Abstract
Objective:
Shift workers have metabolic changes more often than day workers. It is also known that night workers prefer foods high in saturated fat. Such data suggest that shift workers are prone to cardiovascular disease. Therefore, the objective of this study was to propose an animal model to test the effect of high-fat diet (HFD) based on shift workers' diet.
Methods:
This is an experimental study with 20 Wistar rats. Ten rats were allocated to the control group (CG) and were fed standard diet. Ten rats were allocated to the experimental group (EG) and were fed HFD (45% fat). Serum triglycerides (TG), glucose and high-density lipoprotein-cholesterol (HDL-cho) were measured 5, 10 and 15 weeks after the beginning of the study. The amount of visceral adipose tissue (VAT) was determined. Body weight was assessed weekly, and food and water intake were measured daily. Student's t-test was used for independent samples, and P<0.05 was considered significant.
Results:
After 15 weeks of intervention, the EG showed increased serum levels of TG (P=0.001) and glucose (P<0.001) and decreased HDL-cho (P<0.001) when compared with the CG. The EG showed increased VAT (P=0.005) and liver weight (P=0.01). Food intake and water intake were higher in the CG (P<0.001 and P<0.001, respectively), whereas energy intake showed no difference (P=0.48). No difference was found in the weight of adrenal glands (P=0.07) and body weight (P=0.63).
Conclusions:
The experimental diet was effective to show changes in the serum levels of glucose, TG and HDL-cho and visceral fat in spite of no change in body weight in 15 weeks.
Introduction
Over the past few years, there has been a significant increase in the prevalence of nutrition-related diseases, such as obesity, diabetes and cardiovascular diseases. Diet quality may be one of the most important factors associated with hyperglycaemia, hypertriglyceridaemia, hypertension, decreased high-density lipoprotein-cholesterol (HDL-cho) and increased abdominal circumference.
Shift workers are particularly affected by inadequate quality of diet, mainly night workers who often have hypertriglyceridaemia and hyperglycaemia, as well as lower levels of HDL-cho when compared with day workers.1, 2, 3, 4
It is known that night workers, at work, prefer cold snacks containing high concentrations of saturated fat instead of hot food. This population usually keeps this pattern also during the period they are not working.5, 6, 7 Their diet also have several eating events per day,8 including high consumption of fat,9, 10 especially saturated fat.6
It is difficult to distinguish factors related to chronic diseases in human beings; therefore, experimental studies have tried to develop a standardized diet for animal models. Studies with high-fat diets (HFDs) have been conducted in animals, especially in mice and rats, to induce metabolic changes. Some of these studies have linked an HFD with hyperphagia, weight gain, increased adiposity and suppression of hepatic glucose production stimulated by insulin.11, 12, 13 Such conditions may lead to hyperinsulinemia and insulin resistance. These diet models often include 15–65% of fat from different sources (soybean oil, coconut oil, olive oil, fish oil, lard and vegetable shortening, with lard and vegetable shortening being commonly used by food industry) and different times of study (3–32 weeks).14, 15, 16, 17, 18 The type of fat chosen directly influences the biomarkers. Lard and vegetable shortening are equally rich in saturated fatty acids and monounsaturated fatty acids, therefore, they are used in studies for causing metabolic changes effectively. Fat rich in polyunsaturated fatty acids are less harmful because they prevent insulin resistance.12, 19, 20 Most snacks contain fat, but experimental diets focus on only one type of saturated fat, such as lard and coconut oil. A type of snack frequently consumed by shift workers is fried meat stuffed pastry (46% fat, 23.8% saturated fatty acids, 29.3% monounsaturated fatty acids, 37.8% polyunsaturated fatty acids in 100 g).21 Hydrogenated fats, which are commonly used by the food industry, are correlated with cardiovascular diseases, decreasing HDL-cho and increasing low-density lipoprotein-low-density lipoprotein cholesterol (LDL-cho).22
Based on such evidence, it is important to mimic an HFD similar to the diet consumed by shift workers in order to analyse its effects. Therefore, the objective of this study was to test the effect of an HFD on metabolism in an animal model.
Materials and methods
Animals
Twenty male Wistar rats from the Reproduction and Experimentation Centre of Laboratory Animals (CREAL, Centro de Reprodução e Experimentação de Animais de Laboratório; Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil) were used. The animals were 60 days old at the beginning of the study. They were housed in polycarbonate cages, five animals per cage, kept at 22±2 °C with a 12:12 h light:dark cycle (lights on at 0700 hours) according to the Guide to the Use and Care of Laboratory Animals.23, 24 One animal died during the experiment. The study was approved by the Ethics Committee of the Hospital de Clínicas de Porto Alegre, and it was carried out in the Animal Experimental Unit of the same institution. All procedures were performed in such a way as to minimize pain and discomfort.
Diet
All rats were allowed ad libitum access to food and water. The HFD created for this study consisted of 45.5% standard chow, 22.7% lard, 22.7% vegetable shortening and 9% sucrose, whereas the standard diet (control group (CG)) consisted of 100% chow Nuvilab CR-1-Nuvital (São Paulo, Brazil). The chow diet provided 3.97 Kcal g−1 and the HFD provided 6.25 Kcal g−1, 0.18 and 2.84 Kcal g−1 from fat, respectively. The HFD was supplied in pellets similar to those used to offer standard diet. The HFD was prepared every 4 days and stored in a refrigerator under controlled temperature (7 °C, ±2). The amount of fatty acids in each diet is described in Table 1.25, 26, 27
Table 1. Content of fatty acids in the diet.
| CG | EG | |
|---|---|---|
| Saturated fatty acids (SFA) | ||
| Lauric acid (12:0) | 0.10 | 0.04 |
| Myristic acid (14:0) | 1.36 | 1.13 |
| Palmitic acid (16:0) | 22.23 | 19.34 |
| Stearic acid (18:0) | 8.61 | 8.68 |
| Arachidic acid (20:0) | 0.08 | |
| Total% | 32.3 | 29.27 |
| Unsaturated fatty acids | ||
| Palmitoleic acid (16:1) | 0.91 | |
| Oleic acid (18:1) | 35.91 | 39.45 |
| Linoleic acid (18:2) | 31.69 | 20.65 |
| Linolenic acid (18:3) | 0.10 | 0.59 |
| MUFA% | 36.01 | 40.96 |
| PUFA% | 31.69 | 20.65 |
| Total% | 67.7 | 61.6 |
Abbreviations: CG, control group; EG, experimental group; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid. Amounts expressed as percentage (%) in 100 g of diet.
Experimental procedures
The rats were randomized by weight and allowed a 1-week period of adaptation to the laboratory conditions and chow diet. After this, the animals were divided into two groups of 10 animals each: experimental group (EG; receiving HFD) and control group (CG; receiving standart diet). The treatment lasted 15 weeks. Food and water intake were measured daily. Body weight was assessed weekly. Blood samples were collected in non-starved animals at baseline and at 5, 10 and 15 weeks of the treatment period for glucose, HDL-cho and triglycerides (TG) analysis. The samples were obtained from the retro-orbital plexus after sedating the animals with inhaled isoflurane (Isoforine, Cristália Produtos Químicos e Farmacêuticos Ltda., São Paulo, Brazil). At the end of the treatment, the rats were killed by decapitation to facilitate blood collection and no drugs were used before this procedure. Epididymal and retroperitoneal fats were removed from visceral adipose tissue (VAT), liver and adrenal glands and weighed using a digital scale (Marte, model AS5500c, São Paulo, Brazil). This scale was also used to determine the weight of food, water and animal's body weight. The experiment was performed one time by a non-blinded researcher.
Metabolite determinations
Serum levels of glucose, HDL-cho and TG were evaluated. Serum from trunk blood was assayed for TG and glucose using the enzymatic colorimetric assay kits produced by Roche Diagnostics (Mannheim, Germany). Levels of HDL-cho were determined by the homogeneous enzymatic colorimetric assay kits also produced by Roche Diagnostics.
Statistical analysis
Considering a 95% confidence level, 10 animals per group were used to detect differences between the variables. The comparison between CG and EG at baseline considered food, water and energy intake, as well as organ weight. Data were expressed as mean±s.e.m. The statistical analysis was performed using Student's t-test for independent samples. The analysis of CG vs EG at different time points (baseline, 5 weeks, 10 weeks, 15 weeks) was performed using two-way analysis of variance and Tukey's test (post hoc analysis). A P-value <0.05 was considered to be statistically significant. The statistical analysis was carried out using the Statistical Package for the Social Sciences, version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
We investigated two groups of rats (n=20). One group was fed an HFD (45.5% chow) that sought to mimic the diet of shift workers for 15 weeks. The other 10 rats were fed 100% chow (control group).
Baseline
Data for serum glucose, TG and HDL-cho (mean±s.e.m.) are shown in Table 2 and Figure 1. No significant difference was observed between the CG and EG at baseline for body weight, serum glucose, HDL-cho and TG.
Table 2. Characteristics of the sample at baseline and after 15 weeks of a high-fat diet intervention.
| CG (n=9) | EG (n=10) | T | P | |
|---|---|---|---|---|
| Baseline | ||||
| Body weight (g) | 254.23 (±6.89) | 246.66 (±7.76) | 0.72 | 0.47 |
| Serum glucose (mg dl−1) | 152.40 (±4.11) | 166.30 (±7.13) | −1.68 | 0.11 |
| HDL-cholesterol (mg dl−1) | 49.60 (±1.40) | 47.20 (±1.32) | 1.24 | 0.23 |
| Triglycerides (mg dl−1) | 88.40 (±8.68) | 108.00 (±9.21) | −1.54 | 0.14 |
| After 15 weeks | ||||
| Body weight (g) | 402.73 (±13.48) | 391.24 (±19.08) | 0.48 | 0.63 |
| Visceral adipose tissue (g) | 17.12 (±1.76) | 34.22 (±4.70) | −3.26 | 0.005** |
| Liver weight (g) | 11.33 (±1.50) | 13.17 (±0.52) | 2.62 | 0.01** |
| Adrenal gland weight (g) | 0.05 (±0.005) | 0.07 (±0.005) | 1.89 | 0.07 |
| Food intake (g per day per rat) | 12.6 (±0.21) | 7.9 (±0.23) | 24.5 | <0.001*** |
| Water intake (ml per day per rat) | 43.3 (±5.64) | 22.6 (±4.71) | 22.23 | <0.001*** |
| Energy intake (Kcal per day per rat) | 50.6 (±0.65) | 49.4 (±1.49) | 0.70 | 0.48 |
Abbreviations: CG, control group; EG, experimental group; HDL, high-density lipoprotein. Data presented as mean (±s.e.m.). Student's t-test for two independent samples.
Significant P-values: *P<0.05; **P<0.01; ***P<0.001.
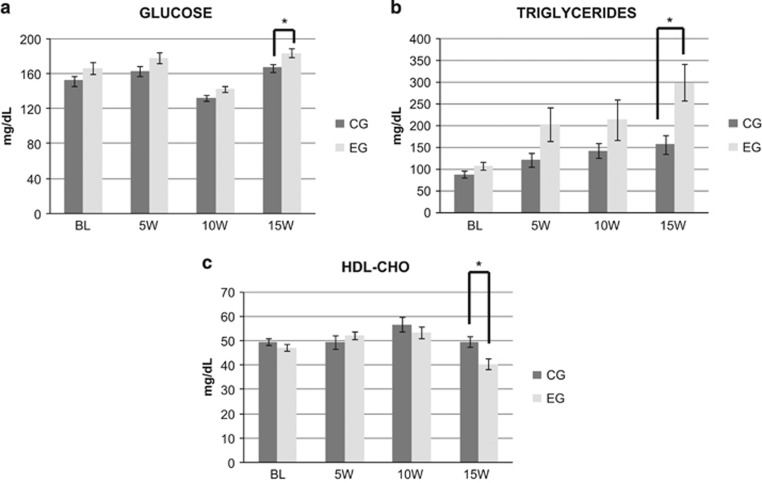
Figure 1.
Values of serum glucose (a), triglycerides (b) and HDL-cholesterol (c) at baseline (BL) and at 5, 10 and 15 weeks in the CG and EG expressed as milligram per decilitre. Asterisk indicates statistical difference between CG and EG. Results are expressed as mean±s.e.m.
Metabolic parameters
The comparison of serum variables (mean±s.e.m.) at baseline, 5, 10 and 15 weeks between EG and CG are presented in Figure 1. Glucose (F19,3=21.768; P<0.001), TG (F19,3=6.570; P=0.001) and HDL-cho (F19,3= 7,409; P<0.001) had statistically significant differences only at week 15. EG showed a significant increase in the levels of serum glucose (CG=166.88±3.64, EG=183.40±4.89) and TG (CG=157.33±21.51, EG=299.10±42.36) and a significant decrease in HDL-cho (CG=49.66±2.19, EG=40.40±2.22).
Organs and tissues
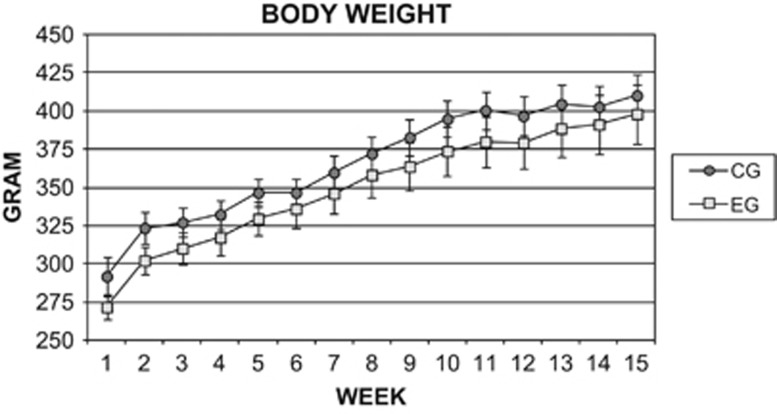
The weights of VAT, liver and adrenal glands (mean±s.e.m.), as well as body weights of the CG and EG are compared in Table 2 and Figure 2. VAT and liver weights of the EG were found to be significantly higher than in the CG. No difference was found between the EG and CG regarding body weight and the weight of adrenal glands.
Figure 2.
Body weight comparison during 15 weeks between the CG and EG expressed as grams. There was no statistical difference between CG and EG. Results are expressed as mean±s.e.m.
Food, water and energy intake
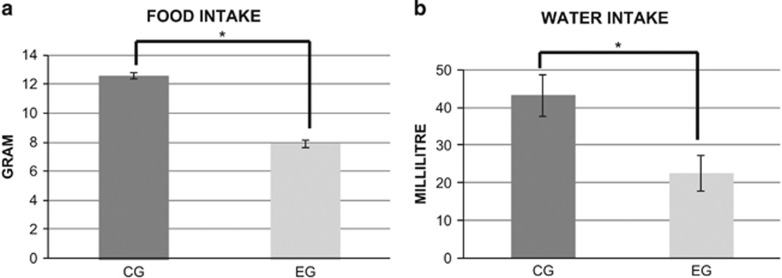
Food and water intake of both groups are shown in Table 2 and Figure 3. Both food and water intake in the EG were lower than in the CG. There was no difference between the groups in terms of energy intake.
Figure 3.
Food intake (a) and water intake (b) per day by animal during 15 weeks between the CG and EG expressed as grams (a) and millilitres (b). Asterisk indicates statistical difference between CG and EG. Results are expressed as mean±s.e.m.
Discussion
The model of diet used in our study caused metabolic changes, but it did not increase body weight. To the best of our knowledge, this is the first study designed to create an animal model diet containing lard and shortening, both saturated fatty acids, which are the most common types of fat found in processed food. Forty-five percent of the energy contained in this diet was supplied by fat compared with 4.5% in the control diet. The metabolic changes confirmed that the quality of the diet composition can have a direct influence on these markers.28, 29
Body weight was not correlated with the amount of VAT. In our study, we demonstrated that there is a non-causal relationship between these variables. Controversy surrounds the results from other studies showing an increase in body weight on an HFD. Unlike our study, which contained shortening and lard, Sampey et al.30 fed Wistar rats a lard-based 45% fat diet for 15 weeks causing higher body weight. It may be that the variety of ingredients used in manipulated HFDs and their flavours are important to determine weight gain. Also, it is a reason for concern that, in spite of the fact that the workers' body mass index is within normal limits, they may have increased visceral adiposity, which is an important risk factor for cardiovascular disease.
In disagreement with other studies, the animals of our study that were fed the HFD did not show hyperphagia because there was lower food intake, thus keeping their body weight and energy intake similar to those in the CG.11, 31 EG's lower food intake may have been due to an HFD with higher caloric density or less palatability. According to Erlanson-Albertsson,32 an HFD can upregulate the expression of hunger and satiety signals and, at the same time, blunt the response to satiety signals. Conversely, it has been reported that rats fed a higher calorie diet required spontaneously increased basal energy expenditure, stimulating thermogenesis as a compensatory mechanism in an attempt to maintain body weight.33, 34 In contrast to another study using streptozotocin combined with an HFD to change metabolic parameters and induce obesity and diabetes, hyperglycaemia was induced only by the HFD, which makes this model more similar to the natural course of disturbed metabolic physiopathology.35
In our research, the latency period for the development of metabolic changes was 15 weeks because of our attempt to trigger the most natural response in established parameters. This longer period allows a longer exposure to this type of food, as seen in shift workers. The changes in our sample were similar to those seen in shift workers after years of shift work. Shift workers have increased serum TG and serum total cholesterol in an intraindividual comparison.36 In terms of waist circumference, shift workers showed more central adiposity than day workers. This risk is increased in those who work night shifts for many years, including visceral fat area.3, 37, 38, 39 Night workers have higher risk to develop diabetes, and in a study with women working night shifts, they showed increased risk to develop type 2 diabetes in 3 years, and the risk gets higher as the working years extend.40, 41
Our study has some limitations. We did not weigh the animals' muscles. Therefore, this could be a potential confounding variable. According to the AIN-93M diet,42 the quantity of protein necessary for long-term studies to support proper growth and its maintenance is 12% of the energy intake. In the present study, the EG received 10% of energy intake, thus it is unlikely that the animals of this group had muscles' atrophy. Furthermore, the experimental diet was intended to mimic the night workers' diet because night workers eat a lower amount of proteins when compared with day workers. They also consume smaller amounts of some vitamins and minerals than day workers, such as vitamin A, calcium and selenium.43 No mineral mixture was added to the HFD. Therefore, there might have been loss of minerals' quantity in the HFD, but unfortunately, we did not measure the quantity of micronutrients present in lard and shortening. Also, we cannot assume that physical activity had a role in weight gain because it was not measured.
This study shows that feeding an HFD to experimental animals for a sustained period can lead to changes in metabolic parameters. Nevertheless, there was no significant increase in body weight although there was an increase in VAT. Body weight per se may not be a good predictor of metabolic change. Metabolic changes are related to the development of chronic diseases, such as a prediabetic state, type 2 diabetes, obesity and nonalcoholic fatty liver disease. In addition, because meal composition may be involved in the genesis of these diseases, studies using HFD can contribute to a better understanding of the metabolic physiopathology. Our results are robust as no difference was observed between the CG and EG at baseline, indicating that the animals were a homogeneous group at the beginning of the study.
In conclusion, the experimental diet was effective in showing that there were changes in serum glucose, TG and HDL-cho levels, as well as fat in the visceral tissue in spite of no change in body weight in 15 weeks.
Acknowledgments
This work was supported by FIPE/HCPA—Fundo de Incentivo a Pesquisa do Hospital de Clínicas de Porto Alegre and Coordenação de Aperfeiçoamento de Profissional do Ensino Superior - CAPES. We are also grateful to Nurse Marta J Giotti Cioato and Vet Fabiola Meyer.
Footnotes
The authors declare no conflict of interest.
References
- Christie AW, McCormick DK, Emmison N, Kraemer FB, Alberti KG, Yeaman SJ. Mechanism of anti-lipolytic action of acipimox in isolated rat adipocytes. Diabetologia 1996; 39: 45–53. [DOI] [PubMed] [Google Scholar]
- Lowden A, Moreno C, Holmbäck U, Lennernäs M, Tucker P. Eating and shift work – effects on habits, metabolism, and performance. Scand J Work Environ Health 2010; 36: 150–162. [DOI] [PubMed] [Google Scholar]
- Guo Y, Rong Y, Huang X, Lai H, Luo X, Zhang Z et al. Shift work and the relationship with metabolic syndrome in Chinese aged workers. PLoS One 2015; 10: e0120632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhang L, Zhang Y, Zhang B, He Y, Xie S et al. Meta-analysis on night shift work and risk of metabolic syndrome. Obes Rev 2014; 15: 709–720. [DOI] [PubMed] [Google Scholar]
- Waterhouse J, Buckley P, Edwards B, Reilly T. Measurement of, and some reasons for, differences in eating habits between night and day workers. Chronobiol Int 2003; 20: 1075–1092. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo L, De Pergola G, Zocchetti C, L'Abbate N, Basso A, Pannacciulli N et al. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord 2003; 27: 1353–1358. [DOI] [PubMed] [Google Scholar]
- Strzemecka J, Bojar I, Strzemecka-Kopytowska E, Owoc A. Dietary habits among persons hired on shift work. Ann Agric Environ Med 2014; 21: 128–131. [PubMed] [Google Scholar]
- De Assis MA, Nahas MV, Bellisle F, Kupek E. Meals, snacks and food choices in Brazilian shift workers with high energy expenditure. J Hum Nutr Diet 2003; 16: 283–289. [DOI] [PubMed] [Google Scholar]
- Balieiro LCT, Rossato LT, Waterhouse J, Paim SL, Mota MC, Crispim CA. Nutritional status and eating habits of bus drivers during the day and night. Chronobiol Int 2014; 31: 1123–1129. [DOI] [PubMed] [Google Scholar]
- Romon M, Edme JL, Boulenguez C, Lescroart JL, Frimat P. Circadian variation of diet-induced thermogenesis. Am J Clin Nutr 1993; 57: 476–480. [DOI] [PubMed] [Google Scholar]
- Dourmashkin JT, Chang GQ, Gayles EC, Hill JO, Fried SK, Julien C et al. Different forms of obesity as a function of diet composition. Int J Obes 2005; 29: 1368–1378. [DOI] [PubMed] [Google Scholar]
- Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Schölmerich J et al. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 2006; 36: 485–501. [DOI] [PubMed] [Google Scholar]
- Prada PO, Zecchin HG, Gasparetti AL, Torsoni MA, Ueno M, Hirata AE et al. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology 2005; 146: 1576–1587. [DOI] [PubMed] [Google Scholar]
- Bargut TC, Silva-E-Silva AC, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur J Nutr 2015; 55: 159–169. [DOI] [PubMed] [Google Scholar]
- Buettner R, Ascher M, Gäbele E, Hellerbrand C, Kob R, Bertsch T et al. Olive oil attenuates the cholesterol-induced development of nonalcoholic steatohepatitis despite increased insulin resistance in a rodent model. Horm Metab Res 2013; 45: 795–801. [DOI] [PubMed] [Google Scholar]
- Coelho DF, Pereira-Lancha LO, Chaves DS, Diwan D, Ferraz R, Campos-Ferraz PL et al. Effect of high-fat diets on body composition, lipid metabolism and insulin sensitivity, and the role of exercise on these parameters. Braz J Med Biol Res 2011; 44: 966–972. [DOI] [PubMed] [Google Scholar]
- Collison KS, Maqbool Z, Saleh SM, Inglis A, Makhoul NJ, Bakheet R et al. Effect of dietary monosodium glutamate on trans-fat-induced nonalcoholic fatty liver disease. J Lipid Res 2009; 50: 1521–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoli AM, Roehrig C, Rotta LN, Kruger AH, Souza KB, Kessler AM et al. Serum and liver lipids in rats and chicks fed with diets containing different oils. Nutrition 2003; 19: 789–793. [DOI] [PubMed] [Google Scholar]
- Jelinek D, Castillo JJ, Arora SL, Richardson LM, Garver WS. A high-fat diet supplemented with fish oil improves metabolic features associated with type 2 diabetes. Nutrition 2013; 29: 1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YY, Kwon EY, Kim HJ, Jeon SM, Lee KT, Choi MS. Differential effect of corn oil-based low trans structured fat on the plasma and hepatic lipid profile in an atherogenic mouse model: comparison to hydrogenated trans fat. Lipids Health Dis 2011; 20: 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.unicamp.br/nepa/taco/contar/taco_4_edicao_ampliada_e_revisada (Research in April 2015).
- Khosla P, Hayes KC. Dietary trans- monounsaturared fatty acids negatively impact plasma lipids in humans: critical review of the evidence. J Am Coll Nutr 1996; 15: 325–339. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals, 1st edn. National Academy Press: Washington DC, USA, 1996. [Google Scholar]
- Luca RR, Alexandre SR, Marques T, Souza NL, Merusse JLB, Neves SP (eds). Colégio Brasileiro de Experimentação Animal - COBEA. Manual Para Técnicos em Bioterismo, 2nd edn. Yellow Graph: São Paulo, Brazil, 1996. [Google Scholar]
- Gunstone FD. Fatty Acid and Lipid Chemistry. Blackie Academic and Professional: London, UK, 1996; 252 pp. [Google Scholar]
- Folador A, Hirabara SM, Bonatto SJ, Aikawa J, Yamazaki RK, Curi R et al. Effect of fish oil supplementation for 2 generations on changes in macrophage function induced by Walker 256 cancer cachexia in rats. Int J Cancer 2007; 120: 344–350. [DOI] [PubMed] [Google Scholar]
- Badolato ESG Aspectos analíticos da determinação de ácidos graxos trans em margarinas e gorduras vegetais hidrogenadas. Dissertação de Mestrado - Faculdade de Ciências Farmacêuticas, Universidade de São Paulo, São Paulo, Brail, 2000.
- Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Di Marzo V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: effect on high fat diets. Mol Cell Endocrinol 2008; 286: 66–78. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Park T. Genes are differentially expressed in the epididymal fat of rats rendered obese by a high-fat diet. Nutr Res 2008; 28: 414–422. [DOI] [PubMed] [Google Scholar]
- Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity 2011; 10: 2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Alexander JT, Zheng P, Yu HJ, Dourmashkin J, Leibowitz SF. Behavioral and endocrine traits of obesity-prone and obesity-resistant rats on macronutrient diets. Am J Physiol 1998; 274: 1057–1066. [DOI] [PubMed] [Google Scholar]
- Erlanson-Albertsson C. How palatable food disrupts appetite regulation. Basic Clin Pharmacol Toxicol 2005; 97: 61–73. [DOI] [PubMed] [Google Scholar]
- Iossa S, Mollica MP, Lionetti L, Barletta A, Liverini G. Hepatic mitochondrial respiration and transport of reducing equivalents in rats fed an energy dense diet. Int J Obes Relat Metab Disord 1995; 19: 539–543. [PubMed] [Google Scholar]
- Lionetti L, Iossa S, Brand MD, Liverini G. The mechanism of stimulations of respiration on isolated hepatocytes from rats fed an energy dense diet. J Nutr Biochem 1996; 7: 571–576. [Google Scholar]
- Kusakabe T, Tanioka H, Ebihara K, Hirata M, Miyamoto L, Miyanaga F et al. Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high-fat diet. Diabetologia 2009; 52: 675–683. [DOI] [PubMed] [Google Scholar]
- Biggi N, Consonni D, Galluzzo V, Sogliani M, Costa G. Metabolic syndrome in permanent night workers. Chronobiol Int 2008; 25: 443–454. [DOI] [PubMed] [Google Scholar]
- Mohebbi I, Shateri K, Seyedmohammadzad M. The relationship between working schedule patterns and the markers of the metabolic syndrome: comparison of shift workers with day workers. Int J Occup Med Environ Health 2012; 25: 383–391. [DOI] [PubMed] [Google Scholar]
- Gu JK, Charles LE, Burchfiel CM, Fekedulegn D, Sarkisian K, Andrew ME et al. Long work hours and adiposity among police officers in a US northeast city. J Occup Environ Med 2012; 54: 1374–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza BB, Monteze NM, de Oliveira FL, de Oliveira JM, de Freitas Nascimento S, Marques do Nascimento Neto R et al. Lifetime shift work exposure: association with anthropometry, body composition, blood pressure, glucose and heart rate variability. Occup Environ Med 2015; 72: 208–215. [DOI] [PubMed] [Google Scholar]
- Toshihiro M, Saito K, Takikawa S, Takebe N, Onoda T, Satoh J. Psychosocial factors are independent risk factors for the development of Type 2 diabetes in Japanese workers with impaired fasting glucose and/or impaired glucose tolerance. Diabet Med 2008; 25: 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimalananda VG, Palmer JR, Gerlovin H, Wise LA, Rosenzweig JL, Rosenberg L et al. Night-shift work and incident diabetes among African-American women. Diabetologia 2015; 58: 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc writing Committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123: 1939–1951. [DOI] [PubMed] [Google Scholar]
- Lennernäs M, Hambraeus L, Akerstedt T. Shift related dietary intake in day and night shift workers. Appetite 1995; 25: 255–265. [DOI] [PubMed] [Google Scholar]