The molecular mechanisms underlying mechanosensory signaling responsible for proprioceptive functions are not completely elucidated. This study provides the first evidence that voltage-gated sodium channels (NaVs) are expressed in the spindle primary sensory ending, where they are found at every site involved in transduction or encoding of muscle stretch. We propose that NaVs contribute to multiple steps in sensory signaling by muscle spindles, as it does in other types of slowly adapting sensory neurons.
Keywords: sensory encoding, muscle spindle, voltage-gated sodium channels, immunohistochemistry
Abstract
Knowledge of the molecular mechanisms underlying signaling of mechanical stimuli by muscle spindles remains incomplete. In particular, the ionic conductances that sustain tonic firing during static muscle stretch are unknown. We hypothesized that tonic firing by spindle afferents depends on sodium persistent inward current (INaP) and tested for the necessary presence of the appropriate voltage-gated sodium (NaV) channels in primary sensory endings. The NaV1.6 isoform was selected for both its capacity to produce INaP and for its presence in other mechanosensors that fire tonically. The present study shows that NaV1.6 immunoreactivity (IR) is concentrated in heminodes, presumably where tonic firing is generated, and we were surprised to find NaV1.6 IR strongly expressed also in the sensory terminals, where mechanotransduction occurs. This spatial pattern of NaV1.6 IR distribution was consistent for three mammalian species (rat, cat, and mouse), as was tonic firing by primary spindle afferents. These findings meet some of the conditions needed to establish participation of INaP in tonic firing by primary sensory endings. The study was extended to two additional NaV isoforms, selected for their sensitivity to TTX, excluding TTX-resistant NaV channels, which alone are insufficient to support firing by primary spindle endings. Positive immunoreactivity was found for NaV1.1, predominantly in sensory terminals together with NaV1.6 and for NaV1.7, mainly in preterminal axons. Differential distribution in primary sensory endings suggests specialized roles for these three NaV isoforms in the process of mechanosensory signaling by muscle spindles.
NEW & NOTEWORTHY The molecular mechanisms underlying mechanosensory signaling responsible for proprioceptive functions are not completely elucidated. This study provides the first evidence that voltage-gated sodium channels (NaVs) are expressed in the spindle primary sensory ending, where NaVs are found at every site involved in transduction or encoding of muscle stretch. We propose that NaVs contribute to multiple steps in sensory signaling by muscle spindles as it does in other types of slowly adapting sensory neurons.
The sensory neurons supplying muscle spindle receptors provide the central nervous system with information that is critical to proprioceptive function (Proske and Gandevia 2012). This information originates from ion channels engaged in mechanotransduction or action potential encoding, and significant recent advances have been made in their identification (Bewick and Banks 2015; Lin et al. 2016; Woo et al. 2015). However, knowledge remains incomplete with regard to the ion channels responsible for sustaining repetitive firing, i.e., tonic firing of spindle afferents in response to static muscle stretch. Some insight was gained from our recent discovery that tonic firing by muscle spindle group Ia afferents can be selectively blocked pharmacologically (Vincent et al. 2015). The block was achieved by two different drugs, riluzole and phenytoin, which apart from their multiple drug effects, share antagonist action on slowly inactivating Na currents, also known as Na-persistent inward currents (INaP; (Lampl et al. 1998; Schuster et al. 2012; Xie et al. 2011; Zeng et al. 2005). INaP is a plausible candidate contributor to tonic firing of muscle spindle receptors, because it participates in sustaining repetitive firing in a wide variety of neurons (Do and Bean 2003; Harvey et al. 2006; Raman et al. 1997), including the large-diameter class of dorsal root ganglia (DRG) somas that give rise to muscle spindle afferents (Baker and Bostock 1997; Xie et al. 2011). Collectively, these observations led us to hypothesize that a NaV in muscle spindle receptors contributes to the sensory encoding mechanisms that produce tonic firing. Here, we test the necessary condition that NaV channels are present in muscle spindle receptors.
Our investigation focused on NaV channels that are TTX-sensitive (TTX-S), because they, unlike TTX resistant (TTX-R) NaV channels, are necessary for the production of muscle stretch-evoked firing by muscle spindle afferents (Hunt et al. 1978). Multiple TTX-S voltage-gated Na channels qualify as potential sources of INaP. NaV1.6 stands out among them, because it produces a particularly large INaP (Chen et al. 2008; Rush et al. 2005) and is expressed by large-diameter DRG neurons, which include Ia afferents (Black et al. 2002). Additionally, NaV1.6 is present in slowly adapting mechanosensitive receptors in skin, gut, and inner hair cells, and it is necessary for tonic firing by stretch-sensitive afferents (Feng et al. 2015; Hossain et al. 2005; Lesniak et al. 2014). Although collectively, these observations point to NaV1.6, it is not the only candidate. NaV1.1 is also expressed by large-diameter DRG neurons and is known to participate in mechanosensation in the skin and the gut (Black et al. 1996; Osteen et al. 2016). NaV1.7 is also present in stretch-sensitive colorectal afferent endings (Feng et al. 2015) and is coexpressed with NaV1.6 at nodes of Ranvier in a subpopulation of small-diameter Aδ myelinated fibers in the sciatic nerve, 40% of which are known to be mechanosensitive only (Black et al. 2012; Cain et al. 2001). Here, we provide, what to our knowledge, is the first demonstration of NaV channel expression in the primary sensory endings of muscle spindle afferents. The presence of NaV1.6 at the heminodes, which are the presumptive spike-encoding regions, potentially provides the primary ending with the capacity to support repetitive firing in response to static mechanical stimulation. The similarity we find in the NaV1.6 expression by primary endings in cats, rats, and mice is consistent with the similarity that we find in the firing profiles of Ia afferents in these three species. In addition to NaV1.6 immunoreactive (IR), we found NaV IR for isoforms 1.1 and 1.7, and the differential distribution of these three suggests specialized roles for each in the process of sensory signaling by muscle spindle group Ia afferents.
METHODS
The IR studies presented here were restricted to tissue recovered from normal animals, and all procedures were approved by the Georgia Institute of Technology Animal Care and Use Committee. The soleus muscles were recovered from nine deeply anesthetized (isoflurane 1.5–2.5% in 100% O2) adult animals, including one female cat (3.0 kg), five female Wistar rats (200–300 g), and four C57BL6/J mice (24–28 g). Animal use approvals by Institutional Care and Use Committees for those used in electrophysiological studies were as follows: Emory University for cats and Wright State University for rats and mice. At the end of all terminal experiments, all animals were overdosed with isoflurane (5%) and exsanguinated.
Immunolabeling
Soleus muscles were placed in 0.1 M PBS containing 4% paraformaldehyde for 40 min, and after a brief wash in a 0.1 M PBS, they were incubated in 0.1 M PBS containing 20% sucrose at 4°C overnight for cryoprotection. Sections of 60-µm thickness were cut using a Cryostat (Leica). Muscle sections were labeled in triplicate with a rabbit polyclonal antibody directed against an epitope of the rat NaV1.6 channel (ASC-009, 1:100; Alomone Laboratories). The specificity of this antibody has been previously demonstrated by complete absence of IR in NaV1.6 KO mice (Black et al. 2002; Royeck et al. 2008). A rabbit polyclonal antibody directed against an epitope of the rat NaV1.1 channel and NaV1.7 channel (ASC-001 and ASC-008, respectively; 1: 100; Alomone Laboratories) were also used. All NaV antibodies were visualized using an Alexa Fluor 488-conjugated secondary antibody (1:100, ThermoFisher Scientific). A rat monoclonal antibody against the fragment 70–89 of the classic human myelin basic protein (MBP) sequence (MAB386, 1:100; Millipore) was used to detect myelin and was visualized using an Cy5-conjugated secondary antibody (1:100, Jackson Immunoresearch Laboratories), and a chicken polyclonal against the heavy-chain (200 kDa) neurofilament protein (NF-H Aves Laboratories) was visualized using a Fluorescein-conjugated secondary antibody (1:100, Aves Laboratory). The slide mounting media (Vectashield) included DAPI in order to label cell nuclei of intrafusal muscle fibers.
Imaging
Z-axis stacks of images of muscle spindle primary endings were constructed by sequentially imaging at high magnification using an Olympus FV1000 confocal microscope with a ×60 objective (NA, 1.35, oil-immersion). Stacks of images were processed and analyzed using Amaris (Bitplane) imaging software. Figures present images as flat projections of the sum of the z-axis optical slices.
Physiological Experiments
Electrophysiological measurement of Ia afferent firing responses to muscle stretch-supplemented comparison of NaV1.6 staining across the three species. These data were collected, although not published, in our previous studies of the cat (Prather et al. 2011) and the rat (Vincent et al. 2016). New data were obtained from four adult mice deeply anesthetized by isoflurane inhalation throughout the entire terminal experiment, beginning with induction in a closed chamber (4–5% in 100% O2) and continuing with delivery via a tracheal cannula (1.5–2.0% in 100% O2). Subcutaneous injections of lactated Ringer solution were given to maintain adequate fluid levels and blood pressure. Respiratory rate, heart rate, oxygen saturation, and Pco2 were monitored to ensure anesthesia and overall animal health. Body temperature was recorded via a rectal probe and was maintained between 36°C and 38°C with heated water pads and a heat lamp.
Surgical Dissection
The left hind limb and lumbosacral spinal cord were surgically exposed, as needed, to record the firing of individual sensory neurons in response to controlled muscle stretch (Bullinger et al. 2011). Briefly, each mouse was placed in a rigid stereotaxic frame, with legs secured and ankle and knee joints fixed at angles of ~90° and 120°, respectively. The left triceps surae muscles (medial and lateral gastrocnemius and soleus muscles) were partially freed from surrounding connective tissue, marked for their resting length measured with the leg in its fixed joint positions, and then detached from the calcaneus. The distal end of the severed Achilles tendon was tied directly to the lever of a force and length-sensing servomotor (model 305B-LR; Aurora Scientific), which provided for the application of controlled muscle stretch, while recording muscle length and force. Triceps surae nerves were freed from the surrounding tissue and were placed on a unipolar stimulating electrode, and other nerves in the left hind limb were crushed, including common peroneal, sural, and posterior tibial nerves. A laminectomy was performed from T10-S1, and the dura mater was removed to expose the spinal cord and dorsal roots. Skin flaps were tied up in the back and hind limb to create pools for mineral oil, to prevent the tissue desiccation.
Data Collection
Action potentials from the axons of individual muscle spindles were recorded with glass microelectrodes (filled with 2 M K+ acetate) driven into dorsal roots supported on bipolar hook electrodes. Sensory axons were randomly sampled and selected for recording when electrical stimulation of the triceps surae nerves evoked orthodromic action potentials. Sensory axons were identified as muscle spindle afferents by the pause in firing observed during electrically evoked twitch contractions of the triceps surae muscles and were further subclassified as Ia when their firing entrained to high-frequency vibration (250 Hz, 80 μm) of the muscles. Firing was then recorded in response to ramp-hold-release stretches (0.25 mm, 50-ms ramp and release, 1-s hold) from resting muscle lengths determined for ankle angle 90° and knee angle 120°. These stretch parameters were matched to those used for cat and rat by their percentage of whole muscle resting lengths; we measured ~88 mm for cat, 44 mm for rat, and 13 mm for mice. Stretch amplitudes of 2 mm, 1 mm, and 0.25 mm for cats, rats, and mice, respectively, achieved 2% strain and 40%/s strain rate for all species. Intra-axonal records of action potentials and of muscle length and force were digitized (20 kHz) and stored on a computer for later analysis using Spike2 software.
Statistical Analysis
Data pooled from individuals of each species were compared statistically using one-way ANOVA (Statistica Software). The level of significance for all statistical tests was set at P < 0.05. All values are reported means ± SD.
RESULTS
The main objective was to identify the distribution of NaV1.6 within primary sensory endings. Results were tested for generalizability across the three mammalian species, in which muscle spindle receptors have been most thoroughly examined (Banks 2006). Study was restricted to the same muscle, the soleus, in all three species, to minimize variability introduced by muscle-specific differences (Banks et al. 2009). We concentrated analysis on 34 muscle spindle primary sensory endings (NaV1.6: rat, n = 17; mouse, n = 8; cat, n = 3; NaV1.1: rat n = 3; NaV1.7: rat n = 3) and overlooked secondary endings, which were less readily resolvable.
Spindle Structure and Ia Afferent Innervation
Findings were entirely consistent with comprehensive descriptions compiled from earlier studies (Bewick and Banks 2015). In all preparations, muscle spindles sensory innervation was identified using a NF-H antibody for the protein known to be highly enriched at this location (Lin et al. 2016; Nahirney and Ovalle 1993). The detailed structure and complex spatial distribution of neurons innervating muscle spindles were clearly labeled by NF-H IR in rat, cat, and mouse (see Figs. 1, 2, and 3, respectively). Primary endings were identified as parent axons and branches innervating sensory terminals expressing their distinctive annulospiral form in association with intrafusal muscle fibers. The parent axons of Ia afferents branched to ultimately innervate multiple sensory terminals (e.g., Figs. 1A1a, 2A1a, and 3A1a). Labeling for MBP IR distinguished myelinated and unmyelinated portions of Ia afferents axons (e.g., Figs. 1A1b and 3A1b, and Fig. 2A1c). Sensory terminals, which are the mechanotransducer sites (Bewick and Banks 2015), and their connecting preterminal axon branches were unmyelinated. Heminodes, the alleged site of action potential initiation and encoding of mechanical stimuli (Banks et al. 1997; Quick et al. 1980), were identified in preterminal axons adjacent to the termination of axonal myelination (e.g., Figs. 1A1b and 3A1b, Fig. 2A1c and 2A1d, arrowheads).
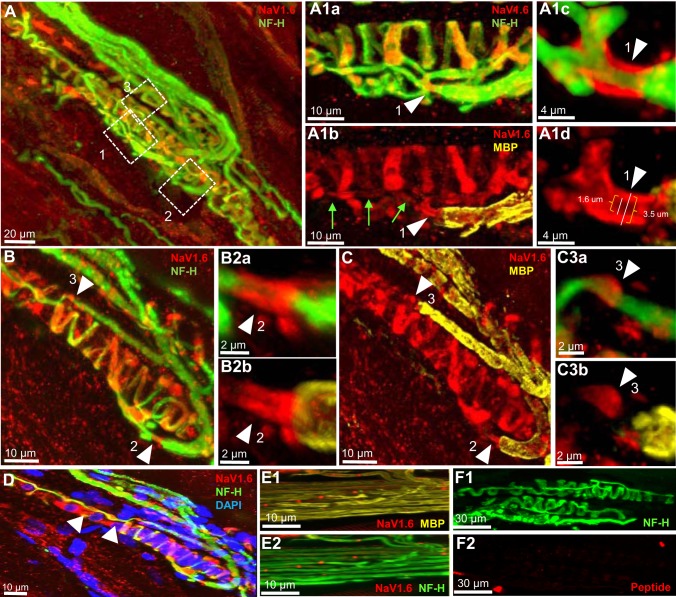
Fig. 1.
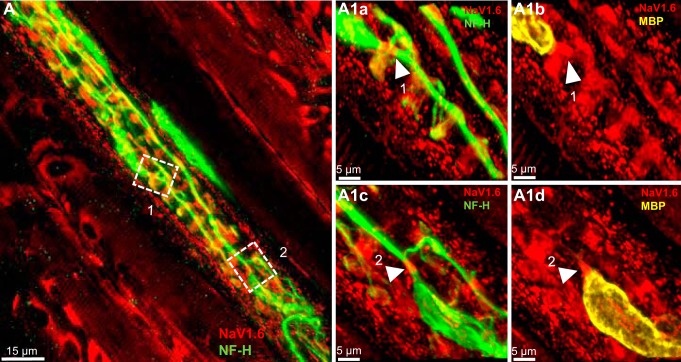
NaV1.6 is present at heminodes and sensory terminals of primary endings of rat muscle spindle. A: Low-magnification of a rat soleus muscle spindle labeled for NF-H (green) and NaV1.6 (red). Boxes 1 through 3 show Ia afferent supplying innervation to three different sensory terminals that form part of the primary ending. A1a and A1b: high magnification of the first order branch shown in box 1. Arrowheads show strong NaV1.6 IR at the heminode, determined by the myelin termination (labeled with MBP in A1b). NaV1.6 was also present in sensory ending originating from this branch (labeled with NF-H in A1a) and in portions of the preterminal axons (green arrows in A1b). A1c and A1d: higher magnification of NaV1.6 IR at this heminode. B and C: Ia afferent branches shown in boxes 2 and 3 in A. The original stack used to create the image in A was optically sliced to provide a clear view of these two branches. At high magnification, NaV1.6 IR is clearly seen at the heminode and sensory endings supplied by the first-order branches shown in box 2 (arrowheads in B2a and B2b) and the first-order branch shown in box 3 (arrowheads in C3a and C3b). D: NaV1.6 IR was also strong in the areas immediately surrounding the cell nuclei of the intrafusal muscle fibers (labeled with DAPI, blue), particularly those located at the end of the equatorial regions of the fibers (arrowheads). E1 and E2: NaV1.6 IR in nodes of Ranvier of a preterminal soleus nerve branch labeled with MBP and NF-H. F1 and F2: a rat muscle spindle labeled with NF-H and NaV1.6 blocking peptide. NaV1.6 IR was almost completely eliminated by the blocking peptide.
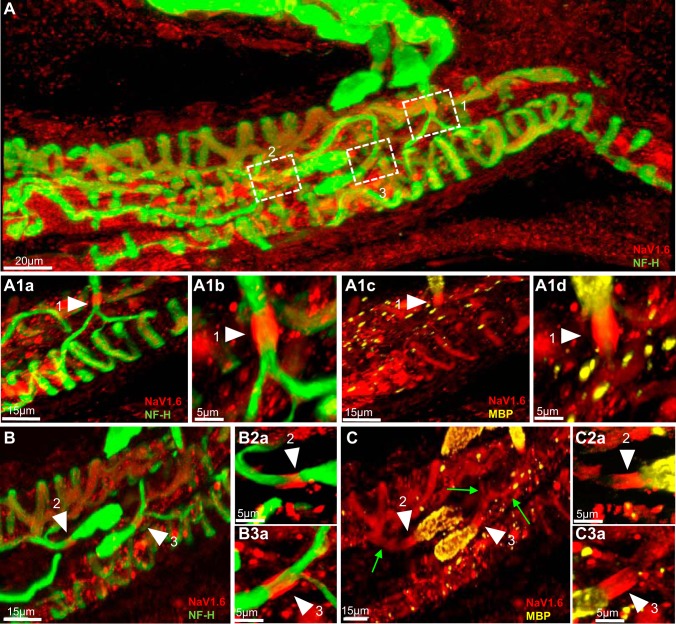
Fig. 2.
NaV1.6 is present at heminodes and sensory terminals of primary endings of cat muscle spindle. A: low magnification of a cat soleus muscle spindle labeled for NF-H (green) and NaV1.6 (red). Boxes 1–3 show Ia afferent supplying innervation to three different sensory terminals that form part of the primary ending. A1a and A1c: first-order branch shown in box 1. Arrowheads show strong Nav1.6 IR at the heminode, determined by myelin termination (labeled with MBP in A1c) and the sensory terminal (labeled with NF-H in A1a) connected to this branch. A1b and A1d: higher magnification of NaV1.6 IR at the heminode shown by arrowheads in panels A1a and A1c. B and C: two Ia afferent branches shown initially in boxes 2 and 3 in A. The original stack used to create A was optically sliced to provide a clear view of the innervation of these two branches. NaV1.6 IR is clearly present at the heminodes, determined by myelin termination (labeled with MBP in C) and sensory terminals (NF-H staining in B) supplied by these two branches. NaV1.6 IR is also clearly present in the preterminal axons (green arrows in C). B2a and B3a, C2a and C3a: higher magnification of NaV1.6 IR at the heminodes is shown by arrowheads in B and C.
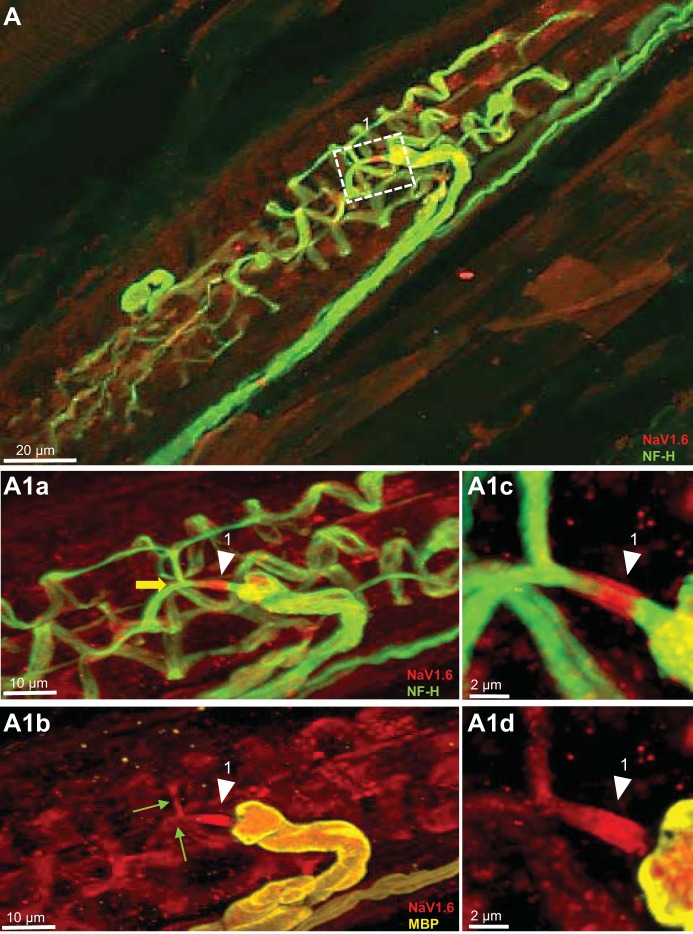
Fig. 3.
NaV1.6 is present at heminodes and sensory terminals of primary endings of mouse muscle spindle. A: low magnification of a mouse soleus muscle spindle labeled for NF-H (green) and NaV1.6 (red). Box contains Ia afferent branches supplying innervation to three different sensory terminals. A1a and A1b: high magnification of branches shown in box in A. Arrowheads show strong NaV1.6 IR at the heminodes, determined by myelin termination (labeled with MBP in A1b). NaV1.6 was also present in the preterminal axons (green arrows in A1b) and the sensory terminals supplied by these two branches (NF-H staining in A1a). Note bifurcation in box 1 of an unmyelinated preterminal branch supplying innervation to two different primary sensory terminals (yellow arrow in A1a). A1c and A1d: higher magnification of NaV1.6 IR at the heminode shown by arrowhead in A1a and A1b.
NaV1.6 IR
Multiple structures within the primary endings stained for NaV1.6. The specificity of the NaV1.6 antibody was demonstrated first by the almost complete absence of NaV1.6 immunoreactivity in muscle spindles stained when the antibody was preabsorbed with the antigenic control peptide (Fig. 1E1 and E2) and by its presence at the nodes of Ranvier of axons located within the soleus muscle nerve (Fig. 1, F1 and F2), where it is known to be the predominant sodium channel (Caldwell et al. 2000).
Heminodes and preterminal axons.
The number of heminodes examined per primary ending ranged from 1 to 4. Every terminal was associated with one heminode, and in many cases, a single heminode was shared by multiple terminals. NaV1.6 IR was concentrated at the heminodes in every primary terminal (n = 34) without exception. The boxes outlined in Fig. 1A and the corresponding insets at higher magnification plainly illustrate NaV1.6 IR at the heminodes in rat primary terminals. For example, Fig. 1, A1c and A1d shows high magnification of a heminode positioned just before the preterminal axons divided to innervate different sensory endings. The same pattern was found in all three species (Fig. 1, A1c, B2a, C3a; Fig. 2, A1b, B2a, B3a; and Fig. 3, A1c). In addition, we commonly observed NaV1.6 IR in the unmyelinated preterminal axons at sites distal to the heminodes (e.g., green arrows in Fig. 1A1b, Fig. 2C, and Fig. 3A1b); however, it was significantly weaker than the one observed for NaV1.7 (see results below).
Sensory terminals and inter-nuclear spaces.
NaV1.6 IR was evident in all sensory endings sampled from all species (Fig. 1, A1b and C, Fig. 2, A1c and C, and Fig. 3A1b). We commonly observed, but did not quantify, that NaV1.6 IR staining was consistently brighter in the sensory terminals associated with intrafusal bag fibers (identified by clustering of nuclei labeled by DAPI) than with chain fibers (See Fig. 1D). NaV IR distribution within the annulospiral sensory terminal was uneven. This pattern of expression is likely due to an uneven antibody penetration occurring during the immunostaining of a large and complex structure, such as the sensory terminal. In other less complicated structures such as the heminode, NaV IR was invariably robust in all primary endings studied. Although the exact structure labeled by NaV1.6 IR within the sensory terminals was not identified in the present study, we determined that it did not completely colocalize with NF-H (Fig. 1, A1a and B; Fig. 2, A1a and B, and Fig. 3, A1a). In all primary endings sampled, we also observed strong NaV1.6 IR between the nuclei of intrafusal muscle fibers, particularly, in juxtaequatorial regions (arrowheads in Fig. 1D).
Ia afferent firing properties in the cat, rat and mouse.
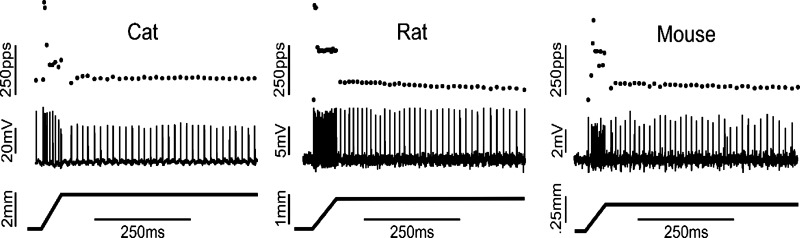
The firing responses of Ia afferents to passive-muscle stretch were compared across species using unpublished data collected from earlier studies in this laboratory, including Prather et al. (2011) and Vincent et al. (2016). Data were selected from a small set of ramp-hold-release stretches having the same strain (2%) and strain rates (40%/s) in each species (see methods). Fig. 4 illustrates representative firing responses. Overall, firing profiles were qualitatively similar, exhibiting a high-frequency initial burst of three or four spikes followed by firing in dynamic stretch that adapted to lower rates during static stretch. Firing properties are compared for pooled samples of Ia afferents in each of the three species in Table 1. Tonic firing extended virtually throughout the full 1 s of the stretch hold phase in all species, corroborating earlier in vivo studies of cat and rat, as well as findings for mouse Ia afferents, which have been studied systematically in vitro (Wilkinson et al. 2012) and to a limited extent in vivo (Nakanishi and Whelan 2012). Remaining parameters exhibit quantitative differences, which in some comparisons, reach statistical significance between species. Measures of dynamic sensitivity (dynamic firing rate and dynamic index) and tonic firing rates were all significantly greater in the mouse. We attribute these differences to species variations, since the responses were studied under similar experimental conditions and in response to muscle stretch applied with identical strain and strain rates in all species. Additional study will be required to determine whether these differences in firing behavior relate to possible differences in levels of NaV expression.
Fig. 4.
Comparable firing profiles of Ia afferents from three species. Representative records taken from Ia afferents in cat, rat, and mouse (three columns). Action potentials (middle traces) and instantaneous firing rates (top traces) were evoked in Ia afferents by ramp-hold-release stretches (bottom trace), all at 2% strain and 40%/s strain rates. Ia afferents from all species generated relatively high firing rates during dynamic (ramp) stretch before adapting but maintaining firing rates throughout to static (hold) stretch.
Table 1.
Firing properties for pooled samples of Ia afferents from cats, rats, and mice
| Cat |
Rat |
Mouse |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Duration, s | 11.0 | 992.5 | 9.8 | 6.0 | 969.7 | 26.8 | 10.0 | 988.8 | 10.2 |
| TFR, pps | 11.0 | 45.4 | 15.1 | 6.0 | 31.9* | 12.7 | 10.0 | 66.7† | 17.9 |
| DFR, pps | 11.0 | 144.7 | 32.4 | 6.0 | 172.3 | 45.5 | 10.0 | 251.9† | 71.6 |
| DI | 11.0 | 100.0 | 33.3 | 6.0 | 113.2 | 56.1 | 10.0 | 186.7† | 75.1 |
Data are expressed as means ± SD. Dynamic firing rate (DFR) was measured at the peak of ramp stretch. Tonic firing rate (TFR) was measured at midpoint of 1-s hold phase of stretch (duration of hold phase firing); dynamic index (DI) is calculated as DFR − TFR. pps, pulse per seconds.
P < 0.5 vs. cat.
P < 0.5 vs. cat and rat.
NaV1.1 and NaV1.7 IR
Heminodes and preterminal axons.
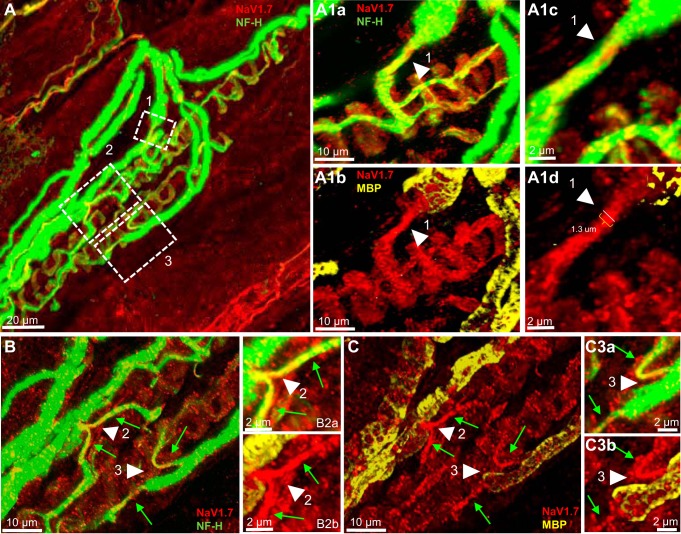
NaV1.1 IR was completely absent at the heminodes and preterminal axons in all primary endings in which it was examined. The box outlined in Fig. 5A and the corresponding inset at higher magnification clearly show the lack of NaV1.1 IR at the termination of axon myelination (Fig. 5, A1b–A1d, B2a, and C2a, white arrowheads), where the heminode normally is positioned and in the preterminal axons innervating the different terminals (green arrows in Fig. 5, A1a). NaV1.7 IR on the other hand was consistently present in the preterminal axons in every primary ending examined (Fig. 6, A1a and A1b, B and C). NaV1.7 IR extended from the end of the axonal myelination, i.e., the heminode area, and along the preterminal axons all the way to the sensory terminals (arrowhead in Fig. 6A1b and green arrows in C). Figures 5 and 6 show at higher magnification that NaV1.7 IR is constrained within the boundaries of the preterminal axon labeled with NF-H (Fig. 6A1c and A1d; B2a and B2b, and C3a and C3b, green arrows). In all primary endings analyzed, the diameter of the preterminal axon stained with NF-H and NaV1.7 at the end of the axonal myelination ranged between 1.1 and 1.3 µm (see Fig. 6A1d, arrowhead). This means that at the heminode, NaV1.7 channels in the axon are likely to be flanked by NaV1.6 channels (cf. Fig. 1A1d and Fig. 6A1D).
Fig. 5.
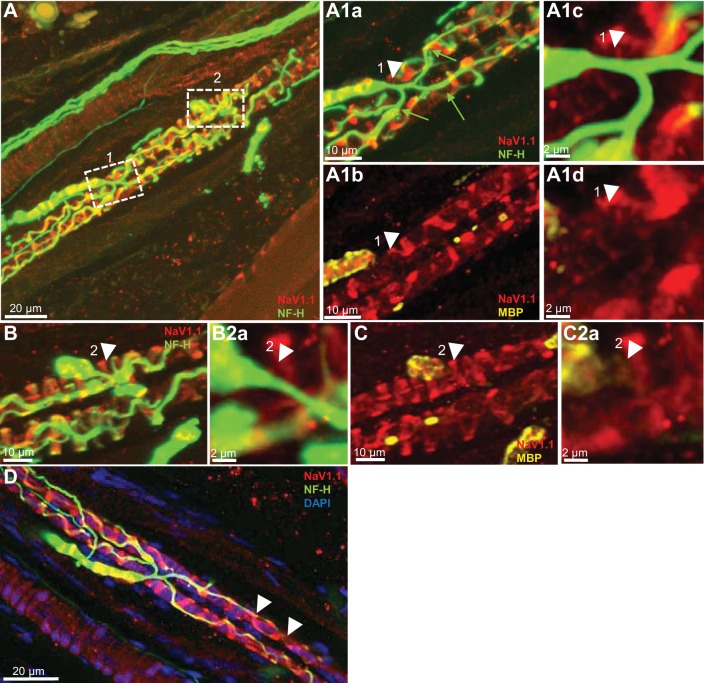
NaV1.1 is present at sensory terminals but not the heminodes of primary endings of rat muscle spindle. A: low magnification of a rat soleus muscle spindle labeled for NF-H (green) and NaV1.1 (red). Boxes 1 and 2 show Ia afferent supplying innervation to two different sensory terminals that form part of the primary ending. A1a and A1b: high magnification of the first-order branch shown in box 1. Arrowheads show complete absence of NaV1.1 IR at the heminode, determined by the myelin termination (labeled with MBP, arrowhead in A1b). NaV1.1 was present only in the sensory ending originating from this branch (labeled with NF-H in A1a). A1c and A1d: higher magnification of the heminode shown by arrowheads in A1a and A1b. Note the complete absence of NaV1.1 IR (arrowhead in A1d). B and C: high magnification of the first-order branch shown initially in box 2 in A. The original stack used to create A was optically sliced to provide a clear view of the innervation of this branch. NaV1.1 IR is clearly absent at the heminode, as determined by the end of myelin termination (labeled with MBP in C) and sensory terminals (NF-H staining in B). NaV1.1 IR, however, is clearly present in sensory terminals innervated by this branch (NF-H in B). B2a and C2a: higher magnification of the heminode shown in B and C. Note the complete absence of NaV1.1 IR (arrowhead in C2a). D: NaV1.1 IR was strong in sensory terminals associated with chain intrafusal fibers (identified by single line of nuclei labeled by DAPI) and clearly seen between the nuclei located in the juxta equatorial regions of these muscle fibers (arrowheads).
Fig. 6.
NaV1.7 is present at sensory terminals but not the heminodes of primary endings of rat muscle spindle. A: low magnification of a rat soleus muscle spindle labeled for NF-H (green) and NaV1.7 (red). Boxes 1–3 show Ia afferent supplying innervations to three different sensory terminals that form part of the primary ending. A1a and A1b: high magnification of the first-order branch shown in box 1 in A. Arrowhead clearly show NaV1.7 IR extends along the preterminal axon from the heminode area, as determined by the myelin termination (labeled with MBP, arrowhead in A1b) to the sensory ending (labeled with NF-H in A1a). NaV1.7 IR was also present in the sensory ending innervated by this axonal branch. A1c and A1d: higher magnification of the heminode area shown by arrowhead in A1a and A1b. Note that NaV1.7 IR is contained within the boundaries of the preterminal axon labeled with NF-H (arrowhead in A1c). B and C: high magnification of two first-order branch shown initially in boxes 2 and 3 in A. The original stack used to create A was optically sliced to provide a clear view of the innervation provided by this branch. NaV1.7 IR is clearly present extending along the preterminal axon from the heminode area, as determined by the end of myelin (labeled with MBP in C) to the sensory terminals (NF-H staining in B). NaV1.7 IR was also present in sensory terminals innervated by these branches (NF-H in B). B2a and B2b, C3a and C3b: higher magnification of the heminode area shown by arrowheads in B and C. Note that NaV1.7 IR is contained within the boundaries of the preterminal axons labeled with NF-H (arrowhead in B2a and C3a).
Sensory terminals and internuclear spaces.
NaV1.1 and NaV1.7 IR were present in all of the sensory endings examined (Figs. 5 and 6, A1b and C). Although we did not quantify NaV1.1 IR, it appeared consistently brighter than NaV1.7 IR in all the sensory terminals associated with intrafusal bag fibers (cf. Fig. 5, A1b and C with Fig. 6, A1b and C). In addition, NaV1.1 IR, like NaV1.6 IR, was clearly seen between the nuclei located in the juxtaequatorial regions of intrafusal muscle fibers (arrowheads in Fig. 5D), and contrary to NaV1.6 and NaV1.7 IR, the IR of this channel was consistently observed in the sensory terminals associated with chain intrafusal fibers (Fig. 5D). Our findings follow those showing the targeting of Na channels to specific regions in sensory axons of other vertebrates (Waxman et al. 1972).
NaV1.6 cross-IR
NaV1.6 IR observed in the preterminal axons and in sensory terminals might have been a product of cross IR with NaV1.1 and/or NaV1.7 antigens. To test this possibility, we preabsorbed the NaV1.6 antibody with the antigenic control peptides for NaV1.1 and NaV1.7 and stained a soleus muscle using the same protocol described in methods. NaV1.6 IR was clearly present and distributed in the patterns described above using the regular protocol (Fig. 7, A1a and A1d, arrowheads), thereby excluding the possibility that NaV1.6 cross-reacted with the other NaV channels analyzed.
Fig. 7.
NaV1.6 IR is present at the heminodes and sensory terminals of primary endings of rat muscle spindle after preincubation in NaV1.1 and NaV1.7 blocking peptides. A: low magnification of a rat soleus muscle spindle labeled for NF-H (green) and NaV1.6 (red). Boxes 1 and 2 show Ia afferent supplying innervations to different sensory terminals that form part of the primary ending. A1a –A1d: high magnification of the two first-order branches shown in boxes 1 and 2 in A. Arrowheads show strong NaV1.6 IR at the heminodes, as determined by the myelin termination (labeled with MBP in panels A1b and A1d) and sensory terminals innervated by these branches (labeled with NF-H in A1a and A1c) despite preincubation of NaV1.6 antibody in NaV1.1 and NaV1.7 blocking peptides.
DISCUSSION
Results presented here provide the first evidence of TTX-S NaV channel expression in muscle spindle primary endings. Study focused on NaV1.6 IR, which we found in cat, rat, and mouse at heminodes, preterminal axons, and sensory terminals, i.e., at every site involved in transduction or encoding of muscle stretch. Its presence, particularly at the spike-encoding region establishes NaV1.6 as a candidate source of INaP and possibly also tonic firing of the primary ending. Consistent with this notion, we find uniformity both in NaV1.6 expression and in the occurrence of tonic firing for all three species. Exploration of additional NaV isoforms in the rat revealed isoforms 1.1 and 1.7 differentially distributed at sites within the primary ending. The NaV expression patterns that we observed suggest a prominent role for these three NaV isoforms in multiple steps of mechanosensory signaling (see Bewick and Banks 2015), and below, we propose how the known biophysical properties of NaV channels might contribute to various steps in sensory signaling by mammalian muscle spindles.
Positive immunostaining for NaV1.6 was found at all heminodes in every spindle primary ending sampled in this study. This finding follows demonstrations of NaV1.6 clustered in the heminodes at two other types of mechanosensory afferents, one that innervates outer hair cells in the cochlea (Hossain et al. 2005) and another that supplies Merkel touch receptors in the skin (Lesniak et al. 2014). Heminodes are thought to be sites where receptor currents representing features of their mechanical stimuli are encoded in the firing rates and patterns of action potential trains (Bewick and Banks 2015; Loewenstein and Rathkamp 1958; Quick et al. 1980). These encoding functions appear similar to those occurring at the axon initial segments, where synaptic currents are integrated and translated into action potential firing (Clark et al. 2009) and where NaV1.6 is also found concentrated in a number of neuron types (Brocard et al. 2016; Hu et al. 2009; Osorio et al. 2010; Royeck et al. 2008). The presumptive encoding function of heminodes appears well served by the three voltage-gated currents mediated by NaV1.6. The transient Na current (INaT) is large, but brief, initiated by small depolarization, and, thereby, well suited to facilitate action potential initiation (Royeck et al. 2008). A resurgent current (INaR) produced by NaV1.6 repriming kinetics is present in some large-diameter DRG neurons (Cummins et al. 2005), has the potential to support firing at high firing rates (Khaliq et al. 2003; Mercer et al. 2007) and can aid in sustaining repetitive firing, as has been shown for Purkinje cells (Raman et al. 1997). Both INaT and INaR are recognized for their capacity to support action potential generation at axon initial segments, where NaV1.6 is shown to aggregate (Hu et al. 2009; Osorio et al. 2010; Royeck et al. 2008). A third current mediated by NaV1.6, INaP, also activates near resting membrane potential threshold, is larger in comparison with some other NaV isoforms (Chen et al. 2008), and persists >600 ms (Crill 1996; Raman et al. 1997). At the heminodes, INaP might support repetitive firing and adjust firing rate in relation to the magnitude of arriving receptor potentials, as it does for synaptic currents in other neurons (e.g., Hultborn et al. 2003). A critical role for NaV1.6 in supporting tonic firing was recently assigned to the sensory endings of stretch-sensitive colorectal afferents (Feng et al. 2015). For spindle primary endings, the presence of NaV1.6 IR aggregations at heminodes qualifies it to mediate inward Na currents, which in other systems described above support both initiation and repetitive occurrence of action potentials.
The robust immunostaining that we found for NaV1.6 in the annulospiral sensory terminals of the spindle primary endings was unexpected. The capacity for NaV1.6 to generate action potentials seems misplaced at these terminals, which are not generally thought to be sites of action potential generation (Banks et al. 1997; Bewick and Banks 2015; Quick et al. 1980). The spindle terminals are instead the probable sites of mechanotransduction, where muscle stretch activates aggregations of mechanically gated ion channels to produce receptor currents (Bewick and Banks 2015). In the absence of evidence for mechanical gating, NaV1.6 is not expected to contribute in mechanotransduction. Nonetheless, we recognize a potential role for NaV1.6 in the sensory terminals that could be critical for sensory signaling. NaV1.6 INaP might assist in conveying the receptor potential from its origin in the unmyelinated sensory terminal to the site of action potential generation in the heminodes. The electrotonic pathway from the sensory terminal through the preterminal axons might result in considerable decrement in receptor current before its arrival at the heminodes. Although the absence of information on the input conductance of spindle sensory terminals precludes estimating the magnitude of current loss, some fraction of the receptor is expected to be lost to electrotonic decay in the terminal’s expansive membrane area of ~17,500 to 18,500 µm2 measured in the adult cat (Banks et al. 1982), and to conductance shunting occurring when mechanically gated channels are activated. INaP has the potential to compensate for electrotonic reduction of the receptor current, just as it is proposed to do for synaptic currents that, without amplification, may be substantially reduced as they spread through the dendritic arbors of neurons (Crill 1996), and therefore, less effective in initiating or modulating neuron firing (Binder 2002; Hultborn et al. 2003). In the sensory terminals of spindle primary endings, NaV1.6 is well positioned through its close proximity to mechanotransducers, to be activated by receptor potentials and to generate INaP that boosts, i.e., amplifies, the receptor current. The amplification might apply over the full time course of the receptor current, owing to the persistence of INaP. In this way, the receptor current’s representation of all phases of a discrete muscle stretch, e.g., early dynamic and later static phases would be preserved. We suggest further that NaV1.6 concentration in segments of preterminal axons might provide additional amplification stations for receptor current along its electrotonic path to the heminodes. Alternatively, NaV1.6 might support action potentials in the preterminal axon (Ito and Ito 1976), as it does in unmyelinated axons of other neurons (Black et al. 2002).
Our IR studies also revealed the presence of two other NaV isoforms in the primary endings of muscle spindles of the rat soleus, the NaV1.1 and NaV1.7 isoforms. We selected these two isoforms because, on the basis of the available evidence, the presence of the others at this location is unlikely. NaV1.2 is mainly expressed in the central nervous system, NaV1.3 is primarily expressed in the central neurons during embryonic and postnatal development, NaV1.4 primarily in skeletal muscle, and NaV1.5 in cardiac muscle (Beckh 1990; Catterall et al. 2005; Goldin 2001; Rush et al. 2007). The other two NaV isoforms, NaV1.8 and NaV1.9, are found mainly in small sensory neurons. Functionally, it seems unlikely that repetitive firing by spindle afferents requires either NaV1.8 or NaV1.9, because both isoforms are resistant to tetrodotoxin (TTX-R; Catterall et al. 2005), which is known to completely block the electrical response of muscle spindles to stretch (Hunt et al. 1978). We acknowledge that although unnecessary for firing, TTX-R NaV channels may play a role, especially since NaV1.8 expression has been shown in dissociated-cultured, large-diameter neurons in DRG, as well as in a large group of neurons with myelinated A fibers (Ramachandra et al. 2012; Shields et al. 2012).
As mentioned earlier, NaV IR for both 1.1 and 1.7 isoforms was clearly observed in the primary endings of Ia afferents of the rat soleus. Their distribution, however, varied significantly. Strong NaV1.1 IR was observed at the sensory terminals of the primary endings of bag fibers, whereas NaV1.7 IR was the weaker of the two isoforms at this location. NaV1.1 IR predominated in the sensory terminal of chain fibers. NaV1.7 IR, on the other hand, was very strong in the presynaptic axon extending from the heminode areas to the sensory terminals where NaV1.1 IR was completely absent. In the sensory terminals NaV1.1 is likely to be another important source of INaP. Purkinje neurons from NaV1.1 KO mice, have significantly reduced INaP and resurgent inward currents compared with neurons from wild-type animals (Kalume et al. 2007). Therefore, and as discussed earlier for NaV1.6, NaV1.1 is also well positioned to boost the receptor currents produced by mechanotransducers, especially in intrafusal chain fibers, where it predominates. It is interesting that deletion of NaV1.1 or NaV1.6 renders mice ataxic, significantly affecting limb coordination and motor reflexes (Kalume et al. 2007; Raman et al. 1997). The motor disorders were associated with the loss of INaP and INaR in Purkinje neurons. Our findings suggest that ataxia in these KO mice might also have resulted from the deletion of NaV1.1 and NaV1.6 from muscle spindle primary endings.
NaV1.7 was clearly the predominant isoform in the preterminal axons. This finding adds to what others have reported in stretch-sensitive colorectal afferent endings (Feng et al. 2015). In addition to its presence in mechanosensitive receptors in the gut, coexpression of NaV1.7 and NaV1.6 has been shown at nodes of Ranvier in a subpopulation of small-diameter Aδ myelinated fibers in sciatic nerve (Black et al. 2012), 40% of which are mechanosensitive only (Cain et al. 2001). Thus, the available evidence, together with our results, points to a probable, but unrecognized role, of this channel in the process of mechanosensory signaling. NaV1.7 is known to produce ramp currents (Cummins et al. 1998) and, via this process, has the capacity to amplify subthreshold potentials, and to decrease the threshold for action potential initiation (Dib-Hajj et al. 2013; Rush et al. 2007).
NaV expression in the muscle spindle was not restricted to primary endings. In a mouse spindle, we were confident in our positive identification of one secondary ending, in which we found clear expression of NaV1.6 in the heminode and terminal endings. This isolated observation may indicate that the roles we propose for NaV1.6 extend to secondary endings. In addition, NaV1.6 and NaV1.1 were observed between nuclei in the juxta regions of bag and chain intrafusal muscle fibers. The intranuclear space is also found to express the provisional mechanotransducing channel, α-ENaC (see Simon et al. 2010). The function of Na channel expression in intranuclear sites at the moment is unknown.
Physiological data demonstrate qualitative similarity among these three species. To our knowledge, this comparison has not been reported. With respect to tonic firing, it establishes for the first time that Ia afferents in these species are indistinguishable. The differences in other parameters are interesting, and we attribute them to species differences, since the responses were obtained using strain and strain rates that were identical in all species.
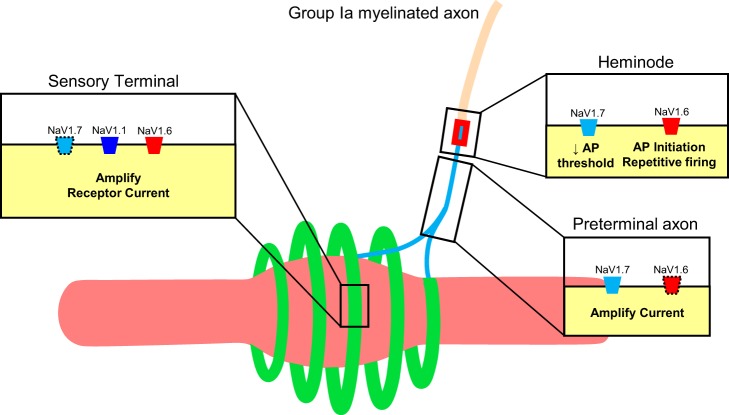
The distribution described above for NaV isoforms is summarized in Fig. 8. This figure is an adapted version of the model advanced by Bewick and Banks (2015), which represented knowledge accumulated up to that time about the molecular mechanisms of primary ending excitability. To this model, we add new details about NaV channel presence in sensory regions involved in encoding by muscle spindles. In the terminals, close proximity to mechanotransducers positions NaV isoforms, primarily 1.1 and 1.6, to detect and amplify receptor potential current. In the preterminal axons, the strong presence of NaV1.7 may serve to boost the receptor current along its electrotonic path to the heminode (Cummins et al. 1998; Rush et al. 2007). Lastly, at the heminode, the presumed site of action potential generation (Bewick and Banks 2015; Loewenstein and Rathkamp 1958; Quick et al. 1980), NaV1.7 may act as a “threshold channel” (Dib-Hajj et al. 2013), while NaV1.6 takes primary responsibility for action potential initiation, as well as for encoding receptor potential features into firing rate modulation and tonic firing.
Fig. 8.
Model of Na voltage-gated channel distribution in muscle spindle Ia primary ending. (model adapted from Bewick and Banks, 2015). The intrafusal muscle fiber (pinkish) was wrapped by an annulospiral ending (green) and a preterminal axon (light blue) that extends unmyelinated from the terminal to the heminode followed by a myelinated axon. Icons represent channels, with dotted outlines for some indicating less prevalent immunoreactivity.
The discussion above attributes particular importance to INaP. This focus originated from our interest in identifying mechanisms that yield the tonic firing that characterizes spindle afferents, i.e., sustained repetitive firing in response to steady depolarization produced by static muscle stretch (Hunt et al. 1978). This mechanism is unknown and is not expressly indicated in ion-channel models of primary spindle endings (cf. Bewick and Banks 2015). The potential importance of INaP to this mechanism is suggested by our recent findings that the drugs riluzole and phenytoin, which share only blockade of INaP among their many effects (Lampl et al. 1998; Urbani and Belluzzi 2000; Xie et al. 2011; Zeng et al. 2005), result in substantial reduction of primary spindle afferent firing in vivo when administered acutely to adult rats (Vincent et al. 2015). We also reported that, as a predicted expression of persistent inward current (Harvey et al. 2006), the threshold for repetitive firing increases for ramp stretch at slow velocity (Vincent et al. 2016). These findings suggested to us that channels mediating INaP are expressed peripherally by muscle spindle afferents, which belong to the group of large-diameter DRG cells that respond to intrasomatic current injection by producing INaP and repetitive firing, both of which are blocked by riluzole (Xie et al. 2011). If INaP does operate in spindle endings, possibly mediated by NaV1.6 and NaV1.1, then considerations presented earlier in the discussion indicate that it might support tonic firing by amplifying sustained depolarization of the receptor potential and/or by supporting repetitive firing at heminodes. Impairment of either process has the potential to produce the reduction of tonic firing that we observe with antiepileptic drugs, as well as with chronic treatment of rats with the anticancer agent oxaliplatin (Bullinger et al. 2011; Vincent et al. 2015, 2016). Therefore, we hypothesize that these agents reduce tonic firing by impairing the INaP mediated by either NaV1.6, NaV1.1, or both in spindle primary endings.
GRANTS
This work was supported, in part, by funding from National Institute of Neurological Disorders and Stroke Grant P01NS-057228.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.C.C. and D.I.C. wrote the manuscript; D.I.C. performed immunohistochemistry and confocal imaging; J.A.V performed electrophysiological recordings.
ACKNOWLEDGMENTS
We gratefully acknowledge the consultations and critical review of this manuscript by Dr. Robert Banks and Dr. Guy Bewick. We also thank Dr. Francisco Alvarez and Dr. Martin Pinter for facilitating some of the equipment that allowed this study to be possible.
REFERENCES
- Baker MD, Bostock H. Low-threshold, persistent sodium current in rat large dorsal root ganglion neurons in culture. J Neurophysiol 77: 1503–1513, 1997. [DOI] [PubMed] [Google Scholar]
- Banks RW. An allometric analysis of the number of muscle spindles in mammalian skeletal muscles. J Anat 208: 753–768, 2006. doi: 10.1111/j.1469-7580.2006.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW, Barker D, Stacey MJ. Form and distribution of sensory terminals in cat hindlimb muscle spindles. Philos Trans R Soc Lond B Biol Sci 299: 329–364, 1982. doi: 10.1098/rstb.1982.0136. [DOI] [PubMed] [Google Scholar]
- Banks RW, Hulliger M, Saed HH, Stacey MJ. A comparative analysis of the encapsulated end-organs of mammalian skeletal muscles and of their sensory nerve endings. J Anat 214: 859–887, 2009. doi: 10.1111/j.1469-7580.2009.01072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW, Hulliger M, Scheepstra KA, Otten E. Pacemaker activity in a sensory ending with multiple encoding sites: the cat muscle spindle primary ending. J Physiol 498: 177–199, 1997. doi: 10.1113/jphysiol.1997.sp021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckh S. Differential expression of sodium channel mRNAs in rat peripheral nervous system and innervated tissues. FEBS Lett 262: 317–322, 1990. doi: 10.1016/0014-5793(90)80218-8. [DOI] [PubMed] [Google Scholar]
- Bewick GS, Banks RW. Mechanotransduction in the muscle spindle. Pflügers Arch 467: 175–190, 2015. doi: 10.1007/s00424-014-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder MD. Integration of synaptic and intrinsic dendritic currents in cat spinal motoneurons. Brain Res Brain Res Rev 40: 1–8, 2002. doi: 10.1016/S0165-0173(02)00183-2. [DOI] [PubMed] [Google Scholar]
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel α-subunit mRNAs. Brain Res Mol Brain Res 43: 117–131, 1996. doi: 10.1016/S0169-328X(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Black JA, Frézel N, Dib-Hajj SD, Waxman SG. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain 8: 82, 2012. doi: 10.1186/1744-8069-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Renganathan M, Waxman SG. Sodium channel Na(v)1.6 is expressed along nonmyelinated axons and it contributes to conduction. Brain Res Mol Brain Res 105: 19–28, 2002. doi: 10.1016/S0169-328X(02)00385-6. [DOI] [PubMed] [Google Scholar]
- Brocard C, Plantier V, Boulenguez P, Liabeuf S, Bouhadfane M, Viallat-Lieutaud A, Vinay L, Brocard F. Cleavage of Na+ channels by calpain increases persistent Na+ current and promotes spasticity after spinal cord injury. Nat Med 22: 404–411, 2016. doi: 10.1038/nm.4061. [DOI] [PubMed] [Google Scholar]
- Bullinger KL, Nardelli P, Wang Q, Rich MM, Cope TC. Oxaliplatin neurotoxicity of sensory transduction in rat proprioceptors. J Neurophysiol 106: 704–709, 2011. doi: 10.1152/jn.00083.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol 85: 1561–1574, 2001. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Nav1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc Natl Acad Sci USA 97: 5616–5620, 2000. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 57: 397–409, 2005. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu FH, Sharp EM, Beacham D, Scheuer T, Catterall WA. Functional properties and differential neuromodulation of Nav1.6 channels. Mol Cell Neurosci 38: 607–615, 2008. doi: 10.1016/j.mcn.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BD, Goldberg EM, Rudy B. Electrogenic tuning of the axon initial segment. Neuroscientist 15: 651–668, 2009. doi: 10.1177/1073858409341973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol 58: 349–362, 1996. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Herzog RI, Waxman SG. Nav1.6 channels generate resurgent sodium currents in spinal sensory neurons. FEBS Lett 579: 2166–2170, 2005. doi: 10.1016/j.febslet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Howe JR, Waxman SG. Slow closed-state inactivation: a novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel. J Neurosci 18: 9607–9619, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Nav1.7 sodium channel: from molecule to man. Nat Rev Neurosci 14: 49–62, 2013. doi: 10.1038/nrn3404. [DOI] [PubMed] [Google Scholar]
- Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron 39: 109–120, 2003. doi: 10.1016/S0896-6273(03)00360-X. [DOI] [PubMed] [Google Scholar]
- Feng B, Zhu Y, La JH, Wills ZP, Gebhart GF. Experimental and computational evidence for an essential role of Nav1.6 in spike initiation at stretch-sensitive colorectal afferent endings. J Neurophysiol 113: 2618–2634, 2015. doi: 10.1152/jn.00717.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol 63: 871–894, 2001. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain WA, Antic SD, Yang Y, Rasband MN, Morest DK. Where is the spike generator of the cochlear nerve? Voltage-gated sodium channels in the mouse cochlea. J Neurosci 25: 6857–6868, 2005. doi: 10.1523/JNEUROSCI.0123-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nat Neurosci 12: 996–1002, 2009. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol 552: 945–952, 2003. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC, Wilkinson RS, Fukami Y. Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. J Gen Physiol 71: 683–698, 1978. doi: 10.1085/jgp.71.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito F, Ito Y. Selective blockage of the sensory terminal activites by micro-application of tetrodotoxin in the frog muscle spindle. Brain Res Bull 1: 363–366, 1976. doi: 10.1016/0361-9230(76)90029-0. [DOI] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci 27: 11065–11074, 2007. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Gouwens NW, Raman IM. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J Neurosci 23: 4899–4912, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl I, Schwindt P, Crill W. Reduction of cortical pyramidal neuron excitability by the action of phenytoin on persistent Na+ current. J Pharmacol Exp Ther 284: 228–237, 1998. [PubMed] [Google Scholar]
- Lesniak DR, Marshall KL, Wellnitz SA, Jenkins BA, Baba Y, Rasband MN, Gerling GJ, Lumpkin EA. Computation identifies structural features that govern neuronal firing properties in slowly adapting touch receptors. eLife 3: e01488, 2014. doi: 10.7554/eLife.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Cheng YR, Banks RW, Min MY, Bewick GS, Chen CC. Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nat Commun 7: 11460, 2016. doi: 10.1038/ncomms11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein WR, Rathkamp R. The sites for mechano-electric conversion in a Pacinian corpuscle. J Gen Physiol 41: 1245–1265, 1958. doi: 10.1085/jgp.41.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JN, Chan CS, Tkatch T, Held J, Surmeier DJ. Nav1.6 sodium channels are critical to pacemaking and fast spiking in globus pallidus neurons. J Neurosci 27: 13552–13566, 2007. doi: 10.1523/JNEUROSCI.3430-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahirney PC, Ovalle WK. Distribution of dystrophin and neurofilament protein in muscle spindles of normal and Mdx-dystrophic mice: an immunocytochemical study. Anat Rec 235: 501–510, 1993. doi: 10.1002/ar.1092350403. [DOI] [PubMed] [Google Scholar]
- Nakanishi ST, Whelan PJ. A decerebrate adult mouse model for examining the sensorimotor control of locomotion. J Neurophysiol 107: 500–515, 2012. doi: 10.1152/jn.00699.2011. [DOI] [PubMed] [Google Scholar]
- Osorio N, Cathala L, Meisler MH, Crest M, Magistretti J, Delmas P. Persistent Nav1.6 current at axon initial segments tunes spike timing of cerebellar granule cells. J Physiol 588: 651–670, 2010. doi: 10.1113/jphysiol.2010.183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteen JD, Herzig V, Gilchrist J, Emrick JJ, Zhang C, Wang X, Castro J, Garcia-Caraballo S, Grundy L, Rychkov GY, Weyer AD, Dekan Z, Undheim EA, Alewood P, Stucky CL, Brierley SM, Basbaum AI, Bosmans F, King GF, Julius D. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 534: 494–499, 2016. doi: 10.1038/nature17976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Nardelli P, Nakanishi ST, Ross KT, Nichols TR, Pinter MJ, Cope TC. Recovery of proprioceptive feedback from nerve crush. J Physiol 589: 4935–4947, 2011. doi: 10.1113/jphysiol.2011.210518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2012. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Quick DC, Kennedy WR, Poppele RE. Anatomical evidence for multiple sources of action potentials in the afferent fibers of muscle spindles. Neuroscience 5: 109–115, 1980. doi: 10.1016/0306-4522(80)90076-7. [DOI] [PubMed] [Google Scholar]
- Ramachandra R, McGrew SY, Baxter JC, Kiveric E, Elmslie KS. Tetrodotoxin-resistant voltage-dependent sodium channels in identified muscle afferent neurons. J Neurophysiol 108: 2230–2241, 2012. doi: 10.1152/jn.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron 19: 881–891, 1997. doi: 10.1016/S0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Royeck M, Horstmann MT, Remy S, Reitze M, Yaari Y, Beck H. Role of axonal Nav1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J Neurophysiol 100: 2361–2380, 2008. doi: 10.1152/jn.90332.2008. [DOI] [PubMed] [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol 579: 1–14, 2007. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J Physiol 564: 803–815, 2005. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster JE, Fu R, Siddique T, Heckman CJ. Effect of prolonged riluzole exposure on cultured motoneurons in a mouse model of ALS. J Neurophysiol 107: 484–492, 2012. doi: 10.1152/jn.00714.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, Dib-Hajj SD. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain 153: 2017–2030, 2012. doi: 10.1016/j.pain.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Simon A, Shenton F, Hunter I, Banks RW, Bewick GS. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol 588: 171–185, 2010. doi: 10.1113/jphysiol.2009.182683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci 12: 3567–3574, 2000. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Vincent JA, Nardelli P, Gabriel HM, Deardorff AS, Cope TC. Complex impairment of IA muscle proprioceptors following traumatic or neurotoxic injury. J Anat 227: 221–230, 2015. doi: 10.1111/joa.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JA, Wieczerzak KB, Gabriel HM, Nardelli P, Rich MM, Cope TC. A novel path to chronic proprioceptive disability with oxaliplatin: distortion of sensory encoding. Neurobiol Dis 95: 54–65, 2016. doi: 10.1016/j.nbd.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Pappas GD, Bennett MV. Morphological correlates of functional differentiation of nodes of Ranvier along single fibers in the neurogenic electric organ of the knife fish Stern archus. J Cell Biol 53: 210–224, 1972. doi: 10.1083/jcb.53.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KA, Kloefkorn HE, Hochman S. Characterization of muscle spindle afferents in the adult mouse using an in vitro muscle-nerve preparation. PLoS One 7: e39140, 2012. doi: 10.1371/journal.pone.0039140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, Patapoutian A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 18: 1756–1762, 2015. doi: 10.1038/nn.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie RG, Zheng DW, Xing JL, Zhang XJ, Song Y, Xie YB, Kuang F, Dong H, You SW, Xu H, Hu SJ. Blockade of persistent sodium currents contributes to the riluzole-induced inhibition of spontaneous activity and oscillations in injured DRG neurons. PLoS One 6: e18681, 2011. doi: 10.1371/journal.pone.0018681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Powers RK, Newkirk G, Yonkers M, Binder MD. Contribution of persistent sodium currents to spike-frequency adaptation in rat hypoglossal motoneurons. J Neurophysiol 93: 1035–1041, 2005. doi: 10.1152/jn.00831.2004. [DOI] [PubMed] [Google Scholar]