Abstract
Gastrointestinal commensal microbiota is a concentrated mix of microbial life forms, including bacteria, fungi, archaea and viruses. These life forms are targets of host antimicrobial defense in order to establish a homeostatic symbiosis inside the host. However, they are also instrumental in shaping the functions of our immune system via a diverse set of communication mechanisms. In the gut, T helper 17, regulatory T and B cells are continuously tuned by specific microbial strains and metabolic processes. These cells in return help to establish a mutually beneficial exchange with the gut microbial contents. Imbalances in this symbiosis lead to dysregulations in the host's ability to control infections and the development of autoimmune diseases. In addition, the commensal microbiota has a significant and obligatory role in shaping both gut intrinsic and distal lymphoid organs, casting a large impact on the overall immune landscape in the host. This review discusses the major components of the microbial community in the gut and how its members collectively and individually exert regulatory roles in the host immune system and lymphoid structure development, as well as the functions of several major immune cell types.
Keywords: commensal fungi, gut microbiota, peripheral lymphoid organs, T cell subtypes
Introduction
Symbiosis is common in biology.1 Although in the cases of large species, this co-existence is often a visual wonder, host microbe symbiosis is deeply rooted in biological systems for more fundamental reasons. Making up 60% of fecal mass,2 gastrointestinal colonization of microbial species in higher animals is likely to first serve as a metabolic complement in food processing, content fermentation and detoxification.3 The digestion of complex sugars by gut microbiota provides not only an additional source of energy to maintain the microbial community but also essential nutrients for gut epithelial cells.4 Germ-free (GF) animals are resistant to high calorie food intake-induced weight gain5 and, accordingly, require 30% more energy to maintain a similar body mass.6 In addition, biochemical evidence indicates that specific bacterial strains located in the intestine promote lipid storage.7 Therefore, energy harvesting is the driving force for mammalian hosts and sets the stage for their microbial companions in the gastrointestinal tract.
While this salubrious ecology is beneficial, the host exerts an enormous effort to contain this cohort of 'guest workers.' By quantitative measurements, the list of immune regulatory factors and cells that are most abundantly found in the gut includes T helper 17 (TH17) cells, γδ T cells and immunoglobulin A (IgA)-producing B cells. The containment of microbiota is accomplished at several levels. The epithelia of the intestinal lumen is covered by a mucus layer. Some proteins in this layer, such as Mucin2, play important roles in downregulating intestinal dendritic cells' (DC) inflammatory responses.8 In the lower intestine where the microbial burden is relatively heavy, this protective layer is particularly thick. The occasional microbial breach of defenses results in microbes that are either phagocytosed by macrophages or taken into the lamina propria (LP) by DCs for prolonged storage and antigen sampling.9 In addition to the barrier function, gut-associated lymphoid organs (GALTs) produce large quantities of IgA, often targeting antigens that are unique in the microbiota.10 In addition, TLR ligands in the gut stimulate Reg3β and Reg3γ antimicrobial molecule production by epithelial cells,11 although in the case of intestinal Paneth cells (specialized in secretion of antimicrobial substances), these molecules are constitutively produced.12
For the host, this defense mechanism is not a zero sum game. Our immune system develops out of the backdrop of a large number of microbial species. This deep interconnection was discovered soon after germ-free animals were developed. While GF animals have life spans that are similar or slightly longer than normally reared counterparts,13 their immune systems are underdeveloped.14 They were found to have very few 'reaction centers' and lymphocytes. Bauer et al.14 reported that in GF Swiss Webster mice, there was a universal reduction in the size of lymph nodes, number of macrophages and 'immune competent cells.' It is now well established that a healthy immune system relies on a well-maintained microbiota. In fact, evolution has very likely applied selection pressure to enrich the diversity of microbiota in higher animals.15 One hypothesis even speculates that the extreme diversity in the gut of more complex animals is linked to the development of adaptive immunity. In contrast, small and short-lived life forms have no such need for immune memory functions.16 Additional investigation is required to determine the veracity of this hypothesis. However, the work in the last decade has revealed a complex pattern of host microbiota exchange, including local immune regulations, overall immune development and dysbiosis in diseases. In this writing, we intend to emphasize two particular points: how the microbiota impacts several important immune cell types (Figure 1) and how it helps to establish secondary lymphoid structures (Figure 2).
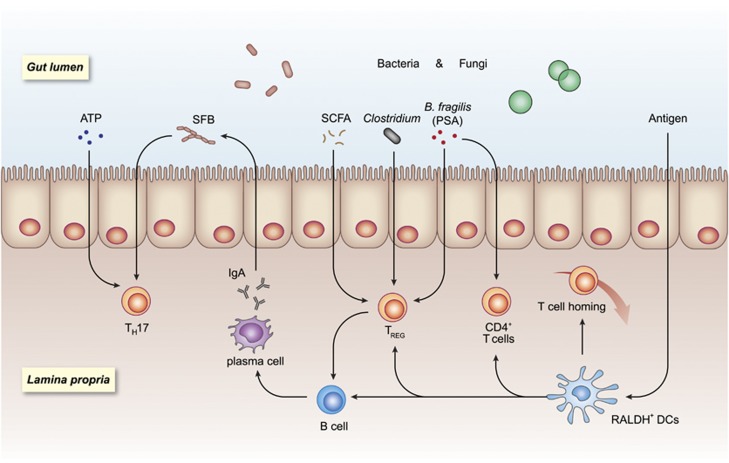
Figure 1.
Gut microbial environment impacts resident lymphocytes. In the steady state, gut microbial contents polarize and fine-tune several types of lymphocytes. The presence of bacterial strains such as segmented filamentous bacteria (SFB) drives B cells to produce IgA, likely assisted by RALDH+ dendritic cells. The resulting immunoglobulin A (IgA) restricts the otherwise unchecked expansion of several bacterial species. Treg cells are induced by Clostridia members and are also strongly linked to the availability of fermentation products such as short-chain fatty acids (SCFAs), while T helper 17 (TH17) cells are mostly driven by SFB and the high concentration of ATP in the intestinal lumen. In parallel to these events, gut microbial antigens are sampled by dendritic cells (DCs) and presented to various types of lymphocytes, leading to a complex balance of activation/inhibition and gut immune homeostasis.
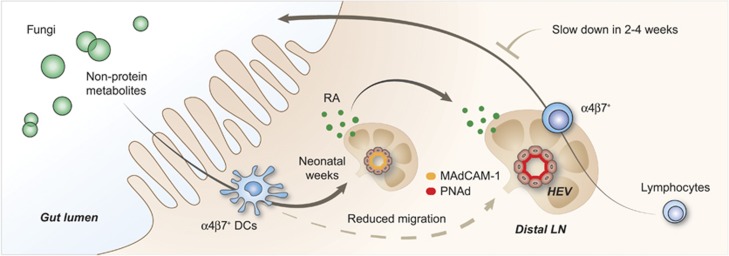
Figure 2.
Peripheral lymphoid volume and gut microbiota. Dendritic cells (DCs) entering secondary lymphoid organs (SLOs) is a critical event to maintaining the proper structure of lymph nodes (LNs). We have found that fungal species drive a group of CD103+CD11b+RALDH+ DCs to leave the gut and enter distal LNs. These fungi mediate the MAdCAM-1 to PNAd addressin profile switch to initiate the adult pattern of lymphocyte homing, resulting in SLO volume expansion. This switch gradually reduces the migration of these DCs to peripheral LNs. In the early weeks, lymphocytes circulating through these LNs are further routed back to the gut, a phenomenon that gradually disappears in the later weeks. The small presence of these DCs is critical to preventing structure attrition in adult LNs.
The microbes in the gut
Amniotic fluid is sterile. While the fetus is exposed to minute amounts of released microbial antigens in gestation, the first inoculation does not take place until environmental exposure after birth. Newborns by vaginal delivery receive the first seeds of fecal microbiota from their mother. In comparison, Cesarean delivery is associated with a slow acquisition of two dominant bacterial divisions, Firmicutes and Bacteroidetes, with the gut inoculation mainly consisting of maternal skin-resident species. This difference leads to significantly higher rates of asthma, connective tissue disorders, arthritis, leukemia and so on, per population statistics over lifetime in the latter.17, 18 After birth, the microbial composition undergoes a period of variation until it reaches a relatively stable mixture. It should be noted that the presence of stable microbiota by itself conveys the generic 'colonization resistance.' In other words, the preexistence of these microbes has the natural ability to limit the expansion of later species, potentially covering pathogenic strains.19 In contrast, antigens present in the microbiota at times act as facilitating triggers to induce autoimmune attacks, such as the case of experimental autoimmune encephalomyelitis (EAE).20
In neonates, members of the Actinobacteria, Proteobacteria and Bacteroidetes dominate first.21 Slowly, the community profile changes and individual differences become evident. In the adult human gut, Firmicutes and Bacteroidetes phyla are highly represented.22 In addition, there are several 'core' phyla that persist in individuals, although the exact composition of the community also evolves over time. As our gut commensals are made of large numbers of anaerobic bacteria and existing culture media are not tailored for individual needs, it is difficult to culture most of them ex vivo, making studies on their metabolism incomplete.23 However, PCR technologies have greatly facilitated our understanding of these distributions. Earlier work was mostly based on 16S RNA sequences, as bacterial rRNAs were sufficiently conserved for common probes, and in addition, they exhibited sufficient taxonomic differences for identification. Then, next generation sequencing (NGS) based on 16S RNA pre-amplification followed by shotgun analysis became available, as did direct sequencing of genomes. The sequences can be clustered and compared to several collected sources, allowing an unprecedented power of resolution. These metagenomic profiles provide rich genetic insights into the diversity of these guest workers. However, mechanism-wise, metabolic function rather than genetic information is more tightly associated with immune regulations. This area of research is more difficult and has only recently moved toward the center of focus.
Overall, depletion of gut bacteria has a significant impact on the immune response to pathogens. Antibiotic treatment reduced both innate and adaptive immune responses against experimental LCMV infection.24 Neomycin-sensitive bacteria were associated with strong antigen-specific (T cell and antibody) responses against influenza infection. However, their functions could be replaced with a provision of TLR ligands.25 This result suggested that canonical PAMP sensing is a contributing factor to the regulation by gut microbiota. Beyond infection models, a great deal of research has focused on the connection between non-infectious/autoimmune diseases and individual species/strains of commensal bacteria. Locally, microbiota dysbiosis has been linked to two forms of inflammatory bowel diseases (IBD): Crohn's disease (CD) and ulcerative colitis (UC). IBDs do not develop in GF mice until the first inoculation, and in some cases, antibiotic treatment helped to reduce the pathological severity.26, 27 A common feature of both types of diseases is the limited diversity in gut commensal bacteria. At the phylum level, there was also a characteristic decrease in the normally dominant Bacteroidetes and Firmicutes with a rise in other relatively underrepresented groups, such as Proteobacteria and Actinobacteria.28, 29, 30 Systemic impact by gut microbiota on autoimmunity is also common. One proposal suggests that the hyperallergic state in modern populations is a result of an overemphasis on 'hygiene,' which limits the diversity of commensals in the gut.31 For more severe aliments in distal organs, a clear link has been established between intestinal bacteria and neurological autoimmunity. EAE models developed limited pathology in GF mice, and antibiotic treatment was beneficial.32 The presence of microbiota and autoantigens in EAE were two critical factors driving disease development.20 In particular, the presence of segmented filamentous bacteria (SFB) was sufficient to trigger EAE via a process involving TH17 cells.33 However, protection against EAE has been associated with Clostridium spp. and Bacteroides fragilis.34, 35, 36 SFB-driven TH17 cells were also involved in the development of autoimmune arthritis.37 Unexpectedly, the same strain was found to suppress type I diabetes.38 These examples clearly indicate the vital yet complex role of gut bacteria in the development of autoimmunity.
Although bacteria are the prototypical residents in the intestinal tract and we are marveled by their sheer magnitude of clonal diversity, a stable microbiota also contains archaea and fungi, as well as viruses. As bacteria are the dominant species in terms of numbers, they attract a lion's share of attention and have received more advanced/dedicated research. For instance, human gut fungi were first identified almost a century ago,39 but analyses of these residents did not take place until the end of the last century.40 In contrast, commercial preparations for bacterial culture were manufactured in the 1800s. A large and comprehensive survey of existing literature on human gut sequencing analyses suggested that approximately 1000 species of bacteria inhabit the gut, with a log higher number of strains,15, 41 while the eukaryotic species are close to 100. Archaea, mostly methanogenic, are still 10-fold lower.42 Some argue that the metabolic contributions from non-bacterial organisms might have been underestimated. For instance, as fungi are, in general, larger in size, the metabolic/regulatory impact may be larger than what would be expected from simply considering the species number.43 In fact, clinical reports often cite fungal imbalance as one of the most common findings in association with gastrointestinal pathologies. In the human colon, the most common fungi are Candida, Aspergillus, Saccharomyces and Cladosporium.44 The abundance of Candida albicans has been positively associated with CD, an observation that was made after stool samples were analyzed from those affected in comparison with their disease-free relatives.45 Candida dominance was also found in UC patients, whose symptoms appeared to be controlled by anti-fungal treatment.46 Fungal β-glucan recognition by C-type lectin receptor Dectin-1 resulted in activation of CARD9 (caspase recruitment domain–containing protein 9), which in turn drove TH17 polarization in the gut.47, 48 Iliev et al.49 reported that in mice, deficiencies in this pathway led to an increased susceptibility to DSS (dextran sodium sulfate)-induced colitis, accompanied by the expansion of Candida and Trichosporon. In humans, a single-nucleotide polymorphism in the CLEC7A gene (Dectin-1), rs2078178, is strongly associated with the severity of UC (medically refractory UC), suggesting a role of anti-fungal immunity in intestinal inflammation.49
The gut virome is also an interesting area of development. The human gut contains ~109/g virions.50 Currently, the direct link between gut viral imbalance and immunological disorders is not well established. One interesting hypothesis is that the phages present in the gut act as a feedback mechanism to control other microbiota. For example, they may kill off the excess expansion of dominant bacterial strains to maintain the population balance.51 Several studies have been published to highlight the importance of gut viruses in shaping the landscape of the microbiome.52 More recently, it was reported that in IBD patients, the gut virome was altered. In contrast to the decreased genetic diversity of bacteria in these patients, Caudovirales bacteriophages showed significant expansion, along with a more diversified virome. At this stage, whether these changes contribute to the disease state or are only secondary consequences of the disease is not clear.53
As research moves deeper into gut microbiota-mediated host immune regulation, the field has moved from simple descriptive analyses to metabolic/molecular investigations of inner mechanisms. The inclusion of regulators from other biological kingdoms vastly increases the complexity and depth of these investigations.
B cells/IgA
GF animals have a reduced number of GALTs.54, 55 Peyer's patches (PP) and isolated lymphoid follicles (ILF) are also diminished.56 An important function of these local structures is to set the stage for IgA production. The significance can be appreciated by two known facts. First, the majority of total antibodies produced in the host is IgA, which is secreted into the intestinal lumen to target gut microbes. Second, the microbiota significantly expands in the absence of IgA, compensated by increased antimicrobial epithelial functions,57 indicating an essential role performed by secreted IgA in gut immune homeostasis. Microbes in the gut were often found to be heavily coated by IgA,10 and the coated microbes exhibited enhanced translocation into lymphoid tissues, facilitating antigen presentation.58 These findings may have implications beyond local immunity. Peterson et al.59 showed that inoculation of GF mice with the single strain Bacteroides thetaiotaomicron induced a strong innate response, and simultaneous introduction of a hybridoma secreting a specific IgA significantly altered the landscape of innate immunity, ranging from complement to FcγR to inflammatory cytokines. The central message is that the IgA response to the gut microbiota sets the threshold for the entire immune system.59 SFB is a well-characterized IgA target. AID (activation-induced cytidine deaminase)-deficient mice produce reduced quantities of antibody due to limited somatic hypermutation and class switch. Parabiosis of regular mice with AID−/− mice significantly suppressed gut SFB outgrowth in the latter.60 IgA production in humans appears to be linked to genetics and food intake. Recently, a comprehensive survey in human twins up to 2 years of age found that the transition from receiving IgA in the breast milk to their own production was affected by several factors. Genetics had the largest impact at the beginning, although this correlation became weaker with age. To complement the human study, some particular strains were isolated and introduced into GF mice; IgA responses that developed thereafter were impacted by food selection.61
For IgA production, intestinal DCs sample the microbial content and translocate to draining MLNs (mesenteric LNs) for the induction of B cell activation. These B cells then move to PP for antibody secretion.9 The secretion is mediated by polymeric Ig receptor (pIgR), which binds and transports IgA from the basolateral to the apical interface.62 DCs from the gut were different from the conventional DCs in their unique ability to induce IgA from activated B cells, an ability at that time attributed to their interleukin-6 (IL-6) production.63 In recent years, particularly with the proper classification of innate lymphoid cells (ILC),64 increased consideration has been given to a group of retinoic acid (RA)-producing RALDH+ (retinaldehyde dehydrogenase) DCs, as they may serve as the conduit to introduce RA to lymphoid tissues. Intake restriction of vitamin A, the precursor of RA, reduced the IgA levels in the small intestine.65, 66 RA itself promoted splenocyte IgA production in vitro;67,68 however, it appeared that in vivo, other cytokines including IL-6 and transforming growth factor-β (TGFβ) were required for the production as well.63, 66, 69 There is also evidence that RA may serve as a specific inducer of IgA class switch.70 Interestingly, in a flagellin-specific T cell receptor (TCR)-transgenic system, depletion of CD4+CD25+ T cells blocked IgA production and introduction of Foxp3+ CD4+ T cells restored the response, suggesting that Tregs can work as conventional CD4+ helper T cells in IgA production.71 Kawamoto et al. and Cong et al. similarly reported that Tregs entered germinal centers to not only increase the quantity of IgA but also to expand the diversity in their BCR genes.71, 72
Unexpectedly, the microbiota may have built-in mechanisms to fine-tune IgA production in MLNs. Diehl et al.73 reported that signaling via Myd88 on a group of CX3CR1hi DCs in the LP inhibited their ability to transport gut bacteria into MLNs, thereby reducing T cell activation and IgA production. This finding likely adds another layer of regulatory complexity to these LNs.
Regulatory T cells
Tregs are from two developmental origins with characteristic expression of Foxp3 as the master switch. Thymic migrants (tTregs) represent a population of self-reactive T cells that escape negative selection74, 75, 76, 77 and provide a steady pool of Tregs available in the periphery. In situ induction of peripheral Tregs (pTregs) is more tuned to local availability of antigens and the state of inflammation and is dynamically regulated.78 These two populations together form the immune suppressive network mediated by a plethora of mechanisms, such as constitutive expression of CTLA-4 and secretion of IL-10 and TGFβ. In a seminal study, Shimon Sakaguchi reported that transfer of CD25+ cell-depleted lymphocytes into Balb/c nude mice led to broad-spectrum autoimmunity, where gastritis was among the most overt.79 Early work by Mottet et al.80 showed that in a CD4+CD45RB+ T cell transfer colitis model, the presence of CD25+ Tregs was essential to suppressing bowel inflammation. These reports all suggested the inhibitory role of Treg-mediated suppression in gut autoimmunity.
Helios and neuropilin 1-negative Tregs, the quintessential pTregs, are hard to detect in other organs but are abundant in the LP of the small intestine.81 Conversely, the number of these peripherally induced Tregs was reduced in the absence of microbiota or dietary antigens.82, 83 In the presence of microbiota, the gut environment can generally be described as anti-inflammatory. Under this general setting, there are a few distinct pathways via which Tregs can be induced. In one modality, DCs are the main driving force behind Treg induction. Coombes et al.84 reported that there was a steady stream of gut CD103+ DCs that moved to the MLNs, where they induced Tregs in an RA-dependent fashion.84 How this induction was linked to gut microbiota was not studied in that report. From the angle of microbial antigen-specific induction of pTregs, it was found that gut Treg TCR usage was different from conventional peripheral T cells. TCRs specific for gut bacterial antigens such as those from Parabacteroides distasonis mediated Treg induction only in the gut but not in the thymus,85 cementing the essential role of the gut environment in Treg induction. Using a chloroform extraction protocol, there has been a series of studies that link Clostridia members to Treg induction in the colon but not in the small intestine or other lymphoid tissues in the gut. Clostridium clusters IV and XIVa can increase the number of Tregs. This increase was associated with TGFβ and indoleamine 2,3-dioxygenase, both known to help pTreg induction.34 Based on this information, the same group isolated 17 strains of clusters IV, XIVa and XVIII of Clostridia from human fecal specimens and inoculated GF mice. In comparison with non-discriminatory human fecal transplant, this selection increased the promotion of Tregs in colonic LP. This inoculation was horizontally transmissible to other GF mice.82 Remarkably, the reduction of those strains was found in multiple sclerosis (MS) patients,35 suggesting the biological relevance of these bacteria in forming a balanced gut immune response.
In a non-mutually exclusive form, Treg induction in the gut can be accomplished by particular bacterial products. Short-chain fatty acids (SCFAs) are fermentation products of complex sugars that are digestible by bacteria present in the fecal content.86 Butyrate, acetate and propionate are the main species with the ability to shape gut immune homeostasis. This production stream leads to a local SCFA concentration approaching 100 mM, a level that is much higher than that required to modulate T cell polarization. In 2013, Smith et al.87 reported that SCFAs increased the number of Foxp3+/IL-10+ colonic Treg cells. The authors suggested that this effect was mediated by SCFAs, such as propionate signaling via GPR43 (G-protein-coupled receptor), which in turn inhibited HDAC6 (histone deacetylase) and HDAC9 activation.87 Arpaia et al.88 extracted SCFAs from gut contents and found mainly that butyrate potentiated the extrathymic de novo transformation from CD4+ T cells to Tregs driven by TGFβ/IL-2 and anti-CD3. Apparently, butyrate also stabilized Foxp3 expression as a consequence of HDAC inhibition.88 Similar findings have been reported by Furusawa et al.,89 which further indicated that these induced Tregs were capable of suppressing the CD4+CD45RB+ T cell transfer colitis model. In addition, propionate was found to increase splenic Treg conversion as well.88 Interestingly, in another report, propionate and acetate, but not butyrate, were found to increase gut tTreg accumulation rather than the conversion from CD4+ T cells.87 These differences may require further analysis to identify subtle discrepancies in experimental protocols. In addition to the direct induction of Tregs, butyrate can signal via GPR109a (also known as the niacin receptor) on macrophages and DCs. These activated cells then mediate the induction of Tregs. This regulation apparently relies on the induction of RALDH1 expression in DCs.90 Independent of GPRs, PSA (polysaccharide A) produced by B. fragiles ameliorated TNBS and Helicobacter hepaticus-induced colitis. This effect was mediated by IL-10-producing pTreg cells and required signaling via TLR2.91, 92, 93 Therefore, microbial products and metabolites modulate a list of regulatory functions to suppress gut inflammation, mainly via the induction of Treg cells.
T helper 17 cells
TH17 cells are a CD4+ T cell polarization in addition to TH1/TH2, characterized by the lineage marker RORγt, in contrast to T-bet and GATA3, which are required for the other two, respectively. This particular lineage strongly produces IL-17A and IL-17F and possesses a greater plasticity in conversion to the TH1 phenotype in vivo.94, 95 IL-17 family cytokines and their signaling via a diverse set of receptors are a prominent feature of gut mucosal immunity. They suppress the outgrowth of several strains of bacteria and fungi and are involved in autoimmune regulation. Additional information was recently published in a recent expert review on the matter.96 TH1 polarization is in general considered proinflammatory. One of the early surprises was that some autoimmunities such as MS and RA (rheumatoid arthritis) were not consistently associated with TH1 polarization.23, 97 It was found that the p40 subunit of IL-12 could be paired with p19 instead of p35 to form the cytokine IL-23, which was sufficient to drive EAE 98 as well as IBDs.99, 100 Most of these effects were found to be regulated by TH17 T cells. Although IL-23 is critical to sustaining the phenotype, it is actually not the cytokine that drives the differentiation. The commitment to this polarization appears to be highly environmentally dependent and can be induced by several combinations of stimulatory factors. TGF-β+IL-6, IL-21 alone and IL-1β+IL-6+IL-23 have all been reported to induce TH17 polarization, and resulting populations displayed interesting functional variations.101 With the molecular regulation of TH17 cells becoming clear, its role in gut homeostasis has come into focus.
TH17 T cells are a significant presence in the LP of the colon. In GF mice, the overall T cell number does not differ from that of controls; however, the percentage of TH17 T cells is reduced.102, 103 This promotion effect is mediated at several levels. First, the presence of microbiota significantly increased the production of several cytokines; among them, the change in IL-17 levels was most profound.102, 103, 104 SFB abundance in the gut also positively regulated TH17 cell expansion.102, 104, 105, 106 It has been reported that compared with C57BL/6 mice from Jackson Laboratories, Taconic strains had much higher SFB. Co-housing these two strains led to higher TH17 in Jackson mice, a phenomenon associated with the increase in gut SFB.104 Inoculation with SFB alone in gnotobiotic mice was sufficient to induce TH17 cells in LP. Strikingly, Yang et al.107 reported that most TH17 cells in the gut expressed TCRs specific for SFB antigens, and the specificities of these TCRs alone were a strong driving force to induce TH17 polarization.107 Sano et al.108 reported that TH17 differentiation occurred in close proximity to SFB colonization on epithelial cells. SFB drove the epithelial production of serum amyloid A (SAA1/2). The latter induced enhanced gene expression of Rorc.108 At the metabolic level, one of the main driving forces for their increase is the ATP in the large intestine. The SPF fecal content contains higher levels of ATP than GF mice.109, 110 The effect of ATP appeared to be induced via CD11clow CD70high DCs that produced IL-6 and TGFβ. These DCs promoted TH17 differentiation by activating STAT3 in the targeted cells.109 It was discovered later that the ATP metabolic enzymes ecto-nucleoside triphosphate diphosphohydrolases (ENTPD1, CD39) were essential for controlling the expansion of TH17 cells. Entpd7-deficient mice were resistant to oral infection with Citrobacter rodentium but had a higher tendency to develop EAE.111 The roles of ATP and the P2X7 receptor have been confirmed in the lesions of human psoriatic skin where ATP-stimulated DCs induced a biased TH17 immune response.110 In addition, acetate and propionate can induce TH17 cell differentiation independent of GPRs. In vitro, acetate and propionate triggered the transcription of IL-17A, IL-17F, RORα, RORγt, T-bet and interferon-γ (IFN-γ), which are genes that are important for TH1 and TH17 T cell differentiation.112
Although TH17 and Tregs are considered functionally antagonistic, Tregs in the gut can express Rorγt. In two Science articles, it was reported that the presence of microbiota expanded a population of Helios-neuropilin- Rorγt+ Tregs, mainly in the colon.113, 114 The deficiency of this gene was associated with reduced pTregs. In one study, the rise of these cells required RA and dendritic cells. The surface expression of CTLA4 on these Tregs suppressed DCs via downregulation of CD80 and CD86, reducing TH2 polarization.114 This suppression is similar to one of the proposed common inhibitory mechanisms by Tregs.115, 116 The other study also found that Rorc was highly upregulated in colonic CD4+Foxp3+ Tregs. However, in contrast to the last study, the authors described a critical regulation on TH1/TH17 cells with no apparent impact on TH2 differentiation.113 Distinct from this population and driven mainly by GATA3, a population of Helios+ Tregs was induced by IL-33 signaling via ST2 (Il1rl1) on their surface.117 This induction was inhibited by IL-23. They significantly expanded when Rorvt+ Tregs were genetically deleted. It should be noted that these cells did not appear to be limited to the gut and might present an intestinal regulation exerted by a systemic Treg population.
Gut microbiota and peripheral immune system development
In addition to their roles in lymphocyte tuning and polarization, the gut microbiota is essential to the establishment of secondary lymphoid organs (SLO). GF animals have no reduction in their thymic outputs, in stark contrast with the multiple immunological deficiencies in the periphery. In GF mice, there is a general deficiency in SLOs, although the lymphoid structural arrest is best studied in the gut. In the intestine, both GALTs and associated MLNs are underdeveloped. Peyer's patches are smaller, and the ILFs are arrested at an immature growth state.56, 118, 119
In the sterile embryo, LN development is independent of microbial presence. However, RA signaling is indispensable. At the distal ends of nerve fibers, the secretion of RA triggers the development of lymphoid anlagen from the primitive lymphoid sacs. This process required a specific population of group 3 innate lymphoid cells, LTi (lymphoid tissue inducer) and lymph toxin signaling.120, 121, 122 After birth, LTs were no longer present. Therefore, the conduit that links the emergence of gut microbes and lymphoid tissue development after birth was not well characterized. Early on, Mazmanian et al.123 reported that PSA could trigger CD4+ T cell activation, which may likely represent an antigen-dependent expansion of these cells. Mebius et al.124 first described a mysterious population of non-T, non-B cells that appeared to be important to maintaining the neonatal LN development. After this appearance, there was a steady movement of CCR7-dependent lymphocyte migration into SLOs, resulting in cellularity increase and volume expansion.125, 126 This process was critically dependent on gut microbiota.118 The most systematic analysis was performed for ILF maturation. After birth, numerous lymph toxin B-dependent crypto patches dynamically form with the emergence of cKit+ and B220+ cells. In the presence of microbiota, they converted to mature ILFs in a CCR7-dependent manner.112, 127 Peptidoglycan from Gram-negative strains signaled through NOD1 and induced robust development of ILFs, also in a CCR7-dependent fashion,56 suggesting that it is an active factor from the microbiota for ILF induction. Disruption of this pathway resulted in 100-fold expansion of Clostridiales, Bacteroides and Enterobacteriaceae.56
Due to the proximity of MLNs and GALTs to the microbial content and lymphatic drainage, it is relatively easy to imagine a close exchange at work. It is, however, more difficult to envision a similar regulation on SLOs that are distal to the gut.128 In GF animals, peripheral challenge with Listeria monocytogenes resulted in a reduced accumulation of lymphocytes.129 Lack of gut microbiota was also associated with reduced responses to influenza infection.24, 25 In viral infections alone, gut microbiota was associated with several specific immune activations.130 The structural underdevelopment is certainly partially responsible for the reduced peripheral immune responses. Thus far, how the gut microbiota sustains the robust responsiveness of the distal immune organs is elusive. A critical event in the neonatal SLO maturation is an addressin profile switch from MAdCAM-1-mediated to PNAd (MECA79, sulfated carbohydrate-decorated adhesion molecules)-mediated lymphocyte homing. The arrival of DCs is essential for this switch. Mossine et al.131 reported that in the steady state, Diphtheria toxin receptor-mediated CD11c+ DC depletion caused a disappearance of MECA79+ on the HEV portal. This reduction limited the migration of peripheral lymphocytes entering the LNs, leading to significant structural attrition.131 There was a simultaneous increase in MAdCAM-1 at HEV, resembling a neonatal addressin profile. They further suggested that LT derived from these DCs was critical for the structural maintenance of LNs. Wendland et al.126 reported that the migration was mediated by CCR7-expressing lymphocytes and relied on CCL21 expression by fibroblastic reticular cells. Although these analyses demonstrated the importance of DCs in supporting peripheral lymphoid volume, they did not indicate any gut microbiota involvement.
Regarding the missing link to the microbiota, we recently reported an interesting mechanism in neonatal mice up to 4 weeks of age; specifically, a group of gut-originated CD103+CD11b+ DCs expressing RALDH2 was driven by the microbiota and traveled to peripheral LNs, leading to local RA production. Injection of these cells into GF mice completely restored the LN development, indistinguishable from SPF mice.132 Arriving at LNs, they mediated the MAdCAM-1 to PNAd (MECA79) transition and increased the HEV portal size and CCL21 expression. This work for the first time directly linked the gut microbiota to peripheral immune development. Three observations were particularly interesting. (1) In the first two weeks, these cells induced the lymphocytes circulating through peripheral LNs to express gut-homing CCR9 and α4β7, resulting in their further migration back to the gut. This process was slowed in adult mice. (2) These cells persisted in low frequencies in adult LNs, and this presence was essential for structural maintenance. (3) Gut fungi, particularly Candida tropicalis, were essential for these cells to leave the gut, suggesting that eukaryotic metabolism within gut microbiota was the critical force for peripheral immune development. Therefore, this presents an interesting scheme whereby those RALDH2+ DCs come to SLOs to turn on an adult-like homing pattern to attract circulating lymphocytes and at the same time drive these cells back to the gut to build an early defense there. It is not until the gut defense is fully completed that they keep a steady stream of incoming lymphocytes to maintain the peripheral lymphoid volume. Our results therefore implicate a potential path linking the gut microbiota, particularly commensal fungi, to peripheral immune volume expansion.
There are many questions to be answered. What are the fungal metabolites driving these DCs to enter the peripheral LNs? Are they unique to Candida tropicalis? What are the molecular regulations that turn on PNAd expression? These are immediate questions for the near future.
Prospective
This review focuses on several individual cell types and processes regulated by gut microbiota, which are individual steps in a multifaceted regulatory process. Even within this confine, there are many other molecular details that are unknown to us. In our view, with understanding deepening and technologies improving, several lines of investigation will be of high priority. (1) The metabolic processes, rather than individual strains, should move to the center of the investigation. The most important are SCFAs, ATP, PSA and those known to play roles in lymphoid function regulations. Additional metabolites and microbial species responsible for their production are also important study subjects. (2) Systematic evaluation of the roles played by life forms other than bacteria. In the case of fungi, clinical diseases are commonly linked to their dysbiosis. Fungal metabolism is similar to that of the host. Therefore, whether their signaling can be directly incorporated into the host immune regulation is an interesting question. (3) As the peripheral lymphoid system is regulated by the gut microbiota, how this regulation impacts all aspects of peripheral immune activation should be thoroughly investigated. (4) As a more distant target, it will be of great value to establish therapeutic protocols to see whether targeting particular microbial strains and their metabolic processes can be used as effective methods to intervene in immunologic dysregulations. It is sobering to note that studies examining immune regulations by gut microbiota as a whole are in the early stage. Only by diving deeper into specific molecular pathways involved will we harness the power of this most fundamental symbiosis in the entire eco-system.
Acknowledgments
YS is supported by the joint Peking-Tsinghua Center for Life Sciences, the National Natural Science Foundation of China General Program (31370878) and by grants from the US NIH (R01AI098995), the Natural Sciences and Engineering Research Council of Canada (RGPIN-355350/396037) and the Canadian Institutes of Health Research (MOP-119295).
Footnotes
The authors declare no conflict of interest.
References
- Relman DA. 'Til death do us part': coming to terms with symbiotic relationships - Foreword. Nat Rev Microbiol 2008; 6: 721–724. [DOI] [PubMed] [Google Scholar]
- Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr 2009; 98: 229–238. [DOI] [PubMed] [Google Scholar]
- Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012; 489: 242–249. [DOI] [PubMed] [Google Scholar]
- Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol 2013; 28: 9–17. [DOI] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 2007; 104: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash HL, Hooper LV. Commensal bacteria shape Intestinal immune system development. ASM News 2005; 71: 77–83. [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004; 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013; 342: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004; 303: 1662–1665. [DOI] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao LM et al. Immunoglobulin A Coating Identifies Colitogenic Bacteria in Inflammatory Bowel Disease. Cell 2014; 158: 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E, Tremaroli V, Lee YS, Koren O, Nookaew I, Fricker A et al. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut 2012; 61: 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 2011; 9: 356–368. [DOI] [PubMed] [Google Scholar]
- Gordon HA, Bruckner-Kardoss E, Wostmann BS. Aging in germ-free mice: life tables and lesions observed at natural death. J Gerontol 1966; 21: 380–387. [DOI] [PubMed] [Google Scholar]
- Bauer H, Horowitz RE, Levenson SM, Popper H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am J Pathol 1963; 42: 471–483. [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006; 124: 837–848. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai M. Adaptive immunity: care for the community. Nature 2007; 445: 153. [DOI] [PubMed] [Google Scholar]
- Sevelsted A, Stokholm J, Bonnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics 2015; 135: e92–e98. [DOI] [PubMed] [Google Scholar]
- Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy 2008; 38: 634–642. [DOI] [PubMed] [Google Scholar]
- Bauer E, Williams BA, Smidt H, Verstegen MW, Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr Issues Intest Microbiol 2006; 7: 35–51. [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011; 479: 538–541. [DOI] [PubMed] [Google Scholar]
- Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta paediatrica 2009; 98: 229–238. [DOI] [PubMed] [Google Scholar]
- Qin JJ, Li RQ, Raes J, Arumugam M, Burgdorf KS, Manichanh C et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464: 59–U70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD et al. Culturing of 'unculturable' human microbiota reveals novel taxa and extensive sporulation. Nature 2016; 533: 543-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012; 37: 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK. Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA 2011; 108: 5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK et al. Antibiotic Therapy in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Am J Gastroenterol 2011; 106: 661–673. [DOI] [PubMed] [Google Scholar]
- Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol 2007; 13: 5581–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage P, Hasler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A et al. Twin Study Indicates Loss of Interaction Between Microbiota and Mucosa of Patients With Ulcerative Colitis. Gastroenterology 2011; 141: 227–236. [DOI] [PubMed] [Google Scholar]
- Frank DN, Amand ALS, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 2007; 104: 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 2012; 9: 599–608. [DOI] [PubMed] [Google Scholar]
- West CE. Gut microbiota and allergic disease: new findings. Curr Opin Clin Nutr Metab Care 2014; 17: 261–266. [DOI] [PubMed] [Google Scholar]
- Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol 2008; 173: 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2011; 108: 4615–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011; 331: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS ONE 2015; 10: e0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol 2010; 185: 4101–4108. [DOI] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010; 32: 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci USA 2011; 108: 11548–11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HW. Yeast-like fungi of the human intestinal tract. J Infect Dis 1917; 21: 341–U18. [Google Scholar]
- Gumbo T, Isada CM, Hall G, Karafa MT, Gordon SM. Candida glabrata fungemia - Clinical features of 139 patients. Medicine (Baltimore) 1999; 78: 220–227. [DOI] [PubMed] [Google Scholar]
- Dimitrov DV. The human gutome: nutrigenomics of the host-microbiome interactions. OMICS 2011; 15: 419–430. [DOI] [PubMed] [Google Scholar]
- Rajilic-Stojanovic M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 2014; 38: 996–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol 2014; 14: 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD et al. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS ONE 2013; 8: e66019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert-Vitse A, Sendid B, Joossens M, Francois N, Vandewalle-El Khoury P, Branche J et al. Candida albicans colonization and ASCA in familial Crohn's disease. Am J Gastroenterol 2009; 104: 1745–1753. [DOI] [PubMed] [Google Scholar]
- Zwolinska-Wcislo M, Brzozowski T, Budak A, Kwiecien S, Sliwowski Z, Drozdowicz D et al. Effect of Candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of colitis ulcerosa. J Physiol Pharmacol 2009; 60: 107–118. [PubMed] [Google Scholar]
- Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol 2012; 13: 246–254. [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 2007; 8: 630–638. [DOI] [PubMed] [Google Scholar]
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 2012; 336: 1314–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer F. Global phage diversity. Cell 2003; 113: 141-. [DOI] [PubMed] [Google Scholar]
- Reyes A, Wu M, McNulty NP, Rohwer FL, Gordon JI. Gnotobiotic mouse model of phage-bacterial host dynamics in the human gut. Proc Natl Acad Sci USA 2013; 110: 20236–20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie LA, Jones BV. The human gut virome: a multifaceted majority. Front Microbiol 2015; 6: 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JM, Handley SA, Baldridge MT, Droit L, Liu CY, Keller BC et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015; 160: 447–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev 1998; 62: 1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 2004; 4: 478–485. [DOI] [PubMed] [Google Scholar]
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008; 456: 507–510. [DOI] [PubMed] [Google Scholar]
- Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med 2011; 17: 1585–U97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen F, Zagato E, Mazzini E, Fosso B, Manzari C, El Aidy S et al. BALB/c and C57BL/6 mice differ in polyreactive IgA abundance, which impacts the generation of antigen-specific IgA and microbiota diversity. Immunity 2015; 43: 527–540. [DOI] [PubMed] [Google Scholar]
- Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2007; 2: 328–339. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA 2004; 101: 1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planer JD, Peng Y, Kau AL, Blanton LV, Ndao IM, Tarr PI et al. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature 2016; 534: 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol 2009; 70: 505–515. [DOI] [PubMed] [Google Scholar]
- Sato A, Hashiguchi M, Toda E, Iwasaki A, Hachimura S, Kaminogawa S. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol 2003; 171: 3684–3690. [DOI] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol 2013; 13: 145–149. [DOI] [PubMed] [Google Scholar]
- Bjersing JL, Telemo E, Dahlgren U, Hanson LA. Loss of ileal IgA+ plasma cells and of CD4+ lymphocytes in ileal Peyer's patches of vitamin A deficient rats. Clin Exp Immunol 2002; 130: 404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 2006; 314: 1157–1160. [DOI] [PubMed] [Google Scholar]
- Tokuyama H, Tokuyama Y. Retinoids enhance Iga production by lipopolysaccharide-stimulated murine spleen-cells. Cell Immunol 1993; 150: 353–363. [DOI] [PubMed] [Google Scholar]
- Tokuyama H, Tokuyama Y. Retinoids enhance IgA production by lipopolysaccharide-stimulated murine spleen cells. Cell Immunol 1993; 150: 353–363. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Sugai M, Nambu Y, Osato M, Hayashi T, Kawaguchi M et al. Requirement for Runx proteins in IgA class switching acting downstream of TGF-beta 1 and retinoic acid signaling. J Immunol 2010; 184: 2785–2792. [DOI] [PubMed] [Google Scholar]
- Seo GY, Jang YS, Kim HA, Lee MR, Park MH, Park SR et al. Retinoic acid, acting as a highly specific IgA isotype switch factor, cooperates with TGF-beta 1 to enhance the overall IgA response. J Leukoc Biol 2013; 94: 325–335. [DOI] [PubMed] [Google Scholar]
- Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci USA 2009; 106: 19256–19261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y et al. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 2014; 41: 152–165. [DOI] [PubMed] [Google Scholar]
- Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 2013; 494: 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol 2013; 14: 307–308. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012; 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunology and cell biology 2011; 89: 45–53. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity 2004; 21: 267–277. [DOI] [PubMed] [Google Scholar]
- Schmitt EG, Williams CB. Generation and function of induced regulatory T cells. Front Immunol 2013; 4: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995; 155: 1151–1164. [PubMed] [Google Scholar]
- Mottet C, Uhlig HH, Powrie F. Cutting edge: Cure of colitis by CD4(+) CD25(+) regulatory T cells. J Immunol 2003; 170: 3939–3943. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med 2012; 209: 1723–1742, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013; 500: 232–236. [DOI] [PubMed] [Google Scholar]
- Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 2016; 351: 858–863. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007; 204: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N et al. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011; 478: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund E, Aura AM, Mattila I, Kosso T, Rouau X, Poutanen K. Formation of phenolic microbial metabolites and short-chain fatty acids from rye, wheat, and oat bran and their fractions in the metabolical in vitro colon model. J Agric Food Chem 2012; 60: 8134–8145. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013; 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013; 504: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504: 446–450. [DOI] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014; 40: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA 2010; 107: 12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008; 453: 620–625. [DOI] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011; 332: 974–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol 2009; 39: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen DQ, Elson CO et al. Late Developmental Plasticity in the T Helper 17 Lineage. Immunity 2009; 30: 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, He X, Li X, Qian Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol Immunol 2016; 13: 418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, TaylorEdwards C, Ridgway W, Dinisco C, Steinman L et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalolmyelitis (EAE). J Immunol 1996; 156: 5–7. [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003; 421: 744–748. [DOI] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, Mckenzie B et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 2006; 116: 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 2010; 33: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett PR, Meyer zu Horste G, Kuchroo VK. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest 2015; 125: 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 2009; 31: 677–689. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008; 4: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih VF, Cox J, Kljavin NM, Dengler HS, Reichelt M, Kumar P et al. Homeostatic IL-23 receptor signaling limits Th17 response through IL-22-mediated containment of commensal microbiota. Proc Natl Acad Sci USA 2014; 111: 13942–13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Littman DR. Segmented filamentous bacteria take the stage. Mucosal Immunol 2010; 3: 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 2014; 510: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell 2015; 163: 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 2008; 455: 808–812. [DOI] [PubMed] [Google Scholar]
- Killeen ME, Ferris L, Kupetsky EA, Falo L Jr, Mathers AR. Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: implications in the pathogenesis of psoriasis. J Immunol 2013; 190: 4324–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusu T, Kayama H, Kinoshita M, Jeon SG, Ueda Y, Goto Y et al. Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J Immunol 2013; 190: 774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 2015; 8: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 2015; 349: 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 2015; 349: 989–993. [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008; 322: 271–275. [DOI] [PubMed] [Google Scholar]
- Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 2011; 332: 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014; 513: 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O, Herbrand H, Friedrichsen M, Velaga S, Dorsch M, Berhardt G et al. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol 2006; 177: 6824–6832. [DOI] [PubMed] [Google Scholar]
- Moreau MC, Corthier G. Effect of the gastrointestinal microflora on induction and maintenance of oral tolerance to ovalbumin in C3h-Hej mice. Infect Immun 1988; 56: 2766–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 2004; 5: 64–73. [DOI] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science 2000; 288: 2369–2373. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 1999; 397: 702–706. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005; 122: 107–118. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Lund FE, Ngo VN, Randall TD, Jansen W, Greuter MJ et al. Initiation of cellular organization in lymph nodes is regulated by non-B cell-derived signals and is not dependent on CXC chemokine ligand 13. J Immunol 2004; 173: 4889–4896. [DOI] [PubMed] [Google Scholar]
- Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol 2006; 176: 803–810. [DOI] [PubMed] [Google Scholar]
- Wendland M, Willenzon S, Kocks J, Davalos-Misslitz AC, Hammerschmidt SI, Schumann K et al. Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity 2011; 35: 945–957. [DOI] [PubMed] [Google Scholar]
- Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol 2002; 168: 57–64. [DOI] [PubMed] [Google Scholar]
- Clarke TB. Microbial programming of systemic innate immunity and resistance to infection. PLoS Pathog 2014; 10: e1004506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H, Suzuki K, Nomoto K, Yoshikai Y. Increased susceptibility to primary infection with Listeria monocytogenes in germfree mice may be due to lack of accumulation of L-selectin(+) CD44(+) T cells in sites of inflammation. Infect Immun 1996; 64: 3280–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Pfeiffer JK. Viruses and the Microbiota. Annu Rev Virol 2014; 1: 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 2011; 479: 542–546. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li J, Zheng W, Zhao G, Zhang H, Wang X et al. Peripheral lymphoid volume expansion and maintenance are controlled by gut microbiota via RALDH+ dendritic cells. Immunity 2016; 44: 330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]