Abstract
Purpose
We compared the efficacy of tamsulosin between 0.2 mg and 0.4 mg in Asian prostatic hyperplasia (BPH) patients using network meta-analysis due to lack of studies with direct comparison.
Methods
The literature search was conducted using the MEDLINE, Embase, and Cochrane Library. Keywords used were “BPH,” “tamsulosin,” “placebo.” Experimental groups were defined as tamsulosin 0.2 mg (Tam 0.2) and 0.4 mg (Tam 0.4) and common control group was defined as placebo for indirect treatment comparison. Mixed treatment comparison was performed including one direct comparison study.
Results
Seven studies met the eligible criteria. Indirect treatment comparison revealed that total International Prostate Symptoms Score (IPSS) and quality of life score of IPSS were not significantly different in Tam 0.2 and Tam 0.4 (P>0.05). There was no significant difference of maximal flow rate and postvoid residual urine volume in Tam 0.2 and Tam 0.4 (P>0.05). Mixed treatment comparison including one direct comparison study showed inconsistency (P<0.001). Therefore, analysis using direct treatment comparison effect sizes of Tam 0.2 vs. placebo and Tam 0.4 vs. placebo was done and there was no significant difference.
Conclusions
Network meta-analysis showed no difference of efficacy between tamsulosin 0.2 mg and 0.4 mg and the evidence of tamsulosin 0.4 mg as initial dose for Asian BPH patient seems to be insufficient. Therefore, initial dose of tamsulosin for Asian BPH patient should be 0.2 mg.
Keywords: Prostatic Hyperplasia, Tamsulosin, Asian, Men, Dose
INTRODUCTION
Tamsulosin is an oral medicine commonly used to treat lower urinary tract symptoms (LUTS) of men with benign prostatic hyperplasia (BPH). Generally, the initial treatment dose is different between Asian and Western men with BPH. Tamsulosin 0.2 mg (Tam 0.2) is recommended as the initial dose for Asian men with BPH, while the recommended dose for Western men is tamsulosin 0.4 mg (Tam 0.4) [1]. The difference of initially recommended doses between Asian and Western men with BPH was based on clinical trials meant to evaluate efficacy and adverse effect; therefore, the initial treatment dose was decided as 0.2 mg for Asian men and 0.4 mg for Western men [2]. In Korea as well as in other Asian countries, men experiencing LUTS due to BPH have an improvement of symptoms and effects after initial treatment with Tam 0.2 mg compared with men treated with other types of alpha-blockers [3,4]. Therefore, the general consensus of a standard initial dose of Tam 0.2 in Asian men is a reasonable estimate for the treatment of LUTS due to BPH.
Recently, Kim et al. [5] reported results comparing the efficacy between Tam 0.2 and Tam 0.4 as an initial dose in Korean men with LUTS due to BPH. The investigators noted that Korean men with BPH receiving Tam 0.4 showed significant improvements on the International Prostatic Symptom Score (IPSS) compared with men receiving Tam 0.2 as their initial treatment dose after 12 weeks of medication. This result is different from those of the above researchers who investigated tamsulosin in Asian men with BPH and used Tam 0.2 as the standard dose for initial treatment [1-4]. Therefore, a reassessment of the efficacy and safety of tamsulosin in Asian men with BPH is necessary to clarify this discrepancy between the recent study [5] and the general consensus [1-4]. However, direct comparison studies to compare the efficacy and safety between Tam 0.2 and Tam 0.4 as the initial treatment dose in Asian men with BPH are insufficient.
Therefore, we compared the effect of Tam 0.2 and Tam 0.4 as the initial treatment dose using network meta-amalysis (NMA).
MATERIALS AND METHODS
This systematic review and network meta-analysis was performed according to the standard PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocol and the Cochrane Collaboration [6].
Data Sources and Literature Searches
The electronic databases screened were MEDLINE (1966 through January 2016) and Cochrane Library (1993 through January 2016). Medical Subject Headings terms were used. The search formula was Search (“tamsulosin” [Supplementary Concept]) OR “tamsulosin”[tiabkw] OR “YM178” [all]) AND (“Placebos”[Mesh] OR “placebo”[tiabkw]) AND (“Lower Urinary Tract Symptoms”[Mesh] OR “Lower Urinary Tract Symptoms”[tiabkw] OR “LUTS”[tiabkw] OR “benign prostatic hyperplasia”[tiabkw] OR “BPH”[tiabkw]). The searches were no language limitation. The same search formula as for Emtree was adopted for the Embase search. Prospective randomized controlled trials (RCTs) using placebo were included in this analysis.
Selection of Studies
Study inclusion criteria were as follows: (1) Interventions were with placebo, Tam 0.2, and/or Tam 0.4 and no dose escalation study from Tam 0.2 to Tam. (2) Participants were diagnosed with BPH. (3) Randomization, blind method, and intention-to-treat (ITT) analysis were performed in RCTs. Only one article using direct treatment comparison (DTC) between Tam 0.2 and Tam 0.4 [4] did not meet selection criteria. However, we input this article to estimate for mixed treatment comparison (MTC) analysis. Two authors (SJK and ISS) independently screened the titles and abstracts of all articles using predefined inclusion and exclusion criteria. The full-text articles were examined independently by another 2 authors (JWK and YSC) to determine whether they met the inclusion criteria. Then, 2 authors (SJK and ISS) independently extracted data using data extraction forms. Final inclusion was determined by the GRADE evaluation discussion using a point estimation system for each article. References and data for each included study were carefully cross-checked to ensure that no overlapping data was present and to maintain the integrity of the meta-analysis.
Types of Interventions and Outcomes
The experimental group received Tam 0.2 or Tam 0.4 and the control group received placebo. The primary outcome was change in BPH as measured by IPSS. Secondary outcomes included quality of life (QoL), maximal flow rate (Qmax), and postvoided residual (PVR) volume.
Indirect Comparison Analysis and Mixed Comparison Analysis: Assessment of Outcome Findings
We conducted all frequentist meta-analyses using the standard techniques of random effects meta-analysis of continuous variables using inverse variance as described by Deeks et al. [7]. We extracted data from all RCTs with Tam 0.2 and Tam 0.4 arms. We combined the 3-arm trials of Tam 0.2, Tam 0.4, and placebo to calculate DTC and indirect treatment comparison (ITC) for the 4 outcomes of interest. For MTC analysis, we use only IPSS because QoL, Qmax, and PVR were lack of data. We combined DTC estimate with the ITC estimate using the method described by Song et al. [8]. The MTC method is not appropriate for the present NMA because of the fail to meet the basic assumption of consistency between DTC and ITC. However, we conducted MTC method after ITC analysis to emphasize for our research hypothesis that the effect size between Tam 0.2 and Tam 0.4 will not be different. A 2-sided P-value of 0.05 or lower was considered to be significant. The abovementioned analyses were conducted with STATA ver. 14 (Stata Corp LP, College Station, TX, USA).
Quality Assessment
The risk of bias and methodological quality were evaluated in duplicate using the Cochrane Collaboration tool. We graded each parameter as unclear, low risk of bias, or high risk of bias throughout the grade evaluation meeting with all authors.
Assessment of Potential Publication Bias
Publication bias was explained by Funnel plot. Asymmetry findings in funnel plots indicate publication bias, but the shape of the plot in the absence of bias depends on the choice of trial arms.
RESULTS
Study Selection Results
The initial search identified a total of 807 articles from electronic databases (PubMed, 62; Cochrane, 97; Embase, 648) and 8 articles from hand-searching. Thirty-nine excluded studies contained overlapping data in more than one database. Upon more detailed screening, an additional 739 papers were excluded for the following reasons: unmatched outcome (243), letter (18), commentary (15), unrelated topic (290), overlapping (43), abstract only (45), review paper (35), and no randomization (50). After screening the titles and abstracts, 37 studies were determined to be eligible for full text review. Among of them, 29 studies were further eliminated because of no prospective clinical trials, no blinding, and insufficient data. Finally, 7 studies [9-15] met our selection criteria and 1 study [4] using direct treatment comparison between Tam 0.2 and Tam 0.4 was inputted to estimate for MTC analysis (Fig. 1). A systematic review of the 8 studies was conducted to assess detailed experimental differences and subject descriptions (Table 1).
Fig. 1.
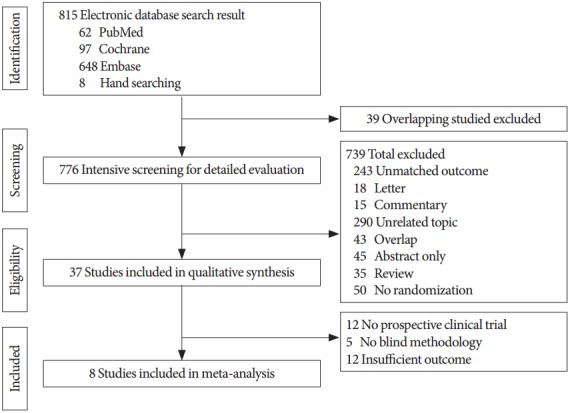
Flowchart of the study selection process.
Table 1.
Characteristics of included studies
| Study | Intervention, dose (No. of patients) | Country | Mean age (yr) | Baseline IPSS | Study duration (wk) |
|---|---|---|---|---|---|
| Nordling 2005 [9] | Placebo (154), Tamsulosin 0.4 mg (158), Finasteride 5 mg (153), Alfuzosin 10 mg (154), Alfuzosin 15 mg (158) | Denmark | 64.5 | 17.6 | 12 |
| Chapple et al. 2011 [10] | Placebo (190), Tamsulosin 0.4 mg (384), Silodosin 8 mg (381) | UK | 65.9 | 19 | 12 |
| Narayan et al. 1998 [11] | Placebo (239), Tamsulosin 0.4 mg (248), Tamsulosin 0.8 mg (244) | USA | NA | NA | 13 |
| Oelke et al. 2012 [12] | Placebo (172), Tamsulosin 0.4 mg (168), Tadalafil 5 mg (171) | Germany | 63.6 | 17.1 | 12 |
| Lepor 1998 [13] | Placebo (254), Tamsulosin 0.4 mg (254), Tamsulosin 0.8 mg (247) | USA | NA | 19.8 | 13 |
| Kawabe et al. 2006 [14] | Placebo (89), Tamsulosin 0.2 mg (192), Silodosin 4 mg (175) | Japan | 65.6 | 17 | 12 |
| Yokoyama et al. 2013 [15] | Placebo (154), Tamsulosin 0.2 mg (152), Tadalafil 2.5 mg (151), Tadalafil 5 mg (155) | Japan | 63.1 | 16.8 | 12 |
| Kim 2016a) | Placebo (160), Tamsulosin 0.4 mg (152), Tamsulosin 0.2 mg (159) | Korea | NA | 19.9 | 12 |
IPSS, International Prostate Symptoms Score; NA, not available.
American Urological Association 2016 abstract, a randomised, double-blind, phase 3 trial in Korean men.
Quality Assessment
Table 2 shows quality assessment of the included studies. All of the studies described randomization, blinding, ITT analysis, and had no selective reporting bias except for the study by Kim et al. [5] using RCT, double-blind method in Korean men is one abstract of American Urological Association 2016.
Table 2.
The quality assessment of included studies
| Study | Random sequence | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Loss of following-up | Intent-to-treat analysis | Selective reporting | Other sources of bias |
|---|---|---|---|---|---|---|---|---|---|
| Nordling 2005 [9] | Low | Unclear | Low | Low | Low | Yes | Yes | Low | Unclear |
| Chapple et al. 2011 [10] | Low | Low | Low | Low | Low | Yes | Yes | Low | Unclear |
| Narayan et al. 1998 [11] | Low | Unclear | Low | Low | Low | Yes | Yes | Low | Unclear |
| Oelke et al. 2012 [12] | Low | Low | Low | Low | Low | Yes | Yes | Low | Unclear |
| Lepor 1998 [13] | Low | Unclear | Low | Low | Low | Yes | Yes | Low | Unclear |
| Kawabe et al. 2006 [14] | Low | Unclear | Low | Low | Low | Yes | Yes | Low | Unclear |
| Yokoyama et al. 2013 [15] | Low | Low | Low | Low | Low | Yes | Yes | Low | Unclear |
| Kim 2016a) | Low | Unclear | Low | Low | Unclear | Unclear | Unclear | Unclear | High |
IPSS, International Prostate Symptoms Score.
American Urological Association 2016 abstract, a randomised, double-blind, phase 3 trial in Korean men.
Outcome Findings
Indirect treatment comparison
A total of 7 trials included 2,767 subjects (placebo, 1,234; Tam 0.2, 344; Tam 0.4, 1,189) in indirect comparison analysis [2,10-15]. Fig. 2 showed all outcomes indirect comparison analysis results between Tam 0.2 and Tam 0.4. The adjusted indirect comparison analysis effect size and heterogeneity statistic P-value of IPSS, QoL, PVR, and Qmax were 0.02 (95% confidence interval [CI], -0.25 to 0.29) and P=0.880, 0.07 (95% CI: -0.23, 0.37) and P=0.663, 0.07 (95% CI, -0.24 to 0.39) and P=0.641, and -0.06 (95% CI, -0.72 to 0.60) and P =0.868, respectively. There were not statistical differences between 2 treatments and there were no evidence of heterogeneity in all outcomes.
Fig. 2.
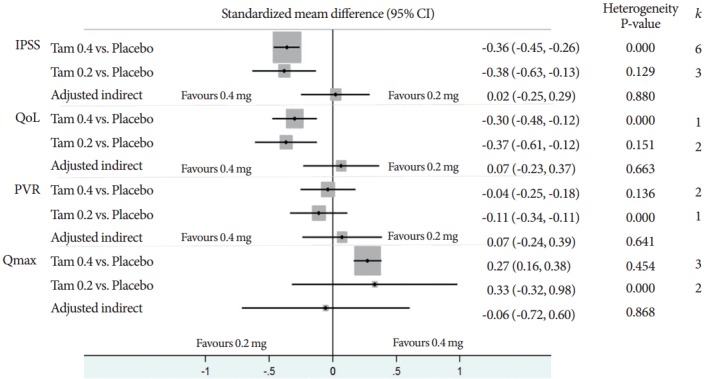
Standardized mean difference of adjusted indirect comparisions. k, number of effect sizes; CI, confidence interval; IPSS, International Prostate Symptoms Score; QoL, quality of life; PVR, postvoid residual; Qmax, maximal flow rate; Tam 0.2, tamsulosin 0.2 mg; Tam 0.4, tamsulosin 0.4 mg.
Mixed treatment comparison
A total of 8 trials included 3,238 subjects (placebo, 1,394; Tam 0.2, 503; Tam 0.4, 1,341) in MTC analysis for IPSS [5,9-15]. Fig. 3 shows the network plot of pairwise comparisons from the included trials (dTam 0.2-placebo [5,14,15], dTam 0.4-placebo [5,9-13], and dTam 0.4-Tam 0.2 [5]). Fig. 4 presented the contribution plot for each direct comparison in columns. The entire influences of direct comparison were 39.8%, 35.2%, and 25.0% for dTam 0.2-placebo, dTam 0.4-Tam 0.2, and dTam 0.4-placebo, respectively.
Fig. 3.
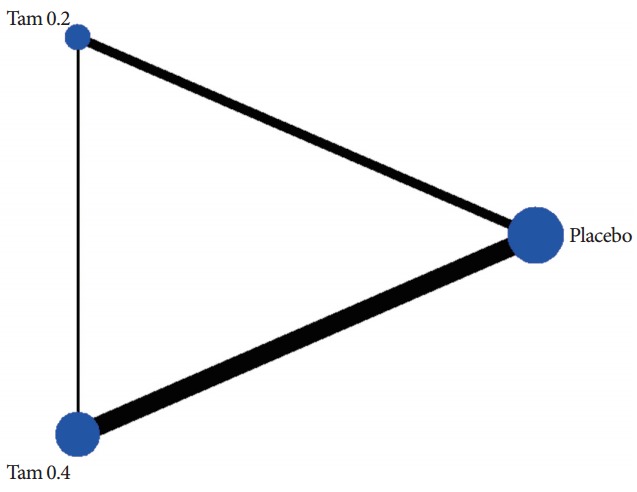
The network plot of pairwise comparisons from the included trials. The lines indicate available direct comparisons from included randomized controlled trials. The width of the lines is proportional to the number of studies for the comparisons; Tam 0.2, tamsulosin 0.2 mg; Tam 0.4, tamsulosin 0.4 mg.
Fig. 4.
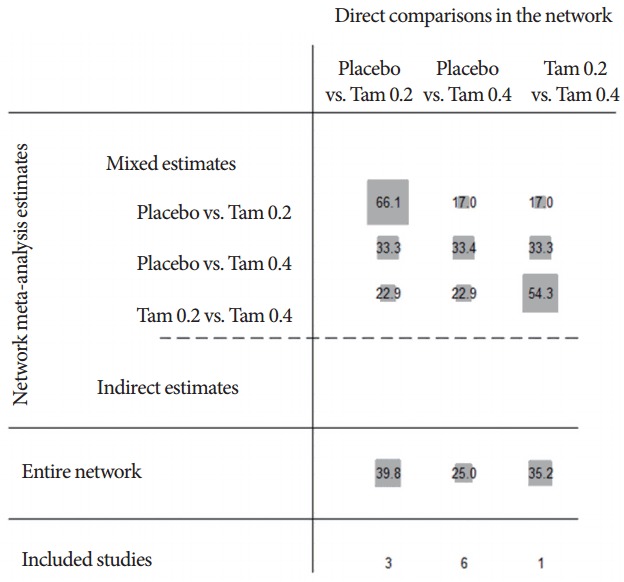
Contribution plot for mixed treatment comparison network. The size of each square is proportional to the weight attached to each direct summary effect (horizontal axis) for the estimation of each network summary effects (vertical axis). The numbers re-express the weights as percentages; Tam 0.2, tamsulosin 0.2 mg; Tam 0.4, tamsulosin 0.4 mg.
The inconsistency test gives a P-value of <0.001, thus we can use only direct comparison analysis effect sizes of Tam 0.2 vs. placebo and Tam 0.4 vs. placebo. However, the standardized mean difference (SMD) within design was -0.79 (95% CI, -1.02 to -0.56) in Tam 0.4 vs. Tam 0.2 that was from only study by Kim et al. [5]. The primary outcome of MTC was that the pooled overall SMD was -0.24 (95% CI, -0.71 to 0.22) in Tam 0.4 vs. Tam 0.2. This mixed comparison analysis result demonstrated that there were no significantly statistical differences between Tam 0.4 and Tam 0.2 even though we input the direct comparison of Tam 0.4 and Tam 0.2 (Fig. 5).
Fig. 5.
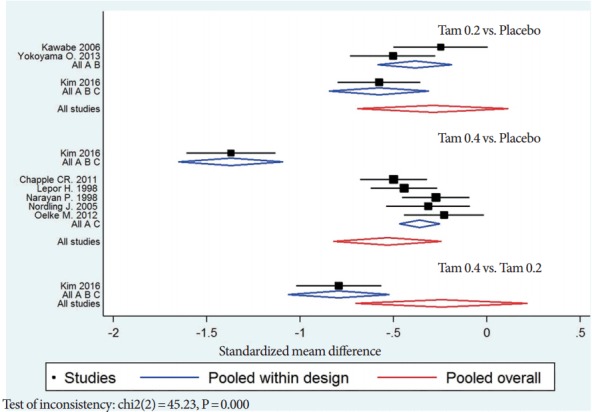
Standardized mean difference of mixed treatment comparisions including direct comparison; Tam 0.2, tamsulosin 0.2 mg; Tam 0.4, tamsulosin 0.4 mg.
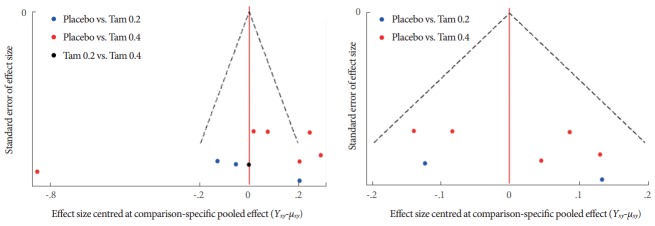
Fig. 6 illustrates the comparison adjusted funnel plot. Left side funnel plot was using all trials that appeared asymmetric. When excluding the direct comparison trial of Tam 0.4 vs. Tam 0.2 by Kim et al. [5], the right side funnel plot appeared more symmetric that implied the absence of small study effects in the network.
Fig. 6.
Comparison-adjusted funnel plot of the included studies; Tam 0.2, tamsulosin 0.2 mg; Tam 0.4, tamsulosin 0.4 mg.
Publicaton bias
Fig. 6 illustrates the comparison adjusted funnel plot. Left side funnel plot was using all trials that appeared asymmetric. When excluding the direct comparison trial of Tam 0.4 vs. Tam 0.2 by Kim et al. [5], the right side funnel plot appeared more symmetric that implied the absence of small study effects in the network.
DISCUSSION
We performed network meta-analysis including indirect and MTCs because of the lack of head-to-head studies comparing the efficacy between Tam 0.2 and Tam 0.4 in Asian BPH patients. No significant differences were noted in subjective and objective parameters treated with Tam 0.2 and Tam 0.4 after 12 to 13 weeks by ITC. These results demonstrate that Tam 0.4 did not show a better treatment effect than Tam 0.2 as the initial treatment dose. In a MTC, there was also no significant difference in the outcomes of those treated with Tam 0.2 vs. Tam 0.4. Based on these results of indirect and MTCs, we conclude that Tam 0.4 did not have better efficacy compared with the standard Tam 0.2 in Asian BPH patients.
The standard therapeutic dose of tamsulsoin is different between Asian and Western men due to the lower body mass index (BMI) of Asian men compared with Western men. Therefore, studies to determine the optimal dose of tamsulosin were done at 0.1, 0.2, and, 0.4 mg/day for Japanese patients and 0.4 and 0.8 mg/day for Western patients in Europe and the United States [2]. Tam 0.2 was adopted as a standard dose for Asian as well as Japanese patients based on a study by Kawabe et al. [16]. According to their study comparing with efficacy of tamsulosin at 0.1–0.4 mg in Japan, obstructive symptoms such as hesitancy, intermittency, and Qmax were significantly improved at the dose of Tam 0.2 mg. Similar to this result, administration with Tam 0.2 improved LUTS in other studies of Asian countries [17-19]. Moreover, Korean BPH patients treated with Tam 0.2 showed improvement of IPSS scores and Qmax comparable to the patients treated with terazosin as the dose was increased from 1 to 5 mg. However, the adverse events associated with treatment were significantly lower in patients administered with Tam 0.2 [2].
Previous studies to evaluate the efficacy and safety of Tam 0.2 as an initial treatment dose demonstrated 23.5%–50.5% of improvement in IPSS scores and approximately 20% of improvement of Qmax after treatment in an Asian population [4]. According to studies in Western men, IPSS scores were improved by 30%–45% and Qmax was increased by 15%–30% compared with the baseline after treatment with Tam 0.4 as an initial dose [20]. These results observed in Western men are similar to the degree of IPSS scores and Qmax in Asians. Therefore, Tam 0.2 as an initial dose in Asian BPH patients with lower BMI seems to be appropriate in light of these previous studies. Likewise, the network meta-analysis indicated that IPSS scores, Qmax, and PVR of the patients treated with Tam 0.2 were not significantly different compared with the patients treated with Tam 0.4 in the present study. Thus, Tam 0.2 as an initial dose is considered to be sufficient to improve BPH associated LUTS in Asian men. A study to analyze the prescription pattern of alpha-blockers in Korea observed that Tam 0.2 was the most frequently recommended medicine among various alpha-blockers. This prescription characteristic in real life might indirectly reflect the efficacy and safety of Tam 0.2 as an initial dose [21]. Without a doubt, Tam 0.2 in Asian men with BPH is the standard therapeutic dose based on the above findings. Only one recent study by Kim et al. [5] reported that patients treated with Tam 0.4 as an initial dose showed better effects compared with men treated with Tam 0.2.
Although the general recommendation for the initial dose of tamsulosin for Asian men is 0.2 mg and efficacy has been demonstrated, there are patients whose LUTS is not improved after treatment with Tam 0.2. A cross-sectional study in Korea demonstrated that 35.5% of BPH patients were not satisfied after treatment with Tam 0.2 [22]. The reason for dissatisfaction was low efficacy and side effects. They found that age and IPSS severity at baseline contributed to the decreases in patient satisfaction. Therefore, there were attempts to investigate the role of dose escalation in BPH patients whose treatment was unsuccessful with the usual dose of Tam 0.2. Kim et al. [23] increased their tamsulosin dose to 0.4 mg, and improvement of IPSS voiding symptom scores and Qmax were observed. They also noted that the patients with older age, severe daytime frequency, and lower Qmax at baseline were more likely to require dose escalation compared with patients who were satisfied with Tam 0.2. This study suggests that dose escalation of tamsulosin could in some cases be applicable to Asian men with BPH, although dose titration is not usually recommended. Moreover, these results also mean that Tam 0.4 was inappropriate as a standard initial dose in Asian men because only patients with older age and more severe symptoms were unsatisfied with Tam 0.2. There was one more study to suggest tamsulosin dose escalation for patients dissatisfied with initial treatment. It was a randomized placebo controlled trial, and the authors compared effects and adverse events after increasing the tamsulosin dose to 0.4 mg in Korean patients who were unsatisfied with the initial treatment of Tam 0.2 [24]. After a 12-week treatment with Tam 0.4, Qmax was significantly improved compared with the placebo, although IPSS scores were not significantly different. In addition, adverse events such as cardiovascular disease and abnormal ejaculation were not different between the dose escalation group and the placebo. This study suggests that the dose escalation to Tam 0.4 could be considered for Asian men who do not show improvement of LUTS after standard treatment with Tam 0.2. Therefore, dose escalation to Tam 0.4 might be worth considering in cases where patients do not respond fully to Tam 0.2.
However, reassessment of the results by Kim et al. [5] observed that better efficacy of Tam 0.4 over Tam 0.2 as an initial dose is necessary before we adopt their findings for initial treatment of BPH in Asian men. Subanalysis of the data showed that initial treatment with Tam 0.4 led to a more significant decrease of IPSS scores especially in patients who complained of more severe LUTS as reported by IPSS scores of more than 20 at baseline. In other words, these results mean that only the patients with severe symptoms who did not respond to the standard therapeutic dose of Tam 0.2 showed a more significant improvement of IPSS. This was similar to the previous study where the patients with severe LUTS at baseline required a dose escalation to Tam 0.4 [23]. As a result, only the patients with severe LUTS in the recent study by Kim et al. [5] could show more significant improvement of IPSS after treatment with Tam 0.4, excluding patients with mild- to moderate-LUTS who could be generally treated with Tam 0.2. Moreover, the report by Kim et al. [5] in 2016 was not an article but an abstract, and therefore, we could obtain from the study very restricted data. For example, only the results after 12 weeks of treatment were provided, even though the authors evaluated IPSS scores, Qmax, and PVR at 4, 8, and 12 weeks. Furthermore, subanalysis data of the baseline symptoms, including mild- to moderateas well as severe LUTS, seems to be necessary to compare the actual efficacy. Therefore, the study by Kim et al. [5] did not provide sufficient evidence to show that Tam 0.4 had better effects in Asian men compared with Tam 0.2.
A recent report about the better effects of Tam 0.4 as compared with the standard dose of Tam 0.2 was contrary to the clinical recommendation of tamsulosin dosage in the Asian BPH population; therefore, reassessment is needed. Network meta-analysis of indirect and direct studies suggested that the efficacy of Tam 0.2 was not significantly different compared with Tam 0.4 as an initial therapeutic dose, and inconsistency was observed between the recent study in 2016 and others. Based on these results, evidence is lacking to support that Tam 0.4 has better therapeutic effects compared with Tam 0.2 as an initial dose for all Asian BPH men. Therefore, initial dose of tamsulosin for Asian BPH patient should be 0.2 mg.
HIGHLIGHTS
- Network meta-analysis with mixed treatment comparison showed no significant difference between tamsulosin 0.2 mg and 0.4 mg as initial dose and the result by a study reported better effect of tamsulosin 0.4 mg was inconsistent with previous studies.
- Therefore, tamsulosin 0.2 mg should be initial standard dose in Asian BPH patients.
Footnotes
Grant/Fund Support
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2015R1C1A1A02036452).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Dunn CJ, Matheson A, Faulds DM. Tamsulosin: a review of its pharmacology and therapeutic efficacy in the management of lower urinary tract symptoms. Drugs Aging. 2002;19:135–61. doi: 10.2165/00002512-200219020-00004. [DOI] [PubMed] [Google Scholar]
- 2.Wilde MI, McTavish D. Tamsulosin. A review of its pharmacological properties and therapeutic potential in the management of symptomatic benign prostatic hyperplasia. Drugs. 1996;52:883–98. doi: 10.2165/00003495-199652060-00012. [DOI] [PubMed] [Google Scholar]
- 3.Shim SR, Kim JH, Chang IH, Shin IS, Hwang SD, Kim KH, et al. Is Tamsulosin 0.2 mg effective and safe as a first-line treatment compared with other alpha blockers?: a meta-analysis and a moderator focused study. Yonsei Med J. 2016;57:407–18. doi: 10.3349/ymj.2016.57.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shim SR, Kim JH, Choi H, Lee WJ, Kim HJ, Bae MY, et al. General effect of low-dose tamsulosin (0.2mg) as a first-line treatment for lower urinary tract symptoms associated with benign prostatic hyperplasia: a systematic review and meta-analysis. Curr Med Res Opin. 2015;31:353–65. doi: 10.1185/03007995.2014.980887. [DOI] [PubMed] [Google Scholar]
- 5.Kim KS, Kim JH, Lee SH, Ha US, Han DH, Chang IH, et al. Efficacy of dose escalation of tamsulosin for the treatment in symptomatic benign prostatic hyperplasia of lower urinary tract symptoms: a randomised, double-blind, phase 3 trial in Korean men. J Urol. 2016;195(4 Suppl):e466 [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in metaanalysis. In: Altman DG, Egger M, Smith GD, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group; 2001. pp. 285–312. [Google Scholar]
- 8.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordling J. Efficacy and safety of two doses (10 and 15 mg) of alfuzosin or tamsulosin (0.4 mg) once daily for treating symptomatic benign prostatic hyperplasia. BJU Int. 2005;95:1006–12. doi: 10.1111/j.1464-410X.2005.05456.x. [DOI] [PubMed] [Google Scholar]
- 10.Chapple CR, Montorsi F, Tammela TL, Wirth M, Koldewijn E, Fernández Fernández E, et al. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: results of an international, randomized, double-blind, placebo-and active-controlled clinical trial performed in Europe. Eur Urol. 2011;59:342–52. doi: 10.1016/j.eururo.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 11.Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol. 1998;160:1701–6. [PubMed] [Google Scholar]
- 12.Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. 2012;61:917–25. doi: 10.1016/j.eururo.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology. 1998;51:892–900. doi: 10.1016/s0090-4295(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 14.Kawabe K, Yoshida M, Homma Y, Silodosin Clinical Study Group Silodosin, a new alpha1A-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006;98:1019–24. doi: 10.1111/j.1464-410X.2006.06448.x. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama O, Yoshida M, Kim SC, Wang CJ, Imaoka T, Morisaki Y, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized placebo- and tamsulosin-controlled 12-week study in Asian men. Int J Urol. 2013;20:193–201. doi: 10.1111/j.1442-2042.2012.03130.x. [DOI] [PubMed] [Google Scholar]
- 16.Kawabe K, Ueno A, Takimoto Y, Aso Y, Kato H. Use of an alpha 1-blocker, YM617, in the treatment of benign prostatic hypertrophy. YM617 Clinical Study Group. J Urol. 1990;144:908–11. doi: 10.1016/s0022-5347(17)39620-9. [DOI] [PubMed] [Google Scholar]
- 17.Lee E, Lee C. Clinical comparison of selective and non-selective alpha 1A-adrenoreceptor antagonists in benign prostatic hyperplasia: studies on tamsulosin in a fixed dose and terazosin in increasing doses. Br J Urol. 1997;80:606–11. doi: 10.1046/j.1464-410x.1997.00411.x. [DOI] [PubMed] [Google Scholar]
- 18.Li NC, Chen S, Yang XH, Du LD, Wang JY, Na YQ, et al. Efficacy of low-dose tamsulosin in chinese patients with symptomatic benign prostatic hyperplasia. Clin Drug Investig. 2003;23:781–7. doi: 10.2165/00044011-200323120-00003. [DOI] [PubMed] [Google Scholar]
- 19.Park CH, Chang HS, Oh BR, Kim HJ, Sul CK, Chung SK, et al. Efficacy of low-dose tamsulosin on lower urinary tract symptoms suggestive of benign prostatic hyperplasia : a nonblind multicentre korean study. Clin Drug Investig. 2004;24:41–7. doi: 10.2165/00044011-200424010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36:1–13. doi: 10.1159/000019919. [DOI] [PubMed] [Google Scholar]
- 21.Kang DH, Lee JY, Chung JH, Cho HJ, Cho JM, Moon HS, et al. Analysis of prescriptions of alpha-blockers and phosphodiesterase 5 inhibitors from the urology department and other departments. Int Neurourol J. 2011;15:216–21. doi: 10.5213/inj.2011.15.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi H, Sim JS, Park JY, Bae JH. Assessment of tamsulosin 0.2 mg for symptomatic bladder outlet obstruction secondary to benign prostatic enlargement: data from a Korean multicenter cross-sectional study. Urol Int. 2015;95:50–5. doi: 10.1159/000366007. [DOI] [PubMed] [Google Scholar]
- 23.Kim JW, Oh MM, Yeo JK, Bae JH, Joo KJ, Choi JB, et al. Efficacy of dose escalation of tamsulosin for the treatment of lower urinary tract symptoms. Low Urin Tract Symptoms. 2012;4:96–102. doi: 10.1111/j.1757-5672.2012.00141.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim JJ, Han DH, Sung HH, Choo SH, Lee SW. Efficacy and tolerability of tamsulosin 0.4 mg in Asian patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia refractory to tamsulosin 0.2 mg: a randomized placebo controlled trial. Int J Urol. 2014;21:677–82. doi: 10.1111/iju.12412. [DOI] [PubMed] [Google Scholar]