.10 | 0.22 | 0.16 | 0.20 | 0.41 | 0.15 | 0.13 | 0.88 | 0.37 | 0.34 |
| 55.91 | Heptadecanoic acid, 10-methyl-, methyl ester | 0.05 | 0.03 | 0.05 | 0.03 | 0.04 | 0.03 | 0.02 | 0.02 | – | – |
| 56.21 | 11-Eicosenamide, (Z)- | – | – | – | – | 0.17 | 0.21 | – | – | 0.26 | 0.29 |
| 56.40 | Palmitoyl chloride | 0.16 | 0.14 | 0.14 | – | 0.25 | 0.13 | 0.11 | 0.13 | 0.09 | 0.10 |
| 57.18 | Octadecanoic acid, ethyl ester | 0.07 | 0.05 | 0.07 | 0.06 | 0.12 | 0.05 | 0.04 | 0.04 | 0.03 | 0.04 |
| 57.47 | Acetic acid n-octadecyl ester | – | – | – | 0.02 | – | 0.02 | – | 0.02 | – | 0.01 |
Table 6.
Effects of light sources on relative content of aroma components of tea (mean ± SD)
| Aroma components | Dark | Ultraviolet light | Yellow light | Blue light | Purple light |
|---|---|---|---|---|---|
| Hydrocarbons | 1.27 ± 0.01Dde | 1.66 ± 0.01BCb | 1.72 ± 0.06Bb | 1.96 ± 0.02Aa | 1.61 ± 0.12BCb |
| Alcohols | 6.14 ± 0.06BCb | 7.29 ± 0.18Aa | 7.05 ± 0.25Aa | 7.05 ± 0.10Aa | 5.69 ± 0.18CDc |
| Acidic and esters | 2.59 ± 0.02CDEde | 2.78 ± 0.02BCDbc | 2.87 ± 0.08Bb | 3.11 ± 0.05Aa | 2.80 ± 0.25BCbc |
| Aldehydes and ketones | 10.44 ± 0.01Aa | 9.38 ± 0.35BCbc | 10.49 ± 0.35Aa | 9.95 ± 0.06ABab | 10.13 ± 0.14ABab |
| Amides | 0.34 ± 0.01Bbc | 0.77 ± 0.06Aa | 0.24 ± 0.00Bbc | 0.31 ± 0.02Bbc | 0.45 ± 0.09Bb |
| Aromatics and heterocyclic compounds | 2.00 ± 0.16BCcd | 2.15 ± 0.03ABab | 2.13 ± 0.05ABabc | 1.90 ± 0.02Cd | 2.04 ± 0.02BCbc |
| Total content | 22.78 ± 0.25ABCbc | 24.03 ± 0.66ABa | 24.50 ± 0.79Aa | 24.29 ± 0.16ABa | 22.70 ± 0.27BCbc |
| Aroma components | White light | Orange light | Red light | Cyan light | Green light |
|---|---|---|---|---|---|
| Hydrocarbons | 1.55 ± 0.13BCbc | 1.42 ± 0.06CDcd | 1.63 ± 0.08BCb | 1.18 ± 0.16De | 1.57 ± 0.18BCbc |
| Alcohols | 5.76 ± 0.09CDc | 5.69 ± 0.17CDc | 7.50 ± 0.01Aa | 5.43 ± 0.26Dc | 6.47 ± 0.34Bb |
| Acidic and esters | 2.41 ± 0.02Ee | 2.5 ± 0.08Ede | 2.54 ± 0.16DEde | 2 ± 0.01Ff | 2.62 ± 0.06BCDEcd |
| Aldehydes and ketones | 7.25 ± 0.05Dd | 10.53 ± 0.16Aa | 9.62 ± 1.12ABCb | 6.87 ± 0.09Dd | 8.87 ± 0.31Cc |
| Amides | 0.30 ± 0.07Bbc | 0.19 ± 0.00Bc | 0.27 ± 0.09Bbc | 0.46 ± 0.08Bb | 0.37 ± 0.08Bbc |
| Aromatics and heterocyclic compounds | 1.53 ± 0.01De | 1.56 ± 0.05De | 2.23 ± 0.13Aa | 1.54 ± 0.02De | 1.57 ± 0.06De |
| Total content | 18.81 ± 0.09De | 21.87 ± 0.51Ccd | 23.80 ± 1.40ABab | 17.47 ± 0.48Df | 21.47 ± 0.88Cd |
SD standard deviation
Each value was the average of three different samples; mean values with the same lowercase letters or capital letters in the same row indicate no significant difference at p = 0.05 or 0.01
Effect of hybrid light withering
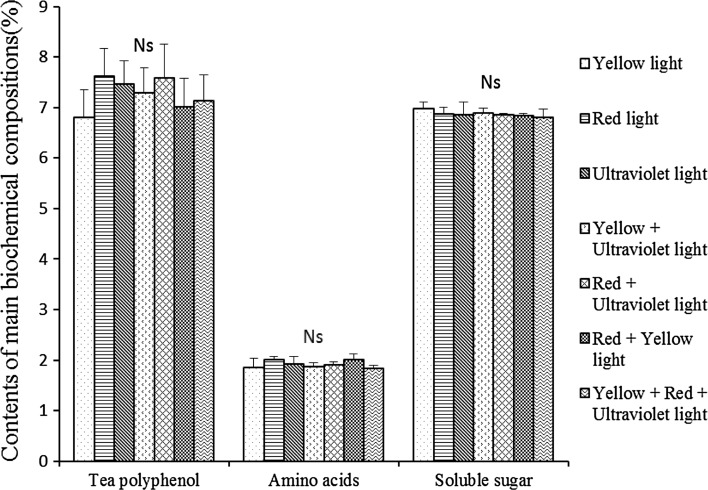
The aforementioned results showed that the red, yellow and ultraviolet light withering could improve the tea quality. Thus the interaction between different lights was investigated by using four hybrid lights. The result in Table 7 showed no significant difference in the sensory quality scores between hybrid lights and monochromatic light, implying that hybrid light withering had little cumulative effect. It was also found that the black tea withered with ultraviolet light, whether monochromatic or hybrid, showed a similar strong astringency. As shown in Fig. 2, no significant difference was found between hybrid and monochromatic light treatments in the content of polyphenol, soluble sugar and amino acids. Therefore, monochromatic light could be suggested to use for withering to improve the quality of black tea.
Table 7.
Sensory quality score of black tea samples withered with hybrid light sources (mean ± SD)
| Samples | Appearance (10) | Aroma (30) | Liquor colour (15) | Taste (35) | Infused leaf (10) | TQSb (100) |
|---|---|---|---|---|---|---|
| Yellow light | 8.8 ± 0.2 | 27.7 ± 0.6 | 13.7 ± 0.6 | 32.3 ± 0.6 | 8.9 ± 0.1 | 91.4 ± 1.5a |
| Red light | 8.8 ± 0.2 | 27.8 ± 0.8 | 13.7 ± 0.6 | 32.5 ± 0.5 | 8.9 ± 0.2 | 91.7 ± 1.0a |
| Ultraviolet light | 8.8 ± 0.3 | 27.3 ± 0.6 | 13.7 ± 0.6 | 31.7 ± 0.6 | 8.9 ± 0.2 | 90.4 ± 1.2a |
| Yellow + Ultraviolet light | 8.8 ± 0.2 | 27.5 ± 0.5 | 13.7 ± 0.6 | 32.3 ± 0.6 | 8.9 ± 0.3 | 91.2 ± 0.5a |
| Red + Ultraviolet light | 8.8 ± 0.1 | 27.5 ± 0.9 | 13.7 ± 0.6 | 32.0 ± 0.5 | 8.9 ± 0.1 | 90.9 ± 1.5a |
| Yellow + Red light | 8.8 ± 0.1 | 27.5 ± 0.5 | 13.8 ± 0.8 | 32.3 ± 0.6 | 8.9 ± 0.1 | 91.4 ± 0.6a |
| Yellow + Red + Ultraviolet light | 8.8 ± 0.2 | 27.3 ± 0.6 | 13.8 ± 0.3 | 32.0 ± 1.0 | 8.9 ± 0.2 | 90.8 ± 1.0a |
SD standard deviation
Each value was the average of three different samples; mean values with the same lowercase letters in the same column indicate no significant difference at p = 0.05
Fig. 2.
Effects of hybrid lights on main biochemical compositions of black tea samples (%) Bars represent standard errors of the mean (n = 3); Ns means not significant
Discussion
Amino acids, soluble sugars, tea polyphenols and their oxidation products (theaflavins) are the main taste contributors to the black tea and are responsible for the brisk, sweet, astringent and strong tastes of black tea (Roberts and Smith 1963; Scharbert et al. 2004). So high-quality black tea requires not only more quantity of these components, but also their suitable proportion. The present study indicated that yellow, orange and red light treatments were likely beneficial to a better taste of black tea, probably due to a higher content of amino acids (P < 0.05), theaflavins (P < 0.01) and soluble sugars (P > 0.01) than the control. While the other treatments like purple, cyan light and dark could also significantly increase the content of amino acids, the lower content of catechins could lead to a slightly lower taste score. Likewise, the ultraviolet light treatment could obviously increase the concentration of polyphenols and catechins, but the relatively lower content of amino acids and soluble sugar could result in an astringent taste with less freshness and sweetness.
The photoreceptors of leaf cells had a selective character in absorbing solar spectra. Photopigment and other compounds in leaf cells can absorb the luminous energy to catalyze the physiological activity of the enzyme, leading to a series of reactions and eventually affecting the black tea quality (Wang et al. 2006). The photoreceptors in tea leaf mainly include photopigment receptors, polyphenols, amino acids and so on. Firstly, chlorophyll and carotenoid are the most important photopigment receptors involved in photosynthesis, indicating that the photosynthesis and its products such as soluble sugar can be influenced by different light treatments. The maximum absorption peaks of chlorophyll and carotenoid are located in the red and orange light area (580–760 nm), followed by the blue and purple light area (390–480 nm) (Smith 1991). The light of these two wavelengths could possibly promote the photosynthesis and therefore increase the soluble sugar content. This result was consistent with the research by Leng et al. (2002) who found that younger ginkgo mulched with yellow, red and blue films could increase the photosynthetic rate and soluble sugar content of leaves. Talbott et al. (1993) also found that, under red light conditions, sucrose levels in guard cells of viciafaba increased to 208% of the initial level after 2 h red light treatment. Similarly, withering the tea leaves with red, yellow and blue lights can significantly increase the sugar content, which is conducive to the sweet taste.
In addition, the luminous energy absorbed by photopigments can also engage in the second photoperiodic reaction to catalyze the increase of reduced nicotinamide adenine dinucleotide phosphate (NADPH), which may provide energy and electron for the synthesis of amino acids and proteins in dark reaction. So the red, yellow and blue lights can also facilitate the synthesis of amino acids and proteins. Kowallik (1982) found the blue light could significantly accelerate the dark respiration rate in mitochondria and the increased organic acids from the respiration could provide more carbon skeletons for the synthesis of amino acids. Another study found that a rise of the blue light proportion could increase nitratereductase activity and respiratoryrate, which respectively provide ammonia materials and carbon skeletons for the synthesis of nitrogen compound, leading to an increase in the concentration of leaf nitrogen, amino acid and protein (Shi et al. 1999). Our results had a similar tendency that the amino acid content was obviously higher than that of the control after yellow, orange, red and blue light withering. However, the ultraviolet light treatment showed a lower content of amino acid, probably because amino acid was also a kind of photopigment, whose absorption area was located in ultraviolet light region (190–290 nm)(Smith 1991), and strong absorption of ultraviolet light induced the decarboxylation and oxidative degradation of amino acids (Wang et al. 2006).
Polyphenols had a strong absorption in the area of 290–390 nm (Smith 1991) which may cause degradation and oxidation and increased the content of catechins indirectly, and this was consistent with the results of blue and ultraviolet light treatments. Similar results were also observed in the yellow and orange light treatments, which may be related to the augment of amino acid. Since phenylalanine is the synthetic source of catechins, the augment of amino acid may probably indirectly increase the catechins content.
Theaflavins are produced as a result of enzymatic oxidation of catechins during black tea processing, indicating that the red, orange, yellow and blue lights increased the content of theaflavins indirectly owing to an increment in catechins. However, the ultraviolet light treatment did not show the same tendency, probably because polyphenols have a strong absorption of ultraviolet light (Smith 1991), leading to the degradation of ester catechins, the precursor of theaflavins.
It has been reported that the aroma could be related to the change of catechins, amino acids and sugars in the processing of black tea. Sanderson et al. (1971) found that oxidation of catechins could lead to the formation of oxidized catechins under the catalysis of polyphenol oxidase, which could accelerate the conversion of β-carotene to β-ionone, an important ketone aroma compound. Thus the red, orange and yellow light treatments can increase not only the content of catechins, but also the synthesis of ketone aroma compounds. Additionally, amino acids could be converted to aldehydes by decarboxylation and oxidative deamination. For example, glycine can be transformed into formaldehyde, alanine into acetaldehyde, valine into isobutyraldehyde, leucine into isovaleraldehyde, and so on (Co and Sanderson 1970). Furthermore, under high temperature conditions, amino acids, sugars and catechins can also produce furan, pyrrole, pyrazine and phenol compounds as well as their corresponding methyl, ethyl, hydroxide radical, acetyl derivatives, acetic acid and so on (Robinson and Owuor 1992). Therefore, the yellow, orange and red lights may probably promote the production of aroma compounds indirectly by increasing the content of amino acids, sugars and catechins.
Acknowledgements
This study was supported by Key Projects in the National Science & Technology Pillar Program during the 25-year Plan Period (2012BAF07B05-2) and the Fundamental Research Funds for the Central Universities (2662015PY136).
Abbreviations
- C
Catechin
- EC
Epicatechin
- ECG
Epicatechingallate
- EDTA
Ethylene diaminetetraacetic acid
- EGC
Epigallocatechin
- EGCG
Epigallocatechingallate
- EI
Electron impact
- GC
Gallocatechin
- GC–MS
Gas chromatography-mass spectrometer
- He
Helium
- HPLC
High performance liquid chromatography
- NADPH
Reduced nicotinamide adenine dinucleotide phosphate
- SDE
Simultaneous distillation and extraction
- TF1
Theaflavin
- TF2A
Theaflavin-3-gallate
- TF2B
Theaflavin-3′-gallate
- TF3
Theaflavin-3, 3′-digallate
Footnotes
Zeyi Ai and Beibei Zhang have contributed equally to this work and should be considered co-first authors.
References
- Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Christie JM. Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science. 1998;282:1698–1701. doi: 10.1126/science.282.5394.1698. [DOI] [PubMed] [Google Scholar]
- Co H, Sanderson GW. Biochemistry of tea fermentation:conversion of amino acid to black tea aroma constituents. J Food Sci. 1970;35:160–164. doi: 10.1111/j.1365-2621.1970.tb12128.x. [DOI] [Google Scholar]
- Fan S, Jin X, Yang Q, Liu D, Du X. Effects on main quality components and enzyme activity of tea leaves by artificial light withering. Hubei Agric Sci. 2012;51(06):1152–1155. [Google Scholar]
- Franklin KA, Larner VS, Whitelam GC. The signal transducing photoreceptors of plants. Int J Dev Biol. 2005;49:653–664. doi: 10.1387/ijdb.051989kf. [DOI] [PubMed] [Google Scholar]
- Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GB (2003) GB/T5009.124-2003. In: Determination of amino acids in foods
- ISO (2005a) ISO 14502-1:2005. In: Determination of substances characteristic of green and black tea—Part 1: content of total polyphenols in tea—colorimetric method using Folin-Ciocalteu reagent
- ISO (2005b) ISO 14502-2:2005. In: Determination of substances characteristic of green and black tea—Part 2: content of catechins in green tea—method using high-performance liquid chromatography
- Kowallik W. Blue light effects on respiration. Annu Rev Plant Physiol. 1982;33:51–72. doi: 10.1146/annurev.pp.33.060182.000411. [DOI] [Google Scholar]
- Leng P, Su S, Wang T, Jiang X, Wang S. Effect of lights intensity and light quality on photosynthesis, flavonol glycoside and terpene lactone content of Ginkgo biloba L. seedlings. J Plant Resour Environ. 2002;11:1–4. [Google Scholar]
- Liang Y, Lu J, Zhang L, Wu S, Wu Y. Estimation of tea quality by infusion colour difference analysis. J Sci Food Agric. 2005;85:286–292. doi: 10.1002/jsfa.1953. [DOI] [Google Scholar]
- Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore AR. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue Iight receptor cryptochrome 2. Proc Natl Acad Sci USA. 1997;95:2686–2690. doi: 10.1073/pnas.95.5.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EAH, Smith RF. The phenolic substances of manufactured tea. IX.—the spectrophotometric evaluation of tea liquors. J Sci Food Agric. 1963;14:689–700. doi: 10.1002/jsfa.2740141002. [DOI] [Google Scholar]
- Robinson JM, Owuor PO. Tea aroma. In: Willson KC, Clifford MN, editors. Tea: cultivation to consumption. Berlin: Springer; 1992. pp. 603–647. [Google Scholar]
- Sanderson GW, Co H, Gonzalez JG. Biochemistry of tea fermentation:the role of carotenes in black tea aroma formation. J Food Sci. 1971;36:231–236. doi: 10.1111/j.1365-2621.1971.tb04031.x. [DOI] [Google Scholar]
- Scharbert S, Jezussek M, Hofmann T. Evaluation of the taste contribution of theaflavins in black tea infusions using the taste activity concept. Eur Food Res Technol. 2004;218:442–447. doi: 10.1007/s00217-004-0888-3. [DOI] [Google Scholar]
- Shi H, Han J, Guan C, Yuan T. Effects of red and blue light proportion on leaf growth, carbon-nitrogen metabolism and quality in tobacco. Acta Agron Sin. 1999;25:215–220. [Google Scholar]
- Smith K. Experiments reprinted from the science of photobiology. In: Valenzeno D, Pottier R, Mathis P, Douglas R, editors. Photobiological techniques. 1. US: Springer; 1991. pp. 347–361. [Google Scholar]
- Talbott LD, Zeiger E. Sugar and organic acid accumulation in guard cells of Vicia faba in response to red and blue light. Plant Physiol. 1993;102:1163–1169. doi: 10.1104/pp.102.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Li D, Zhang Z, Xia T, Ling T, Chen Q. Research advance on tea biochemistry. J Tea Sci. 2015;35(1):1–10. [Google Scholar]
- Wang T, Wang H, Meng F, Zhang L. The application of PVC coloured mulching films in ginseng. J Chin Med Mater. 1989;12:5–8. [Google Scholar]
- Wang D, Zhang L, Mao M, Jiang S. Effects of withered with different light-wave bands on qualities of Dancong tea. J Food Sci Biotechnol. 2006;25:56–59. [Google Scholar]
- Wu Q, Dong Q, Sun W, Huang Y, Wang Q, Zhou W. Discrimination of Chinese teas with different fermentation degrees by stepwise linear discriminant analysis (S-LDA) of the chemical compounds. J Agric Food Chem. 2014;62:9336–9344. doi: 10.1021/jf5025483. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang S, Luo J, Ye Z, Li S. Advance in research on fruit anthoyanin synthesis. J Fruit Sci. 2004;21:456–460. [Google Scholar]
- Zhao D, Li M, Xing J, Tong Z. Effects of light on cell growth and flavonoids biosynthesis in Callus cultures of Saussurea medusa maxim. Acta Phytophysiol Sin. 1999;25:127–132. [Google Scholar]
- Zhong L. Physical and chemical analysis on tea quality. Shanghai: Shanghai Scientific & Technical Publishers; 1989. [Google Scholar]