Macrophage; Microparticles; HPV infection Although macrophages are very important in antiviral immune responses, various types of viruses such as the human immunodeficiency virus, hepatitis B virus, hepatitis C virus, human cytomegalovirus, and poliovirus are capable of infecting macrophages. However, the underlying mechanisms remain incompletely understood. Recent studies highlight that cells during activation or apoptosis may shed components of the plasma membranes encapsulating cytoplasmic elements into the extracellular space. These released vesicles are known as microparticles (MPs) with 0.1–1 μm sizes.1 MPs can function as vectors to deliver information materials to other cells,2,3,4 implying a possibility of MP-mediated virus infection of macrophages. In fact, MPs are proposed to serve as a ‘Trojan horse'-like mechanism to transfer infectious particles such as the HIV virus to cells.5,6 Here, we provide evidence that human papillomavirus (HPV) may infect macrophages through the MP-mediated pathway.
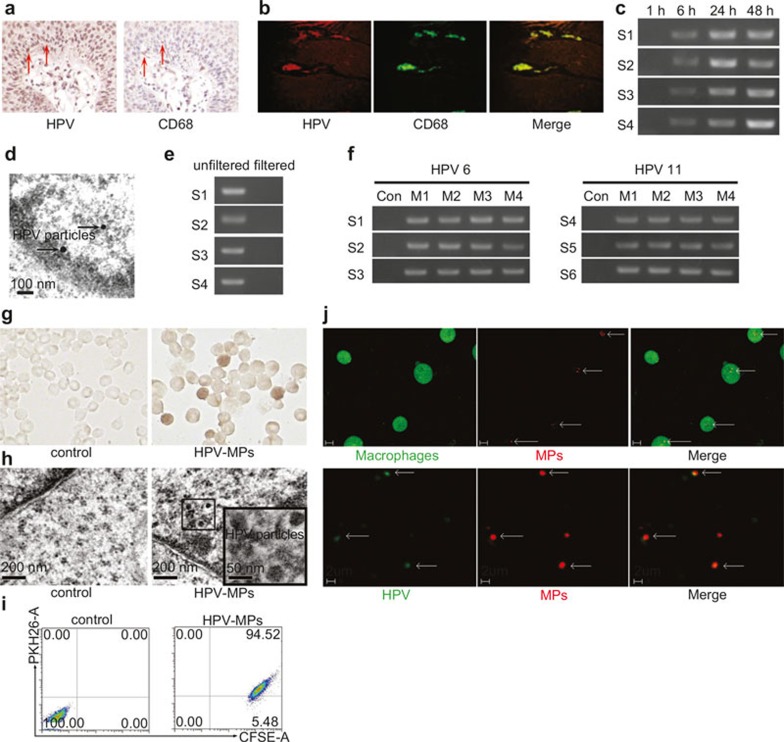
To determine whether macrophages can be infected by HPV, macrophages were first analyzed in patients with genital warts by immunohistochemical staining as well as immunofluorescent methods. Both results showed that CD68+ cells could merge with HPV+ cells (Figure 1a and b), suggesting that macrophages might be infected by HPV. Given the existence of HPV type 6 or 11 in genital warts, collagenase and hyaluronidase were used to digest genital wart tissues freshly isolated from clinical patients. The generated wart supernatants were incubated with primary macrophages derived from peripheral blood mononuclear cells (PBMCs) of healthy donors. Six hours later, the media were refreshed for further culture. After 1, 6, 24, and 48 hours, macrophages were harvested respectively to detect HPV E6 mRNA expression by RT-PCR. The 1-hour re-culture did not show the amplified E6 PCR production; however, E6 PCR products were observed after 6-, 24-, and 48-hour re-culture (Figure 1c). Meanwhile, the 48-hour culture supernatants were used to further incubate with new isolated macrophages. Similarly, the media were refreshed 6 hours later and the macrophages were continuously cultured for HPV E6 mRNA detection at different time points. These macrophages were named M2 and the previous macrophages were named as M1. Consistently, HPV mRNA was detected after 6-hour re-culture (Figure 1c). We used the 48-hour re-culture M2 supernatants to incubate with new macrophages and found that HPV E6 mRNA was also expressed in the designated M3 macrophages (Figure 1c). Again, HPV E6 mRNA was also expressed in M4 macrophages (Figure 1c), suggesting that HPV infects and replicates in macrophages. In line with these data, the virus particles in 24-hour re-cultured macrophages were observed under an electron microscope (Figure 1d). To further validate the above data, another six wart tissues were collected from patients and used to conduct the above experiments. Similar results were obtained from all the samples (Supplementary Table 1). Together, these data suggested that HPV may enter macrophages and results in the infection through a certain pathway.
Figure 1.
Macrophages can be infected by HPV. (a and b) Analysis of macrophages in genital warts by immunohistochemical staining (a) and immunofluorescent methods (b). (c) Macrophages derived from PBMCs were incubated with genital wart supernatants for 6 hours. The incubation media were washed away and macrophages were cultured in fresh RPMI 1640 culture medium for different times. The expression of HPV E6 mRNA in macrophages was detected by RT-PCR. S1–S4 represented four samples, respectively. (d) Virus particles in 24-hour re-cultured macrophages were observed under an electron microscope. (e) Genital wart supernatants were filtered with 100 nm size filters and used for incubation with macrophages for 6 hours. The incubation media were washed away and macrophages were cultured in fresh RPMI 1640 culture medium for 24 hours. The expression of HPV E6 mRNA in macrophages was detected by RT-PCR. S1–S4 represented four samples, respectively. (f) MPs were isolated from genital wart supernatants and incubated with macrophages for 6 hours. The incubation media were washed away and macrophages were cultured in fresh RPMI 1640 culture medium for 24 hours. The expression of HPV E6 mRNA in macrophages was detected by RT-PCR. S1–S6 represented four samples, respectively. MPs isolated from healthy donor peripheral blood were used as control. M1–M4 represented microparticles generated from four generations of macrophages, respectively. (g and h) HPVs were observed in macrophages in experimental group by immunocytochemistry (g) and electron microscopy (h). MPs isolated from healthy donor peripheral blood were used as negative control group. (i) The uptake of MPs by macrophages were analyzed by flow cytometry. (j) Two-photon microscopic analysis. Upper row: macrophages took up MPs. Lower row: HPV was included in MPs.
Next, we wondered the pathway by which HPV entered into macrophages. Since HPV particles are ∼50 nm in diameter, the above genital wart-derived supernatants were filtered with 100 nm size filters to get rid of larger nonviral components. However, the filtered supernatants did not cause the HPV infection of macrophages (Figure 1e), suggesting HPV particles might not infect macrophages directly. Given that cell membrane-derived MPs with 100–1000 nm in size can transfer various materials between cells,2,3 we speculated that infection of keratinocytes by HPV resulted in the release of MPs that encapsulated viral particles, thereby mediating HPV infection of macrophages. To test this, we isolated MPs from the wart supernatants according to previous reports,7,8 and then incubated the MPs with macrophages for 6 hours and then refreshed the medium. Twenty-four hours later, macrophages were harvested for HPV E6 mRNA detection by RT-PCR. As expected, HPV mRNA was detected in the experiment group, but not in the control group in which MPs were isolated from healthy peripheral blood (Figure 1f). Meanwhile, the supernatants were used to isolate the second generation of MPs to further incubate with new macrophages. Again, the third and fourth generations of MPs were prepared for macrophage co-culture with the same protocol. Consistently, all those macrophages were found to express HPV mRNA (Figure 1f). Corroborating these findings, macrophages containing HPV were also confirmed by immunocytochemistry (Figure 1g), and HPV particles in macrophages were observed under an electron microscope (Figure 1h). More convincingly, additional five samples from five genital wart patients we tested. The repeated experiments all obtained the same results (Figure 1f). Taken together, these data suggested that the HPV may infect macrophages through the MP pathway, though how MPs mediated HPV infection of macrophages remains unanswered.
Two lines of evidence were obtained. First, macrophages were found to effectively take up MPs according to flow cytometric analysis (Figure 1i); second, these MPs contained HPV based on results from a two-photon microscopic study (Figure 1j). Thus, MPs package HPV and macrophages take up MPs, leading to the entry of HPV into macrophages. Phagocytosis is the classical routine wherein macrophages take up and degrade exogenous and endogenous materials. On the basis of the data that HPV particles could not infect macrophages (Figure 1e), we speculate that macrophages phagocytose-free HPV particles and destroy HPV in phagolysosomes, thereby avoiding the viral infection; however, the MP pathway avoids the lysosome-mediated degradation of HPV components and releases HPV in the cytoplasm, leading to the survival of the HPV in macrophages.
HPV, the small double-stranded DNA virus, commonly infects keratinocytes and mucosal epidermis, leading to papilloma and carcinoma in large human populations.9 However, the pathway of HPV infection remains incompletely understood. The data here show that MPs, by virtue of their lipid bilayer membranous structures and large vesicle sizes, are capable of packaging HPV and are easily taken up by macrophages through non-phagocytosis pathways, resulting in the transferal of live HPV to macrophages. Besides the classic receptor-mediated virus infection pathways,10 our data suggest an alternative general biological pathway for virus entry into cells via a non-receptor-mediated pathway. Considering the infiltration and the long survival of macrophages in infected tissues, this study might provide a new insight into macrophage as a source for chronic viral infections.
Competing Interests
The authors have no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81472000), National Basic Research Program of China (2014CB542103), National Science Fund for Distinguished Young Scholars of China (81225021).
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res 2003; 59: 277–287. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 2006; 20: 1487–1495. [DOI] [PubMed] [Google Scholar]
- Bargeron Clark K, Hsiao HM, Noisakran S, Tsai JJ, Perng GC. Role of microparticles in dengue virus infection and its impact on medical intervention strategies. Yale J Biol Med 2012; 85: 3–18. [PMC free article] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008; 10: 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci USA 2003; 100: 10592–10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes DGJ, Raab-Traub N. Microvesicles and viral infection. J Virol 2011; 85: 12844–12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun 2012; 3: 1282. [DOI] [PubMed] [Google Scholar]
- Tang K, Liu J, Yang Z, Zhang B, Zhang H, Huang C et al. Microparticles mediate enzyme transfer from platelets to mast cells: a new pathway for lipoxin A4 biosynthesis. Biochem Biophys Res Commun 2010; 400: 432–436. [DOI] [PubMed] [Google Scholar]
- Chow KY, Brotin É , Ben Khalifa Y, Carthagena L, Teissier S, Danckaert A et al. A pivotal role for CXCL12 signaling in HPV-mediated transformation of keratinocytes: clues to understanding HPV-pathogenesis in WHIM syndrome. Cell Host Microbe 2010; 8: 523–533. [DOI] [PubMed] [Google Scholar]
- Grove J, Marsh M. The cell biology of receptor-mediated virus entry. J Cell Biol 2011; 195: 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.