Abstract
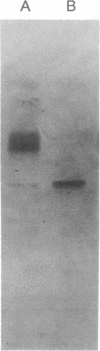
Glyoxal oxidase (GLOX) is an extracellular H2O2-generating enzyme produced by ligninolytic cultures of Phanerochaete chrysosporium. The production, purification, and partial characterization of GLOX from agitated cultures are described here. High-oxygen levels are critical for GLOX production as for lignin peroxidase. GLOX purified by anion-exchange chromatography appears homogeneous by NaDod-SO4/PAGE (molecular mass = 68 kDa). However, analysis by isoelectric focusing indicates two major bands (pI 4.7 and 4.9) that stain as glycoproteins as well as for H2O2-producing activity in the presence of methylglyoxal. Purified GLOX shows a marked stimulation in activity when incubated with Cu2+; full activation takes more than 1 hr with 1 mM CuSO4 at pH 6. The steady-state kinetic parameters for the GLOX oxidation of methylglyoxal, glyceraldehyde, dihydroxyacetone, glycolaldehyde, acetaldehyde, glyoxal, glyoxylic acid, and formaldehyde, were determined by using a lignin peroxidase coupled-assay at pH 4.5. Of these substrates, the best is the extracellular metabolite methylglyoxal with a Km of 0.64 mM an apparent rate of catalysis, kcat, of 198 s1 under air-saturated conditions. The Km for oxygen is greater than the concentration of oxygen possible at ambient pressure--i.e., >1.3 mM at 25 degrees C. Importantly, oxygen-uptake experiments show that purified GLOX is inactive unless coupled to the peroxidase reaction. With this coupled reaction, for each mol of methylglyoxal, veratryl alcohol (a lignin peroxidase substrate), and oxygen consumed, 1 mol each of pyruvate and veratraldehyde is produced. The importance of these results is discussed in relation to the physiology of lignin biodegradation and possible extracellular regulatory mechanisms for the control of oxidase and peroxidase activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Lev S. S., Kirk T. K. Effects of molecular oxygen on lignin degradation by Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1981 Mar 31;99(2):373–378. doi: 10.1016/0006-291x(81)91755-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Gold M. H., Kuwahara M., Chiu A. A., Glenn J. K. Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1984 Nov 1;234(2):353–362. doi: 10.1016/0003-9861(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Greene R. V., Gould J. M. Fatty acyl-coenzyme A oxidase activity and H2O2 production in Phanerochaete chrysosporium mycelia. Biochem Biophys Res Commun. 1984 Jan 30;118(2):437–443. doi: 10.1016/0006-291x(84)91322-6. [DOI] [PubMed] [Google Scholar]
- Greene R. V., Gould J. M. Substrate-induced H2O2 production in mycelia from the lignin-degrading fungus Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Nov 30;117(1):275–281. doi: 10.1016/0006-291x(83)91571-1. [DOI] [PubMed] [Google Scholar]
- Haemmerli S. D., Leisola M. S., Sanglard D., Fiechter A. Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. J Biol Chem. 1986 May 25;261(15):6900–6903. [PubMed] [Google Scholar]
- Jäger A., Croan S., Kirk T. K. Production of Ligninases and Degradation of Lignin in Agitated Submerged Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Nov;50(5):1274–1278. doi: 10.1128/aem.50.5.1274-1278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Kirk T. K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987 May;169(5):2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- RACKER E. The mechanism of action of glyoxalase. J Biol Chem. 1951 Jun;190(2):685–696. [PubMed] [Google Scholar]
- Tien M., Kirk T. K., Bull C., Fee J. A. Steady-state and transient-state kinetic studies on the oxidation of 3,4-dimethoxybenzyl alcohol catalyzed by the ligninase of Phanerocheate chrysosporium Burds. J Biol Chem. 1986 Feb 5;261(4):1687–1693. [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M. Properties of ligninase from Phanerochaete chrysosporium and their possible applications. Crit Rev Microbiol. 1987;15(2):141–168. doi: 10.3109/10408418709104456. [DOI] [PubMed] [Google Scholar]
- Whittaker M. M., Whittaker J. W. The active site of galactose oxidase. J Biol Chem. 1988 May 5;263(13):6074–6080. [PubMed] [Google Scholar]