Abstract
Different adiposity measures have been associated with increased risk of atrial fibrillation, however, results have previously only been summarized for BMI. We therefore conducted a systematic review and meta-analysis of prospective studies to clarify the association between different adiposity measures and risk of atrial fibrillation. PubMed and Embase databases were searched up to October 24th 2016. Summary relative risks (RRs) were calculated using random effects models. Twenty-nine unique prospective studies (32 publications) were included. Twenty-five studies (83,006 cases, 2,405,381 participants) were included in the analysis of BMI and atrial fibrillation. The summary RR was 1.28 (95% confidence interval: 1.20–1.38, I2 = 97%) per 5 unit increment in BMI, 1.18 (95% CI: 1.12–1.25, I2 = 73%, n = 5) and 1.32 (95% CI: 1.16–1.51, I2 = 91%, n = 3) per 10 cm increase in waist and hip circumference, respectively, 1.09 (95% CI: 1.02–1.16, I2 = 44%, n = 4) per 0.1 unit increase in waist-to-hip ratio, 1.09 (95% CI: 1.02–1.16, I2 = 94%, n = 4) per 5 kg increase in fat mass, 1.10 (95% CI: 0.92–1.33, I2 = 90%, n = 3) per 10% increase in fat percentage, 1.10 (95% CI: 1.08–1.13, I2 = 74%, n = 10) per 5 kg increase in weight, and 1.08 (95% CI: 0.97–1.19, I2 = 86%, n = 2) per 5% increase in weight gain. The association between BMI and atrial fibrillation was nonlinear, p nonlinearity < 0.0001, with a stronger association at higher BMI levels, however, increased risk was observed even at a BMI of 22–24 compared to 20. In conclusion, general and abdominal adiposity and higher body fat mass increase the risk of atrial fibrillation.
Electronic supplementary material
The online version of this article (doi:10.1007/s10654-017-0232-4) contains supplementary material, which is available to authorized users.
Keywords: Obesity, BMI, Waist circumference, Hip circumference, Waist-to-hip ratio, Fat mass, Fat percentage, Atrial fibrillation, Meta-analysis
Introduction
The prevalence of overweight and obesity has increased rapidly over the last decades in all areas of the world [1]. Overweight and obesity are important risk factors for a wide range of chronic diseases, including cardiovascular diseases, type 2 diabetes, gallbladder disease, total mortality and several types of cancer [2–11], and the current trends are a major challenge for public health both in terms of reduced quality of life and increased medical costs [12].
Atrial fibrillation is the most common arrhythmia diagnosed in clinical practice [13] and globally there was an estimated 5 million incident cases in 2010 [14], while the prevalence was estimated at 33 million in 2015 [15]. The prevalence of atrial fibrillation has been projected to increase 2.5-fold in the next 50 years, mainly due to an aging population, but also due to an increased incidence of the disease [13]. Patients with atrial fibrillation are at increased risk of cardiovascular diseases including ischemic heart disease, heart failure, sudden cardiac death, stroke, as well as chronic kidney disease and all cause mortality [16]. The economic costs due to atrial fibrillation in the US has been estimated at more than $6 billion annually [17]. Overweight and obesity have been associated with increased risk of atrial fibrillation in several studies [18, 19]. Some studies suggested a J-shaped dose–response relationship between BMI and atrial fibrillation [20, 21], however, other studies suggested a linear association [22–28]. In addition, it is not clear whether other measures of body fatness such as waist circumference [26, 29–32], hip circumference [30, 32, 33], waist-to-hip ratio [29, 30, 32, 33], fat mass [30–32, 34], or body fat percentage [30, 31, 34] are associated with risk of atrial fibrillation or if the association differs by geographic location or ethnicity. Although a meta-analysis from 2008 found that both overweight and obesity as measured by body mass index (BMI) was associated with increased risk of atrial fibrillation [35], at least 20 additional studies involving >78,000 atrial fibrillation cases and >2.2 million participants have been published since that meta-analysis [20, 21, 23–31, 33, 34, 36–42]. Given the large number of additional studies that have been published since the previous meta-analysis and the availability of data regarding other adiposity measures as well, we conducted a systematic review and dose–response meta-analysis of prospective studies that investigated the association between body mass index, waist circumference, waist-to-hip ratio, or other measures of adiposity (hip circumference, fat mass, weight, weight gain) and the risk of atrial fibrillation.
Methods
Search strategy and inclusion criteria
We searched the PubMed and Embase databases up to October 24th 2016 for eligible studies (DA, SS and AS). A list of the search terms used are provided in Supplementary Tables 1 and 2. We followed standard criteria (MOOSE Guidelines) for reporting meta-analyses [43]. In addition, we searched the reference lists of previous meta-analyses [2, 35, 44] and the reference lists of the relevant publications for further studies. Study quality was assessed using the Newcastle–Ottawa scale [45].
Study selection
We included prospective and retrospective cohort studies and nested case–control studies of the association between adiposity measures (BMI, waist circumference, and waist-to-hip ratio, hip circumference, body fat mass, fat percentage, weight, weight gain) and risk of atrial fibrillation that were published in English. Studies in high-risk populations (patient populations), abstract only publications, grey literature and unpublished studies were excluded. Adjusted relative risk (RR) estimates (hazard ratios, risk ratios, or odds ratios) had to be available with the 95% confidence intervals (95% CIs) in the publication and for the dose–response analysis, a quantitative measure of adiposity and the total number of cases and person-years or non-cases for at least 3 categories of the adiposity variable or on a continuous scale had to be available in the publication. When multiple publications were available from the same study we used the study with the largest number of cases, but when data on different anthropometric measures were covered by different publications from the same study both were included, but each study was only included once in each analysis. A list of the excluded studies and exclusion reasons are found in the Supplementary Table 3.
Data extraction
We extracted the following data from each study: The first author’s last name, publication year, country where the study was conducted, study period, sample size, number of cases/controls, exposure variable, exposure level, relative risks and 95% confidence intervals for the highest versus the lowest level of the exposure variable and variables adjusted for in the analysis. Data were extracted by one reviewer (DA) and checked for accuracy by a second reviewer (AS).
Statistical analysis
We calculated summary RRs and 95% CIs for a 5 unit increment in BMI, 10 cm increment in waist and hip circumference, a 0.1 unit increment in waist-to-hip ratio, and a 5 kg increment in fat mass and weight, 10% increase in body fat percentage, and 5% increase in weight gain using a random effects model [46]. For the primary analysis we used the model from each study that had the greatest degree of control for potential confounding with the exception of when potential intermediate risk factors were adjusted for in a separate step (as an exploration of how much of the association might be mediated by cholesterol for example). The average of the natural logarithm of the RRs was estimated and the RR from each study was weighted according to the method of DerSimonian and Laird [46]. A two-tailed p < 0.05 was considered statistically significant. If studies reported results separately for men and women or other subgroups we combined the subgroup-specific estimates using a fixed-effects model to generate an overall estimate so that each study was only represented once in the main analysis, but sex-specific results are presented separately in subgroup analyses.
The method described by Greenland and Longnecker [47] was used for the dose–response analysis and we calculated study-specific slopes (linear trends) and 95% CIs from the natural logs of the reported RRs and CIs across categories of each adiposity measure. The mean or median level of each adiposity measure in each category was assigned to the corresponding relative risk for every study and for studies that reported the exposures in ranges we calculated the average of the upper and the lower cut-off point which was used as a midpoint. When the lowest or highest category was open-ended or had an extreme range we used the width of the adjacent interval to calculate an upper or lower confidence interval. A potential nonlinear dose–response relationship between BMI and waist circumference and risk of atrial fibrillation was examined by using fractional polynomial models [48]. We determined the best fitting second order fractional polynomial regression model, defined as the one with the lowest deviance. A likelihood ratio test was used to assess the difference between the nonlinear and linear models to test for nonlinearity [48].
Subgroup analyses stratified by sex, measurement versus self-report of adiposity measures, duration of follow-up, geographic location, number of cases, study quality scores, and adjustment for confounders (age, smoking, alcohol, and physical activity) and potential intermediates (hypertension, blood pressure, cholesterol, diabetes mellitus, coronary heart disease, heart failure, and left ventricular hypertrophy) were conducted to investigate potential sources of heterogeneity and heterogeneity between studies was quantitatively assessed by the Q test and I2 [49]. Meta-regression analyses were used to examine between subgroup differences in the summary estimates. Small study effects, such as publication bias, were assessed by inspecting the funnel plots for asymmetry and with Egger’s test [50] and Begg’s test [51] with the results considered to indicate small study effects when p < 0.10. Sensitivity analyses excluding one study at a time were conducted to clarify whether the results were simply due to one large study or a study with an extreme result.
Results
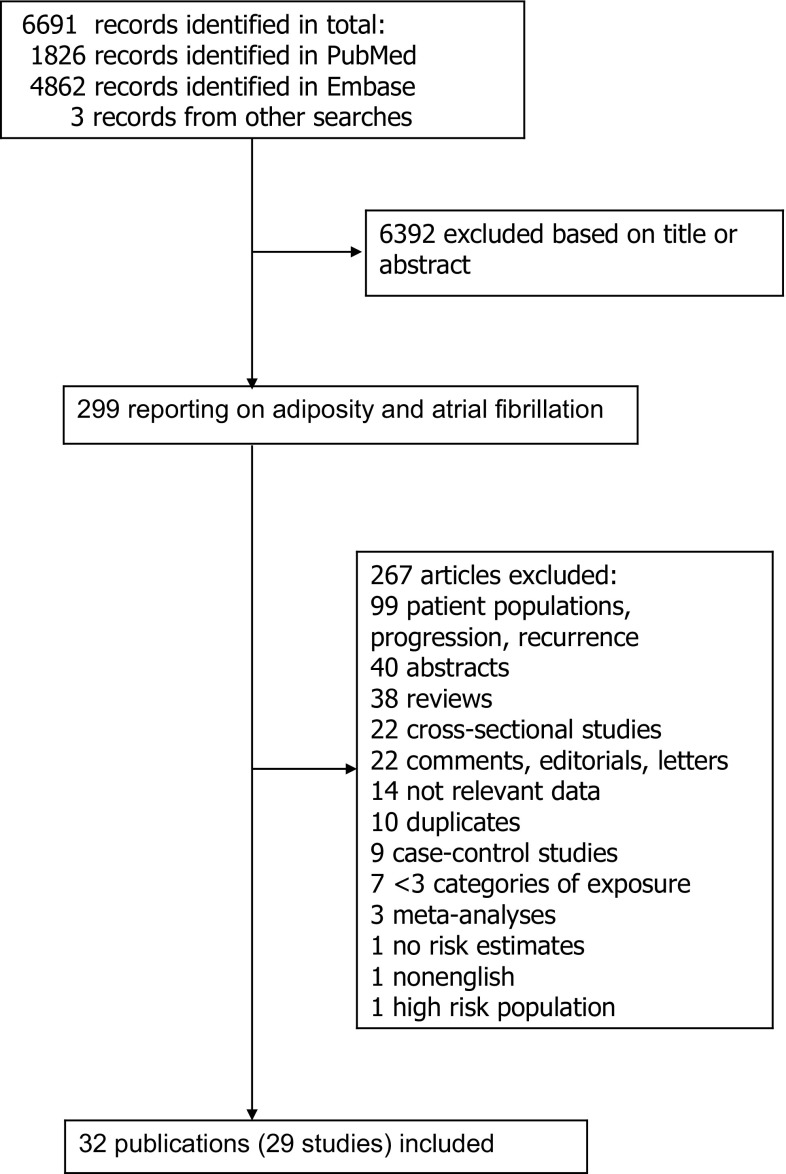
We identified 29 prospective studies (32 publications) that were included in the systematic review of BMI, waist circumference, hip circumference, waist-to-hip ratio, body fat mass, body fat percentage, weight, and weight gain and risk of atrial fibrillation (Supplementary Table 4, Fig. 1) [18–34, 36–42, 52–59]. Only one study reported on pericardial fat, intrathoracic fat, and abdominal visceral fat and atrial fibrillation, thus it was not possible to conduct meta-analyses for these measures [52]. In addition, only one study reported on BMI and mortality from atrial fibrillation, thus the study was excluded from the main analysis, but it was included in a sensitivity analysis [54]. Fourteen studies were from Europe, eight were from the USA, four were from Asia, and three were from Australia (Supplementary Table 4).
Fig. 1.
Flow-chart of study selection
Body mass index
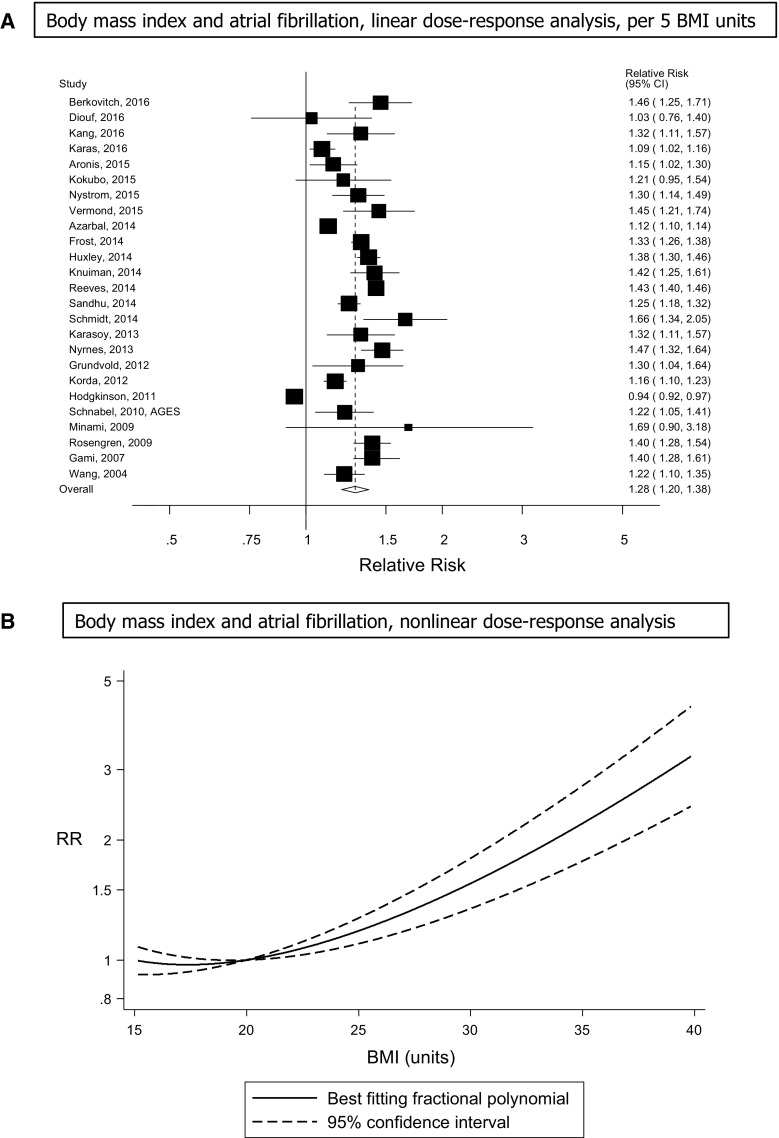
Twenty-five prospective studies (25 publications) [18–21, 23–34, 36–40, 42, 55–57] including two nested case–control studies (were included in the dose–response analysis of BMI and atrial fibrillation incidence and included 83,006 incident cases among 2,405,381 participants. The summary RR for a 5 unit increment in BMI was 1.28 (95% confidence interval: 1.20–1.38, I2 = 97%, p heterogeneity < 0.0001; Fig. 2a, Supplementary Table 7 ), and it was similar when stratified by gender, but the heterogeneity was lower among men (I2 = 37%) compared to women (I2 = 98%; Table 1). All but one of the studies found increased risk, but the strength of the association differed between studies. In sensitivity analyses excluding the most influential studies, the summary RR ranged from 1.27 (95% CI: 1.18–1.37) when excluding the Danish Military Conscripts study [28] to 1.30 (95% CI: 1.23–1.38) when excluding the UK General Practice Research Database Study [20]. There was no indication of publication bias with Egger’s test, p = 0.31, or with Begg’s test, p = 0.44, however, by inspection of the funnel plot there was some evidence of asymmetry with potentially smaller negative studies missing (Supplementary Fig. 1). There was evidence of a nonlinear association between BMI and atrial fibrillation, p nonlinearity < 0.0001 (Fig. 2b, Supplementary Table 5) with a steeper increase in risk at higher BMI values. In a sensitivity analysis, one study of BMI and atrial fibrillation mortality [54] was included in the analysis, but the results remained similar, summary RR = 1.29 (95% CI: 1.20–1.38, I2 = 97%, p heterogeneity < 0.0001) per 5 BMI units.
Fig. 2.
BMI and atrial fibrillation, linear and nonlinear dose–response analysis
Table 1.
Subgroup analyses of BMI and atrial fibrillation
| BMI | ||||||
|---|---|---|---|---|---|---|
| n | RR (95% CI) | I a (%) | P bh | P ch | ||
| All studies | 25 | 1.28 (1.20–1.38) | 96.8 | <0.0001 | ||
| Sex | ||||||
| Men | 9 | 1.39 (1.30–1.48) | 37.2 | 0.12 | 0.05/0.21 | |
| Women | 7 | 1.30 (1.14–1.48) | 98.1 | <0.0001 | ||
| Men and women | 11 | 1.25 (1.11–1.39) | 94.3 | <0.0001 | ||
| Assessment of weight/height | ||||||
| Measured | 19 | 1.27 (1.18–1.37) | 95.2 | <0.0001 | 0.66 | |
| Self-reported | 4 | 1.28 (1.13–1.45) | 94.9 | <0.0001 | ||
| Not available | 2 | 1.41 (1.26–1.58) | 0 | 0.57 | ||
| Duration of follow-up | ||||||
| <5 years | 3 | 1.28 (1.12–1.47) | 78.2 | 0.01 | 0.70 | |
| 5 ≤ 10 years | 7 | 1.37 (1.27–1.47) | 38.8 | 0.13 | ||
| 10 ≤ 15 years | 8 | 1.23 (1.13–1.33) | 91.0 | <0.0001 | ||
| 15 ≤ 20 years | 3 | 1.33 (1.23–1.44) | 72.3 | 0.03 | ||
| ≥20 years | 4 | 1.29 (0.96–1.73) | 96.8 | <0.0001 | ||
| Geographic location | ||||||
| Europe | 10 | 1.34 (1.16–1.56) | 98.5 | <0.0001 | 0.47 | |
| America | 8 | 1.22 (1.14–1.31) | 89.8 | <0.0001 | ||
| Australia | 3 | 1.22 (1.04–1.44) | 78.1 | 0.01 | ||
| Asia | 4 | 1.37 (1.23–1.52) | 0 | 0.53 | ||
| Number of cases | ||||||
| Cases < 250 | 5 | 1.38 (1.22–1.57) | 43.4 | 0.13 | 0.10 | |
| Cases 250 ≤ 1000 | 10 | 1.32 (1.24–1.40) | 47.2 | 0.05 | ||
| Cases ≥ 1000 | 10 | 1.23 (1.11–1.36) | 98.7 | <0.0001 | ||
| Study quality | ||||||
| 0–3 | 0 | 0.92 | ||||
| 4–6 | 3 | 1.29 (1.08–1.54) | 78.3 | 0.01 | ||
| 7–9 | 22 | 1. 28 (1.19–1.38) | 97.2 | <0.0001 | ||
| Adjustment for confounders | ||||||
| Age | Yes | 23 | 1.27 (1.18–1.37) | 97.0 | <0.0001 | 0.24 |
| No | 2 | 1.45 (1.15–1.83) | 71.8 | 0.06 | ||
| Smoking | Yes | 14 | 1.21 (1.11–1.32) | 98.1 | <0.0001 | 0.01 |
| No | 11 | 1.40 (1.34–1.47) | 0 | 0.56 | ||
| Alcohol | Yes | 12 | 1.26 (1.11–1.42) | 98.2 | <0.0001 | 0.53 |
| No | 13 | 1.30 (1.21–1.40) | 86.1 | <0.0001 | ||
| Physical activity | Yes | 10 | 1.30 (1.18–1.43) | 97.2 | <0.0001 | 0.66 |
| No | 15 | 1.28 (1.16–1.42) | 94.3 | <0.0001 | ||
| Adjustment for potential intermediates | ||||||
| Hypertension | Yes | 11 | 1.24 (1.13–1.35) | 96.3 | <0.0001 | 0.18 |
| No | 14 | 1.33 (1.23–1.43) | 88.0 | <0.0001 | ||
| Blood pressure | Yes | 7 | 1.26 (1.17–1.35) | 32.4 | 0.18 | 0.76 |
| No | 18 | 1.29 (1.19–1.40) | 97.7 | <0.0001 | ||
| Cholesterol | Yes | 6 | 1.28 (1.15–1.43) | 93.6 | <0.0001 | 0.99 |
| No | 19 | 1.29 (1.17–1.42) | 97.2 | <0.0001 | ||
| Diabetes mellitus | Yes | 11 | 1.24 (1.14–1.36) | 96.6 | <0.0001 | 0.29 |
| No | 14 | 1.32 (1.22–1.42) | 88.2 | <0.0001 | ||
| Coronary heart disease | Yes | 13 | 1.27 (1.16–1.38) | 96.3 | <0.0001 | 0.58 |
| No | 12 | 1.30 (1.20–1.41) | 90.6 | <0.0001 | ||
| Heart failure | Yes | 8 | 1.18 (1.06–1.31) | 96.7 | <0.0001 | 0.02 |
| No | 17 | 1.33 (1.26–1.41) | 86.8 | <0.0001 | ||
| Left ventricular hypertrophy | Yes | 2 | 1.19 (1.10–1.28) | 0 | 0.48 | 0.39 |
| No | 23 | 1.29 (1.20–1.39) | 97.1 | <0.0001 | ||
n denotes the number of studies
a P for heterogeneity within each subgroup
b P for heterogeneity between subgroups
c P for heterogeneity between men and women (excluding men/women combined)
Waist circumference
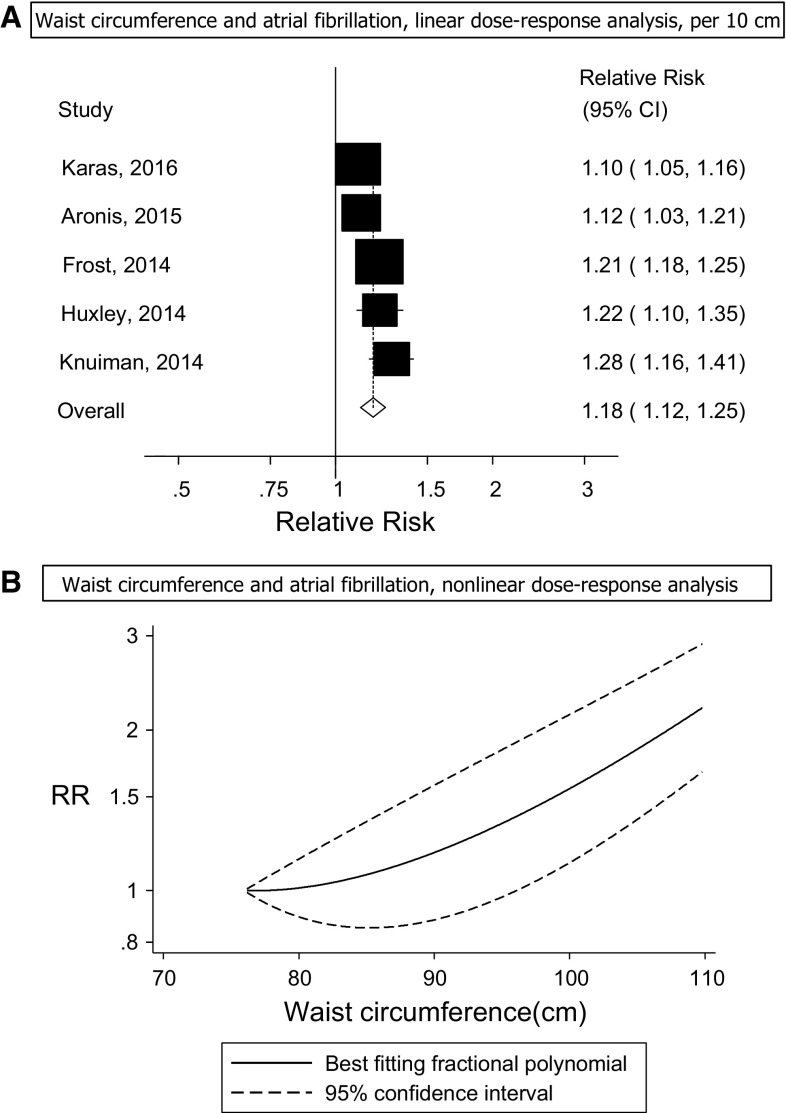
Five prospective studies (5 publications) [26, 29–32] were included in the analysis of waist circumference and risk of atrial fibrillation incidence and included 6120 cases among 80,752 participants. Three studies were from the USA, one from Denmark and one from Australia (Supplementary Table 4). The summary RR for a 10 cm increase in waist circumference was 1.18 (95% CI: 1.12–1.25, I2 = 73%, p heterogeneity = 0.005) (Fig. 3a, Supplementary Table 7). The summary RR ranged from 1.16 (95% CI: 1.10–1.23) when the Busselton Health Study [29] was excluded to 1.20 (95% CI: 1.15–1.26) when the Cardiovascular Health Study [32] was excluded. There was no evidence of publication bias with Egger’s test, p = 0.85 or Begg’s test, p = 0.99, although the number of studies was limited. There was no evidence of a nonlinear association between waist circumference and atrial fibrillation incidence (p nonlinearity = 0.09; Fig. 3b, Supplementary Table 6).
Fig. 3.
Waist circumference and atrial fibrillation, linear and nonlinear dose–response analysis
Waist-to-hip ratio, hip circumference, weight, body fat mass, body fat percentage, pericardial fat, intrathoracic fat, and abdominal visceral fat.
Four prospective studies (4 publications) [29, 30, 32, 33] were included in the analysis of waist-to-hip ratio and risk of atrial fibrillation (4259 cases and 67,837 participants) and the summary RR for a 0.1 unit increment in waist-to-hip ratio was 1.09 (95% CI: 1.02–1.16, I2 = 44%, pheterogeneity = 0.15) (Fig. 4a, Supplementary Table 7).
Fig. 4.
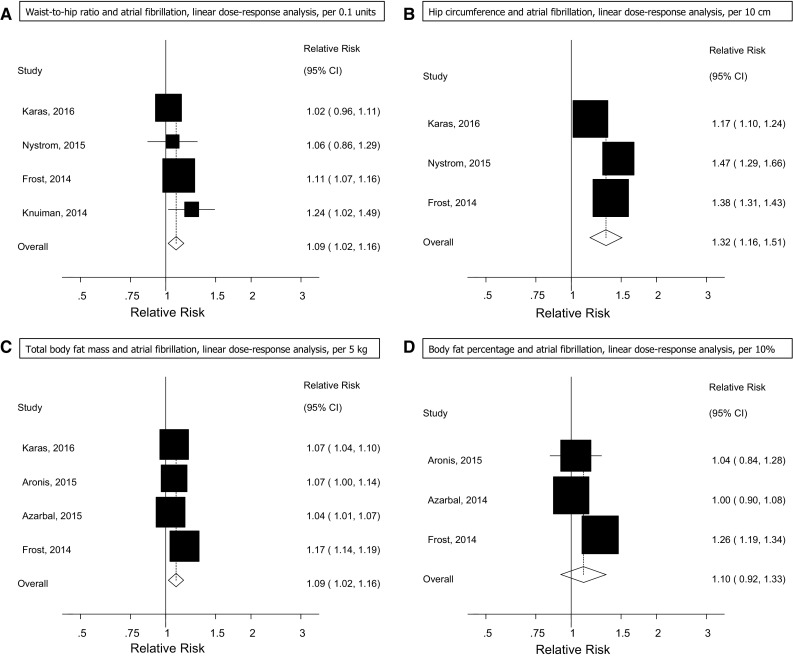
Waist-to-hip ratio, hip circumference, total body fat mass, and body fat percentage and atrial fibrillation
Three prospective studies (3 publications) [30, 32, 33] were included in the analysis of hip circumference and risk of atrial fibrillation (3916 cases and 63,570 participants) and the summary RR for a 10 cm increase in hip circumference was 1.32 (95% CI: 1.16–1.51, I2 = 91%, p heterogeneity < 0.0001; Fig. 4b, Supplementary Table 7).
Four prospective studies (3 publications) [29, 30, 32, 33] were included in the analysis of total body fat mass and atrial fibrillation (5037 cases and 71,098 participants), and the summary RR for a 5 kg increase in body fat mass was 1.09 (95% CI: 1.02–1.16, I2 = 94%, p heterogeneity < 0.0001) (Fig. 4c, Supplementary Table 7).
Three prospective studies (3 publications) [29, 30, 33] were included in the analysis of body fat percentage and risk of atrial fibrillation (2952 cases and 57,990 participants) and the summary RR per 10% increase in fat percentage was 1.10 (95% CI: 0.92–1.33, I2 = 90% p heterogeneity < 0.0001) (Fig. 4d, Supplementary Table 7).
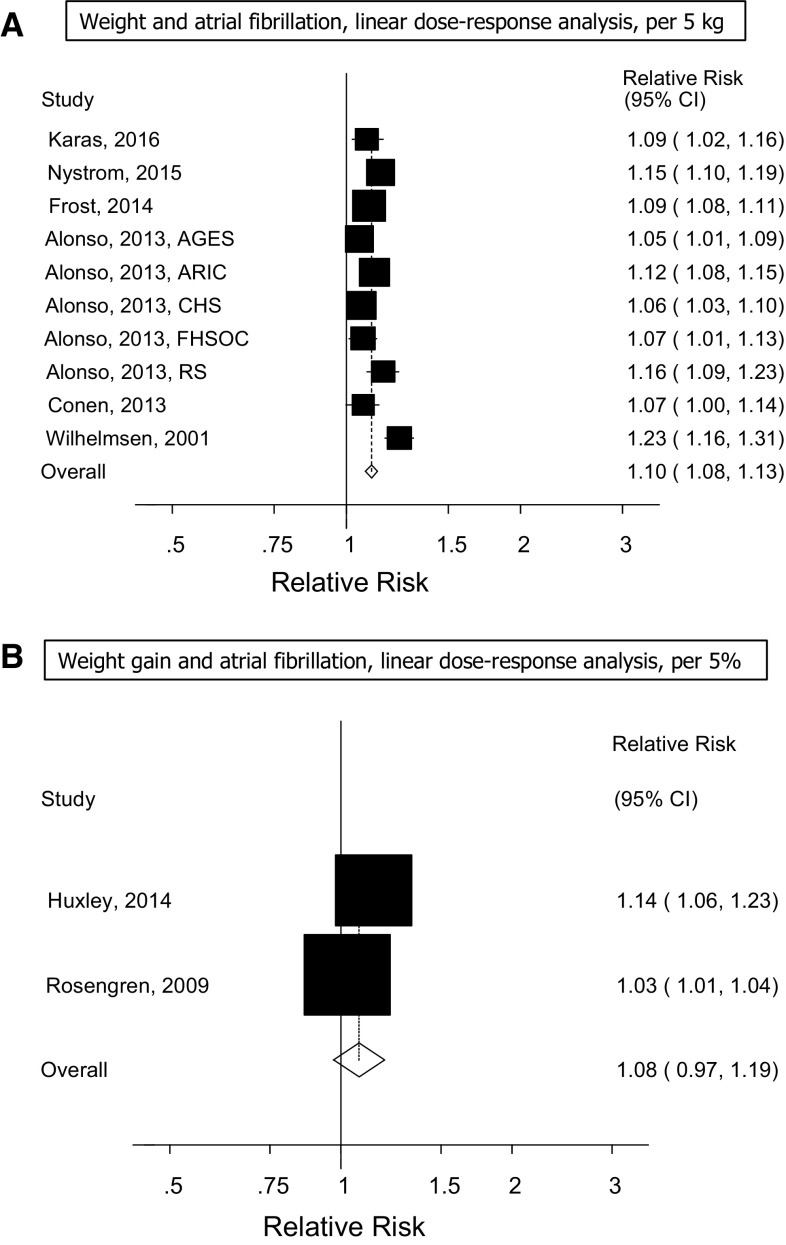
Ten prospective studies (6 publications) [30, 32, 33, 53, 58, 59] were included in the analysis of weight and the risk of atrial fibrillation (7237 cases and 132,006 participants) and the summary RR for a 5 kg increment in weight was 1.10 (95% CI: 1.08–1.13, I2 = 74%, p heterogeneity < 0.0001) (Fig. 5a, Supplementary Table 7). There was no evidence of publication bias with Egger’s test, p = 0.52, or Begg’s test, p = 0.59.
Fig. 5.
Weight and weight change and atrial fibrillation
Two prospective studies [23, 26] were included in the analysis of weight gain and the risk of atrial fibrillation (3028 cases and 21,122 participants) and the summary RR was 1.08 (95% CI: 0.97–1.19, I2 = 86% p heterogeneity = 0.007) (Fig. 5b) per 5% increase in weight gain. Only one study reported on pericardial fat, intrathoracic fat, abdominal visceral fat and the risk of atrial fibrillation and found hazard ratios of 1.13 (95% CI: 0.99–1.30), 1.19 (95% CI: 1.01–1.40), 1.09 (95% CI: 0.93–1.28), respectively [52].
Subgroup and sensitivity analyses and study quality
The positive association between BMI, and risk of atrial fibrillation persisted in almost all subgroup analyses defined by gender, assessment of weight and height, duration of follow-up, geographic location, number of cases, study quality and adjustment for confounding and potential intermediate factors and there was little evidence of heterogeneity between any of these subgroups with meta-regression analyses (Table 1). In further subgroup analyses of two studies that reported data stratified by ethnicity [31, 37], the summary RR per 5 BMI units was 1.14 (95% CI: 1.06–1.22) among Caucasians and 1.23 (95% CI: 1.09–1.39) for African Americans, with no significant heterogeneity between subgroups, p = 0.39. When the studies of BMI and atrial fibrillation were stratified by study design, the summary RR was 1.15 (95% CI: 0.67–1.98, I2 = 69.5%) for the nested case–control studies and 1.30 (95% CI: 1.23–1.38, I2 = 94.3%) for the cohort studies. Study quality was high with a mean (median) score of 7.7 (8) out of 9 points in the analysis of BMI and atrial fibrillation.
Discussion
This is to our knowledge the first meta-analysis to assess multiple adiposity measures in relation to risk of atrial fibrillation. There was a 28% increase in the relative risk per 5 units increase in BMI, a 18% increase in relative risk per 10 cm increase in waist circumference, a 9% increase in the relative risk per 0.1 unit increase in waist-to-hip ratio, a 32% increase in relative risk per 10 cm increase in hip circumference, a 9% increase in the relative risk per 5 kg increase in body fat mass, and a 10% increase in the relative risk per 5 kg increase in body weight, but no significant association was observed for body fat percentage or weight gain, although the number of studies was very low in these analyses. There was evidence of a nonlinear association between BMI and atrial fibrillation, with a slightly steeper association at higher BMI levels, however, there was evidence of increased risk even within the normal BMI range (22–24) compared to a BMI of around 20, although the increased risk was most pronounced in the obese and severely obese BMI ranges. The association between waist circumference and atrial fibrillation was approximately linear. The positive association between BMI and atrial fibrillation was observed across all geographic locations and both in Caucasians and African Americans, suggesting that adiposity is a risk factor for atrial fibrillation across populations.
The current findings are consistent with a previous meta-analysis of 5 cohort studies which found a 39 and 87% increase in the relative risk of atrial fibrillation among overweight and obese, respectively, compared to normal weight subjects [35]. However, the current analysis has a much larger number of studies and cases and participants (25 studies with 83,006 incident cases among 2,405,381 participants compared to 5 studies with 2114 cases and 78,602 participants) and thus provides a much more robust estimate of the association, in addition to a more comprehensive assessment of different adiposity measures in relation to risk of atrial fibrillation. Although a recent randomized trial did not find a statistically significant reduction in risk of atrial fibrillation among individuals with type 2 diabetes with a weight loss intervention, the study may have had too low power to detect a moderate reduction in risk [60]. The hazard ratio for the highest quintile of weight loss was 0.70 (95% CI: 0.41–1.18), thus a moderate reduction in risk cannot be excluded based on this trial. Another recent study of obese patients undergoing bariatric surgery found a reduced risk of developing atrial fibrillation with a hazard ratio of 0.71 (95% CI: 0.60–0.83) compared to the control group, providing additional evidence that adiposity is related to increased risk of atrial fibrillation [61]. Our findings of an increased risk of atrial fibrillation with higher hip circumference is somewhat in contrast to previous studies that have found an inverse association between hip circumference and cardiovascular disease [62], however, the inverse associations were only observed after further adjustment for BMI and waist circumference while none of the studies in the current meta-analysis made further adjustments for BMI and waist circumference. Further studies are therefore needed to clarify whether hip circumference, waist circumference and BMI are independently associated with atrial fibrillation.
Our meta-analysis has some limitations that need to be mentioned. Confounding by other risk factors may have influenced the results. However, the association between BMI and atrial fibrillation persisted in subgroup analyses when studies were stratified by whether they adjusted for confounding factors such as age, smoking, alcohol, and physical activity. In addition, the association persisted among studies that adjusted for potential intermediates including hypertension, blood pressure, serum cholesterol, diabetes, coronary heart disease, heart failure, and left ventricular hypertrophy. There was some evidence of heterogeneity between the subgroups of studies that adjusted for heart failure, with weaker, but still significant associations among studies with such adjustment. This could indicate that part of the association between adiposity and atrial fibrillation may be mediated by heart failure. This is consistent with our previous finding of an increased risk of heart failure related to both general and abdominal adiposity [7] and with the increased risk of atrial fibrillation among patients with heart failure [53]. Although the heterogeneity between studies was high, this appeared to be largely due to different effect sizes between studies, rather than differences in the direction of the association, as all but one study found a positive association. Exclusion of the study which showed an inverse association in the linear dose–response analysis did not substantially reduce the heterogeneity.
Measurements of weight, height, waist and hip circumferences may have been affected by measurement errors, however, the association for BMI was similar among studies that used measured weight and height compared to those that used self-reported weight and height. Validation studies have reported high correlations between self-reported and measured anthropometric measures [63–66]. BMI is an imperfect measure of body fatness as it does not distinguish between body fat and muscle mass. However, studies have shown high correlations between BMI and waist measures and body fat as measured by dual-energy X-ray absorptiometry (DXA) [67, 68]. Importantly, the association between adiposity and atrial fibrillation was in the direction of increased risk for all adiposity measures analysed, and the association with body fat mass (measured by DXA) did not appear to be stronger than that for BMI or waist circumference, supporting the use of these measures for the measurement of adiposity and for prediction of atrial fibrillation. Although publication bias or small study bias can affect the findings of meta-analyses of published literature, we found no evidence of such bias with Egger’s or Begg’s test. However, power was low for these tests in the analyses apart from BMI and weight because the number of studies was low.
Several potential mechanisms could explain an association between body fatness and risk of atrial fibrillation. Adiposity is associated with increased risk of hypertension [2], insulin resistance [69], diabetes [70], obstructive sleep apnea [71], coronary heart disease [72], and heart failure [7], which are established risk factors for atrial fibrillation [53, 73, 74]. Adiposity is associated with increased risk of left ventricular hypertrophy [75–77] and left atrial size [78, 79], and the latter may be due to hypertension, volume overload, left ventricular diastolic abnormalities, autonomic dysfunction and enhanced neurohormonal activation [80, 81]. In an experimental animal study weight gain resulted in atrial remodeling and increased atrial volumes, left atrial and systemic pressures, ventricular mass, pericardial fat volumes, increased atrial interstitial fibrosis, inflammation, myocardial lipid accumulation, and conduction abnormalities with slowing of atrial conduction and increased conduction heterogeneity [82]. Adiposity is related to low-grade inflammation [83, 84] which is strongly associated with atrial fibrillation [85]. Overweight and obesity is also related to greater epicardial fat thickness [86–88] which has been associated with alterations in atrial electrophysiology [89] and risk of atrial fibrillation [90, 91]. The findings of a recent Mendelian Randomisation study of genetic obesity and atrial fibrillation is consistent with a causal interpretation of the positive association found in the current meta-analysis between adiposity and atrial fibrillation [92].
Our meta-analysis has several strengths including the prospective design of the included studies which avoids recall bias and reduces the possibility for selection bias, the large number of cohort studies with >83,000 cases and >2.4 million participants in the BMI analysis which provided statistical power to detect moderate associations, the detailed dose–response analyses which clarified the shape of the dose–response relationship, the observation of a similar association between BMI and atrial fibrillation in different geographic regions, and robustness of the findings in multiple subgroup analyses as well as the high study quality of the included studies.
The findings have important clinical implications for the prevention of atrial fibrillation as a previous meta-analysis only analysed BMI, but not other fat measures in relation to the risk of atrial fibrillation [35], and have not assessed the dose–response relationship between adiposity and atrial fibrillation in as much detail as the current analysis. In addition, we found in subgroup analyses that higher BMI was associated with increased risk of atrial fibrillation in studies from Europe, North America, Australia and Asia, as well as in Caucasian and African American participants suggesting that avoidance of excess weight is important across populations. The current analysis suggests that both general and abdominal adiposity measures as well as increased hip circumference and total body fat mass is related to increased risk of atrial fibrillation and that being relatively slim as assessed by BMI, waist circumference and other adiposity measures may confer the lowest risk of atrial fibrillation. However, to what degree different fat measures independently of each other predict atrial fibrillation risk is not clear from the current data as few studies reported mutually adjusted results, but this requires further study. Because of the moderate number of studies in the analyses of other adiposity measures than BMI and weight further studies are needed of these measures. These findings have important public health implications because of the increasing prevalence of overweight and obesity worldwide [1] and because of the consistency of the results across populations. Thus if current trends continue unabated it might contribute to an increased incidence of atrial fibrillation and associated complications globally [16].
In conclusion, our findings confirm that overweight, obesity, abdominal fatness and high body fat mass increase the risk of atrial fibrillation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work has been supported by funding from the Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU) and the Imperial College National Institute of Health Research (NIHR) Biomedical Research Centre (BRC). The study sponsor had no role in the study design, collection of data, analysis, and interpretation of data. D. Aune takes primary responsibility for the integrity of the data and the accuracy of the data analysis. We thank Darren C. Greenwood (Biostatistics Unit, Centre for Epidemiology and Biostatistics, University of Leeds, Leeds, United Kingdom) for providing the Stata code for the nonlinear dose–response analysis.
Author’s contributions
Conceived and designed the research: DA, TN, ST, LJV. Acquired the data: DA, AS. Analyzed and interpreted the data: DA, AS, SS, TN, IJ, PR, ST, ER, LJV. Performed statistical analysis: DA. Handled funding and supervision: LJV, ST. Drafted the manuscript: DA, AS. Made critical revision of the manuscript for intellectual content: DA, AS, SS, TN, IJ, PR, ST, ER, LJV.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aune D, Norat T, Vatten LJ. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur J Epidemiol. 2015;30:1009–1019. doi: 10.1007/s10654-015-0081-y. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose–response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global BMI Mortality Collaboration Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aune D, Sen A, Norat T, et al. Body mass index, abdominal fatness and heart failure incidence and mortality: a systematic review and dose–response meta-analysis of prospective studies. Circulation. 2016;133:639–649. doi: 10.1161/CIRCULATIONAHA.115.016801. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund/American Insitute for Cancer Research . Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington: AICR; 2007. [Google Scholar]
- 9.Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose–response meta-analysis of prospective studies. Ann Oncol. 2012;23:843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 10.Aune D, Navarro Rosenblatt DA, Chan DS, et al. Anthropometric factors and ovarian cancer risk: a systematic review and nonlinear dose–response meta-analysis of prospective studies. Int J Cancer. 2014;136:1888–1898. doi: 10.1002/ijc.29207. [DOI] [PubMed] [Google Scholar]
- 11.Aune D, Navarro Rosenblatt DA, Chan DS, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose–response meta-analysis of prospective studies. Ann Oncol. 2015;26:1635–1648. doi: 10.1093/annonc/mdv142. [DOI] [PubMed] [Google Scholar]
- 12.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 14.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 17.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 19.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 20.Hodgkinson JA, Taylor CJ, Hobbs FD. Predictors of incident atrial fibrillation and influence of medications: a retrospective case–control study. Br J Gen Pract. 2011;61:e353–e361. doi: 10.3399/bjgp11X578034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karasoy D, Bo JT, Hansen ML, et al. Obesity is a risk factor for atrial fibrillation among fertile young women: a nationwide cohort study. Europace. 2013;15:781–786. doi: 10.1093/europace/eus422. [DOI] [PubMed] [Google Scholar]
- 22.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish diet, cancer, and health study. Am J Med. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J. 2009;30:1113–1120. doi: 10.1093/eurheartj/ehp076. [DOI] [PubMed] [Google Scholar]
- 24.Korda RJ, Liu B, Clements MS, et al. Prospective cohort study of body mass index and the risk of hospitalisation: findings from 246,361 participants in the 45 and up study. Int J Obes (Lond). 2012;37:790–799. doi: 10.1038/ijo.2012.155. [DOI] [PubMed] [Google Scholar]
- 25.Reeves GK, Balkwill A, Cairns BJ, Green J, Beral V. Hospital admissions in relation to body mass index in UK women: a prospective cohort study. BMC Med. 2014;12:45. doi: 10.1186/1741-7015-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huxley RR, Misialek JR, Agarwal SK, et al. Physical activity, obesity, weight change, and risk of atrial fibrillation: the atherosclerosis risk in communities study. Circ Arrhythm Electrophysiol. 2014;7:620–625. doi: 10.1161/CIRCEP.113.001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandhu RK, Conen D, Tedrow UB, et al. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc. 2014;3:e000916. doi: 10.1161/JAHA.114.000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt M, Botker HE, Pedersen L, Sorensen HT. Comparison of the frequency of atrial fibrillation in young obese versus young nonobese men undergoing examination for fitness for military service. Am J Cardiol. 2014;113:822–826. doi: 10.1016/j.amjcard.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 29.Knuiman M, Briffa T, Divitini M, et al. A cohort study examination of established and emerging risk factors for atrial fibrillation: the Busselton health study. Eur J Epidemiol. 2014;29:181–190. doi: 10.1007/s10654-013-9875-y. [DOI] [PubMed] [Google Scholar]
- 30.Frost L, Benjamin EJ, Fenger-Gron M, Pedersen A, Tjonneland A, Overvad K. Body fat, body fat distribution, lean body mass and atrial fibrillation and flutter. A Danish cohort study. Obesity (Silver Spring) 2014;22:1546–1552. doi: 10.1002/oby.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aronis KN, Wang N, Phillips CL, et al. Associations of obesity and body fat distribution with incident atrial fibrillation in the biracial health aging and body composition cohort of older adults. Am Heart J. 2015;170:498–505. doi: 10.1016/j.ahj.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karas MG, Yee LM, Biggs ML, et al. Measures of body size and composition and risk of incident atrial fibrillation in older people: the cardiovascular health study. Am J Epidemiol. 2016;183:998–1007. doi: 10.1093/aje/kwv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nystrom PK, Carlsson AC, Leander K, de Faire U, Hellenius ML, Gigante B. Obesity, metabolic syndrome and risk of atrial fibrillation: a Swedish, prospective cohort study. PLoS ONE. 2015;10:e0127111. doi: 10.1371/journal.pone.0127111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azarbal F, Stefanick ML, Salmoirago-Blotcher E, et al. Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc. 2014;3:e001127. doi: 10.1161/JAHA.114.001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J. 2008;155:310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Minami M, Kobayashi Y, Toyokawa S, Inoue K, Takeshita Y. Risk factors for new-onset atrial fibrillation during routine medical checkups of Japanese male workers. Int Heart J. 2009;50:457–464. doi: 10.1536/ihj.50.457. [DOI] [PubMed] [Google Scholar]
- 37.Schnabel RB, Aspelund T, Li G, et al. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170:1909–1917. doi: 10.1001/archinternmed.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grundvold I, Skretteberg PT, Liestol K, et al. Low heart rates predict incident atrial fibrillation in healthy middle-aged men. Circ Arrhythm Electrophysiol. 2013;6:726–731. doi: 10.1161/CIRCEP.113.000267. [DOI] [PubMed] [Google Scholar]
- 39.Nyrnes A, Toft I, Njolstad I, et al. Uric acid is associated with future atrial fibrillation: an 11-year follow-up of 6308 men and women—the Tromso study. Europace. 2014;16:320–326. doi: 10.1093/europace/eut260. [DOI] [PubMed] [Google Scholar]
- 40.Vermond RA, Geelhoed B, Verweij N, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol. 2015;66:1000–1007. doi: 10.1016/j.jacc.2015.06.1314. [DOI] [PubMed] [Google Scholar]
- 41.Toren K, Schioler L, Soderberg M, Giang KW, Rosengren A. The association between job strain and atrial fibrillation in Swedish men. Occup Environ Med. 2015;72:177–180. doi: 10.1136/oemed-2014-102256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kokubo Y, Watanabe M, Higashiyama A, et al. Interaction of Blood pressure and body mass index with risk of incident atrial fibrillation in a Japanese urban cohort: the Suita study. Am J Hypertens. 2015;28:1355–1361. doi: 10.1093/ajh/hpv038. [DOI] [PubMed] [Google Scholar]
- 43.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 44.Wong CX, Sun MT, Mahajan R, et al. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626,603 individuals in 51 studies. JACC. 2015 doi: 10.1016/j.jacep.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Wells G, Shea B, O’Connell D. et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 13 Aug 2014.
- 46.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 47.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 48.Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose–response data, with an application to alcohol and mortality. Am J Epidemiol. 2004;159:1077–1086. doi: 10.1093/aje/kwh142. [DOI] [PubMed] [Google Scholar]
- 49.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 50.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 52.Lee JJ, Yin X, Hoffmann U, Fox CS, Benjamin EJ. Relation of pericardial fat, intrathoracic fat, and abdominal visceral fat with incident atrial fibrillation (from the Framingham heart study) Am J Cardiol. 2016;118:1486–1492. doi: 10.1016/j.amjcard.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15,000 middle-aged men and women (the Renfrew–Paisley study) Eur Heart J. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 55.Kang SH, Choi EK, Han KD, et al. Underweight is a risk factor for atrial fibrillation: a nationwide population-based study. Int J Cardiol. 2016;215:449–456. doi: 10.1016/j.ijcard.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 56.Diouf I, Magliano DJ, Carrington MJ, Stewart S, Shaw JE. Prevalence, incidence, risk factors and treatment of atrial fibrillation in Australia: the Australian diabetes, obesity and lifestyle (AusDiab) longitudinal, population cohort study. Int J Cardiol. 2016;205:127–132. doi: 10.1016/j.ijcard.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 57.Berkovitch A, Kivity S, Klempfner R, et al. Body mass index and the risk of new-onset atrial fibrillation in middle-aged adults. Am Heart J. 2016;173:41–48. doi: 10.1016/j.ahj.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250:382–389. doi: 10.1046/j.1365-2796.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 59.Conen D, Glynn RJ, Sandhu RK, Tedrow UB, Albert CM. Risk factors for incident atrial fibrillation with and without left atrial enlargement in women. Int J Cardiol. 2013;168:1894–1899. doi: 10.1016/j.ijcard.2012.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alonso A, Bahnson JL, Gaussoin SA, et al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the look AHEAD randomized trial. Am Heart J. 2015;170:770–777. doi: 10.1016/j.ahj.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jamaly S, Carlsson L, Peltonen M, Jacobson P, Sjostrom L, Karason K. Bariatric surgery and the risk of new-onset atrial fibrillation in Swedish obese subjects. J Am Coll Cardiol. 2016;68:2497–2504. doi: 10.1016/j.jacc.2016.09.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 63.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Bes-Rastrollo M, Sabate J, Jaceldo-Siegl K, Fraser GE. Validation of self-reported anthropometrics in the adventist health study 2. BMC Public Health. 2011;11:213. doi: 10.1186/1471-2458-11-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weaver TW, Kushi LH, McGovern PG, et al. Validation study of self-reported measures of fat distribution. Int J Obes Relat Metab Disord. 1996;20:644–650. [PubMed] [Google Scholar]
- 66.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5:561–565. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 67.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blew RM, Sardinha LB, Milliken LA, et al. Assessing the validity of body mass index standards in early postmenopausal women. Obes Res. 2002;10:799–808. doi: 10.1038/oby.2002.108. [DOI] [PubMed] [Google Scholar]
- 69.Everson SA, Goldberg DE, Helmrich SP, et al. Weight gain and the risk of developing insulin resistance syndrome. Diabetes Care. 1998;21:1637–1643. doi: 10.2337/diacare.21.10.1637. [DOI] [PubMed] [Google Scholar]
- 70.Abdullah A, Peeters A, de Court M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89:309–319. doi: 10.1016/j.diabres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 71.Bazzano LA, Hu T, Bertisch SM, et al. Childhood obesity patterns and relation to middle-age sleep apnoea risk: the Bogalusa heart study. Pediatr Obes. 2016;11:535–542. doi: 10.1111/ijpo.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danaei G, Lu Y, Singh GM, et al. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634–647. doi: 10.1016/S2213-8587(14)70102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goudis CA, Korantzopoulos P, Ntalas IV, Kallergis EM, Ketikoglou DG. Obesity and atrial fibrillation: a comprehensive review of the pathophysiological mechanisms and links. J Cardiol. 2015;66:361–369. doi: 10.1016/j.jjcc.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;169:207–214. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 75.Cuspidi C, Rescaldani M, Sala C, Grassi G. Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J Hypertens. 2014;32:16–25. doi: 10.1097/HJH.0b013e328364fb58. [DOI] [PubMed] [Google Scholar]
- 76.Brady TM. The role of obesity in the development of left ventricular hypertrophy among children and adolescents. Curr Hypertens Rep. 2016;18:3. doi: 10.1007/s11906-015-0608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Falkner B, DeLoach S, Keith SW, Gidding SS. High risk blood pressure and obesity increase the risk for left ventricular hypertrophy in African–American adolescents. J Pediatr. 2013;162:94–100. doi: 10.1016/j.jpeds.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ayer JG, Almafragy HS, Patel AA, Hellyer RL, Celermajer DS. Body mass index is an independent determinant of left atrial size. Heart Lung Circ. 2008;17:19–24. doi: 10.1016/j.hlc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 79.Ayer JG, Sholler GF, Celermajer DS. Left atrial size increases with body mass index in children. Int J Cardiol. 2010;141:61–67. doi: 10.1016/j.ijcard.2008.11.157. [DOI] [PubMed] [Google Scholar]
- 80.Pelat M, Verwaerde P, Merial C, et al. Impaired atrial M(2)-cholinoceptor function in obesity-related hypertension. Hypertension. 1999;34:1066–1072. doi: 10.1161/01.HYP.34.5.1066. [DOI] [PubMed] [Google Scholar]
- 81.Engeli S, Sharma AM. The renin–angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med (Berl) 2001;79:21–29. doi: 10.1007/s001090000144. [DOI] [PubMed] [Google Scholar]
- 82.Abed HS, Samuel CS, Lau DH, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. doi: 10.1016/j.hrthm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 83.Fontana L, Hu FB. Optimal body weight for health and longevity: bridging basic, clinical, and population research. Aging Cell. 2014;13:391–400. doi: 10.1111/acel.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest. 2007;30:210–214. doi: 10.1007/BF03347427. [DOI] [PubMed] [Google Scholar]
- 85.Dewland TA, Vittinghoff E, Harris TB, et al. Inflammation as a mediator of the association between race and atrial fibrillation: results from the health, aging, and body composition study. JACC Clin Electrophysiol. 2015;1:248–255. doi: 10.1016/j.jacep.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bazzocchi A, Diano D, Vicennati V, et al. Relationships between total and regional adiposity and epicardial fat in obese women: How can dual-energy X-ray absorptiometry be associated with echocardiographic epicardial fat measurements? Clin Obes. 2013;3:132–140. doi: 10.1111/cob.12027. [DOI] [PubMed] [Google Scholar]
- 87.Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2014;12:31–42. doi: 10.1089/met.2013.0107. [DOI] [PubMed] [Google Scholar]
- 88.Fernandez Munoz MJ, Basurto AL, Cordova PN, et al. Epicardial adipose tissue is associated with visceral fat, metabolic syndrome, and insulin resistance in menopausal women. Rev Esp Cardiol (Engl Ed) 2014;67:436–441. doi: 10.1016/j.recesp.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 89.Lin YK, Chen YC, Chang SL, et al. Heart failure epicardial fat increases atrial arrhythmogenesis. Int J Cardiol. 2013;167:1979–1983. doi: 10.1016/j.ijcard.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Yorgun H, Canpolat U, Aytemir K, et al. Association of epicardial and peri-atrial adiposity with the presence and severity of non-valvular atrial fibrillation. Int J Cardiovasc Imaging. 2015;31:649–657. doi: 10.1007/s10554-014-0579-5. [DOI] [PubMed] [Google Scholar]
- 91.Iacobellis G, Zaki MC, Garcia D, Willens HJ. Epicardial fat in atrial fibrillation and heart failure. Horm Metab Res. 2014;46:587–590. doi: 10.1055/s-0034-1367078. [DOI] [PubMed] [Google Scholar]
- 92.Chatterjee NA, Giulianini F, Geelhoed B et al. Genetic obesity and the risk of atrial fibrillation-causal estimates from mendelian randomization. Circulation. 2016. [Epub Ahead of Print]. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.