Abstract
Context
Recent advances within the field of genetics are currently changing many of the methodologies in which medicine is practiced. These advances are also beginning to influence the manner in which physical therapy services are rendered. Rotator cuff pathology is one of the most common diagnoses treated by the sports physical therapist. The purpose of this commentary is to educate sports physical therapists on the recent advances regarding how genetics influences rotator cuff pathology, including rotator cuff tears, and provide a perspective on how this information will likely influence post-operative shoulder rehabilitation in the near future.
Evidence Acquisition
A comprehensive review of the literature was completed using the Medline database along with individual searches of relevant physical therapy, surgical, cell biology, and sports medicine journals. Search terms included: shoulder, rotator cuff pathology, genetics, apoptosis, and physical therapy. Search results were compiled and evaluated; relevant primary studies and review articles were gathered; the results from this comprehensive review are summarized here.
Study Design
Clinical Commentary, Review of the Literature
Results
Recent advances within the understanding of rotator cuff pathology have further elucidated the cellular and molecular mechanisms associated with rotator cuff tears. There appears to be a hypoxic-induced apoptotic cellular pathway that contributes to rotator cuff tears. Activation of specific proteins termed matrix metalloproteinases appear to be involved in not only primary rotator cuff tears, but also may influence the re-tear rate after surgical intervention. Further advancements in the understanding of the cellular mechanisms contributing to rotator cuff tears and postoperative techniques to help prevent re-tears, may soon influence the methodology in which physical therapy services are provided to patients sustaining a rotator cuff injury.
Conclusions
At this time continued research is required to more fully develop a comprehensive understanding of the role of genetic variables both within primary rotator cuff tears and their influences on post-operative rehabilitation from rotator cuff repair surgery.
Level of Evidence
Level 5
Keywords: Apoptosis, matrix metalloproteinases, post-operative rehabilitation, shoulder
INTRODUCTION
The power of genetics has already greatly altered the landscape in which medicine is practiced in many fields. An improved understanding of the influence of genetic variability among individuals is contributing to personalized medicine directed toward a patient's specific genetic profile. A specific example of these recent advancements is within pharmacogenomics where the ultimate goal is to design pharmaceuticals personalized to an individual's genetic make-up in order to improve outcomes and decrease risks.1 The utility of genetic information to personalize pharmacological interventions is logical, however, the impact of genetic information on the delivery of physical therapy services is a bit more challenging to conceptualize.
Improving the awareness of how the cellular and molecular processes influence tissue homeostasis can enhance a clinician's ability to deliver optimal care through the appropriate therapeutic intervention. The concept of how genetic information will influence the delivery of physical therapy interventions was highlighted in a review article within the December 2009 issue of PT in Motion Magazine.2 This review article summarized the opinions of several physical therapists regarding the impact of genetics on the field of physical therapy, and the necessity to expand the education of physical therapists to understand the implications of genetics for optimal patient care. These earlier predictions on the impact of genetics within the field of physical therapy seemed very futuristic, but more recent advances in both genetics and physical therapy are now making this relationship more of a reality than a theoretical dream.
The importance of genetic information and its influence on clinical decision-making for physical therapists was recently highlighted in a two part series on regenerative rehabilitation and genomics in Physical Therapy Journal in 2016.3-5 Norland et al, stressed the importance of staying abreast with medical advances, beginning with the initial coursework of graduate physical therapy programs.3 More importantly, their survey revealed that many academic programs have yet to adequately incorporate new medical knowledge and technology regarding regenerative medicine into their respective doctor of physical therapy (DPT) curriculum. As physical therapy education has transitioned to a doctorate degree, a greater understanding of the basic science of genetics, and its subsequent influence on a person's response to medical interventions is required. This dearth of fundamental genetic education within many DPT curriculums was also highlighted by Goldberg.6 This position paper emphasized the necessity of a physical therapist to understand the influence of genetic factors in maintenance of health and development of disease. Furthermore, the relevance of regenerative rehabilitation topics on the future of physical therapy practice was rated as being strongly relevant by 71.3% of polled DPT students and 67.3% of DPT faculty.3 Indeed, the need for the physical therapy profession to further advance its understanding of fundamental genetics and its influence on the development of disease was elucidated in an editorial by Ambrosio and Kleim, who proclaimed the ‘Genomic Era’ is already impacting the practice of physical therapy.4
Norland et al reported that it is likely many physical therapists are unaware of the influence of genetics on a patient's response to medical interventions.3 It is also likely that therapists may not recall the basic science behind genetics from the four-letter DNA code through translation to proteins that constitute the fundamental makeup of an individual. Curtis et al reviewed the basic science of genetics and its implications for physical therapists.5 Even the American Physical Therapy Association has embraced the genomic era by developing a webpage that provides information about the role of genetic technology within patient care.7 Certainly, providing a comprehensive review of the basic science of genetics and the overall influence on the field of physical therapy is beyond the scope of this manuscript. However, readers are encouraged to reference these two sources for an excellent overview of the basic concepts behind genetics.5,7
The purpose of this literature review and clinical commentary is to educate sports physical therapists on the recent advances regarding how genetics influences rotator cuff pathology, including rotator cuff tears, and provide a perspective on how this information will likely influence post-operative shoulder rehabilitation in the near future. Moreover, physical therapists are an integral part of the post-op recovery from a rotator cuff repair; therefore, it is important that physical therapists begin to understand how genetic variables can influence a patient's overall recovery.
GENETIC INFLUENCES ON ROTATOR CUFF PATHOLOGY
Family History of Rotator Cuff Tears
A complete understanding of the exact etiology of rotator cuff tears remains elusive, however, there are likely several factors, both genetic and environmental, contributing to these tears. It has previously been determined that there is an increased risk of rotator cuff pathology among first and second degree relatives.8 Harvey et al showed siblings of individuals diagnosed with a rotator cuff tear had more than twice the relative risk for developing a rotator cuff tear.9 This finding was also supported by Tashjian et al who reported relatives with similar genetic profiles are afflicted with rotator cuff tendinopathies at a higher rate.10 Interestingly, they also found spouses also display an increased prevalence of rotator cuff pathology, suggesting an ‘environmental’ factor may also be present, as an individual's spouse would have a dissimilar genetic profile, but likely engage in similar activities. The interplay of genetic predisposition and environmental factors appears to play a role in the development and progression of rotator cuff pathology.11 The exact genetic profile responsible for being more susceptible to a rotator cuff tear is not currently entirely understood, but researchers have discovered a collection of genes that contribute to rotator cuff pathology.12
Cellular Mechanisms Orchestrating Rotator Cuff Pathology
The confined position of the supraspinatus tendon within the subacromial space makes this tendon especially susceptible to degenerative changes. Impingement of the tendon has been suggested to cause mechanical damage and failure of the tendon.13,14 As noted previously by authors of the hereditary studies, mechanical strain is not exclusively responsible for the high prevalence of rotator cuff tears. Recent studies on rotator cuff tendinopathy reveal the process of ‘apoptosis’ is regularly involved in tendon degeneration.15-17
Apoptosis is a highly regulated cellular process during which cellular contents are recycled and remodeled.18 Apoptosis is an integral aspect of cellular homeostasis and directly influences important cellular processes such as embryonic development. For example, apoptosis is responsible for the coordinated elimination of tissue between fingers during the development of a defined hand within a vertebrate limb.18 Apoptosis not only occurs during development, but throughout the lifespan in order to promote tissue remodeling and turnover. Repressed apoptosis can result in pathology such as cancer where cellular tissue continues to expand unregulated since the processes of apoptosis are unable to effectively prevent continued expansion of a tumor.19 Conversely, excessive apoptosis can result in premature degradation of tissue such as that which occurs during autoimmune diseases.20 By comparison, excessive apoptosis within the rotator cuff tendon can alter the balance of normal tissue turnover and promote increased soft tissue degradation leading to tissue tearing. Yuan et al has shown an increased prevalence of apoptotic tissue within the edges of torn supraspinatus tissue compared to the control subscapularis tendon.21 Consequently, the management of apoptosis is highly regulated at the cellular level and is dependent on a variety of cellular signals to ensure the proper balance of apoptosis for normal tissue homeostasis.
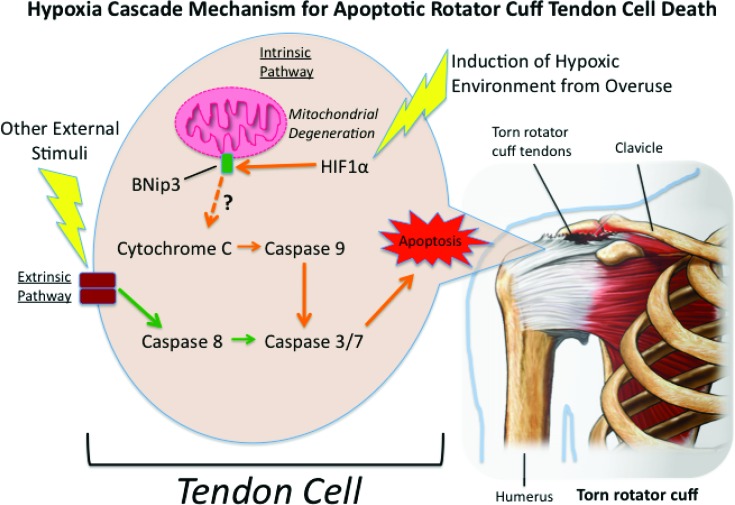
For example, the regulation of apoptosis is coordinated by cellular signaling proteins (such as cytochrome C) that are responsible for activating a family of protease enzymes called caspases (Figure 1). Caspases in turn promote the degradation of cellular contents during apoptosis.22 Individuals with a rotator cuff tear display increased expression of cytochrome C and caspase 3/7, 8 and 9 at not only the distal aspect of the tear, but also within regions more proximal to the tear.17 This expression response was not observed in control patients undergoing surgery for proximal humeral fractures. Millar et al also described that an increase in caspase expression was noted in both animal and human subjects with rotator cuff tears.23 They suggested that these cytokines may play a role in oxidative-stress induced apoptosis, however, the exact mechanisms and signaling that initiate the cascade toward apoptosis is not fully understood.
Figure 1.
Apoptotic cascade initiated by a hypoxic environment. Briefly, the regulation of apoptosis is coordinated by cellular signaling proteins starting with the initiation of HIF1 α. Once activated, HIF1 α is then believed to activate BNip3 (a Bcl-2 Nineteen kilodalton interacting protein) at the mitochondrial membrane. After the activation of BNip3, Cytochrome C is subsequently activated which in turn then promotes a pro-apoptotic response through the activation of caspases. However, the connection between BNip3 and Cytochrome C activation has not yet been fully elucidated. This apoptotic response is believed to promote the initiation and subsequent propagation of rotator cuff tears. Hypoxia Inducible Factor 1α (HIF 1α); Bcl-2 Nineteen kilodalton interacting protein (BNip3); (Picture of Torn Rotator cuff purchased from Nucleus Medical Art Inc / Alamy Stock Photo in accordance with their license agreement).
The poor blood supply to the distal supraspinatus tendon has long been established.24 As a consequence, it has been proposed that one possible mechanism of excessive apoptosis within the rotator cuff tendon may be due in part to poor vascularity, which may cause a hypoxia trigger creating a cascade of signals initiating apoptosis. For example, two proteins, Hypoxia Inducible Factor 1 α (HIF1 α) and Bcl-2 Nineteen kilodalton interacting protein (BNip3), have been shown to promote a pro-apoptotic response in cells (Figure 1).25 It has also been established that over-expression of HIF1 α, can cause a successive expression of BNip3.26 Thus it appears that HIF1 α is an upstream regulator of BNip3 as part of a signaling cascade that elicits apoptosis within a hypoxic environment. Downstream of these signaling proteins are the previously mentioned cytochrome C and caspases, which further propagate the cellular signaling associated with apoptosis within rotator cuff tissue.25 (Figure 1) However, the connection between BNip3 and pro-apoptotic pathway via a cytochrome C and caspase dependent pathway is still controversial.27,28
A study exploring the ‘hypoxia/cascade mechanism’ of apoptosis activation, investigated rotator cuff tissue from individuals experiencing various degrees of impingement, various stages of rotator cuff tears, and control individuals undergoing surgery for a shoulder stabilization procedure.29 Interestingly, Benson et al found that indeed a high level of expression of the protein, HIF1 α, could be observed in the partial, small, medium, large, and massive rotator cuff tears. Also, a concomitant increase in expression of the protein BNip3 in these tissues was seen, except for the massive tears, where BNip3 actually decreases. The reduction of BNip3 in the massive tears was hypothesized to be due to an adaptation of the remaining tenocytes into chondrocyte-like cells, which would tolerate the hypoxic environment better. No increased expression was noted within the control subjects. Interestingly, Benson et al also saw a spike in expression of HIF1 α in the mild impingement group without a subsequent increase in BNip3. This may be due to vascularity changes during the early stages of impingement and enough regulatory processes within the cell to inhibit the apoptotic pathway from progressing further. Consequently, this study supports the early initiation of conservative care such as physical therapy to help improve oxygen flow to the tissue and open the subacromial space through stretching of the pectoralis minor musculature and strengthening of the periscapular musculature.30,31 Early intervention of physical therapy may prevent the initiation of an ‘aggressive apoptosis cascade’ which could progress toward a full blown apoptotic response within the rotator cuff tissue.
Another component of apoptosis within the rotator cuff is the expression of proteins called matrix metalloproteinases (MMP). Once activated, these enzymes degrade all components of connective tissue.32 This enzymatic breakdown of connective tissue is an essential component to apoptosis, but is also deleterious to conservative care and post-operative healing of rotator cuff pathology.33 MMPs are a family of 23 proteins precisely regulated by endogenous inhibitors and induced by factors such as physical stress and cytokines.34,35 Therefore, the balance between suppression and induction of the MMPs can determine the overall level of degradation within the connective tissue extracellular matrix. Specifically regarding rotator cuff degeneration, multiple authors have described alterations in protein expression levels of MMP1, MMP2, and MMP3, with the majority of studies finding increased expression of MMP1 within torn supraspinatus tissue.36-38 Castagna et al found these increased enzyme levels not only in the region of the torn supraspinatus tissue, but also in intact portions of the medial supraspinatus and the subscapularis, which suggests a more global breakdown of tissue may be occurring.37 The global expression of MMPs within this study was suggested as a possible precursor for subsequent rotator cuff tearing. The exact role in which MMPs are regulated is not fully understood, but again oxidative stress is predicted to induce a signaling cascade.39 The contribution of MMPs in the degradation of rotator cuff tissue support the role of early intervention by physical therapist to promote an environment within the distal supraspinatus tendon to help prevent progression of this hypoxic induced apoptotic cascade.
Individual Differences in Cellular Driven Apoptotic Rotator Cuff Degeneration
With a more complete understanding of some of the cellular and molecular mechanisms contributing to rotator cuff pathology, the next question is whether there are unique differences within an individual's genetic makeup increasing propensity and /or risk for rotator cuff pathology. Genetic differences uniquely distinguish one individual from the next. The variability of these differences at the DNA level have been reported to be very small, approximately 0.1% between individuals.40,41 Although a small percentage, with the human genetic code being three billion letters long, these differences account for much of the variability among people. The variances in the genetic code between individuals are termed single nucleotide polymorphisms (SNPs).42,43 These SNPs can account for everything from a person's response to medication to their susceptibility to numerous medical conditions including, asthma, cancer and diabetes to name a few.44-48 Tashjian et al determined there are two SNPs in genes associated with apoptosis that may make an individual more susceptible to a rotator cuff tear.49 Their study involved genetic analysis of 311 subjects with a full thickness rotator cuff tear. Those with a partial thickness tear were excluded from the study. The subjects were compared to a control database of 3293 individuals. The results showed a statistically significant association of SNPs within the genes of SAP30BP and SASH1, both of which are associated with the apoptotic process. The authors concluded that alterations within these genes might promote increased protein activity, thus leading to a higher tendency of tissue breakdown and subsequent rotator cuff tears in individuals with these SNPs.
In summary, the cellular and molecular mechanisms involved with rotator cuff tears are both highly regulated and complex. Researchers are just beginning to understand the milieu of cellular signaling that induces apoptosis within the rotator cuff tissue contributing to an intrinsically driven process that promotes both the initiation and subsequent propagation of a rotator cuff tear.11,12 This understanding now has the potential to help implement specific interventions to prevent the initiation of a rotator cuff tear and also help advance treatment strategies to optimize post-operative outcomes.
GENETIC INFLUENCES ON POST-OPERATIVE ROTATOR CUFF REPAIR
A recent commentary by Dr. Theodore Blaine states: “the molecular therapeutics and targeted gene therapies are the new frontier in treatment of rotator cuff disease”.50 (e163) Several recent studies have investigated how the cellular processes regarding the intrinsic formation of rotator cuff tears can also influence the post-operative outcome of surgically repaired rotator cuff tissue.51-53
Tashjian et al recently identified a SNP within the estrogen-related receptor beta (ESRRB) gene that appears to promote increased susceptibility to re-tears after a rotator cuff repair.51 SNPs within ESRRB have previously been shown to correlate with increased prevalence of rotator cuff tearing.54-56 Indeed, the ESRRB protein is believed to promote increased HIF activity through an upregulation of HIF transcription.57 As noted previously, HIF activity is associated with the process of apoptosis. The Tashjian et al study examined 72 patients undergoing an arthroscopic repair of a full thickness rotator cuff tear. They then completed MRI analysis at least one year post-operatively and detected a 42% re-tear rate. The patients with a re-tear were found to display a statistically significant increased prevalence of a SNP within the ESRRB gene compared to patients that did not re-tear. Additionally, they did not find any difference in age, supraspinatus muscle quality, or single versus double row repair type in tears that healed or re-tore. This finding indicates that the genetic profile, i.e. presence or absence of the SNP within the ESRRB gene, may be a better predictor of future rotator cuff post-operative re-tearing than some of the traditional factors such as muscle quality or age.
The negative implications of increased MMP protein expression on the integrity of rotator cuff tissue has been established.33,36-38 For example, Gotoh et al investigated the presence of MMP gene expression during rotator cuff repairs by harvesting a marginal section of the torn tendon and analyzing expression activity of various MMPs and MMP inhibitors.52 Twenty-four patients were included in the study and repeat imaging was completed at greater than one year post-op and revealed that six patients experienced a re-tear. Within the patients with a re-tear they found a statistically significant increase in MMP3 gene expression compared to individuals within the study that did not experience a re-tear. One limitation to this study was that they were measuring gene expression and not actual protein activity. Another limitation noted is the patients that experienced re-tear also demonstrated an increased duration of time from injury to surgical intervention compared to the group that did not display a re-tear. This finding emphasizes a potential urgency of surgical intervention following the initial injury. Furthermore, if there is such a delay, then it seems as if the expression of MMP3 may need to be monitored to help predict patients that may re-tear.
Robertson et al examined 30 patients with a supraspinatus tear in a similar study, with the tear being classified as full thickness or high-grade partial-thickness (>80% torn), and massive tears were excluded.53 At the time of surgery, a tissue sample of the torn supraspinatus was harvested along with a sample of the subscapularis to be used as a control. Several genes involved in inflammation and tendon degradation were then analyzed for expression levels in the harvested tissues. At greater than six months post-op, ultrasound was used to detect any re-tears and seven patients were found to have re-tears. Within this study, they found an increase in MMP1 and MMP9 gene expression within the patients that re-tore, compared to the group that displayed good healing. Unlike the Gotoh et al study, no difference in duration of symptoms was noted between the defect group and the healed group. Interestingly, they also found increased MMP9 gene expression within the healthy subscapularis tendon of the re-tear group which was not found in the healed group. MMP3 gene expression was not examined within this study.
From these three studies,51-53 it can be concluded that it may be possible to predict which patients will respond well to rotator cuff repairs and which patients will face an increased risk of re-tear based on genetic profiles and from tissue samples. This information has led several researchers to investigate if exogenous inhibitors of MMPs applied during tendon repairs in an animal model could improve repair strength. One study utilized the MMP inhibitor, α-2-macroglobulin protein, and applied this protein to the tendon-bone interface during a rotator cuff repair in rats. Increased collagen organization and reduction in collagen degradation was noted at two and four-weeks compared to the control group where this inhibitor was not utilized.58 The antibiotic doxycycline is another inhibitor of MMPs and an animal study by Pasternak et al found that rat Achilles tendons repaired with doxycycline coated sutures resulted in improved suture-holding capacity compared to a control group with uncoated sutures.59 Furthermore, rats who underwent a detachment and immediate repair of the supraspinatus displayed improved healing enthesis when started on oral doxycycline preoperatively or at post-operative day five compared to control animals where doxycycline was not administered.60 This study also found a reduction of MMP13 protein activity at post-operative day eight in the doxycycline treated rats compared to controls, suggesting that inhibition of this MMP promoted aspects of improved rotator cuff healing. At this time MMP inhibitors have not been investigated in human subjects for surgical repair of the rotator cuff.
Ling et al took an alternative approach by not examining the expression level of the MMP3 gene, but rather investigated the presences of SNPs within the MMP3 gene and also interleukin 6(IL-6) gene.61 IL-6 is an inflammatory cytokine, which has been associated with the presence of subacromial bursitis.62,63 In contrast to previous studies, they focused on the influence of SNPs within these two genes on post-operative stiffness rather than the rate of post-operative re-tearing. Ling et al examined 188 patients undergoing a mini-open rotator cuff repair and found the presence of SNPs within both IL-6 and MMP3 genes that significantly correlated with increased post-operative stiffness. These SNPs may result in different isoforms of the MMP3 protein, where a structurally similar protein from the same gene may display slightly different functions between people based on the SNP that is present. The authors suggest the presence of these SNPs in a post-operative patient may justify a more aggressive post-operative rehabilitation approach to help prevent excessive post-operative stiffness. This study contrasts previous reports that indicate excessive expression of the MMP3 gene may result in an increased presence of re-tear rates in individuals having a rotator cuff repair. Therefore, one can conclude from this study that not only the expression level, but also the unique isoform of the MMP protein may need to be examined to determine if a patient can be categorized into an increased risk of re-tear or post-operative stiffness susceptible group. The SNP within the MMP3 gene of this recent study may have influenced the activity of the MMP3 protein, thus preventing normal collagen remodeling, resulting in excessive collagen deposition and subsequent stiffness. The role of this SNP on MMP3 protein function was not explored within this study, therefore, the cause of the increased tightness within patients displaying this SNP remains hypothetical. Ling et al highlight how further research is required to more fully elucidate how the complex interplay between these numerous proteins can influence a patient's post-operative recovery and return to a high level of function.
CLINICAL REHABILITATION IMPLICATIONS
It is not understood whether changing the biological environment by improving vascularity or alleviating mechanical stress has any effect on the apoptotic cascade once it is initiated. However, the authors argue in favor of utilizing physical therapy interventions to address impairments contributing to rotator cuff impingement. This includes, but is not limited to: thoracic mobilizations, capsular stretching/mobilizations of the shoulder girdle joints, scapular stabilization exercises, and rotator cuff strengthening.
Kokmeyer et al outlined several rotator cuff repair prognostic indicators and recommended post-operative protocols based on several key factors, however the inclusion of genetic factors was not considered with these initial recommendations.64 The future of sports medicine is to consider these genetic factors when choosing a rehabilitation protocol (Table 1). It is not known if altering a rotator cuff repair protocol based on genetic factors will ultimately improve surgical outcomes, but we advocate that it is reasonable to modify the rehabilitation progression in light of the patient's unique genetic presentation (Table 2). Complications following arthroscopic rotator cuff repair are common. One of the most common complications is post-operative stiffness.65-67 Huberty et al outlined several risk factors for developing post-operative stiffness 68 (Table 3). The presence of SNPs within MMP3 and IL-6 genes could be added to this list of risk factors. Meijden et al presented evidence-based guidelines for rehabilitation following arthroscopic rotator cuff repair.69 The “moderate” rehabilitation protocol is designed for the young patient with good tissue quality or a small tear. The moderate protocol calls for PROM to begin immediately without restrictions. Koo et al presented a modified accelerated rehabilitation program beginning with active assisted table slides immediately.70 They stated that this modification helped to keep the rate of stiffness low (<1%) in the high-risk group of patients. Uhl et al showed the prayer stretch position used to regain forward elevation ROM has minimal supraspinatus and infraspinatus activation, with only 2-10% maximum voluntary isometric contraction (MVIC).71 Adding table slides and/prayer stretch immediately in the group with high risk of developing post-operative stiffness, including those with SNPs within the MMP3 and IL-6 genes, is recommended by the authors of this clinical commentary to avoid this complication.
Table 1.
Rotator Cuff Repair Rehabilitation Protocols Classified by Prognostic Factors
| Moderate | Intermediate | Conservative | |
|---|---|---|---|
| Age | < 50 | 50-60 | >60 |
| Bone Mass Density | > -1 | −2.4 to −1 (osteopenia) | <−2.5 (osteoporosis) |
| Fatty Infiltrate, Atrophy | Stage 0 | Stage 0-1 | Stage 1-2 |
| Diabetes Mellitus | + | + | - |
| Body Mass Index | <25 | 25-30 | >30 |
| Smoker | - | - | + |
| Tear Size | Partial Thickness - Small | Small - Medium | Large - Massive |
| Retraction | None | In-between | >Glenoid |
| Tissue Quality | Good | Fair | Poor |
| Pre-Op Strength | Good | Fair | Poor |
| SNPs in IL-6 and/or MMP3 | Present | Absent | Absent |
| SNPs in SAP30BP, SASH1 and/or ESRRB | Absent | Absent | Present |
| Increased expression of MMP 1, 3, 9 | Absent | Absent | Present |
Adapted from Kokmeyer's Prognostic Spectrum.58
SNP=single nucleotide polymorphism; MMP=matrix metalloproteinase; IL-6=interleukin 6 gene; SAP30BP=30kDa Sin3-associated binding protein gene; SASH1=sterile alpha motif and sarcoma homologous 3 domain-containing protein 1 gene; ESRRB=estrogen-related receptor beta gene
Table 2.
Rotator Cuff Repair Prognosis-Based Rehabilitation Protocols
| Moderate | Intermediate | Conservative | |
|---|---|---|---|
| Sling | 0-2 weeks | 4-6 weeks | 6 + weeks |
| PROM | Begin immediately Full PROM | Begin 0-4 weeks 30 ER, 90 Abd, 120 FE Full PROM 4-6 weeks | Begin 4-6 weeks 30 ER, 90 Abd, 120 FE Full PROM 6-8 weeks |
| AAROM | 0-2 weeks | 4 weeks | 6 weeks |
| AROM | 0-2 weeks | 4-6 weeks | 6-8 weeks |
| Strengthening | 4-6 weeks | 8-10 weeks | 10-12 weeks |
Adapted from Kokmeyer's Prognosis-based Rehabilitation.58
PROM=passive range of motion; AAROM=active assistive range of motion; AROM=active range of motion; ER=external rotation; Abd=abduction; FE=forward elevation
Table 3.
Post-Operative Stiffness Risk Factors
|
|
Possible genetic risk factors:
|
|
Adapted from Huberty et al.62
PASTA=partial articular supraspinatus tendon avulsion; SNP=single nucleotide polymorphism; IL-6=interleukin 6 gene; MMP3=matrix metalloproteinase 3
Another common complication of arthroscopic rotator cuff repair is re-tear of the repaired tendons. Galatz et al report re-tear rates as high as 94%.72 Thomazeau et al state that, in the presence of rotator cuff atrophy, recurrent rotator cuff tears occur in 25% of patients.73 Koo et al report those with a large tear (>5cm) or involving more than two tendons also have an elevated risk of re-tear.70 Patients with increased MMP gene expression are at a higher risk for recurrent tear of the repaired rotator cuff. Meijden et al outline a conservative protocol for the older patient with poor tissue quality or large tears.69 Their protocol outlines the patient wears a sling for six to seven weeks with PROM beginning at week three. PROM in the conservative protocol is restricted to 120 degrees of flexion, 30 degrees of external rotation, internal rotation to the belly and 90 degrees of abduction until week five. AROM is delayed until week six to seven in the conservative protocol. There are several genetic risk factors for recurrent tear following a rotator cuff repair (Table 4). When a dysfunctional apoptotic cascade is present, such as increased expression of MMP1, 3, 9 genes or the presence of SNPs within SAP30BP, SASH1 or ESRRB genes, the authors of this commentary recommend following the conservative protocol. Dockery et al investigated seven passive shoulder motion modes and found CPM, Codman's and therapist-assisted PROM, generated the lowest percentage of MVIC of rotator cuff activity.74 Therefore, such exercises or mobility interventions are recommended for the PROM stage. At six weeks post-surgery AAROM should begin progressing slowly to AROM. This progression should account for progressive increases in rotator cuff activation. Wise et al demonstrated vertical wall slides have relatively low rotator cuff activation (8-13%) whereas unsupported vertical slides increases cuff activation (10-17%).75 Therefore, considering not only the rate of exercise progression, but the selection of exercise within the post-operative rehabilitation process, is vital to help prevent re-tearing in patients that may be susceptible to re-tearing due to their specific genetic profile.
Table 4.
Possible Post-Operative Re-Tear Genetic Risk Factors
Increased expression of the following MMPs:
|
|
Presence of SNPs in apoptotic genes:
| |
Possible prophylactic interventions:
|
MMP=matrix metalloproteinase; SNP=single nucleotide polymorphism; SAP30BP=30kDa Sin3-associated binding protein gene; SASH1=sterile alpha motif and sarcoma homologous 3 domain-containing protein 1 gene; ESRRB=estrogen-related receptor beta gene
CONCLUSION
The techniques used to repair torn rotator cuff tendons has evolved immensely since Karl Hüter performed the first rotator cuff repair in 1870.76 The innovation of utilizing arthroscopic techniques on the shoulder in the 1980s was a major breakthrough in the advancement in addressing shoulder pathology and more specifically rotator cuff tears. The next such innovation appears to be within the realm of utilization of genomic information to improve outcomes.
One concern about utilization of genetic information is the potential increased cost associated with analyzing a patient's genetic profile and gene expression levels during rotator cuff repair surgery. With exploding healthcare costs, this is a very real concern. However, as the understanding of the cellular processes associated with rotator cuff tears has advanced, so has the technology associated with sequencing DNA samples. Recent advances have reduced the cost of sequencing a million base pairs of DNA from thousands of dollars to mere cents.77 Genomic sequencing has become such commonplace that individuals now have the liberty of examining part of their genetic profile at home through kits available from various websites for a moderate fee.78,79 If individuals are willing to pay for genetic information from these sites out of curiosity, they would also likely be willing to accept extra expenses associated with this analysis during a rotator cuff repair surgery. Subsequently, if the utilization of this genetic information allows a physician and therapist to categorize a patient into a particular treatment group to optimize their outcome, and potentially prevent a re-tear with subsequent revision surgery, this will likely outweigh the extra costs and time associated with this analysis. However, further research is required to determine any potential clinical impact of patient stratification based on their genetic profile, prior to routinely utilizing this technology in the care of patients with rotator cuff tears.
It is clear there are numerous genetic factors that influence normal cell homeostasis within the rotator cuff tendon. These factors also seem to have an influence on post-operative repair of torn rotator cuff tendons. A comprehensive understanding of the complex interplay of all of the proteins that influence both rotator cuff tears and subsequent healing is not yet fully understood, but research is rapidly advancing the understanding of the regulation of this process. With rotator cuff repair re-tear rates being reported from 10% to as high as 94%,72.73,80,81 it will be important to fully understand how the genetic influences of rotator cuff healing can facilitate a positive post-operative outcome following a rotator cuff repair. Understanding this information will be important for physical therapists as patients may soon be categorized into various treatment groups based on their genetic profile, such as an aggressive stretching group based on presence of IL6 and MMP3 SNPs, or a more conservative group based on increased gene expression levels of MMP1, 3, 9 or the presence of SNPs within SAP30BP, SASH1 or ESRRB genes found at the time of repair. Using genetic information may optimize selection of treatment interventions to promote superior patient outcomes.
Consequently, sports physical therapists should stay abreast of recent advancements within the medical field and be willing to utilize these new discoveries to create practices that promote improved post-operative rotator cuff repair outcomes. The emphasis on evidence-based practice has prompted sports physical therapist to routinely utilize advances in knowledge on surgical techniques and exercise prescription to help optimize patient outcomes. It is clearly evident that the ‘Genomic Era’ has arrived and it is crucial that sports physical therapists are ready to embrace a new framework for understanding not only the structural aspects of a rotator cuff repair, but also the cellular mechanisms that facilitate a successful surgical outcome. Adopting this knowledge into routine patient care may optimize post-operative outcomes and help substantially reduce post-operative re-tear rates.
REFERENCES
- 1.Shabaruddin FH, Fleeman DN, Payne K. Economic evaluations of personalized medicine: existing challenges and current developments. Pharmgenomics Pers Med. 2015;8:115-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ries E. Rooting out genetic links: advances in genetics research have profound implications for patient/client management, creating an educational need. PT in Motion. 2009;1(3):18-23. [Google Scholar]
- 3.Norland R, Muchnick M, Harmon Z, et al. Opportunities for regenerative rehabilitation and advanced technologies in physical therapy: perspective from academia. Phys Ther. 2016;96(4): 550-7. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosio F, Kleim JA. Regenerative rehabilitation and genomics: frontiers in clinical practice. Phys Ther. 2016;96(4):430-2. [DOI] [PubMed] [Google Scholar]
- 5.Curtis CL, Goldberg A, Kleim JA, et al. Translating genomic advances to physical therapists practice: a closer look at the nature and nurture of common diseases. Phys Ther. 2016;96(4):570-80. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg A. Genetics education for the physical therapy profession. J Phys Ther Educ. 2005;19(1):8-14. [Google Scholar]
- 7.American Physical Therapy Association. Genetics website: http://www.apta.org/Genetics/. Updated July 29, 2014. Accessed December 20, 2016.
- 8.Tashjian RZ, Farnham JM, Albright FS, et al. Evidence for an inherited predisposition contributing to the risk for rotator cuff disease. J Bone Joint Surg Am. 2009; 91(5):1136-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvie P, Ostlere SJ, Teh J et al. Genetic influence in the aetiology of tears of the rotator cuff. Sibling risk of a full-thickness tear. J Bone Joint Surg Br. 2004;86(5):696-700. [DOI] [PubMed] [Google Scholar]
- 10.Tashjian RZ, Farnham JM, Granger EK, et al. Evidence for an environmental and inherited predisposition contributing to the risk for global tendonopathies or compression neuropathies in patients with rotator cuff tears. Ortho J Sports Med. 2016;12(4):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nho S, Yadav H, Shindle M K. et al. Basic science update: rotator cuff degeneration: etiology and pathogenesis. Amer J Sports Med. 2008;36(5):987-93. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhury S, Carr AJ. Lessons we can learn from gene expression patterns in rotator cuff tears and tendinopathies. J Shoulder Elbow Surg. 2012;21(2)191-99. [DOI] [PubMed] [Google Scholar]
- 13.Mayerhoefer ME, Breitenseher MJ, Wuring C, et al. Shoulder impingement: relationship of clinical symptoms and imaging criteria. Clin J Sport Med. 2009;19(2):83-9. [DOI] [PubMed] [Google Scholar]
- 14.Natsis K, Tsikaras P, Totlis T, et al. Correlation between the four types of acromion and the existence of enthesophytes: a study on 423 dried scapulas and review of the literature. Clin Anat. 2007;20(3):267-72. [DOI] [PubMed] [Google Scholar]
- 15.Yuan J, Wang MX, Murrell GA. Cell death and tendinopathy. Clin Sports Med. 2003;22(4):693-701. [DOI] [PubMed] [Google Scholar]
- 16.Maffulli N, Longo UG, Berton A, et al. Biological factors in the pathogenesis of rotator cuff tears. Sports Med Arthrosc Rev. 2011;19(3):194-201. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Kim YS, Ok JH, et al. Apoptosis occurs throughout the diseased rotator cuff. Am J Sports Med. 2013;41(10):2249-55. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Garijo A, Steller H. Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease. Development. 2015;142(19):3253-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez J, Tait SW. Mitochondrial apoptosis: killing cancer using the enemy within. Br J Cancer. 2015;112(6):957-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn A, Wenzel J, Weyd H. Photosensitivity, apoptosis, and cytokines in the pathogenesis of lupus erythematosus: a critical review. Clin Rev Allergy Immunol. 2014; 47(2):148-62. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J, Murrell GA, Wei AQ, et al. Apoptosis in rotator cuff tendonopathy. J Orthop Res. 2002;20(6):1372-9. [DOI] [PubMed] [Google Scholar]
- 22.Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspases family proteinases. J Biol Chem. 1999;274(29);20049-52. [DOI] [PubMed] [Google Scholar]
- 23.Millar NL, Wei AQ, Molloy TJ, et al. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg Br. 2009;91(3):417-24. [DOI] [PubMed] [Google Scholar]
- 24.Rathbun JB, Macnab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg Br. 1970;52(3):540-53. [PubMed] [Google Scholar]
- 25.Vasagiri N, Kutala VK. Structure, function, and epigenetic regulation of BNIP3: a pathophysiological relevance. Mol Biol Rep. 2014;41(11):7705-14. [DOI] [PubMed] [Google Scholar]
- 26.Guo K, Searfoss G, Krolikowski D, et al. Hypoxia induces the expression of the pro apoptotic gene BNIP3. Cell Death Differ. 2001;8(4)367-76. [DOI] [PubMed] [Google Scholar]
- 27.Vande Velde C, Cizeau J, Dubik D et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20(15):5454-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JY, Cho JJ, Ha J, et al. The carboxy terminal C-tail of BNip3 is crucial in induction of mitochondrial permeability transition in isolated mitochondria. Arch Biochem Biophys. 2002;398(2):147-52. [DOI] [PubMed] [Google Scholar]
- 29.Benson RT, McDonnell SM, Knowles HJ, Rees et al. Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. J Bone Joint Surg Br. 2010; 92(3):448-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hegedus EJ, Cook C, Brennan M, et al. Vascularity and tendon pathology in the rotator cuff: a review of literature and implications for rehabilitation and surgery. Br J Sports Med. 2010;44(12):83-47. [DOI] [PubMed] [Google Scholar]
- 31.Page P. Shoulder muscle imbalance and subacromial impingement syndrome in overhead athletes. Int J Sports Phys Ther. 2011;6(1):51-8. [PMC free article] [PubMed] [Google Scholar]
- 32.Birkedal-Hansen H, Moore WGI, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197-250. [DOI] [PubMed] [Google Scholar]
- 33.Del Buono A, Oliva F, Longo UG, et al. Metalloproteases and rotator cuff disease. Shoulder Elbow Surg. 2012; 21(2): 200-8. [DOI] [PubMed] [Google Scholar]
- 34.Nagase H, Woessner JF. Matrix Metalloproteinases. J Biol Chem. 1999;274(31):21491-94. [DOI] [PubMed] [Google Scholar]
- 35.Birkedal-Hansen H, Yamada S, Winsor J, et al. Matrix metalloproteinases. Curr Protoc Cell Biol. 2008:Chapter 10 Unit 10.8. [DOI] [PubMed] [Google Scholar]
- 36.Riley GP, Curry V, DeGroot J, et al. Matrix metalloproteinase activities and their relationship with collage remodeling in tendon pathology. Matrix Biol. 2002;21(2):185-95. [DOI] [PubMed] [Google Scholar]
- 37.Castagna A, Cesari E, Garofalo R. Matrix metalloproteases and their inhibitors are altered in torn rotator cuff tendons, but also in the marcoscopically and histologically intact portion of those tendons. Muscles Ligaments Tendons J. 2013;3(3):132-8. [PMC free article] [PubMed] [Google Scholar]
- 38.Choo A, McCarthy M, Pichika R, et al. Muscle gene expression patterns in human rotator cuff pathology. J Bone Joint Surg Am. 2014;96(18):1558-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Longo UG, Berton A, Papapietro N, et al. Epidemiology, genetic and biological factors of rotator cuff tears. Med Sport Sci. 2012;57:1-9. [DOI] [PubMed] [Google Scholar]
- 40.Jorde LB, Wooding SP. Genetic variation, classification and ‘race’. Nat Genet. 2004;36 (11 Suppl):S28-33. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg NA, Pritchard JK, Weber JL et al. Genetic structure of human populations. Science. 2002;298(5602):2381-85. [DOI] [PubMed] [Google Scholar]
- 42.Katsonis P, Koire A, Wilson SJ, et al. Single nucleotide variations: biological impact and theoretical interpretation. Protein Sci. 2014; 23(12):1650-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patnala R, Clements J, Batra J. Candidate gene association studies: a comprehensive guide to useful in silico tools. BMC Genet. 2013;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katara P. Single nucleotide polymorphism and its dynamics for pharmacogenomics. Interdiscip Sci. 2014;6(2):85-92. [DOI] [PubMed] [Google Scholar]
- 45.Tizaoui K, Kaabachi W, Hamzaoui K, et al. Association of single nucleotide polymorphisms in toll-like receptor genes with asthma risk: a systematic review and meta-analysis. Allergy Asthma Imunol Res. 2015;7(2):130-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maxwell KN, Nathanson KL. Common breast cancer risk variants in the post-COGS era: a comprehensive review. Breast Cancer Res. 2013;15(6):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qui CJ, Ye XZ, Yu XJ, et al. Association between FABP2 Ala54Thr polymorphisms and type 2 diabetes mellitus risk: a HuGE review and meta-analysis. J Cell Mol Med. 2014;18(12):2530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stankov K, Denc D, Draskovic D. Genetic and epigenetic factors in etiology of diabetes mellitus type 1. Pediatrics. 2013;132(6):1112-22. [DOI] [PubMed] [Google Scholar]
- 49.Tashjian RZ, Granger EK, Farnham JM, et al. Genome wide association study for rotator cuff tears identifies two significant single nucleotide polymorphisms. J Shoulder Elbow Surg. 2016;25(2):174-9. [DOI] [PubMed] [Google Scholar]
- 50.Blaine TA, Basic training in shoulder surgery: investigating molecular mechanism of rotator cuff disease. J Bone Joint Surg Am. 2014;96(18):e163(1-2). [DOI] [PubMed] [Google Scholar]
- 51.Tashjian RZ, Granger EK, Zhang Y, et al. Identification of a genetic variant with rotator cuff repair healing. J Shoulder Elbow Surg. 2016;25(6):865-72. [DOI] [PubMed] [Google Scholar]
- 52.Gotoh M, Mitsui Y, Shibata H, et al. Increased matrix metalloprotease-3 gene expression in ruptured rotator cuff tendons is associated with postoperative tendon retear. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1807-12. [DOI] [PubMed] [Google Scholar]
- 53.Robertson CM, Chen CT, Shindle MK, et al. Failed healing of rotator cuff repair correlates with altered collagenase and gelatinase in supraspinatus and subscapularis tendons. Am J Sports Med. 2012;40(9):1993-2001. [DOI] [PubMed] [Google Scholar]
- 54.Bonato LL, Quinelato V, Pinheiro AR, et al. ESRRB polymorphisms are associated with comorbidity of temporomandibular disorders and rotator cuff disease. Int J Oral Maxillofac Surg. 2016;45(3):323-31. [DOI] [PubMed] [Google Scholar]
- 55.Motta GR, Amaral MV, Rezende E, et al. Evidence of genetic variations associated with rotator cuff disease. J Shoulder Elbow Surg. 2014;23(2):227-35. [DOI] [PubMed] [Google Scholar]
- 56.Teerlink CC, Cannon-Albright LA, Tashjian RZ. Significant association of full-thickness rotator cuff tears and estrogen-related receptor-ß (ESRRB). J Shoulder Elbow Surg. 2015;24(2):e31-5. [DOI] [PubMed] [Google Scholar]
- 57.Ao A, Wang H, Kamarajugadda S, et al. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc Natl Acad Sci USA 2008;105(22):7821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bedi A, Kovacevic D, Hettrich C, et al. The effect of matrix metalloproteinase inhibition on tendon-to-boan healing in a rotator cuff repair model. J Shoulder Elbow Surg. 2010; 19(3):384-91. [DOI] [PubMed] [Google Scholar]
- 59.Pasternak B, Missios A, Askendal A, et al. Doxycycline-coated sutures improve the suture-holding capacity of the rat Achilles tendon. Act Orthopaedica. 2007;78(5):680-6. [DOI] [PubMed] [Google Scholar]
- 60.Bedi A, Fox AJ, Kovacevic D, et al. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010; 38(2):308-17. [DOI] [PubMed] [Google Scholar]
- 61.Ling Y, Peng C, Liu C, et al. Gene polymorphism of IL-6 and MMP-3 decreases passive range of motion after rotator cuff repair. Int J Clin Exp Pathol. 2015;8(5):5709-14. [PMC free article] [PubMed] [Google Scholar]
- 62.Rossi JF, Lu ZY, Jourdan M, et al. Interleukin-6 as a therapeutic target. Clin Cancer Res. 2015;21(6):1248-57. [DOI] [PubMed] [Google Scholar]
- 63.Blaine TA, Kim YS, Voloshin I, et al. The molecular pathophysiology of subacromial bursitis in rotator cuff disease. J Shoulder Elbow Surg. 2005;14:84S-89S. [DOI] [PubMed] [Google Scholar]
- 64.Kokmeyer D, Dube E, Millett PJ. Prognosis driven rehabilitation after rotator cuff repair surgery. Open Orthop J. 2016;10:339-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Severud EL, Ruotolo C, Abbott DD, et al. All-arthroscopic versus mini-open rotator cuff repair: a long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234-8. [DOI] [PubMed] [Google Scholar]
- 66.Flurin PH, Landreau P, Gregory T, et al. Arthroscopic repair of full-thickness cuff tears: a multicentric retrospective study of 576 cases with anatomical assessment. Rev Chir Orthop Reparatrice Appar Mot. 2005;91(S8):31-42. [DOI] [PubMed] [Google Scholar]
- 67.Tauro JC. Stiffness and rotator cuff tears: incidence, arthroscopic findings, and treatment results. Arthroscopy. 2006;22(6):581-6. [DOI] [PubMed] [Google Scholar]
- 68.Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-90. [DOI] [PubMed] [Google Scholar]
- 69.Meijden OA, Westgard P, Chandler Z, et al. Rehabilitation after arthroscopic rotator cuff repair: current concepts review and evidence-based guidelines. Int J Sports Phys Ther. 2012;7(2):197-218. [PMC free article] [PubMed] [Google Scholar]
- 70.Koo SS, Burkhart SS. Rehabilitation following arthroscopic rotator cuff repair. Clin Sports Med. 2010;29(2):203-211. [DOI] [PubMed] [Google Scholar]
- 71.Uhl TL, Carver TJ, Mattacola CG, et al. Shoulder muscle activation during upper extremity weight-bearing exercise. J Orthop Sports Phys Ther. 2003;33(3):109-17. [DOI] [PubMed] [Google Scholar]
- 72.Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-24. [DOI] [PubMed] [Google Scholar]
- 73.Thomazeau H, Boukobza E, Morcet N, et al. Predication of rotator cuff repair results by magnetic resonance imaging. Clin Orthop Relat Res. 1997;344:275-83. [PubMed] [Google Scholar]
- 74.Dockery ML, Wright TW, LaStayo PC. Electromyography of the shoulder: an analysis of passive modes of exercise. Orthopedics. 1998;21(11):1181-4. [DOI] [PubMed] [Google Scholar]
- 75.Wise MB, Uhl TL, Mattacola CG, et al. The effect of limb support on muscle activation during shoulder exercises. J Shoulder Elbow Surg. 2004;13(6):614-20. [DOI] [PubMed] [Google Scholar]
- 76.Randelli P, Cucchi D, Ragone V, et al. History of rotator cuff surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(2):344-62. [DOI] [PubMed] [Google Scholar]
- 77.Shendure J, Aiden EL. The expanding scope of DNA sequencing. Nat Biotechnol. 2012;30(11);1084-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ancestry DNA. The leader in DNA testing for family history website. https://www.ancestry.com/dna/ 1997-2016. Accessed December 4, 2016.
- 79.23andMe website. http://www.23andme.com. Copyright 2016. Accessed December 4, 2016.
- 80.Ahmad S, Haber M, Bokor D. The influence of intraoperative factors and postoperative rehabilitation compliance on the integrity of the rotator cuff after arthroscopic repair. J Shoulder Elbow Surg. 2015; 24(2):229-35. [DOI] [PubMed] [Google Scholar]
- 81.Abtahi AM, Granger EK, Tasjian RT. Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop 2015; 6(2):211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]