Saccades, which repeatedly realign the line of sight, introduce spurious signals in retinal images that normally go unnoticed. In part, this happens because of perisaccadic suppression of visual sensitivity, which is known to depend on spatial frequency. We discovered that a specific subtype of superior colliculus (SC) neurons demonstrates spatial-frequency-dependent suppression. Curiously, it is the neurons that help mediate the saccadic command itself that exhibit such suppression, and not the purely visual ones.
Keywords: saccades, microsaccades, superior colliculus, saccadic suppression, perceptual stability
Abstract
Saccades cause rapid retinal-image shifts that go perceptually unnoticed several times per second. The mechanisms for saccadic suppression have been controversial, in part because of sparse understanding of neural substrates. In this study we uncovered an unexpectedly specific neural locus for spatial frequency-specific saccadic suppression in the superior colliculus (SC). We first developed a sensitive behavioral measure of suppression in two macaque monkeys, demonstrating selectivity to low spatial frequencies similar to that observed in earlier behavioral studies. We then investigated visual responses in either purely visual SC neurons or anatomically deeper visual motor neurons, which are also involved in saccade generation commands. Surprisingly, visual motor neurons showed the strongest visual suppression, and the suppression was dependent on spatial frequency, as in behavior. Most importantly, suppression selectivity for spatial frequency in visual motor neurons was highly predictive of behavioral suppression effects in each individual animal, with our recorded population explaining up to ~74% of behavioral variance even on completely different experimental sessions. Visual SC neurons had mild suppression, which was unselective for spatial frequency and thus only explained up to ~48% of behavioral variance. In terms of spatial frequency-specific saccadic suppression, our results run contrary to predictions that may be associated with a hypothesized SC saccadic suppression mechanism, in which a motor command in the visual motor and motor neurons is first relayed to the more superficial purely visual neurons, to suppress them and to then potentially be fed back to cortex. Instead, an extraretinal modulatory signal mediating spatial-frequency-specific suppression may already be established in visual motor neurons.
NEW & NOTEWORTHY Saccades, which repeatedly realign the line of sight, introduce spurious signals in retinal images that normally go unnoticed. In part, this happens because of perisaccadic suppression of visual sensitivity, which is known to depend on spatial frequency. We discovered that a specific subtype of superior colliculus (SC) neurons demonstrates spatial-frequency-dependent suppression. Curiously, it is the neurons that help mediate the saccadic command itself that exhibit such suppression, and not the purely visual ones.
a long-standing question in visual neuroscience has been about how we normally experience a sense of perceptual stability despite incessant eye movements (Wurtz 2008). Saccadic eye movements, in particular, dramatically alter retinal images several times per second. During each saccade, retinal images undergo rapid motion, which can be beyond the range of motion sensitivity of many neurons. Such motion ought, at least in principle, to cause a brief period of “gray out” every time a saccade occurs (Campbell and Wurtz 1978; Matin 1974; Wurtz 2008; Wurtz et al. 2011), much like the gray out experienced by persons while standing near train tracks as high-speed trains sweep by.
Several theories about why we do not experience saccade-related visual disruptions have been debated in the literature. On the one hand, purely visual mechanisms, such as masking (Matin et al. 1972), can be sufficient to suppress perception of saccade-induced gray out and/or motion (Wurtz 2008). Consistent with this, people are not entirely “blind” during saccades, as long as spatiotemporal properties of perisaccadic stimuli remain within sensitivity ranges of visual neurons (Burr and Ross 1982; Castet et al. 2001; Castet and Masson 2000; García-Pérez and Peli 2011; Ilg and Hoffmann 1993; Matin et al. 1972; Ross et al. 1996). On the other hand, extraretinal mechanisms (Sperry 1950; von Holst and Mittelstaedt 1950) for suppression are supported by the lack of suppression during simulated image displacements (Diamond et al. 2000), the dependence of suppression on spatial frequency (Burr et al. 1982, 1994; Hass and Horwitz 2011; Volkmann et al. 1978), and the observation of saccade-related modulation of neural excitability in the absence of visual stimulation (Rajkai et al. 2008).
Although it is likely that a combination of visual and extraretinal mechanisms coexist (Wurtz 2008), further understanding of neural mechanisms is needed to resolve some of the debates surrounding saccadic suppression. We were particularly interested in potential mechanisms for extraretinal suppression, whose sources remain elusive. For example, it was suggested from behavioral studies that selective suppression of low spatial frequencies is evidence for selective magnocellular (achromatic) pathway suppression (Burr et al. 1994). However, in lateral geniculate nucleus (LGN) and primary visual cortex (V1), two early visual areas possessing clear magno- and parvocellular segregations, selective magnocellular suppression is not established (Hass and Horwitz 2011; Kleiser et al. 2004; Ramcharan et al. 2001; Reppas et al. 2002; Royal et al. 2006). In addition, a hypothesis about a source of saccadic suppression is that a “corollary” of saccade commands in visual motor and motor neurons of the superior colliculus (SC) is fed back to superficial purely visual neurons to suppress their sensitivity and to jumpstart a putative feedback pathway for cortical suppression through pulvinar (Berman and Wurtz 2008; Berman and Wurtz 2010; Berman and Wurtz 2011; Isa and Hall 2009; Lee et al. 2007; Phongphanphanee et al. 2011; Wurtz 2008; Wurtz et al. 2011). However, evidence for an SC saccadic suppression pathway from visual motor/motor neurons to visual neurons comes primarily from rodent SC slices (Isa and Hall 2009; Lee et al. 2007; Phongphanphanee et al. 2011). In the awake, behaving primate, findings of stronger suppression in visual motor rather than visual neurons (Chen et al. 2015; Hafed et al. 2015; Hafed and Krauzlis 2010) suggest a more nuanced set of mechanisms. Moreover, spatial-frequency-specific suppression of visual sensitivity in either visual or visual motor SC neurons has not yet been investigated.
In this study, we visited the question of neural loci for saccadic suppression in the SC by looking for spatial frequency specificity of visual suppression. We have previously shown that SC neurons exhibit time courses of saccadic suppression remarkably similar to those of perceptual effects in humans (Hafed and Krauzlis 2010). However, our previous experiments did not investigate any potential spatial frequency dependence in saccadic suppression, as might be expected from earlier human experiments (Burr et al. 1994). Our earlier experiments only presented a white bar stimulus within a neuron’s visual response field (RF). Thus, in this study, we adapted our behavioral paradigm from (Hafed and Krauzlis 2010) to first establish selectivity in saccadic suppression during this paradigm, and we then asked whether visual neural modulations in either purely visual or visual motor SC neurons would reflect such selectivity. Contrary to what we might have predicted on the basis of a suppressive pathway from deep to superficial layers (Isa and Hall 2009; Lee et al. 2007; Phongphanphanee et al. 2011), we observed spatial-frequency-specific saccadic suppression only in the deeper visual motor neurons. Visual neurons showed mild suppression, but this suppression was not modulated as a function of spatial frequency. Moreover, we recorded local field potentials (LFPs) as a proxy for population and synaptic activity around our isolated neurons (Hafed and Chen 2016; Ikeda et al. 2015), and we found evidence that the visual suppression of firing rates that we observed in isolated neurons may have been mediated by the presence of modulatory signals in the SC associated with the motor generation of saccades, and particularly in the visual motor layers. Our results suggest that the SC may indeed be relevant for spatial-frequency-specific saccadic suppression, which has been reported previously in humans (Burr et al. 1994), but that the putatively extraretinal modulatory signal mediating suppression may already be established in the visual motor neurons.
From a technical standpoint, we exploited microsaccades to study saccadic suppression in this study because microsaccades offer important experimental advantages while at the same time being mechanistically similar to larger saccades (Hafed 2011; Hafed et al. 2009; Hafed et al. 2015; Zuber et al. 1965). First, microsaccades are small (median amplitude in our data: ~7.5 min arc). Thus pre- and postmovement visual RFs are not displaced by much, minimizing the problem of dramatic spatial image shifts caused by saccades (Wurtz 2008; Wurtz et al. 2011). Experimentally, this meant presenting the same stimulus at the same screen location with and without microsaccades to isolate suppression effects. Second, microsaccades have velocities significantly <100 deg/s (median peak velocity in our data: ~17.7 deg/s). Thus image motion caused by microsaccades is well within the range of motion sensitivity, even for small features (Thiele et al. 2002), allowing us to study suppression even when no motion-induced gray out is expected to occur. Third, we have previously shown, with simple white bars, that SC visual sensitivity exhibits pre-, peri-, and postmicrosaccadic suppression that is similar in time course and amplitude to perceptual saccadic suppression in humans with larger saccades, and we also have demonstrated a sensitive behavioral paradigm for the same phenomenon (Hafed and Krauzlis 2010). Fourth, and more importantly, we avoided potential masking effects by only presenting stimuli immediately after microsaccades. This allowed us to study suppression after saccades, which is known to still occur (Chen et al. 2015; Hafed and Krauzlis 2010; Zuber et al. 1966), and to ensure comparison of “no-microsaccade” to “microsaccade” conditions without the latter involving saccade-induced retinal image motion. Finally, it was established long ago that at the behavioral level, microsaccades are associated with similar suppression to larger saccades (Zuber et al. 1966) and that saccadic suppression is also expected to occur far away from the movement end point (Knöll et al. 2011); this meant that using microsaccades as a model system for saccadic suppression was reasonable. Thus the logic of all of our experiments was to present high-contrast gratings (80% contrast), which were highly visible and well within the saturation regime of SC contrast sensitivity curves (Chen et al. 2015; Hafed and Chen 2016; Li and Basso 2008), and to ask whether either behavioral or visual neural responses to these gratings were altered if the gratings appeared immediately after a microsaccade.
MATERIALS AND METHODS
Animal Preparation
Ethics committees at regional governmental offices in Tuebingen approved experiments. Monkeys N and P (male, Macaca mulatta, age 7 yr) were prepared as detailed earlier (Chen and Hafed 2013; Chen et al. 2015; Hafed and Chen 2016; Hafed and Ignashchenkova 2013). Briefly, under isoflurane anesthesia and aseptic conditions, we first attached a head holder to the skull. The head holder consisted of a titanium implant that was embedded under the skin and attached to the skull using titanium screws. In a subsequent surgery, we made a small skin incision on top of the head and attached a metal connector to the previously implanted head holder. This connector acted as the interface for fixing the head to a standard position in the laboratory during data collection. In the same surgery, a scleral search coil was implanted in one eye to allow measurement of eye movements with high temporal and spatial precision using the magnetic induction technique (Fuchs and Robinson 1966; Judge et al. 1980). After the animals completed the behavioral training and experimental sessions, we implanted recording chambers to access the SC. The chambers were placed on the midline, aimed at 1 mm posterior to and 15 mm above the interaural line. Chambers were tilted posterior to vertical (by 35° and 38° for monkeys N and P, respectively).
Behavioral Tasks
In all tasks, the monkeys initially fixated a small, white spot presented over a gray background (Chen and Hafed 2013; Chen et al. 2015; Hafed and Ignashchenkova 2013). Spot and background luminance values were 72 and 21 cd/m2, respectively.
Behavioral tests.
Trials started with an initial fixation interval of random duration (between 600 and 1,500 ms). After this interval, we initiated a real-time process to detect microsaccades (Chen and Hafed 2013). Briefly, this process evaluated instantaneous radial eye velocity on the basis of recently sampled eye positions, and it flagged the presence of a microsaccade when this velocity exceeded a user-defined threshold. If a microsaccade was detected within 500 ms, a stationary vertical Gabor grating (having 80% contrast relative to background luminance) appeared at 3.5° to the right or left of fixation, and the fixation spot was removed simultaneously. Monkeys oriented to the grating using a saccadic eye movement, and saccadic reaction time (RT) served as a sensitive behavioral measure of SC visual response strength (Boehnke and Munoz 2008; Hafed and Chen 2016; Hafed and Krauzlis 2010; Hafed et al. 2015; Tian et al. 2016; also see discussion). Because of their extensive training on visually guided saccades, our monkeys were likely making speeded reactions to the gratings, further justifying the use of RT. Grating onset occurred ~25, 50, 75, 100, 150, or 200 ms after online microsaccade detection, and we later measured precise times of microsaccade onset during data analysis for all results presented in this article (see Data Analysis). Our choice of times to sample (listed above) was based on earlier observations that saccadic suppression effects in the SC subside by ~100 ms after the movements (Chen et al. 2015; Hafed and Krauzlis 2010). If no microsaccade was detected during our 500-ms online detection window, a grating was presented anyway, and the data contributed to “baseline” measurements (i.e., ones with the stimulus appearing without any nearby microsaccades). The grating was 2° in diameter. Spatial frequency in cycles per degree (cpd) was one of five values: 0.56, 1.11, 2.22, 4.44, or 11.11 (Hafed and Chen 2016), and phase was randomized. Our monitor resolution allowed display at the highest spatial frequency without aliasing and distortion. We collected 8,153 and 7,117 trials from monkeys N and P, respectively. We removed trials with an intervening microsaccade between fixation spot removal and the orienting saccade.
Neural recordings.
We isolated single neurons online, and we identified their RF locations and sizes using standard saccade tasks (Chen et al. 2015; Hafed and Chen 2016). We then ran our main experimental paradigm. In each trial, monkeys fixated while we presented a vertical grating similar to the one we used in the behavioral tests described above (i.e., with similar contrast and spatial frequency ranges), but the grating was now inside the recorded neuron’s RF. Grating size was optimized for the recorded neuron and was specifically chosen to fill as much of the RF as possible (and showing >1 cycle of the lowest spatial frequency). Task timing was identical to that in Chen et al. (2015); briefly, a grating was presented for 250 ms while monkeys fixated, and the monkeys never generated any saccadic or manual responses to the grating (they simply maintained fixation, during which they generated microsaccades, and they were rewarded at the end of the 250-ms stimulus presentation phase for maintaining fixation). We collected data from 90 neurons (n = 39 from monkey N and n = 51 from monkey P), covering 1°–24° eccentricities. We classified neurons as purely visual neurons or visual motor neurons by using previous criteria from visually guided and memory-guided saccade tasks (Chen et al. 2015; Hafed and Chen 2016). To ensure sufficient microsaccades for statistical analyses (i.e., with sufficient trials having stimulus onset within the critical postmovement intervals that we analyzed), we collected >800 trials per neuron. We then separated trials as ones having no microsaccades within ±100 ms from grating onset (>100 trials per neuron; mean: 289 trials per neuron; median: 191 trials per neuron) or ones with grating onset within 50 ms after microsaccades (>25 trials per neuron; mean: 79 trials per neuron; median: 79 trials per neuron). The former trials provided an estimate of “baseline” responses without the influence of saccadic suppression, and the latter trials provided an estimate of the suppressed responses due to saccadic suppression. Moreover, the times chosen were justified on the basis of previous descriptions of the time courses of saccadic suppression (e.g., Hafed and Krauzlis 2010; Zuber et al. 1966). Some of our analyses also included grating onsets up to 100 ms after microsaccades.
It is important to note that for all neurons reported in this article, we never observed a microsaccade-related movement burst (Hafed et al. 2009; Hafed and Krauzlis 2012). Thus, even for stimuli appearing immediately after a microsaccade, the neural responses that we analyzed were visual bursts in response to stimulus onset, and not movement-related saccade or microsaccade bursts. The only difference between purely visual and visual motor neurons in this study was that visual motor neurons would, in principle, exhibit a saccade-related burst if the monkeys were to hypothetically generate saccades toward the RF location (but not if they generated smaller microsaccades during fixation). Thus any neural modulations that we report in this study are not direct microsaccade-related motor bursts.
It also is important to note that our monkeys did not generate any targeting saccades to the gratings during recordings. We were simply studying visual sensitivity if a stimulus appeared near an eye movement. Our approach was thus very similar to classic ways of studying neural correlates of saccadic suppression (i.e., monkeys make saccades while neurons are visually stimulated; e.g., Bremmer et al. 2009; Hafed and Krauzlis 2010; Zanos et al. 2016).
Data Analysis
In all figures, we plotted mean values (along with suitable measures of variance, such as SE) for the parameters that we were visualizing; we used the mean in the figures because this is a standard way of presenting data. However, in quantitative descriptions in the text, we sometimes report median values in addition to mean values, and for statistical analyses, we always performed nonparametric statistical tests because our neural and behavioral data were not always normally distributed.
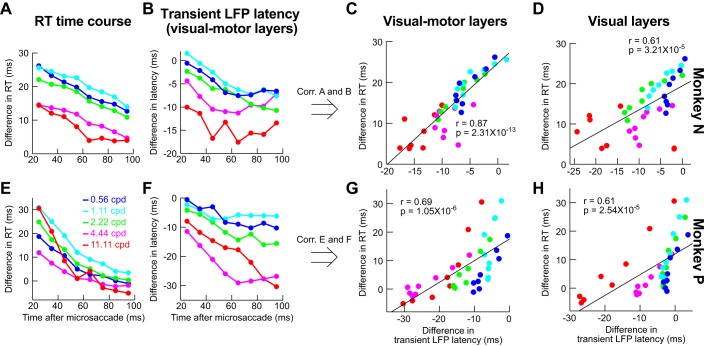
In all neural data analyses, we combined results from both monkeys. This was justified because the two monkeys showed consistent results with each other, and also consistent results with the prior literature (e.g., Chen et al. 2015; Hafed and Krauzlis 2010). However, for relating neural activity to behavior, it was unfair to compare the behavior of an individual monkey with neural data combined from both animals. Thus, only when relating neural activity to behavior, we separated the neural data into individual monkey data. This had the added advantage of demonstrating the consistency of neural results across individual monkeys, justifying our pooling of the animals for the summary figures of neural data analyses.
Behavioral analyses.
For behavior, we measured reaction time (RT) as a function of spatial frequency and time of grating onset relative to microsaccades. We also counted “express saccade” RT trials, which we defined as trials with RT <100 ms (Fischer and Boch 1983).
During offline analysis, we re-detected microsaccades using previously described methods (Hafed et al. 2009), because we could now use noncausal filters for better estimates of eye velocity and because we could also refine the time of movement onset/end on the basis of eye acceleration. We used such detection to identify grating onset time relative to microsaccade onset or offset. We defined no-microsaccade trials as trials with no microsaccades <250 ms from grating onset. RT on these trials constituted our baseline.
Firing rate analyses.
For neural data, we measured stimulus-evoked firing rate after the onset of a given spatial frequency grating under two scenarios: 1) when the grating appeared without any nearby microsaccades within ±100 ms and 2) when the grating appeared immediately after a microsaccade. Baseline, no-microsaccade spatial frequency tuning curves (i.e., responses for each given spatial frequency) were described recently (Hafed and Chen 2016), but in this study we analyzed microsaccadic influences on these curves. We did not analyze trials with grating onset immediately before or during microsaccades, to avoid premovement modulations (Chen et al. 2015; Hafed 2013) and retinal image shift effects caused by movement of the eyes, but previous studies have demonstrated suppression also during these intervals (Hafed and Krauzlis 2010).
To analyze stimulus-evoked firing rate, we measured peak visual response 20–150 ms after grating onset. To compare visual sensitivity on microsaccade and no-microsaccade trials, we created a “normalized firing rate” modulation index for each individual spatial frequency. We measured firing rate on microsaccade trials (i.e., trials with grating onset within 50 ms after microsaccades) and divided it by rate on no-microsaccade trials (i.e., trials with no microsaccades within <100 ms from grating onset). A value <1 indicates suppression. Note that we only considered neurons with a >5 spikes/s stimulus-evoked response (even on 11.11 cpd trials, which frequently had the lowest firing rates), thus avoiding “divide by zero” problems. Also, note that this modulation index isolates changes in visual sensitivity associated with saccadic suppression, regardless of how visual sensitivity itself might depend on spatial frequency without microsaccades. For example, visual responses in general are expected to be weaker for high spatial frequencies (Hafed and Chen 2016); however, our modulation index would normalize activity within a given spatial frequency to isolate any further suppression of visual sensitivity due to saccadic suppression.
In our analyses (including behavioral analyses), we combined microsaccades toward or away from the grating because suppression is not direction dependent in the postmovement interval that we focused on (Chen et al. 2015). However, we also confirmed this when analyzing the present data set (e.g., see Fig. 5). Our population analyses also combined neurons representing different eccentricities. We did so because we found that suppression is independent of eccentricity during the postmovement interval that we focused on (Chen et al. 2015).
Fig. 5.
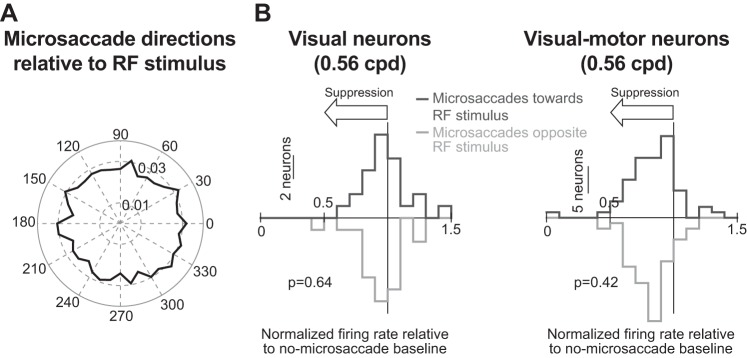
Lack of dependence of microsaccadic suppression on movement direction. A: normalized histogram of microsaccade directions relative to stimulus location (i.e., with all neuronal hotspot locations rotated to be aligned with 0 as in Chen et al. 2015). Across our population, microsaccade directions were evenly distributed relative to the location of the RF stimulus, similar to the results of Chen et al. (2015). Thus our results from Fig. 4 are not due to biased sampling of microsaccade directions. B: for each visual (left) or visual motor neuron (right), we calculated a suppression index (as in Fig. 4), but only for trials in which a microsaccade was directed either toward (dark gray) or opposite (light gray) the location of the stimulus (“toward” and “opposite” microsaccades were defined as in Chen et al. 2015). Across the population of either visual or visual motor neurons, the suppression index was similar for toward and opposite microsaccades, suggesting that suppression was not dependent on movement direction. Similar observations were made with large saccades in (Knöll et al. 2011). Each graph in B shows the P value obtained from a rank sum test comparing neural suppression indexes for toward and opposite trials. Note that we also repeated the analysis shown in B for all other spatial frequencies (and for either visual or visual motor neurons), and we always obtained similar suppression values for toward and opposite microsaccades (P > 0.07 for each performed test).
To investigate the relationship between neural modulations and behavioral effects, we correlated behavioral patterns of saccadic suppression from the behavioral tests to neural modulations obtained from the recordings. For example, we related visual response firing rate strength to mean RT as a function of time of grating onset after microsaccades. The mean RT was obtained from all collected behavioral trials (i.e., including the minority of express RT trials; see results) because visual responses are expected to affect overall behavior, without being specifically “labeled” in the brain as belonging to either a potential express RT trial or a regular trial.
For all analyses with time courses, we used bin steps of 10 ms and bin widths of 50 ms (except for Fig. 2, G, H, J, and K with both bin steps and bin widths of 25 ms).
Fig. 2.
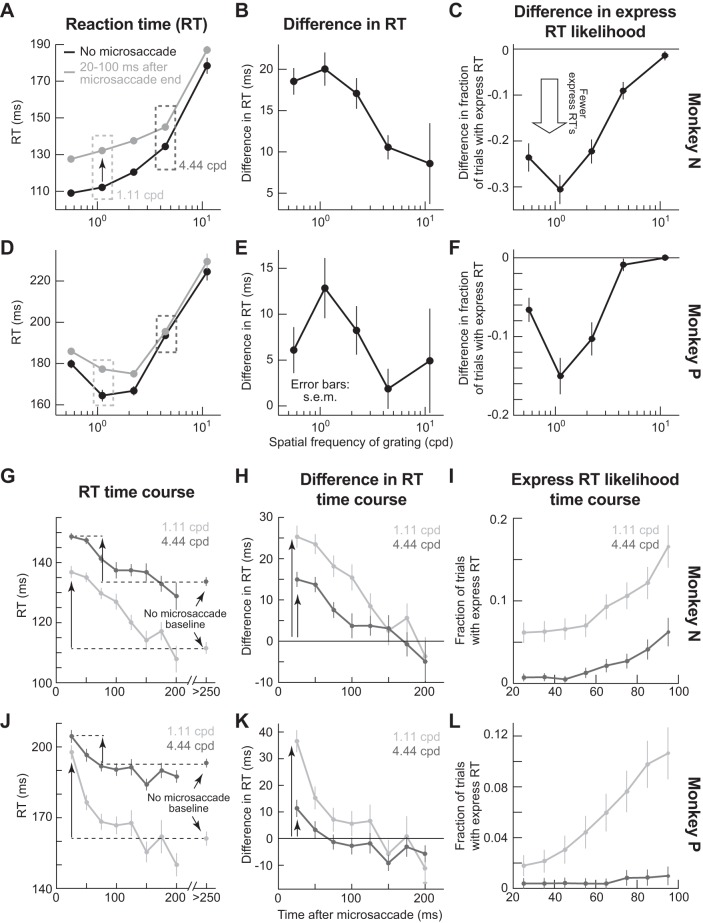
Spatial-frequency-selective microsaccadic suppression in behavior. A: mean RT as a function of spatial frequency. On no-microsaccade trials (black), RT increased with spatial frequency, consistent with dependence of visual response dynamics on spatial frequency (Breitmeyer 1975). If the same gratings appeared ~20–100 ms after microsaccades (gray), RT increased relative to no-microsaccade trials (a behavioral correlate of suppressed visual sensitivity), but more dramatically for low rather than high spatial frequencies (compare gray and black curves at different spatial frequencies). B: difference in RT between microsaccade and no-microsaccade trials (i.e., difference between gray and black curves in A), demonstrating the diminishing effects of microsaccades on RT behavioral costs with increasing spatial frequency. C: difference in the likelihood of express RT trials between microsaccade and no-microsaccade trials, demonstrating diminishing effects of microsaccades on reducing the likelihood of express RTs. D–F: same analyses as in A–C but for a second monkey. G and H: time courses of mean RT (G; as in A) or difference in RT (H; as in B) as a function of the time of grating onset after microsaccade end. The time courses are from 2 sample spatial frequencies (complete time courses from all spatial frequencies, and for each animal individually, are also shown in Fig. 6). For the difference in RT time course, RTs on trials with no microsaccades within <250 ms from grating onset were taken as the baseline. The initial RT cost caused by microsaccades was weaker for higher spatial frequency gratings (compare vertical arrows, consistent with A). I: likelihood of express RT trials as a function of time after microsaccade end, for the same spatial frequencies as in G and H. Immediately after microsaccades, there was an express RT cost (i.e., fewer express RTs), with gradual recovery in time. Moreover, the recovery dynamics were different for different spatial frequencies, as with overall RT (G and H). Also, note that the baseline fraction of express RTs (i.e., long after microsaccades) was different for different spatial frequencies so that the recovery for different spatial frequencies is toward different absolute values (as in G). J–L: same analyses as in G–I but for a second monkey. Error bars, when visible, denote SE; n = 8,153 trials for monkey N, and n = 7,117 for monkey P.
Local field potential analyses.
To analyze LFPs, we sampled neurophysiological activity at 40 KHz. The signal was first filtered in hardware (0.7–6 kHz). We then removed 50-, 100-, and 150-Hz line noise using an IIR notch filter and then applied a zero-phase lag low-pass filter (300-Hz cutoff). We finally downsampled to 1 kHz. We analyzed filtered LFP traces like firing rates (Hafed and Chen 2016; Ikeda et al. 2015), and we classified electrode track locations as visual or visual motor according to the neurons isolated from these tracks in the same sessions (Hafed and Chen 2016).
To obtain a measure of intrinsic perimicrosaccadic modulation of LFPs independent of visual stimulation, we took all microsaccades occurring in a prestimulus interval (20–100 ms before grating onset). We then aligned LFP traces on either microsaccade onset or end, to uncover any systematic LFP modulation time-locked to the movement execution. To compare these data to baseline, we took analysis intervals of identical length, again from prestimulus periods, but with no microsaccades occurring anywhere within these intervals.
To correlate LFP responses to behavioral dynamics of saccadic suppression (similar to what we did with firing rates), we measured peak transient LFP deflection as the minimum in the stimulus-evoked LFP trace 20–150 ms after grating onset. We created a “field potential index” by dividing this measurement on microsaccade trials by that on no-microsaccade trials. An index >1 indicates enhancement. For a control analysis, we computed the index after correcting for a microsaccade-related LFP level shift that may have happened due to intrinsic perimicrosaccadic modulation of the LFP independent of visual stimulation. We did this according to the following procedure. On microsaccade trials, we measured the average LFP value −25 to 25 ms from grating onset. We then subtracted the peak stimulus-evoked LFP deflection from this baseline measurement before dividing by the no-microsaccade trials. If an intrinsic perimicrosaccadic LFP modulation explained our results of LFP enhancement with increasing spatial frequency (see results), then the baseline-shifted index should show no enhancement.
We also analyzed transient stimulus-evoked LFP deflection latency. We found the first time at which the LFP was >2 SD away from baseline LFP (calculated as the mean LFP value −25 to 25 ms from grating onset), and there also had to be >5 ms of continuous >2 SD deviation from baseline. We did this separately for microsaccade and no-microsaccade trials, and we subtracted the measurements to obtain the influences of saccadic suppression on stimulus-evoked LFP deflection latency. If the LFP transient deflection occurs faster on microsaccade trials, then the subtraction gives a negative value.
RESULTS
Selective Microsaccadic Suppression of Low Spatial Frequencies in Behavior
Isolation of spatial-frequency-specific saccadic suppression requires demonstrating a selective form of suppression in behavior and subsequently asking which neurons reflect such selectivity. Thus we first developed a behavioral measure demonstrating selective suppression, which was based on our earlier results (Hafed and Krauzlis 2010). We did so for microsaccades because they are mechanistically similar to larger saccades while at the same time providing important experimental advantages (see Introduction). Monkeys fixated, and we initiated a computer process for real-time microsaccade detection (Chen and Hafed 2013). After such detection by a programmable delay, we presented a stationary vertical Gabor grating (80% contrast). The monkeys oriented toward the grating as fast as possible. Because SC visual bursts are strongly correlated with RT (Boehnke and Munoz 2008; Chen et al. 2015; Hafed and Chen 2016; Hafed and Krauzlis 2010; Hafed et al. 2015; Tian et al. 2016), we used RT changes in this task as a sensitive measure of microsaccadic influences on visual sensitivity (Hafed and Krauzlis 2010; Tian et al. 2016; also see discussion).
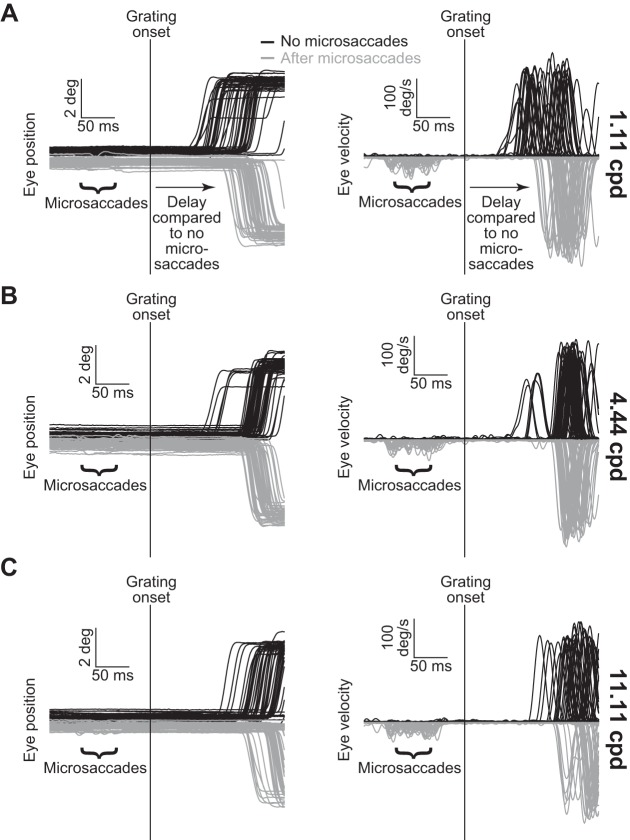
Similar to previously reported perceptual effects with large saccades (Burr et al. 1994) and also microsaccades (Hass and Horwitz 2011), grating onset after microsaccades had a strong, yet selective impact on behavior in our monkeys. Figure 1A shows example eye position (left) and velocity traces (right) recorded from one monkey while we presented a 1.11 cpd grating. The black traces show trials without microsaccades <250 ms from grating onset, and the gray traces show trials with grating onset ~20–100 ms after microsaccades. There was a marked increase in RT during microsaccade trials (Fig. 1A). However, when we presented 4.44 (Fig. 1B) or 11.11 cpd gratings (Fig. 1C), RTs on microsaccade and no-microsaccade trials were more similar to each other (compare the gray and black distributions in each panel). Thus the microsaccadic suppressive effect (causing slower RTs relative to no-microsaccade baselines) was diminished for higher frequency gratings. These sample trial results demonstrate a correlate in our monkeys of selective perceptual suppression of low spatial frequencies by large saccades and also microsaccades (Burr et al. 1982, 1994; Hass and Horwitz 2011; Volkmann et al. 1978), even though we used a different behavioral measure.
Fig. 1.
Behavioral measure of microsaccadic suppression across spatial frequencies. A: eye position (left) and radial eye velocity (right) traces from 100 sample trials from monkey N during a stimulus detection task. A 1.11 cpd grating appeared during fixation either with no nearby microsaccades (black; n = 50 randomly selected trials) or ~20–100 ms after microsaccades (gray; n = 50 randomly selected trials), and the monkey had to orient as fast as possible to the grating. Reaction time (RT) on the microsaccade trials was slower than on the no-microsaccade trials. Note that we flipped the gray position and velocity traces around the horizontal axis to facilitate comparison to the black traces, and we also displaced the initial fixation position in the position traces. The microsaccades are more visible in the velocity traces because they constitute spikes of eye velocity. B: same analysis as in A, but from 100 randomly selected trials having a higher spatial frequency grating (4.44 cpd). RTs in this case were more similar between the microsaccade and no-microsaccade trials, suggesting that the effect in A disappears with increasing spatial frequency. C: observations similar to those in B were also made for 11.11 cpd gratings. Note that RTs in this case were longer than in A and B, meaning that some traces were truncated either before saccade onset or midway through saccades. Also, note that results of statistical tests for this and other figures are detailed in the text.
Across behavioral sessions, both monkeys showed selective RT increases for low spatial frequencies (Fig. 2, A and D). On no-microsaccade trials (black curves), mean RT increased with increasing spatial frequency, as expected from dynamics of the early visual system (Breitmeyer 1975) and SC (unpublished observations). For example, mean RT for 0.56 cpd gratings was 109.1 ± 1.37 ms (mean ± SE) in monkey N and 179.8 ± 2.12 ms in monkey P, whereas it was 178.4 ± 4.37 ms in monkey N and 224.5 ± 4.2 ms in monkey P for 11.11 cpd. This effect was statistically significant (P < 0.01 for monkey N and P < 0.01 for monkey P, Kruskal-Wallis test with spatial frequency as the main factor). However, with gratings appearing ~20–100 ms after microsaccades, the RT cost relative to no-microsaccade trials (i.e., the difference in RT between microsaccade and no-microsaccade trials) was strongest for the lowest spatial frequencies (Fig. 2, B and E; P < 0.01 for monkey N and P < 0.01 for monkey P, Kruskal-Wallis test with spatial frequency as the main factor). This effect was not a ceiling effect on RT, because it was still possible for RT to increase even more at higher spatial frequencies. For example, at 4.44 cpd, RT on microsaccade and no-microsaccade trials was similar (Fig. 2, A and D; dark gray dashed boxes), but it got even slower for 11.11 cpd regardless of eye movements. This effect is also shown in the raw black traces of Fig. 1C, exhibiting longer RT values than the black traces of Fig. 1B. Importantly, even at 11.11 cpd, RT on microsaccade trials was modestly longer than on no-microsaccade trials in both animals (Fig. 2, A and D), suggesting that the impact of microsaccades could still be visible even when RT itself was very long because of high spatial frequencies. Thus the reduction in RT differences between microsaccade and no-microsaccade trials for high spatial frequencies (Fig. 2, B and E) was suggestive of a selective suppression of low spatial frequencies, and not necessarily a ceiling effect on RT.
On a small subset of the trials in Fig. 2, A and D, our monkeys’ RT values fell within a so-called “express” range (which we defined as trials having RT <100 ms). Overall, 11.05% and 6.34% of all trials in monkeys N and P, respectively, were express. These trials formed a small but distinct peak in RT distributions typical of express saccades (although this small peak appeared to merge with regular RT distributions for the lowest spatial frequency in monkey N because of this monkey’s low overall RT values). We thus additionally analyzed how these specific express responses were affected by microsaccades occurring near grating onset. In both monkeys (Fig. 2, C and F), there was a reduction in express RT trials (i.e., the small low-latency peak in RT distributions was further reduced); moreover, the change in express RT trial likelihood between microsaccade and no-microsaccade trials was largest for low spatial frequencies, consistent with the spatial frequency-specific lengthening of RTs in Fig. 2, A, B, D, and E. Thus the spatial frequency-specific microsaccadic influence that we describe in this study affected our monkeys’ behavior even during express RT trials.
Our behavioral paradigm also provided rich information about saccadic suppression dynamics, which we could later use to relate to SC neural modulations. For example, we evaluated microsaccadic suppression time courses across different spatial frequencies. Figure 2, G, H, J, and K, illustrates this by plotting mean RT from Fig. 2, A and D as a function of when a 1.11 or 4.44 cpd grating appeared after microsaccades. Microsaccadic occurrence had a clear time course of RT costs for each spatial frequency, with both monkeys showing lower RT costs for the higher spatial frequency immediately after microsaccades and then a gradual return toward the baseline no-microsaccade performance for a given frequency. Similarly, when we only focused on the subset of express RT trials, we found that the likelihood of express RTs was decreased immediately after microsaccades and gradually recovered (i.e., increased), and the magnitude of the recovery was again spatial frequency specific (Fig. 2, I and L).
Therefore, using a behavioral measure sensitive to SC visual response strength (Boehnke and Munoz 2008; Hafed and Chen 2016; Hafed and Krauzlis 2010; Hafed et al. 2015), we found a robust and selective pattern of microsaccadic suppression, which we think is analogous to perceptual suppression in humans with large saccades (Burr et al. 1982; Burr et al. 1994; Volkmann et al. 1978). Note that our results are also consistent with spatial frequency-specific suppression of contrast detection performance in monkeys around the time of microsaccades (Hass and Horwitz 2011), which confirms that microsaccades have similar effects to larger saccades and that our RT measures in the present study were indeed sufficient to establish a behavioral effect in our animals. We were now in a position to evaluate neural correlates of this behavioral effect and to specifically test whether spatial-frequency-specific suppression would emerge in purely visual SC neurons, as we might predict from a previously published hypothesis about an SC circuit model for saccadic suppression (Berman and Wurtz 2008, Berman and Wurtz 2010; Berman and Wurtz 2011; Isa and Hall 2009; Lee et al. 2007; Phongphanphanee et al. 2011; Wurtz 2008; Wurtz et al. 2011).
Selective Suppression of Low Spatial Frequencies in Visual Motor but not Visual SC Neurons
Using the same animals but in completely different experimental sessions not requiring any saccadic responses at all (see materials and methods), we recorded the activity of purely visual SC neurons (24 neurons; located 680 ± 95 μm below SC surface) or visual motor neurons (66 neurons; 1,159 ± 66 μm below SC surface). Both types of neurons exhibit robust visual responses, but the question remains as to which would show spatial-frequency-specific suppression. We presented gratings similar to those used in Figs. 1 and 2 inside each neuron’s RF (see materials and methods). However, the task was now a fixation task with no saccadic eye movements toward the gratings; we only analyzed either no-microsaccade trials or trials in which the gratings appeared immediately after microsaccades (see materials and methods).
Ensuring fixation during the recordings was especially important to demonstrate behavioral relevance of our neural modulations. Specifically, one of our goals was to directly correlate neural dynamics to behavior in each animal (as will be presented later). Showing that a specific SC cell class is highly correlated with behavior compared with another cell class, even when the correlations are made across completely independent sessions and tasks, would demonstrate the behavioral relevance of the cell class. Moreover, demonstrating that neural suppression dynamics appear on visual responses, even in the complete absence of an overt response, shows that it is sensory responses that matter during saccadic suppression. Finally, ensuring fixation avoided influences on visual sensitivity that take place during tasks requiring monkeys to generate a subsequent saccade to the presented stimulus (Li and Basso 2008).
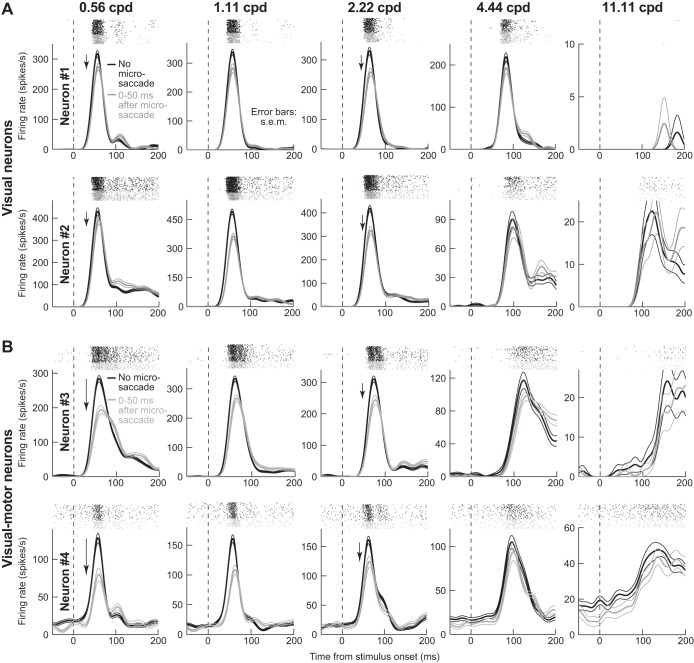
Visual motor SC neurons showed the strongest saccadic suppression, and in a spatial-frequency-selective manner. Figure 3A shows the activity of two sample pure visual neurons (one per row) during presentations of different spatial frequencies (across columns), and Fig. 3B shows the activity of two sample visual motor neurons (in the same format). In each graph, black traces show activity with no microsaccades <100 ms from grating onset, and gray traces show activity when the same grating was presented within 50 ms after microsaccades. In no-microsaccade trials, all neurons showed expected visual bursts, but burst strength varied with spatial frequency (Fig. 3, black). This is suggestive of spatial frequency tuning (Hafed and Chen 2016), but our purpose was to investigate suppression relative to no-microsaccade responses; thus we scaled the y-axis in each panel such that, across panels, no-microsaccade curves visually appeared to be roughly equal in height. With the use of such scaling, visual burst suppression (Fig. 3, gray) was rendered clearer (quantitatively, we always measured suppression relative to the no-microsaccade responses within each given spatial frequency independently, and not across spatial frequencies; see materials and methods). Importantly, there were differences in suppression patterns between visual and visual motor neurons. For the visual neurons (Fig. 3A), suppression was mild and relatively inconsistent across spatial frequencies; for the visual motor neurons (Fig. 3B), there was strong suppression for the lowest spatial frequency (neuron 3: ~32%; neuron 4: ~38%; P < 0.01 for each neuron, Wilcoxon rank sum test), and there was also a systematic reduction in suppression strength with increasing frequency (by 4.44 and 11.11 cpd, there was no suppression left; P = 0.49 for 4.44 cpd and P = 0.41 for 11.11 cpd in neuron 3, and P = 0.15 for 4.44 cpd and P = 0.99 for 11.11 cpd in neuron 4, Wilcoxon rank sum test). Importantly, the eye movement associated with suppression in all panels had ended before grating onset. Thus the suppression cannot be attributed to blurring of the gratings by eye movements.
Fig. 3.
Spatial-frequency-selective microsaccadic suppression of visual motor SC neurons. A: neural activity as a function of time after grating onset for 2 sample purely visual SC neurons (1 per row). Each graph in a row shows activity after presentation of a specific spatial frequency (indicated above each graph). Rasters above each firing rate curve show individual action potentials emitted by the neuron across individual trials. We divided trials into ones in which there was no microsaccade within <100 ms from grating onset (black; n ≥ 38 trials per spatial frequency in these sample neurons) and ones in which the grating appeared immediately after microsaccades (gray; n ≥ 30 trials per spatial frequency). The y-axis was scaled in each panel such that the no-microsaccade firing rates visually appear to have approximately similar heights across panels, allowing easier comparison of suppression effects. Both neurons showed moderate microsaccadic suppression, with no clear pattern across spatial frequencies. B: same format as A, but for 2 sample visual motor neurons. The neurons showed stronger suppression at the lowest spatial frequency, and the suppression gradually decreased in strength with increasing spatial frequency (as in behavior); by 4.44 and 11.11 cpd, there was no suppression left. For these neurons, n ≥ 28 trials per spatial frequency for no microsaccade trials (black), and n ≥ 22 trials per spatial frequency trials for microsaccade trials (gray).
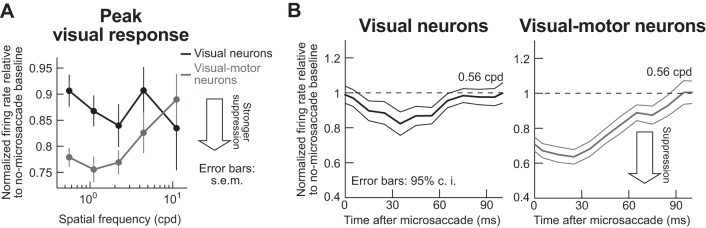
Across neurons, there was selective suppression of visual sensitivity as a function of spatial frequency, but only in visual motor neurons. Figure 4A summarizes these findings by plotting a suppression index (see materials and methods) as a function of spatial frequency. Peak visual response was suppressed in both visual and visual motor neurons (suppression index <1). However, the suppression was not spatial frequency selective and was weaker in visual neurons; in visual motor neurons, there was strong suppression for the lowest spatial frequencies, and the effect gradually dissipated away with increasing frequency. Quantitatively, the average suppression value in visual neurons was 11% across spatial frequencies, and it was 22% in visual motor neurons. When the data are separated for low and high spatial frequencies, the average suppression value in visual neurons for the lowest two spatial frequencies or the highest two spatial frequencies was 11%, meaning that the suppression value was similar for the two groups of frequencies (P = 0.77, Wilcoxon rank sum test). On the other hand, visual motor neurons were suppressed by 23% for the lowest two spatial frequencies and 17% for the highest two spatial frequencies, and the difference between the groups of spatial frequencies was significant (P < 0.01, Wilcoxon rank sum test).
Fig. 4.
Spatial-frequency-dependent microsaccadic suppression of visual bursts in visual motor but not visual SC neurons. A: we measured peak stimulus-evoked visual burst after grating onset (e.g., from traces like those in Fig. 3) and plotted it as a function of grating spatial frequency. We grouped neurons as purely visual (dark gray) or visual motor (light gray). Visual neurons showed only ~10% suppression, and there was no consistent spatial frequency dependence of this suppression. Visual motor neurons showed ~25% suppression in the low spatial frequencies, and this effect gradually decreased with increasing spatial frequency (as in behavior). Error bars denote SE. Note that the error bars for the highest spatial frequency are larger than for other frequencies because some neurons completely stopped responding at 11.11 cpd, which reduced population size in this spatial frequency (see materials and methods). B: time courses of microsaccadic suppression in visual (left) and visual motor neurons (right) for a sample spatial frequency. We performed an analysis similar to that described in Chen et al. (2015) but aligning on microsaccade end. For each time window after microsaccade end in which a grating appeared (x-axis; 50-ms bins in 10-ms steps), we measured peak firing rate evoked by grating onset (see materials and methods), and we normalized it by peak firing rate on no-microsaccade trials. Visual motor neurons showed stronger suppression than visual neurons (compare y-axis in both graphs), and both neuron types experienced recovery with increasing time after microsaccades (consistent with behavioral effects). Note that the time course of visual motor neuron suppression is similar to the time course of behavioral effects (e.g., Fig. 2, H and K) and is also similar to the time course of saccadic suppression in the earlier literature (e.g., Diamond et al. 2000; Hafed and Krauzlis 2010; Ibbotson and Krekelberg 2011). Figure 6 shows individual monkey time courses, other spatial frequencies, and relationships between neural time courses and the respective monkey’s behavioral performance dynamics. n = 66 visual motor neurons, and n = 24 visual neurons.
A difference between visual and visual motor neurons also appeared in suppression temporal dynamics, again showing weaker suppression in the visual neurons (Fig. 4B). Thus there are differences in saccadic suppression strength between visual and visual motor SC neurons, and visual motor neuron suppression selectivity appears more similar to behavioral effects, both in our own experiments (Figs. 1 and 2) and in the literature of human perceptual effects (Burr et al. 1982; Burr et al. 1994; Volkmann et al. 1978) and monkey contrast detection thresholds (Hass and Horwitz 2011).
Even though our previously published results revealed no differences in postmicrosaccadic suppression in the SC as a function of microsaccade direction (Chen et al. 2015), we nonetheless analyzed movement directions in the present study, as well. Across our population, the direction of a microsaccade relative to the location of a neuron’s RF hotspot was fairly uniformly distributed (Fig. 5A; similar to Chen et al. 2015). This means that our results in Figs. 3 and 4 described above are not an artifact of biased sampling of microsaccade directions. Moreover, for each spatial frequency, and for each of either visual or visual motor neurons, we computed the suppression index of Fig. 4, but now separately for microsaccades either toward or opposite the RF location (with “toward” and “opposite” being defined as in Chen et al. 2015). Figure 5B shows the results of this analysis for an example spatial frequency. As shown, for either visual or visual motor neurons, the suppression values observed were statistically similar whether the microsaccade occurring before stimulus onset was directed toward or away from the grating location (P = 0.64 for visual neurons and P = 0.42 for visual motor neurons, Wilcoxon rank sum test). This result also held for all other spatial frequencies (P > 0.07 for either visual or visual motor neurons, Wilcoxon rank sum test). Because of this, we combined microsaccade directions in all subsequent analyses.
Better Correlation Between Visual Motor Neuron Dynamics and Behavior than Between Visual Neuron Dynamics and Behavior
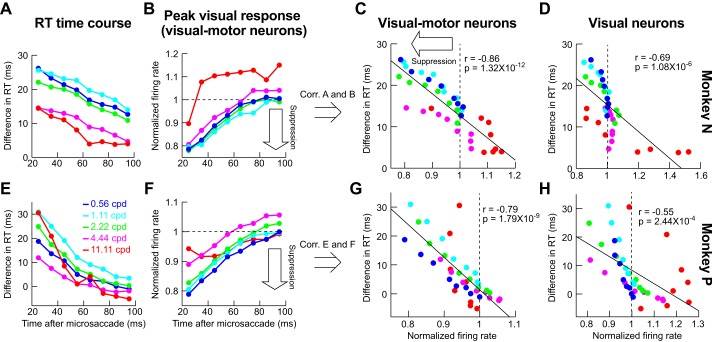
To further explore the apparent similarity between visual motor neuron suppression patterns (Fig. 4) and behavior (Fig. 2), we used the dynamics of our recorded population as a proxy for how the SC might be engaged in our behavioral task of Figs. 1 and 2. We plotted the time course of behavioral suppression (similar to Fig. 2, H and K) for each spatial frequency and each monkey individually (Fig. 6, A and E), and we also plotted the neural time course of visual motor neuron suppression, again for each monkey individually (Fig. 6, B and F; an example time course for purely visual neurons is also shown in Fig. 4B). For this comparative analysis, we used the same binning windows in both behavioral and neural data (50-ms bin widths in steps of 10 ms starting at 0 ms after microsaccade end), and we next correlated the two time courses: we plotted all samples of the behavioral time course against all samples of the neural time course irrespective of spatial frequency or time after microsaccades (Fig. 6, C and G). There was high correlation between visual burst strength in SC visual motor neurons and the behavioral effect of microsaccadic suppression: whenever visual bursts were weaker, RT costs increased, and vice versa, regardless of spatial frequency or time after microsaccades. This high correlation is particularly remarkable given that the behavioral and neural data were collected in completely different sessions and with different behavioral tasks, and even with imperfect matching of neuron locations relative to the grating location used in the behavioral study.
Fig. 6.
Correlating behavioral microsaccadic suppression with neural microsaccadic suppression on completely different experimental sessions. A: time course of difference in RT from baseline (e.g., Fig. 2, H and K; see materials and methods) as a function of time of grating onset after microsaccade end in our behavioral experiments (monkey N). Different color curves show different spatial frequencies. Immediately after microsaccades, there was a strong cost in RT for low spatial frequencies and a more moderate cost for high spatial frequencies. In all spatial frequencies, the RT cost associated with microsaccadic suppression slowly dissipated in time. B: analysis similar to that in A but for the peak visual response in our neural experiments, on completely different sessions from the behavioral data, and only for visual motor neurons. C: correlation (Corr.) between the data points in A and those in B, regardless of time or spatial frequency. There was strong correlation between visual burst strength and RT cost, even on completely different experimental sessions, suggesting that visual motor neurons are modulated during microsaccadic suppression in a manner that could be relevant for the phenomenon reported by Burr et al. (1994). D: we correlated the behavioral points in A with similar points, but for visual neuron time courses (e.g., Fig. 4B). The correlation with behavior was worse than in visual motor neurons. E–H: observations similar to those in A–D for a second monkey (monkey P). n = 24 visual motor neurons for monkey N, and n = 42 visual motor neurons for monkey P; n = 15 visual neurons for monkey N, and n = 9 visual neurons for monkey P.
The highest correlation between neural patterns and behavior was observed only when we used peak visual response of visual motor SC neurons as the behavioral predictor (Fig. 6, C and G). When we correlated behavioral time courses with peak visual response of purely visual neurons, the correlations were significantly weaker (Fig. 6, D and H; P = 0.02 for monkey N and P = 0.02 for monkey P, Steiger’s Z-test; actual correlation values are shown in Fig. 6). Thus a most simple linear readout of visual motor neurons would fare better at predicting behavior than a similarly simple readout of purely visual neurons.
The results of Fig. 6 suggest that saccadic suppression in visual motor neurons is more in line with our behavioral effects than in purely visual neurons. However, one possible confound could be in the distribution of preferred spatial frequencies in visual motor neurons. For example, if only the preferred spatial frequency of a neuron experiences the strongest suppression, and if visual motor neurons only had low preferred spatial frequencies, then the selective suppression of Fig. 4A would emerge, because there would be more visual motor neurons preferring low spatial frequencies than visual neurons. However, we found no clear differences in patterns of preferred spatial frequencies between visual and visual motor neurons. Specifically, across our population, both visual and visual motor neurons spanned a wide range of preferred spatial frequencies (from 0.56 to 4.54 cpd in visual neurons and from 0.56 to 4.82 cpd in visual motor neurons), with large overlap between the two neuron types; this meant that there was no statistically significant difference in preferred spatial frequencies between our visual and visual motor neurons (P = 0.996, Wilcoxon rank sum test).
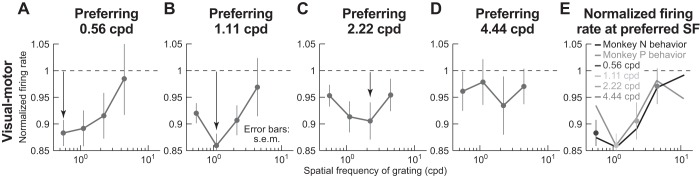
To further investigate the above potential confound, we also explicitly analyzed suppression profiles of visual motor neurons as a function of the neurons’ preferred spatial frequencies. For each spatial frequency, we took only neurons preferring this spatial frequency, and we checked how these neurons were suppressed. Figure 7, A–D, shows the results of this analysis. There was indeed a tendency for the preferred spatial frequency of a neuron to experience the strongest suppression relative to other frequencies (e.g., black arrows). However, this strongest suppression still became progressively weaker and weaker with increasing spatial frequency (e.g., compare Fig. 7, A and B with Fig. 7, C and D). This is further demonstrated by Fig. 7E, in which we took the maximal suppression frequency from each of the panels in Fig. 7, A–D, and plotted them with an indication of the behavioral microsaccadic suppression profile (obtained as the graphical inverse of RT modulation profiles from Fig. 2, B and E, with arbitrary y-axis scaling). Importantly, we again made sure that the neural suppression data in Fig. 7 were analyzed in an identical manner to behavioral analyses (i.e., we considered the same interval of stimulus onsets happening 20–100 ms after microsaccade end as in the behavioral analyses). As shown in Fig. 7E, there was a clear match between neural and behavioral effects in both animals (the correlation between neural suppression and behavioral suppression was 0.99 for monkey N and 0.89 for monkey P). Thus the selective suppression of Figs. 3–6 was not an artifact of potential biased spatial frequency tuning properties of only visual motor neurons.
Fig. 7.
Selective low-frequency suppression in visual motor neurons independent of preferred spatial frequency. A–D: in each panel, we selected only neurons preferring a single spatial frequency on no-microsaccade trials (see materials and methods). We then repeated the analysis of Fig. 4A. The preferred spatial frequency tended to experience the strongest suppression compared with other spatial frequencies (black arrows). However, the strength of the suppression even for the preferred spatial frequency consistently decreased with increasing spatial frequency (compare arrows in individual panels). Note that we did not have neurons preferring 11.11 cpd in this analysis, and thus we do not show this spatial frequency in this figure. E: we collected the maximally suppressed spatial frequency from each panel A–D (see key), and we plotted them together. The monkey N and monkey P behavior lines are a copy of the behavioral RT microsaccadic suppression curves of Fig. 2, B and E, but are inverted (and with arbitrary y-axis scaling) to match the neural suppression curves. As can be seen, even if the preferred spatial frequency of neurons always experienced maximal suppression, this maximal suppression was still decreased with increasing spatial frequency. Thus the spatial frequency selectivity of visual motor neural suppression was still correlated with behavior. Error bars denote SE; n = 26, 19, 8, and 5 neurons in A, B, C, and D, respectively.
Taken together, our results so far suggest that spatial-frequency-specific SC saccadic suppression is localized in the visual motor neurons, with visual neurons only showing modest and nonselective suppression.
Influence of a Putative Microsaccadic Source Signal on Local SC Population Activity During Suppression
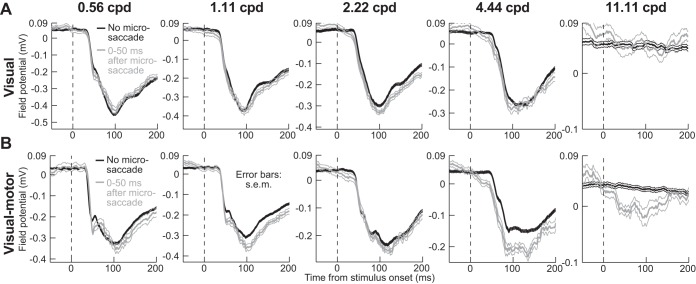
To demonstrate that there may indeed be a saccadic source signal associated with suppressed SC visual bursts (i.e., putative corollary discharge associated with the movement command), we analyzed local field potentials (LFPs) around our electrodes (see materials and methods). Stimulus onset in no-microsaccade trials caused a negative-going “stimulus-evoked” LFP deflection for both visual and visual motor electrode tracks (Hafed and Chen 2016; Ikeda et al. 2015). For example, Fig. 8 shows LFP traces (in a format similar to Fig. 3) as a function of spatial frequency for an example superficial track (i.e., among visual neurons; Fig. 8A) and an example deeper track (among visual motor neurons; Fig. 8B). Remarkably, on microsaccade trials, stimulus-evoked LFP response was not suppressed for any of the spatial frequencies. In fact, for the visual motor electrode track (Fig. 8B), LFP response was enhanced, and more so with increasing spatial frequency (Fig. 9A; P < 0.01, Kruskal-Wallis test on the modulation index with spatial frequency as the main factor). Given that LFPs reflect not only local population spiking activity but also putative synaptic inputs, these results suggest the existence of a possible microsaccade-related input modulating visual bursts. This effect, an enhanced LFP response with increasing spatial frequency, was again stronger in visual motor than visual electrode tracks, as summarized in Fig. 9A. However, it is important to emphasize that this signal was not a direct microsaccade command, because none of our neurons at all electrode locations in this study exhibited microsaccade-related movement bursts (see materials and methods).
Fig. 8.
LFP modulations during microsaccadic suppression. A and B are formatted similarly to Fig. 3, except that LFP modulations are plotted around a sample electrode track near visual (A) or visual motor neurons (B). There was no evidence of a reduced LFP-evoked response for trials with grating onset after microsaccades (faint colors). If anything, the peak evoked response and the latency to evoked response were stronger and shorter, respectively (see Fig. 9). This effect was not explained by an intrinsic perimicrosaccadic modulation of LFP (see Figs. 9A and 10), but it is consistent with an additional movement-related modulatory signal associated with saccade execution that influences stimulus-evoked spiking activity. Error bars denote SE. For the visual track (A), n ≥ 113 trials per spatial frequency on no-microsaccade trials (black), and n ≥ 25 trials per spatial frequency on microsaccade trials (gray). For the visual motor track (B), n ≥ 140 trials per spatial frequency on no-microsaccade trials (black), and n ≥ 12 trials on microsaccade trials (gray).
Fig. 9.
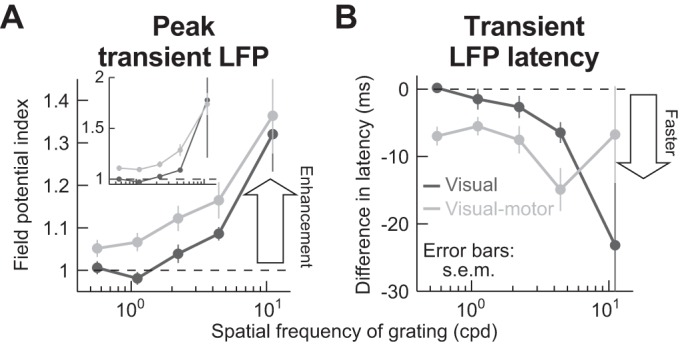
Lack of microsaccadic suppression in LFP stimulus-evoked responses. A: we performed an analysis similar to that shown in Fig. 4A, but on LFPs. We measured peak LFP response with and without microsaccades and then obtained a modulation index (see materials and methods). The inset shows the modulation index from raw measurements, whereas the main panel shows the same analysis but after a baseline shift was subtracted from the microsaccade trials. Specifically, data shown in Fig. 10 suggest that there is a negativity in LFPs after microsaccades that and stimulus onset in the microsaccade trials came after a previous microsaccade. Thus we measured the peak LFP stimulus-evoked response on microsaccade trials as the difference between the raw LFP stimulus-evoked negativity minus the baseline LFP value that was present at the time of grating onset (see materials and methods). In both the inset and the main panel, there was no suppression in the stimulus-evoked LFP response, contrary to firing rate results (Fig. 4A). Rather, there was response enhancement, which progressively increased with increasing spatial frequency, and this happened for both visual and visual motor electrode track locations (P < 0.01 for either baseline-corrected or raw measurements and for each of visual-only or visual motor electrode tracks; Kruskal-Wallis test with spatial frequency as the main factor). B: analyses similar to those in A but measuring the latency to LFP stimulus-evoked response, which decreased on microsaccade trials (y-axis values <0 ms; P < 0.02 for visual electrode tracks and P = 0.07 for visual motor electrode tracks; Kruskal-Wallis test with spatial frequency as the main factor). Thus, when a stimulus appeared immediately after a microsaccade, the stimulus-evoked LFP response started earlier than without a microsaccade. Error bars denote SE.
Our interpretation of an increased LFP negativity as reflecting a possible movement-related input mediating firing rate suppression effects is consistent with the enhanced LFP response shown in Figs. 8 and 9A for high spatial frequencies. These frequencies evoke the weakest visual activity (Figs. 3 and 8, black). Thus, if the LFP signal reflects both visual inputs associated with the stimulus onset as well as movement-related modulatory inputs to the population associated with movement execution (which do not depend on visual response strength), then the influence of the modulatory input (i.e., the putative saccadic source signal for suppression) should become increasingly more obvious in the LFP with increasing spatial frequency (Fig. 9A). However, we cannot tell from these data whether the two signals integrated in the LFP reflect pure superposition of visual and modulatory inputs, or whether a more complex integration takes place. In any case, combined with earlier firing rate results, our LFP analyses reveal that visual motor SC neurons may be closely associated with a movement-related source for spatial-frequency-specific saccadic suppression.
One possible confound with the above result is that microsaccades (even though they ended before stimulus onset) might cause long-lasting LFP modulations, which would be superimposed on a stimulus-evoked LFP deflection in Fig. 8. In other words, the evoked response could potentially still be suppressed, but it could be level-shifted because it rides on a microsaccade-induced LFP modulation. We thus tested for intrinsic microsaccade-induced LFP modulation. During simple fixation without any other visual stimuli, both visual and visual motor SC electrode locations exhibited prolonged microsaccade-related LFP modulations, involving a subtle negativity after microsaccade end, as shown in Fig. 10 (additional evidence of such negativity can also be seen in the prestimulus interval of Fig. 8, but it is washed out because of alignment to stimulus onset rather than to microsaccades). We wondered whether this modulation is sufficient to explain the lack of LFP suppression in stimulus-evoked LFPs (Fig. 8). We corrected for a baseline shift at grating onset (see materials and methods), and we still found no suppression in the strength of the stimulus-evoked LFP response (Fig. 9A). Thus, as represented in Figs. 8–10, we believe that we have uncovered evidence for a putative microsaccade-related modulatory input at the time of visual burst suppression in both SC visual and visual motor neurons. This input does not itself necessarily trigger microsaccades (see discussion); it may instead mediate visual burst suppression in firing rates, although the exact mechanisms remain to be explored. Moreover, the modulatory input shows differential modulation between superficial and intermediate electrode tracks (Fig. 9A), consistent with our firing rate results.
Fig. 10.
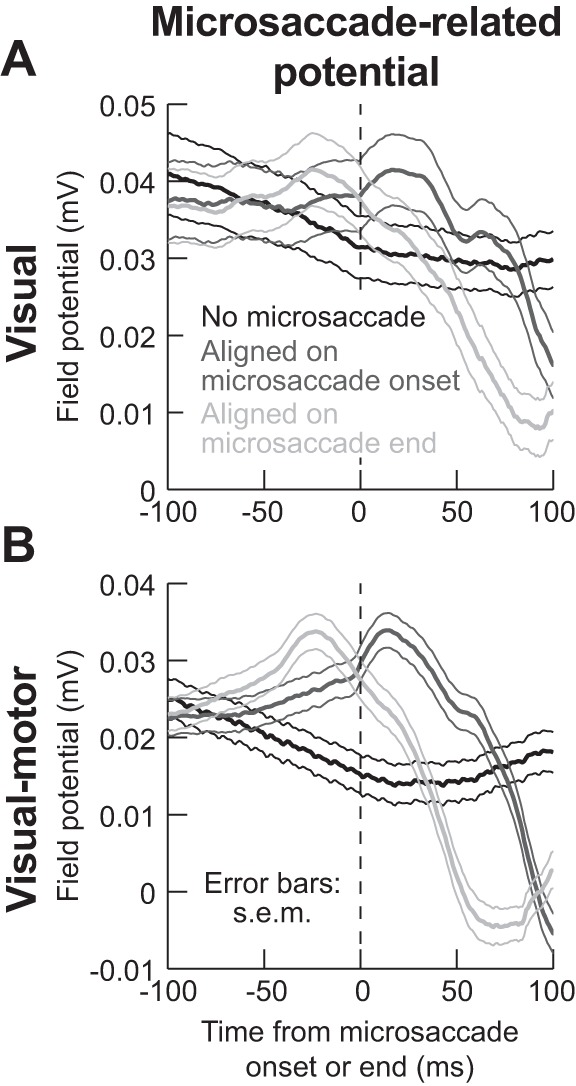
Microsaccade-related LFP modulations in the absence of an RF stimulus. We aligned LFP activity to either microsaccade onset (dark gray) or microsaccade end (light gray) during a baseline fixation interval with no RF stimulus at all (see materials and methods). The black curves show LFP activity during equally long control intervals, again with no RF stimulus, but also with no microsaccade occurrence. Even though there was no microsaccade-related spiking at all the sites investigated in this study, microsaccades caused systematic modulations in both visual (A) and visual motor (B) electrode locations in the SC, even though our electrodes were primarily placed in extrafoveal SC representations far from the movement end points. Thus these LFP modulations, similar to previously reported saccade-related LFP modulations (Liu et al. 2009), reflect a potential microsaccade-related modulatory signal that can mediate microsaccadic suppression of firing rates in extrafoveal SC neurons. Also, note how the effect on visual motor layers (B) is more systematic and robust than in visual layers (A). This is further evidence of a putative extraretinal signal in the SC visual motor layers that might mediate saccadic suppression (and explain the data in Fig. 6), and it also makes it unlikely that the LFP modulations in this figure are due to ocular muscle artifacts. Error bars denote SE; n = 66 electrode tracks visual motor layers (B), and n = 24 electrode tracks for visual layers (A).
Enhanced stimulus-evoked LFP response amplitudes (Fig. 9A) were also accompanied by slightly faster LFP responses (Fig. 9B), again consistent with a movement-related source modulating neural firing rates at the time visual burst occurrence (because the movement happened before stimulus onset). It is also interesting to note that, like firing rate time courses, time courses of stimulus-evoked LFP modulations for stimuli appearing after microsaccades were also correlated with behavioral microsaccadic suppression dynamics (as in Fig. 6). In the LFPs, the best behavioral predictor was the latency of stimulus-evoked LFP deflection (Fig. 11, formatted similarly to Fig. 6), and visual motor electrode tracks again showed higher correlation values with behavior (Fig. 11, C and G) than visual electrode tracks (Fig. 11, D and H). For monkey N, this effect was significant (P < 0.01, Steiger’s Z-test), but it did not reach significance in monkey P (P = 0.38).
Fig. 11.
Correlation between LFP modulation parameters and behavioral effects of suppression. This figure is formatted similarly to Fig. 6, except that we have plotted LFP time courses instead of firing rate time courses. Specifically, in B and F, we plotted the time course of LFP stimulus-evoked response latency (e.g., Fig. 9B) as a function of spatial frequency and time after microsaccades. The correlation between this latency in visual motor layers and behavior was better (C and G) than in visual layers (D and H). Thus it is again the visual motor layers that are better predictors of behavior, as in Fig. 6, although firing rates (Fig. 6) showed higher correlations to behavior in general. Note that we also measured correlations between behavior and LFP stimulus-evoked response strength rather latency (data not shown), but the LFP response latency always showed the better correlations with behavior.
Our results combined demonstrate that visual motor neurons are more in line with selective effects of saccadic suppression, in both humans (Burr et al. 1982, 1994; Volkmann et al. 1978) and monkeys (Fig. 2; also see Hass and Horwitz 2011), than purely visual neurons. This suggests that the mechanisms for saccadic suppression in the SC are more complicated than those suggested by a hypothesized pathway of a simple inhibitory relay to superficial SC layers from deeper centers of the saccade motor command.
DISCUSSION
We found spatial-frequency-selective saccadic suppression in SC visual motor neurons, and the neural dynamics of visual motor neuron suppression were well correlated with behavior. Visual neurons showed weaker suppression overall, which also was not dependent on spatial frequency. These results suggest that SC visual motor neurons are among the neural loci for spatial-frequency-specific saccadic suppression. Because spatial frequency specificity is a robust characteristic of saccadic suppression (Burr et al. 1994; Hass and Horwitz 2011), identifying neural loci for this phenomenon is important. In what follows, we discuss our methodological choices, the implications of our results, and how these results fit within our current understanding of saccades, active vision, and the SC.
Our results are in line with interpretations of saccadic suppression as a reduction in response gain (Chen et al. 2015; Guez et al. 2013; Hafed and Krauzlis 2010). Consistent with this, we have recently found that SC neural contrast thresholds are increased after microsaccades (Chen et al. 2015). We also have found that for SC neurons possessing some baseline activity in the absence of a stimulus, there was very modest perimicrosaccadic modulation of activity (see Fig. S2 of Chen et al. 2015) compared with the modulations in stimulus-evoked visual bursts that we observed in the present study and earlier (Chen et al. 2015; Hafed and Krauzlis 2010). We believe that observations like these place constraints on the potential sources and mechanisms of extraretinal modulation often invoked in theories of saccadic suppression.
There have been few successful demonstrations of spatial-frequency-specific patterns of saccadic suppression in neural activity. In early visual areas, selective magnocellular pathway suppression is not clear (Hass and Horwitz 2011; Kleiser et al. 2004; Ramcharan et al. 2001; Reppas et al. 2002; Royal et al. 2006), even though behavioral effects strongly predicted them (Burr et al. 1982, 1994; Hass and Horwitz 2011; Volkmann et al. 1978). Rather, there is mild suppression, regardless of magno- or parvocellular pathway. Higher areas, primarily in the dorsal stream, do show saccadic suppression dynamics (Bremmer et al. 2009; Han et al. 2009; Ibbotson et al. 2007, 2008; Krock and Moore 2016; Thiele et al. 2002; Zanos et al. 2016), but the origins of such suppression remain elusive. In fact, it has been suggested that suppression in motion-related areas MT and MST (Bremmer et al. 2009; Ibbotson et al. 2007, 2008; Thiele et al. 2002) may be inherited from earlier visual areas (Ibbotson et al. 2007, 2008), which themselves have weak and unselective suppression. Thus there is a pressing need for better understanding of saccadic suppression mechanisms.
The fact that primarily motion areas have been shown to exhibit the most convincing suppression additionally does not help account for the fact that saccadic suppression may be useful for perception even if the “motion problem” (Wurtz 2008) caused by saccades, which we described in the Introduction, is solved. For example, suppression could help regularize processing of stimuli after saccades, regardless of the image shift itself. Consistent with this, we saw SC suppression for microsaccades, even though both the retinal-image motion and displacement caused by these eye movements are quite mild. Moreover, we saw suppression even with purely stationary gratings.
Related to the above, the fact that we saw any effects with microsaccades at all is interesting in its own regard, because it adds to the microsaccade literature, but the real advantage to studying microsaccades was that they allowed better experimental control. Microsaccades are mechanistically similar to larger saccades (Hafed 2011; Hafed et al. 2009, 2015; Zuber et al. 1965), making them an extremely viable tool to understanding saccadic suppression. However, these movements simplify several challenges associated with large saccades. For example, studies with large saccades have to contend with large image shifts caused by eye movements. As a result, full field stimuli often become necessary (Ibbotson et al. 2007, 2008). In our case, we could use stimuli identical to how normal experiments might stimulate RFs. More importantly, microsaccades allowed us to dissociate the location of saccadic suppression from the movement end-point location, as is known to happen with large saccades (Knöll et al. 2011). This has allowed us to make the intriguing observation of movement-related LFP modulations (Fig. 10) even in extrafoveal SC (i.e., with no microsaccade-related bursting neurons). These modulations, similarly to saccade-related LFP modulations in human SC (Liu et al. 2009), can potentially explain recently observed perimicrosaccadic alterations in neural activity and behavior at eccentricities much farther than microsaccade amplitudes (Chen et al. 2015; Hafed 2013; Hafed et al. 2015; Tian et al. 2016).
Another experimental advantage was the fact that SC shows suppression after saccades in our type of paradigm (Chen et al. 2015; Hafed and Krauzlis 2010). This allowed us to avoid probing neurons during the eye movements themselves. Of course, saccadic suppression would be even stronger during the microsaccades themselves, as we have recently shown (Chen et al. 2015; Hafed and Krauzlis 2010), which is further evidence of a consistency between our visual motor neural modulations and classic perceptual effects of saccadic suppression in humans (e.g., Zuber et al. 1966). Thus our choice to focus on postmovement modulations was one of exploiting the experimental advantages of doing so as opposed to one of a conceptual difference between our visual motor neural modulations and the phenomenon itself.
Concerning superficial visual neurons, one can speculate about their source of mild and unselective suppression. This suppression could reflect retinal effects, because the superficial SC receives retinal projections (Pollack and Hickey 1979). Indeed, retinal outputs do show transient perturbations in response to saccade-like image displacements (Roska and Werblin 2003). Additionally, the effect could be inherited from V1, which does not show selectivity (Hass and Horwitz 2011). Regardless of the source, suppression in visual neurons is not selective for spatial frequency as is known in perception (e.g., Burr et al. 1994). Of course, such suppression could still be functional. For example, a collicular-thalamic-cortical pathway from superficial SC may selectively target motion-related areas (Berman and Wurtz 2008, 2010, 2011; Wurtz et al. 2011). As a result, superficial SC may still contribute to saccadic suppression of motion (Bridgeman et al. 1975; Burr et al. 1982); in this case, selectively suppressing motion by superficial SC neurons would arise not necessarily because the neurons themselves are selective in their suppression profiles, but instead because of selectivity in their connections to cortical targets. Although this idea is consistent with similarities of neural saccadic suppression dynamics between superficial SC neurons and MT neurons (Berman et al., in press), it receives substantially less support from SC inactivation experiments in the same study (Berman et al., in press). In these experiments, inactivating the superficial SC did not reduce MT suppression effects, whereas inactivating the deeper SC layers did. In this regard, we believe that the pathway from intermediate SC layers to frontal eye field (FEF) via thalamus (Sommer and Wurtz 2004) is the more likely source of cortical saccadic suppression in general, not only in MT but also in other cortical areas such as FEF (Krock and Moore 2016) and V4 (Han et al. 2009; Zanos et al. 2016). This is consistent with our present results showing that saccadic suppression may already be established in the intermediate SC layers themselves without the need for an internal inhibitory relay to superficial layers. This inhibitory relay (Isa and Hall 2009; Lee et al. 2007; Phongphanphanee et al. 2011) could be used for other functions, perhaps in coordination with an excitatory relay in parallel, which has also been identified (Ghitani et al. 2014).
Our observation of a lack of suppression selectivity in purely visual neurons also helps address an important question regarding the nature of our selective visual motor neuron modulations. Specifically, it may be argued that (peripheral) SC neurons may preferentially over-sample low spatial frequencies in their tuning curves (Hafed and Chen 2016), meaning that they exhibit higher sensitivity for low spatial frequencies even without microsaccades. This, in turn, could mean that we only saw stronger suppression at low spatial frequencies (in the visual motor neurons) simply because the baseline visual responses were stronger. However, our visual neurons preferred similar ranges of spatial frequencies as our visual motor neurons. If our effects are explained by the dependence of suppression on baseline visual sensitivity in the absence of microsaccades, then our visual neurons should have shown the same patterns of selective suppression as the visual motor neurons, but they did not (Figs. 3 and 4). Second, we specifically examined suppression within each spatial frequency relative to the no-microsaccade baseline of the same frequency, to isolate the suppression effect independent of baseline response strength. This avoided questions of absolute firing sensitivity across spatial frequencies. Third, in Fig. 7, we explicitly examined suppression as a function of preferred spatial frequency and still found diminishing returns in suppression strength with increasing spatial frequency even when each spatial frequency bin only included the neurons preferring that frequency. Finally, because the visual system is inherently generally low pass anyway (especially in the periphery), even a mechanism in which suppression simply scales with visual sensitivity of a given spatial frequency would still explain the well-known perceptual phenomenon of selective suppression of low spatial frequencies in humans.
There also may be an additional potential counter-interpretation of our results. Specifically, it may be argued that we uncovered a highly specific effect only modulating saccadic RTs and that SC modulations are irrelevant for other forms of behavior (e.g., not requiring saccadic responses). However, this is unlikely. First, the SC contributes to behavior even with nonsaccadic outputs. For example, during attentional tasks with button presses, SC lesions impair performance (Sapir et al. 1999), suggesting that it is sensory and/or cognitive modulations that are relevant. Consistent with this, the SC contributes to attentional paradigms with a variety of response modalities (Lovejoy and Krauzlis 2010; Zénon and Krauzlis 2012). Second, we only looked at the earliest visual responses and uncovered strong correlations to behavior observed in separate experiments. This indicates that it was the sensory response that mattered. Consistent with this, we have recently found that the occurrence of a microsaccade near the time of stimulus onset affected both manual and saccadic RTs in a similar fashion despite the different motor response modalities (Tian et al. 2016). Third, our behavioral effects on RT are themselves remarkably similar to perceptual effects of saccadic suppression in humans, but with different perceptual measures and response modalities (Burr et al. 1982, 1994; Volkmann et al. 1978). Fourth, we found that monkey P had a stronger suppression effect in behavior than monkey N at the low spatial frequencies (compare the light gray curves in Fig. 2, H and K) even though monkey P had significantly longer saccadic RTs to begin with (compare the black no-microsaccade curves in Fig. 2, A and D). If our behavioral and neural effects were restricted to limits on saccadic RT, perhaps due to potential saccadic refractory periods between successive saccades and microsaccades, then monkey P should have shown weaker behavioral suppression than monkey N because this monkey’s saccadic system had plenty of time to recover from the previous generation of a microsaccade before having to generate the next saccadic RT. Given all of the above, as well as further arguments in Hafed and Krauzlis (2010), we find it unlikely that our modulations are only specific to modulating saccadic RTs.
If that is the case, then why might the SC be among the neural substrates for spatial-frequency-specific saccadic suppression? We think that the SC has several appealing features to place it well within a hypothetical saccadic suppression system. For example, the SC contributes to triggering the saccade command. Thus a source of corollary discharge is already present in the visual motor layers, as demonstrated by our differential firing rate (Figs. 3–7) and LFP effects (Figs. 8–10). Second, proximity of the SC to motor outputs confers an additional advantage: SC suppression, besides having potential perceptual effects, could help to regularize how often subsequent saccades are made to sample the visual world. That is, in reality, suppression could serve to control the temporal structure of saccades, which can be very important both behaviorally (Tian et al. 2016) and cortically (Lowet et al. 2016). This becomes even more interesting in light of the strong prevalence of low spatial frequencies in natural scene statistics (Field 1987), suggesting that selective suppression of low spatial frequencies is indeed functional. Moreover, controlling the temporal structure of saccades might explain refractory periods between successive movements, which we briefly alluded to above. Specifically, it is known that signal delays from the retina to the eye muscles can be much shorter than typically observed intersaccadic intervals. For example, SC neurons receive visual responses within ~50 ms after stimulus onset, and SC stimulation can trigger saccades within ~20 ms (i.e., a total of ~70 ms); on the other hand, typical RT values or intersaccadic intervals are at least twice as long (Boch et al. 1984; Robinson 1972; Schiller and Stryker 1972; Wurtz and Mohler 1976). This has led to talk of saccadic refractory periods (e.g., Becker and Jürgens 1979), but the mechanisms for such refractory periods are not known. If the SC is desensitized after every saccade, then this can delay subsequent saccades, introducing refractoriness and also more generally controlling the temporal structure of eye movement generation.
GRANTS
This work was funded by the Werner Reichardt Centre for Integrative Neuroscience (CIN) at the Eberhard Karls University of Tuebingen. The CIN is an Excellence Cluster funded by the Deutsche Forschungsgemeinschaft (DFG) within the framework of the Excellence Initiative (EXC 307).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.-Y.C. and Z.M.H. conceived and designed research; C.-Y.C. and Z.M.H. performed experiments; C.-Y.C. and Z.M.H. analyzed data; C.-Y.C. and Z.M.H. interpreted results of experiments; C.-Y.C. and Z.M.H. prepared figures; C.-Y.C. and Z.M.H. drafted manuscript; C.-Y.C. and Z.M.H. edited and revised manuscript; C.-Y.C. and Z.M.H. approved final version of manuscript.
REFERENCES
- Becker W, Jürgens R. An analysis of the saccadic system by means of double step stimuli. Vision Res 19: 967–983, 1979. doi: 10.1016/0042-6989(79)90222-0. [DOI] [PubMed] [Google Scholar]
- Berman RA, Cavanaugh J, McAlonan K, Wurtz RH. A circuit for saccadic suppression in the primate brain. J Neurophysiol. In press. doi: 10.1152/jn.00679.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH. Exploring the pulvinar path to visual cortex. Prog Brain Res 171: 467–473, 2008. doi: 10.1016/S0079-6123(08)00668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH. Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci 30: 6342–6354, 2010. doi: 10.1523/JNEUROSCI.6176-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH. Signals conveyed in the pulvinar pathway from superior colliculus to cortical area MT. J Neurosci 31: 373–384, 2011. doi: 10.1523/JNEUROSCI.4738-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch R, Fischer B, Ramsperger E. Express-saccades of the monkey: reaction times versus intensity, size, duration, and eccentricity of their targets. Exp Brain Res 55: 223–231, 1984. doi: 10.1007/BF00237273. [DOI] [PubMed] [Google Scholar]
- Boehnke SE, Munoz DP. On the importance of the transient visual response in the superior colliculus. Curr Opin Neurobiol 18: 544–551, 2008. doi: 10.1016/j.conb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG. Simple reaction time as a measure of the temporal response properties of transient and sustained channels. Vision Res 15: 1411–1412, 1975. doi: 10.1016/0042-6989(75)90200-X. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Kubischik M, Hoffmann KP, Krekelberg B. Neural dynamics of saccadic suppression. J Neurosci 29: 12374–12383, 2009. doi: 10.1523/JNEUROSCI.2908-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgeman B, Hendry D, Stark L. Failure to detect displacement of the visual world during saccadic eye movements. Vision Res 15: 719–722, 1975. doi: 10.1016/0042-6989(75)90290-4. [DOI] [PubMed] [Google Scholar]
- Burr DC, Holt J, Johnstone JR, Ross J. Selective depression of motion sensitivity during saccades. J Physiol 333: 1–15, 1982. doi: 10.1113/jphysiol.1982.sp014434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature 371: 511–513, 1994. doi: 10.1038/371511a0. [DOI] [PubMed] [Google Scholar]
- Burr DC, Ross J. Contrast sensitivity at high velocities. Vision Res 22: 479–484, 1982. doi: 10.1016/0042-6989(82)90196-1. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Wurtz RH. Saccadic omission: why we do not see a grey-out during a saccadic eye movement. Vision Res 18: 1297–1303, 1978. doi: 10.1016/0042-6989(78)90219-5. [DOI] [PubMed] [Google Scholar]
- Castet E, Jeanjean S, Masson GS. ‘Saccadic suppression’- no need for an active extra-retinal mechanism. Trends Neurosci 24: 316–318, 2001. doi: 10.1016/S0166-2236(00)01828-2. [DOI] [PubMed] [Google Scholar]
- Castet E, Masson GS. Motion perception during saccadic eye movements. Nat Neurosci 3: 177–183, 2000. doi: 10.1038/72124. [DOI] [PubMed] [Google Scholar]
- Chen CY, Hafed ZM. Postmicrosaccadic enhancement of slow eye movements. J Neurosci 33: 5375–5386, 2013. doi: 10.1523/JNEUROSCI.3703-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Ignashchenkova A, Thier P, Hafed ZM. Neuronal response gain enhancement prior to microsaccades. Curr Biol 25: 2065–2074, 2015. doi: 10.1016/j.cub.2015.06.022. [DOI] [PubMed] [Google Scholar]
- Diamond MR, Ross J, Morrone MC. Extraretinal control of saccadic suppression. J Neurosci 20: 3449–3455, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field DJ. Relations between the statistics of natural images and the response properties of cortical cells. J Opt Soc Am A 4: 2379–2394, 1987. doi: 10.1364/JOSAA.4.002379. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res 260: 21–26, 1983. doi: 10.1016/0006-8993(83)90760-6. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966. [DOI] [PubMed] [Google Scholar]
- García-Pérez MA, Peli E. Visual contrast processing is largely unaltered during saccades. Front Psychol 2: 247, 2011. doi: 10.3389/fpsyg.2011.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitani N, Bayguinov PO, Vokoun CR, McMahon S, Jackson MB, Basso MA. Excitatory synaptic feedback from the motor layer to the sensory layers of the superior colliculus. J Neurosci 34: 6822–6833, 2014. doi: 10.1523/JNEUROSCI.3137-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez J, Morris AP, Krekelberg B. Intrasaccadic suppression is dominated by reduced detector gain. J Vis 13: 4, 2013. doi: 10.1167/13.8.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM. Mechanisms for generating and compensating for the smallest possible saccades. Eur J Neurosci 33: 2101–2113, 2011. doi: 10.1111/j.1460-9568.2011.07694.x. [DOI] [PubMed] [Google Scholar]
- Hafed ZM. Alteration of visual perception prior to microsaccades. Neuron 77: 775–786, 2013. doi: 10.1016/j.neuron.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Chen C-Y. Sharper, stronger, faster upper visual field representation in primate superior colliculus. Curr Biol 26: 1647–1658, 2016. doi: 10.1016/j.cub.2016.04.059. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Chen C-Y, Tian X. Vision, perception, and attention through the lens of microsaccades: mechanisms and implications. Front Syst Neurosci 9: 167, 2015. doi: 10.3389/fnsys.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. A neural mechanism for microsaccade generation in the primate superior colliculus. Science 323: 940–943, 2009. doi: 10.1126/science.1166112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Ignashchenkova A. On the dissociation between microsaccade rate and direction after peripheral cues: microsaccadic inhibition revisited. J Neurosci 33: 16220–16235, 2013. doi: 10.1523/JNEUROSCI.2240-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Krauzlis RJ. Microsaccadic suppression of visual bursts in the primate superior colliculus. J Neurosci 30: 9542–9547, 2010. doi: 10.1523/JNEUROSCI.1137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Krauzlis RJ. Similarity of superior colliculus involvement in microsaccade and saccade generation. J Neurophysiol 107: 1904–1916, 2012. doi: 10.1152/jn.01125.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Xian SX, Moore T. Dynamic sensitivity of area V4 neurons during saccade preparation. Proc Natl Acad Sci USA 106: 13046–13051, 2009. doi: 10.1073/pnas.0902412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass CA, Horwitz GD. Effects of microsaccades on contrast detection and V1 responses in macaques. J Vis 11: 1–17, 2011. doi: 10.1167/11.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibbotson M, Krekelberg B. Visual perception and saccadic eye movements. Curr Opin Neurobiol 21: 553–558, 2011. doi: 10.1016/j.conb.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibbotson MR, Crowder NA, Cloherty SL, Price NS, Mustari MJ. Saccadic modulation of neural responses: possible roles in saccadic suppression, enhancement, and time compression. J Neurosci 28: 10952–10960, 2008. doi: 10.1523/JNEUROSCI.3950-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibbotson MR, Price NS, Crowder NA, Ono S, Mustari MJ. Enhanced motion sensitivity follows saccadic suppression in the superior temporal sulcus of the macaque cortex. Cereb Cortex 17: 1129–1138, 2007. doi: 10.1093/cercor/bhl022. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Boehnke SE, Marino RA, White BJ, Wang CA, Levy R, Munoz DP. Spatio-temporal response properties of local field potentials in the primate superior colliculus. Eur J Neurosci 41: 856–865, 2015. doi: 10.1111/ejn.12842. [DOI] [PubMed] [Google Scholar]
- Ilg UJ, Hoffmann KP. Motion perception during saccades. Vision Res 33: 211–220, 1993. doi: 10.1016/0042-6989(93)90159-T. [DOI] [PubMed] [Google Scholar]
- Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol 102: 2581–2593, 2009. doi: 10.1152/jn.00498.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kleiser R, Seitz RJ, Krekelberg B. Neural correlates of saccadic suppression in humans. Curr Biol 14: 386–390, 2004. doi: 10.1016/j.cub.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Knöll J, Binda P, Morrone MC, Bremmer F. Spatiotemporal profile of peri-saccadic contrast sensitivity. J Vis 11: 15, 2011. doi: 10.1167/11.14.15. [DOI] [PubMed] [Google Scholar]
- Krock RM, Moore T. Visual sensitivity of frontal eye field neurons during the preparation of saccadic eye movements. J Neurophysiol 116: 2882–2891, 2016. doi: 10.1152/jn.01140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Sooksawate T, Yanagawa Y, Isa K, Isa T, Hall WC. Identity of a pathway for saccadic suppression. Proc Natl Acad Sci USA 104: 6824–6827, 2007. doi: 10.1073/pnas.0701934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Basso MA. Preparing to move increases the sensitivity of superior colliculus neurons. J Neurosci 28: 4561–4577, 2008. doi: 10.1523/JNEUROSCI.5683-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Nachev P, Wang S, Green A, Kennard C, Aziz T. The saccade-related local field potentials of the superior colliculus: a functional marker for localizing the periventricular and periaqueductal gray. J Clin Neurophysiol 26: 280–287, 2009. doi: 10.1097/WNP.0b013e3181b2f2c1. [DOI] [PubMed] [Google Scholar]
- Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci 13: 261–266, 2010. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowet E, Roberts MJ, Bosman CA, Fries P, De Weerd P. Areas V1 and V2 show microsaccade-related 3-4-Hz covariation in gamma power and frequency. Eur J Neurosci 43: 1286–1296, 2016. doi: 10.1111/ejn.13126. [DOI] [PubMed] [Google Scholar]
- Matin E. Saccadic suppression: a review and an analysis. Psychol Bull 81: 899–917, 1974. doi: 10.1037/h0037368. [DOI] [PubMed] [Google Scholar]
- Matin E, Clymer AB, Matin L. Metacontrast and saccadic suppression. Science 178: 179–182, 1972. doi: 10.1126/science.178.4057.179. [DOI] [PubMed] [Google Scholar]
- Phongphanphanee P, Mizuno F, Lee PH, Yanagawa Y, Isa T, Hall WC. A circuit model for saccadic suppression in the superior colliculus. J Neurosci 31: 1949–1954, 2011. doi: 10.1523/JNEUROSCI.2305-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack JG, Hickey TL. The distribution of retino-collicular axon terminals in rhesus monkey. J Comp Neurol 185: 587–602, 1979. doi: 10.1002/cne.901850402. [DOI] [PubMed] [Google Scholar]
- Rajkai C, Lakatos P, Chen CM, Pincze Z, Karmos G, Schroeder CE. Transient cortical excitation at the onset of visual fixation. Cereb Cortex 18: 200–209, 2008. doi: 10.1093/cercor/bhm046. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. The effects of saccadic eye movements on the activity of geniculate relay neurons in the monkey. Vis Neurosci 18: 253–258, 2001. doi: 10.1017/S0952523801182106. [DOI] [PubMed] [Google Scholar]
- Reppas JB, Usrey WM, Reid RC. Saccadic eye movements modulate visual responses in the lateral geniculate nucleus. Neuron 35: 961–974, 2002. doi: 10.1016/S0896-6273(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res 12: 1795–1808, 1972. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Rapid global shifts in natural scenes block spiking in specific ganglion cell types. Nat Neurosci 6: 600–608, 2003. doi: 10.1038/nn1061. [DOI] [PubMed] [Google Scholar]
- Ross J, Burr D, Morrone C. Suppression of the magnocellular pathway during saccades. Behav Brain Res 80: 1–8, 1996. doi: 10.1016/0166-4328(96)00012-5. [DOI] [PubMed] [Google Scholar]
- Royal DW, Sáry G, Schall JD, Casagrande VA. Correlates of motor planning and postsaccadic fixation in the macaque monkey lateral geniculate nucleus. Exp Brain Res 168: 62–75, 2006. doi: 10.1007/s00221-005-0093-z. [DOI] [PubMed] [Google Scholar]
- Sapir A, Soroker N, Berger A, Henik A. Inhibition of return in spatial attention: direct evidence for collicular generation. Nat Neurosci 2: 1053–1054, 1999. doi: 10.1038/15977. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Stryker M. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol 35: 915–924, 1972. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol 91: 1381–1402, 2004. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43: 482–489, 1950. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Thiele A, Henning P, Kubischik M, Hoffmann KP. Neural mechanisms of saccadic suppression. Science 295: 2460–2462, 2002. doi: 10.1126/science.1068788. [DOI] [PubMed] [Google Scholar]
- Tian X, Yoshida M, Hafed ZM. A microsaccadic account of attentional capture and inhibition of return in Posner cueing. Front Syst Neurosci 10: 23, 2016. doi: 10.3389/fnsys.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann FC, Riggs LA, White KD, Moore RK. Contrast sensitivity during saccadic eye movements. Vision Res 18: 1193–1199, 1978. doi: 10.1016/0042-6989(78)90104-9. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. Das Reafferenzprincip: Wechselwirkungen zwischen Zentralnervensystem und Peripherie. Naturwissenschaften 37: 464–476, 1950. doi: 10.1007/BF00622503. [DOI] [Google Scholar]
- Wurtz RH. Neuronal mechanisms of visual stability. Vision Res 48: 2070–2089, 2008. doi: 10.1016/j.visres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH, Joiner WM, Berman RA. Neuronal mechanisms for visual stability: progress and problems. Philos Trans R Soc Lond B Biol Sci 366: 492–503, 2011. doi: 10.1098/rstb.2010.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH, Mohler CW. Organization of monkey superior colliculus: enhanced visual response of superficial layer cells. J Neurophysiol 39: 745–765, 1976. [DOI] [PubMed] [Google Scholar]
- Zanos TP, Mineault PJ, Guitton D, Pack CC. Mechanisms of saccadic suppression in primate cortical area V4. J Neurosci 36: 9227–9239, 2016. doi: 10.1523/JNEUROSCI.1015-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zénon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature 489: 434–437, 2012. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber BL, Stark L, Cook G. Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science 150: 1459–1460, 1965. doi: 10.1126/science.150.3702.1459. [DOI] [PubMed] [Google Scholar]
- Zuber BL, Stark L, Lorber M. Saccadic suppression of the pupillary light reflex. Exp Neurol 14: 351–370, 1966. doi: 10.1016/0014-4886(66)90120-8. [DOI] [PubMed] [Google Scholar]