Abstract
The tripartite motif-containing (TRIM) proteins represent the largest E3 ubiquitin ligase family. The multifaceted roles of TRIM38 in innate immunity and inflammation have been intensively investigated in recent years. TRIM38 is essential for cytosolic RNA or DNA sensor-mediated innate immune responses to both RNA and DNA viruses, while negatively regulating TLR3/4- and TNF/IL-1β-triggered inflammatory responses. In these processes, TRIM38 acts as an E3 ubiquitin or SUMO ligase, which targets key cellular signaling components, or as an enzymatic activity-independent regulator. This review summarizes recent advances that highlight the critical roles of TRIM38 in the regulation of proper innate immune and inflammatory responses.
Keywords: Inflammation, Innate Immunity, Signaling transduction, TRIM38, Type I Interferon
INTRODUCTION
The innate immune system is the first line of host defense against infection of microbial pathogens. Host cells express several types of germline-encoded pattern-recognition receptors (PRRs), which sense a wide range of pathogenic components that are named pathogen-associated molecular patterns (PAMPs), including nucleic acids, lipids, proteins and so on.1 According to their subcellular locations and structures, PRRs can be grouped in several families, including plasma or endosomal membrane-bound Toll-like receptors (TLRs), cytosolic RIG-I-like receptors (RLRs), cytosolic DNA sensors and cytosolic NOD-like receptors (NLRs).2, 3, 4, 5 The membrane-bound TLRs are mainly expressed in immune cells, while RLRs and DNA sensors recognize RNA or DNA in a variety of cell types, including both immune and non-immune cells.6 The NLR family contains more than 20 members. Several members of this family form inflammasomes and trigger inflammatory responses, including the secretion of interleukin (IL)-1β via the activation of caspase-1, in response to various pathogenic stimulations.7, 8 The signaling through TLRs, RLRs, DNA sensors and NLRs culminates in the expression of downstream host defense genes, such as type I interferons (IFNs) and inflammatory cytokines, to inhibit replication of pathogens, clear pathogen-infected cells, and facilitate adaptive immune response.9
Various mechanisms regulate innate immune and inflammatory responses. In the past years, increasing evidence suggests that members of the tripartite motif (TRIM) family, which is the largest family of RING domain-containing E3 ligases, have critical regulatory roles in innate immunity and inflammation.10, 11 Although most TRIM family members are E3 ubiquitin ligases, some members of the TRIM family have been suggested to confer E3 ligase activity for ubiquitin-like modifiers (UbLs), such as SUMO, NEDD8 and ISG15.12, 13, 14, 15, 16, 17 Among the TRIM family members, TRIM38 has been demonstrated to have important regulatory roles through distinct mechanisms in various innate immune and inflammatory pathways, which are the focus of this review.
STRUCTURE OF TRIM38
The TRIM proteins derive their names from their common N-terminal tripartite RBCC motif, which consists of a RING domain, one or two BBox domains and a coiled-coil domain (CCD).11 The BBox exhibits zinc-finger structure that is highly similar to the RING domain.18, 19 Because of the high similarity of BBox to RING domain, it has been suggested that BBox offers an E2 binding site similar to RING and thereby confers E3 ligase activity to some TRIM proteins lacking a RING domain. For example, a recent work has demonstrated that TRIM16, a TRIM protein lacking a RING domain, confers E3 ubiquitin ligase activity in vitro.20 The CCD is necessary and sufficient for oligomerization of TRIM proteins.21, 22, 23 In addition, systematic studies have demonstrated that TRIM hetero-oligomers are formed at least in vitro, which increases the spectrum of their biological functions.20, 24 The C-terminal domains found in TRIM proteins are quite diverse. The most universal C-terminal domain is PRY-SPRY (B30.2), which is present in most TRIM proteins, including TRIM38. The reported functions of the PRY-SPRY domain are divergent. Predominantly, this domain mediates protein-protein interactions,25 which entails the binding of ubiquitination substrates and determining E3 ligase specificity. In addition, the PRY-SPRY domain is critical for the direct antiviral restriction activity of certain TRIM proteins such as TRIM5.26 TRIM38 is a typical TRIM protein and contains a RING, two BBoxes, a CCD and a PRY-SPRY domain.27 It has been shown that amino acids C16 and C31 in the RING are critical for the optimal catalytic activity of TRIM38, and mutation of either of these cysteines severely impairs TRIM38-mediated polyubiquitination of its substrates.28, 29, 30, 31
INDUCIBLE EXPRESSION OF TRIM38
TRIM38 is ubiquitously expressed in different cell types, such as various human and murine cell lines (HEK293, HeLa, HCT116, A549, THP-1, and RAW264.7), mouse lung fibroblasts (MLFs), bone marrow-derived macrophages (BMDMs) and dendritic cells (BMDCs).27, 28, 29, 30, 31 Expression of TRIM38 can be induced by various stimuli, such as TLR ligands, type I IFNs, and viral infection, suggesting that TRIM38 is a potential interferon-stimulating gene (ISG).28, 29, 30
NEGATIVE REGULATION OF TRIM38 on TLR-MEDIATED SIGNALING PATHWAYS
TLRs recognize a set of pathogenic components and have critical roles in host defenses against certain microbes. So far, 10 TLRs have been reported in humans (TLR1–10), while there are 12 known TLRs in mice (TLR1-9 and TLR11–13).2, 32 TLRs contain an extracellular domain to which ligands bind, a transmembrane domain, and a conserved cytoplasmic Toll/IL-1R (TIR) domain, which acts as a platform for the recruitment of downstream TIR domain-containing adapter proteins and other signaling components upon ligand stimulation.2 Among the TLRs, TLR3 recognizes viral dsRNA, as well as its synthetic analog polyinosinic-polycytidylic acid [poly(I:C)] in the endosomes.33 Upon activation, TLR3 recruits a co-receptor MEX3B, an accessory protein WDFY1, and the critical adapter TIR domain-containing adapter TRIF (also called TICAM-1).2, 34, 35 TRIF, in turn, recruits TRAF2/6 and two kinase complexes: the IKK complex to activate NF-κB and the TBK1 complex to activate IRF3, leading to subsequent induction of proinflammatory cytokines, such as TNF and IL-1β, type I IFNs and ISGs.1 Most other TLRs trigger signaling through the MyD88-TRAF6-IKK axis to activate NF-κB but not IRF3.1 TLR4, which recognizes lipopolysaccharides (LPS) of gram-negative bacteria, is the only receptor that signals through MyD88-dependent pathways to activate NF-κB and TRIF-dependent pathways to activate both NF-κB and IRF3.36
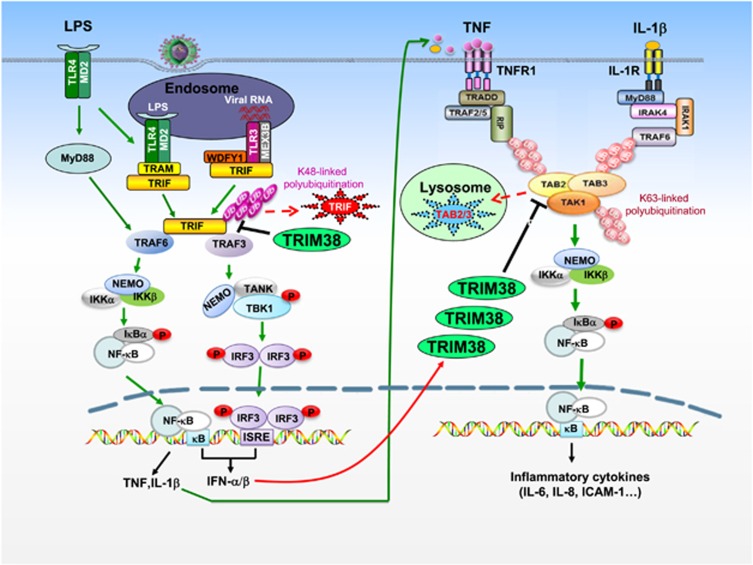
In mouse RAW264.7 cells, it has been demonstrated that Trim38 (referred to as the mouse ortholog of human TRIM38) negatively regulates TLR3/4-mediated NF-κB activation by targeting TRAF6 for proteasomal degradation.30 Furthermore, Trim38 also targets NAP1 for proteasomal degradation, which leads to negative regulation of TLR3/4-mediated IRF3 activation and type I IFN induction.29 An independent study demonstrates that TRIM38 negatively regulates TLR3-mediated activation of IRF3 and induction of type I IFNs by mediating proteasomal degradation of TRIF in human cell lines,31 which represents a distinct mechanism from the previous report. Mouse gene knockout studies suggest that Trim38-deficiency potentiates poly(I:C)- and LPS-induced, but not R848 (a ligand for TLR7)- or PGN (a ligand for TLR2)-induced expression of type I IFNs and proinflammatory cytokines in BMDMs, BMDCs and MLFs.28 Trim38-deficiency also increases the serum cytokine levels induced by poly(I:C) and LPS, as well as susceptibility to body weight loss and death triggered by administration of poly(I:C) or LPS or infection with S. typhimurium.28 Biochemical experiments indicate that Trim38-deficiency abolishes K48-linked polyubiquitination of Trif and markedly upregulates the protein level of Trif, suggesting that Trim38 targets Trif for K48-linked polyubiquitination and degradation.28 The mechanisms responsible for the negative regulatory roles of TRIM38 on TLR3/4-mediated signaling are illustrated in Figure 1. Previously, it has been reported that another E3 ubiquitin ligase, WWP2, also targets TRIF for K48-linked polyubiquitination and proteasomal degradation.37 However, WWP2 functions differently with TRIM38. WWP2 specifically regulates TLR3- but not TLR4-mediated innate immune and inflammatory responses. Furthermore, WWP2-deficiency increases TLR3-mediated induction of cytokines in BMDMs but not in BMDCs, whereas Trim38-deficiency increases TLR3/4-mediated induction of cytokines in both cell types. It is possible that Trim38 and WWP2 function in different cell types and distinct pathways.
Figure 1.
TRIM38-mediated negative regulation of TLR3/4-mediated and TNF/IL-1-triggered signaling. After the activation of TLR3/4, TRIM38 is recruited to the adapter protein TRIF, leading to its K48-linked polyubiquitination and degradation, therefore negatively regulating TLR3/4-mediated induction of proinflammatory cytokines and type I IFNs. In the early phase of infection, type I IFNs induce the expression of TRIM38, which in turn mediates the degradation of TAB2/3 by a lysosomal pathway, leading to negative regulation of TNF- and IL-1-triggered signaling and inflammatory response.
POSITIVE REGULATION OF RLR-MEDIATED INNATE IMMUNE RESPONSE BY TRIM38
RLRs, including RIG-I and MDA5, contain two N-terminal tandem CARD domains, an RNA helicase domain and a C-terminal domain (CTD), and recognize RNAs of different RNA viruses.38 In the absence of viral infection, RIG-I and MDA5 are phosphorylated in their respective CARDs to suppress their activation in resting cells.39, 40, 41 After recognition of cytosolic viral RNA, RIG-I and MDA5 undergo conformational changes and recruit PP1 for their dephosphorylation,41 leading to their further recruitment of several E3 ubiquitin ligases, including TRIM25, RNF135 and TRIM4 for K63-linked polyubiquitination,42, 43, 44 followed by their translocation to the outer membrane of mitochondria, where they activate the central adapter VISA (MAVS, CARDIF and IPS-1).45, 46, 47, 48 VISA, together with WDR5 and TRIM14, in turn recruits TRAF3, the TANK-TBK1-NAP1 complex and the transcriptional factor IRF3.49, 50 A recent study has indicated that MSX1 is critical for optimal assembly of the TANK-TBK1-NAP1 complex.51 Furthermore, in this process, GSK3β is recruited to TBK1 and promotes the self-association and trans-phosphorylation of TBK1, followed by TBK1-mediated phosphorylation of IRF3, leading to the dimerization and translocation of IRF3 to the nucleus.52 VISA also recruits TRAF2/6 and the IKK complex, which then phosphorylate IκBα and activate the transcriptional factor NF-κB, leading to translocation of NF-κB to the nucleus. The translocated IRF3 and NF-κB cooperatively drive the transcription of type I IFN genes.53, 54 In the late phase of viral infection, RIG-I and MDA5 as well as VISA are regulated by K48-linked polyubiquitination and degradation to avoid their sustained activation.55, 56, 57, 58, 59, 60, 61
In addition to polyubiquitination and phosphorylation, sumoylation of RIG-I and MDA5 has also been reported.62, 63 Our recent study suggests that TRIM38 is associated with MDA5 and RIG-I and positively regulates MDA5- and RIG-I-mediated induction of downstream antiviral genes.64 Gene knockout in mice suggests that Trim38 is essential for efficient induction of type I IFNs, proinflammatory cytokines and other downstream antiviral genes as well as for host defense against RNA viruses in vivo.64 Biochemical analysis suggests that Trim38 acts as an E3 SUMO1 ligase for Mda5 (referred to as the mouse ortholog of human MDA5) and Rig-I (referred to as the mouse ortholog of human RIG-I). Trim38 catalyzes the sumoylation of Mda5 at K43/K865 and Rig-i at K96/K889. In uninfected cells, K43 of Mda5 is basally sumoylated. Upon viral infection, the sumoylation at K43 is enhanced, and K865 is further sumoylated. Similarly, K889 of Rig-i is basally sumoylated in uninfected cells, and K96 is further sumoylated upon viral infection. Trim38-mediated sumoylations of Mda5 and Rig-i are important for antagonizing their K48-linked polyubiquitination and degradation in uninfected and early-infected cells. Previously, it has been demonstrated that dephosphorylation of Mda5 at S88 and Rig-I at S8/T177 by the phosphatase PP1 following viral infection is critical for their activation.41 Mutagenesis suggests that sumoylation of Mda5 at K43 and Rig-i at K96 is essential for their dephosphorylation and activation following viral infection.64 These studies provide solid evidence that sumoylation of Mda5 and Rig-i is essential for the efficient onset of innate immune response to RNA viruses. In addition, these findings suggest that Mda5 and Rig-i are regulated by Trim38-mediated sumoylation with similar mechanisms. Interestingly, it is reported that prolonged EV71 infection causes degradation of TRIM38 in human cells, implying that inactivation of TRIM38 following viral infection is an important immune evasion strategy of RNA viruses.65
POSITIVE REGULATION OF THE cGAS-MITA/STING PATHWAYS BY TRIM38
Cytosolic DNA derived from viruses, bacteria and the damaged host cells induces innate immune responses.66 Although several DNA sensors have been reported to recognize various DNAs in different cell types, it is widely believed that the cyclic GMP-AMP (cGAMP) synthase (cGAS) is the major sensor of cytosolic DNA in divergent cell types.67, 68, 69, 70, 71, 72, 73, 74, 75, 76
After binding of cytosolic viral or cellular DNA, cGAS undergoes oligomerization and utilizes ATP and GTP for the synthesis of cyclic GMP–AMP (cGAMP), which then acts as a second messenger to bind to and activate the central adapter protein MITA (also called STING).77, 78, 79, 80 MITA/STING is translocated from the ER to ER-Golgi intermediate compartments (ERGIC) and the Golgi apparatus.81 In this process, it has been shown that the ER-associated protein ZDHHC1 facilitates the oligomerization and optimal activation of MITA/STING.82 In addition, another ER-associated protein, called iRhom2, facilitates translocation and stability of MITA/STING.83 At ERGIC/Golgi apparatus, the kinases TBK1 and IKK are recruited to the MITA/STING-associated complex in which they phosphorylate IRF3 and IκBα, respectively, leading to the induction of downstream antiviral genes.84 In the late phase of viral infection, MITA/STING is further translocated to perinuclear microsomes, in which it is degraded via a lysosome-dependent pathway.81, 85
Post-translational modifications, including phosphorylation, polyubiquitination and glutamylation, have important roles in regulating the cGAS-MITA/STING pathways.9, 86, 87, 88 For example, TTLL4/6 catalyzes the glutamylation of cGAS, which impairs its DNA-binding and synthase activity in resting cells. Upon viral infection, the glutamylation modification of cGAS is removed by CCP5/6, leading to the activation of cGAS.87 AKT1 phosphorylates cGAS at K305, which inhibits DNA binding to cGAS and avoids its sustained activation.89 MITA/STING is also regulated by phosphorylation. ULK1 phosphorylates MITA/STING at S366 upon DNA or cGAMP stimulation, leading to attenuated IRF3 activation.85 TBK1 has been shown to phosphorylate MITA/STING at the same residue but positively regulates MITA/STING-mediated signaling.90 In addition to phosphorylation, MITA/STING is also modified by various types of polyubiquitin chains, which distinctly regulate the activity of MITA/STING. TRIM56 and TRIM32 catalyze K63-linked polyubiquitination of MITA/STING, leading to its activation.91, 92 AFMR catalyzes K27-linked polyubiquitination of MITA/STING, which forms a platform for TBK1 recruitment.93 RNF5 catalyzes K48-linked polyubiquitination of MITA/STING, causing the proteasomal degradation of MITA/STING.94 RNF26 catalyzes K11-linked polyubiquitination of MITA/STING, which unlocks its K48-linked polyubiquitination and prevents its proteasomal degradation. RNF26 also appears to negatively regulate innate immune signaling in a temporal fashion.95
Recently, it has been demonstrated that TRIM38 is a SUMO ligase for both cGAS and MITA/STING.96 Sumoylation of cGAS and STING kinetically regulates the innate immune response to DNA viral infection (Figure 2). In uninfected cells, Trim38 catalyzes sumoylation of cGas at K217, which antagonizes its K48-linked polyubiquitination at K271 and degradation by the ubiquitin-proteasomal pathway. Upon viral infection, Trim38 further catalyzes the sumoylation of cGas at K464, which prevents it from K48-linked polyubiquitination at the same residue and degradation by the ubiquitin-proteasomal pathway. These processes ensure the proper level of cGas and its activation in the early phase of viral infection. The activated cGas catalyzes the synthesis of cGAMP, which binds to and activates the ER-associated Mita/Sting. In the early phase of viral infection, Trim38 also targets Mita/Sting for sumoylation at K337, which promotes its CTT-mediated oligomerization and prevents its degradation by the CMA pathway. These actions result in optimal activation of Mita/Sting as well as induction of downstream antiviral genes. In the late phase of infection, cGas and Mita/Sting are desumoylated by SENP2, leading to their K48-linked polyubiquitination-proteasomal and CMA degradation, respectively. Therefore, the temporal sumoylation of cGas/Mita/Sting by Trim38 and their desumoylation by Senp2 provide important regulatory mechanisms for efficient innate antiviral response at the early phase of infection and its timely termination at the late phase of infection.
Figure 2.
Sumoylation promotes the stability of cGas and Sting and regulates the kinetics of the response to a DNA virus. In uninfected or early-infected cells, cGas and Sting are sumoylated, which promotes their stability and activation by inhibiting K48-linked polyubiquitin-proteasomal and chaperone-mediated degradation (CMA) pathways, respectively, thus promoting an efficient innate immune response to a DNA virus. In the late phase of viral infection, Senp2 mediates the desumoylation of cGas and Sting, leading to their degradation by the proteasomal pathway and CMA, respectively, therefore turning off the innate immune response.
REGULATION OF THE TNF/IL-1β-TRIGGERED INFLAMMATORY RESPONSE BY TRIM38
The proinflammatory cytokines TNF and IL-1β have central roles in many diseases, such as inflammation, autoimmunity and cancers. After the binding of TNF to TNF receptor 1 (TNFR1), the receptor recruits TRADD, TRAF2/5, cIAP1/2 and RIP1 to form a large receptor-associated complex, in which RIP1 undergoes K63-linked polyubiquitination. The TAK1-associated chaperones TAB2 and TAB3 bind to K63-linked polyubiquitin chains of RIP1, which in turn activates downstream kinases, leading to the activation of transcription factors NF-κB and AP1.97, 98
IL-1β binds to IL-1 receptor (IL-1R), leading to recruitment of the IL-1R accessory protein (IL-1RAcP) and the adapter protein MyD88. MyD88 further recruits IRAK1/4 and TRAF6 to the receptor complex, in which TRAF6 catalyzes K63-linked autoubiquitination and/or the synthesis of unanchored K63-linked polyubiquitin chains. These polyubiquitin chains recruit the TAK1-TAB1-TAB2/3 complex, causing TAK1 autophosphorylation and activation. TAK1 ultimately activates NF-κB and MAPKs, leading to the induction of various proinflammatory cytokines and chemokines.97, 99
TNF- and IL-1β-triggered signaling is timely downregulated or terminated to avoid excessive inflammatory responses in host cells. Several proteins have been reported to terminate TNF and IL-1β signaling by targeting various signaling components in the pathways. For example, MARCH8, cIAP1/2 and RBCK1 have been shown to induce K48-linked polyubiquitination and proteasome-dependent degradation of ILRAcP, RIP1 and TAB2/3, respectively.100, 101, 102 TRIM5α interacts with the TAK1-TAB1-TAB2/3 complex and promotes TAB2 degradation via a lysosome-dependent approach.103 Several deubiquitinating enzymes have also been shown to have negative regulatory roles in TNF and IL-1β signaling. A20, USP2a, USP4 and USP20 mediate deubiquitination of TRAF6,104, 105, 106, 107 whereas CYLD deubiquitinates TRAF6 and IKKγ.108, 109 In addition, A20, CYLD, and USP4 mediate deubiquitination of RIP1, TRAF2 and TAK1, respectively.108, 110, 111 It has also been shown that DUSP14 catalyzes dephosphorylation of TAK1, leading to suppression of TNF and IL-1β-triggered signaling.112 It is possible that different enzymes target distinct signaling components in various cell types following TNF or IL-1β stimulation.
Recently, it has been shown that TRIM38 negatively regulates TNF- and IL-1β-triggered activation of NF-κB and MAPKs as well as inflammatory responses.27 TRIM38 promotes the degradation of TAB2/3 through a lysosomal-dependent pathway, which requires its C-terminal PRY-SPRY but not the RING domain.27 The degradation of TAB2/3 inhibits recruitment of TAK1 to upstream adapters RIP1 and TRAF6, leading to inhibition of NF-κB and MAPKs and the expression of inflammatory cytokines.27 TRIM38 is highly induced by type I IFNs and negatively regulates TNF/IL-1β signaling in IFN-β-primed but not unprimed mouse immune cells.28 These findings suggest that TRIM38 probably has important negative regulatory roles in the late phase of inflammatory response to various pathogenic stimuli, which trigger induction of type I IFNs at the early phase of infection (Figure 1).
TRIM38 IN AUTOIMMUNE DISEASES
Several TRIM family proteins have been reported to be associated with certain autoimmune diseases.113, 114 A recent study clearly demonstrates that TRIM38 is a valid target for auto-antibodies in primary Sjögren's syndrome (SS).115 In primary SS, the presence of anti-TRIM21 has been associated with increased disease severity.116 It has also been shown that anti-TRIM38 positivity is significantly associated with the presence of auto-antibodies to TRIM21. However, how TRIM38 is involved in autoimmune diseases is still unknown. It has been shown that mouse TRIM21-reactive antibodies penetrate live salivary gland cells in a mouse model.117 It is possible that anti-TRIM38 penetrates live cells, causing dysregulated inflammatory responses.
Several studies suggest that constitutive activation of MDA5 and RIG-I derived from viral infection or their genetic mutations is associated with three different types of autoimmune diseases including Aicardi-Goutières syndrome, systemic lupus erythematosus and Singleton–Merten syndrome.118, 119, 120, 121, 122 Deregulation of the cGAS-MITA/STING pathway also causes lethal autoimmune diseases such as Aicardi-Goutières Syndrome and STING-associated vasculopathy with onset in infancy (SAVI). Considering the important roles of TRIM38-mediated sumoylation in the regulation of the activation and stability of MDA5, RIG-I, cGAS and MITA/STING, this enzyme may serve as a potential target for drug development for autoimmune diseases.
CONCLUDING REMARKS
Recent studies have demonstrated multifaceted roles of TRIM38 in innate immune and inflammatory responses. TRIM38 acts as a SUMO ligase and targets RIG-I/MDA5 and cGAS/STING for SUMO1 modification, which is essential for their optimal activation and stability. Therefore, TRIM38 is essential for efficient innate immune responses to both RNA and DNA viruses. In these responses, TRIM38 functions with similar biochemical mechanisms, in that sumoylation of the RNA and DNA sensors by TRIM38 prevents their degradation. TRIM38 can also act as a ubiquitin ligase that targets TRIF for K48-linked polyubiquitination and degradation and therefore negatively regulates TLR3/4-mediated innate immune responses. In addition, TRIM38 can mediate lysosomal degradation of TAB2/3 in an enzymatic activity-independent manner to negatively regulate TNF/IL-1β-triggered inflammatory responses. Because TRIM38 is induced by type I IFNs and its inhibitory effects on TLR3/4-mediated or TNF/IL-1β-triggered inflammatory responses require its high expression level, it is possible that TRIM38 promotes innate immune responses at the early phase of viral infection, while inhibiting inflammatory responses at the late phase to avoid host damage. These multifaceted roles of TRIM38 qualify it as a critical regulator of proper innate immune and inflammatory responses against viral infection. One important question that remains unanswered is how TRIM38 is regulated to exert distinct enzymatic activities in different signaling pathways. In addition, the detailed mechanism on TRIM38-mediated lysosomal degradation of TAB2 and how TRIM38 is involved in autoimmunity are still elusive. Because innate immune response is essential for adaptive immunity, the function of TRIM38 in the activation of adaptive immunity should be of great interest. Further investigations into these and other outstanding questions will help to better explain the delicate regulatory mechanisms of innate immune and inflammatory responses and to evaluate whether TRIM38 is a proper target for drug development against infectious and autoimmune diseases.
Acknowledgments
We thank members of the Shu laboratory for helpful discussions. The work in the authors' laboratory is supported by grants from the Ministry of Science and Technology of China (2016YFA0502102, 2014CB910103), the National Natural Science Foundation of China (3163000013, 31521091, and 91429304) and National Postdoctoral Program for Innovative Talents (BX201600116).
Footnotes
The authors declare no conflict of interest.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006; 124: 783–801. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34: 637–650. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity 2007; 27: 549–559. [DOI] [PubMed] [Google Scholar]
- Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell 2014; 54: 289–296. [DOI] [PubMed] [Google Scholar]
- Ran Y, Shu HB, Wang YY. MITA/STING: a central and multifaceted mediator in innate immune response. Cytokine Growth Factor Rev 2014; 25: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140: 805–820. [DOI] [PubMed] [Google Scholar]
- Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T et al. Inflammasomes: current understanding and open questions. Cell Mol Life Sci 2011; 68: 765–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 2011; 29: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HB, Wang YY. Adding to the STING. Immunity 2014; 41: 871–873. [DOI] [PubMed] [Google Scholar]
- Ozato K, Shin DM, Chang TH, Morse HC 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol 2008; 8: 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg GA, Benke S, Garcia-Sastre A, Rajsbaum R. InTRIMsic immunity: positive and negative regulation of immune signaling by tripartite motif proteins. Cytokine Growth Factor Rev 2014; 25: 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Iwai K, Ciechanover A. The NEDD8 pathway is essential for SCF(beta -TrCP)-mediated ubiquitination and processing of the NF-kappa B precursor p105. J Biol Chem 2002; 277: 23253–23259. [DOI] [PubMed] [Google Scholar]
- Arimoto K, Konishi H, Shimotohno K. UbcH8 regulates ubiquitin and ISG15 conjugation to RIG-I. Mol Immunol 2008; 45: 1078–1084. [DOI] [PubMed] [Google Scholar]
- Begitt A, Droescher M, Knobeloch KP, Vinkemeier U. SUMO conjugation of STAT1 protects cells from hyperresponsiveness to IFNgamma. Blood 2011; 118: 1002–1007. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Hwang SY, Imaizumi T, Yoo JY. Negative feedback regulation of RIG-I-mediated antiviral signaling by interferon-induced ISG15 conjugation. J Virol 2008; 82: 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regad T, Chelbi-Alix MK. Role and fate of PML nuclear bodies in response to interferon and viral infections. Oncogene 2001; 20: 7274–7286. [DOI] [PubMed] [Google Scholar]
- Vatsyayan J, Qing G, Xiao G, Hu J. SUMO1 modification of NF-kappaB2/p100 is essential for stimuli-induced p100 phosphorylation and processing. EMBO Rep 2008; 9: 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnayer L, Khuri S, Merheby HA, Meroni G, Elsas LJ. A structure-function study of MID1 mutations associated with a mild Opitz phenotype. Mol Genet Metab 2006; 87: 198–203. [DOI] [PubMed] [Google Scholar]
- Short KM, Cox TC. Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J Biol Chem 2006; 281: 8970–8980. [DOI] [PubMed] [Google Scholar]
- Bell JL, Malyukova A, Holien JK, Koach J, Parker MW, Kavallaris M et al. TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members. PLoS One 2012; 7: e37470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L et al. The tripartite motif family identifies cell compartments. EMBO J 2001; 20: 2140–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cainarca S, Messali S, Ballabio A, Meroni G. Functional characterization of the Opitz syndrome gene product (midin): evidence for homodimerization and association with microtubules throughout the cell cycle. Hum Mol Genet 1999; 8: 1387–1396. [DOI] [PubMed] [Google Scholar]
- Cao T, Borden KL, Freemont PS, Etkin LD. Involvement of the rfp tripartite motif in protein-protein interactions and subcellular distribution. J Cell Sci 1997; 110: 1563–1571. [DOI] [PubMed] [Google Scholar]
- Napolitano LM, Meroni G. TRIM family: Pleiotropy and diversification through homomultimer and heteromultimer formation. IUBMB Life 2012; 64: 64–71. [DOI] [PubMed] [Google Scholar]
- Nisole S, Stoye JP, Saib A. TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol 2005; 3: 799–808. [DOI] [PubMed] [Google Scholar]
- Yap MW, Nisole S, Stoye JP. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr Biol 2005; 15: 73–78. [DOI] [PubMed] [Google Scholar]
- Hu MM, Yang Q, Zhang J, Liu SM, Zhang Y, Lin H et al. TRIM38 inhibits TNFalpha- and IL-1beta-triggered NF-kappaB activation by mediating lysosome-dependent degradation of TAB2/3. Proc Natl Acad Sci USA 2014; 111: 1509–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MM, Xie XQ, Yang Q, Liao CY, Ye W, Lin H et al. TRIM38 negatively regulates TLR3/4-mediated innate immune and inflammatory responses by two sequential and distinct mechanisms. J Immunol 2015; 195: 4415–4425. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang L, Zhang M, Wang P, Yuan C, Qi J et al. Tripartite motif-containing protein 38 negatively regulates TLR3/4- and RIG-I-mediated IFN-beta production and antiviral response by targeting NAP1. J Immunol 2012; 188: 5311–5318. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang L, Zhang M, Yuan C, Gao C. E3 ubiquitin ligase tripartite motif 38 negatively regulates TLR-mediated immune responses by proteasomal degradation of TNF receptor-associated factor 6 in macrophages. J Immunol 2012; 188: 2567–2574. [DOI] [PubMed] [Google Scholar]
- Xue Q, Zhou Z, Lei X, Liu X, He B, Wang J et al. TRIM38 negatively regulates TLR3-mediated IFN-beta signaling by targeting TRIF for degradation. PLoS One 2012; 7: e46825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester SN, Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol 426: 1246–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Wang G, Lei X, Flavell RA. Mex3B: a coreceptor to present dsRNA to TLR3. Cell Res 2016; 26: 391–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang SY, Huang ZF, Zou HM, Yan BR, Luo WW et al. The RNA-binding protein Mex3B is a coreceptor of Toll-like receptor 3 in innate antiviral response. Cell Res 2016; 26: 288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YH, Zhang Y, Jiang LQ, Wang S, Lei CQ, Sun MS et al. WDFY1 mediates TLR3/4 signaling by recruiting TRIF. EMBO Rep 2015; 16: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001; 2: 675–680. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liao B, Wang S, Yan B, Jin Y, Shu HB et al. E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation. Proc Natl Acad Sci USA 2013; 110: 5115–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity 2008; 29: 178–181. [DOI] [PubMed] [Google Scholar]
- Nistal-Villan E, Gack MU, Martinez-Delgado G, Maharaj NP, Inn KS, Yang H et al. Negative role of RIG-I serine 8 phosphorylation in the regulation of interferon-beta production. J Biol Chem 2010; 285: 20252–20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Nistal-Villan E, Inn KS, Garcia-Sastre A, Jung JU. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J Virol 2010; 84: 3220–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wies E, Wang MK, Maharaj NP, Chen K, Zhou S, Finberg RW et al. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity 2013; 38: 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 2007; 446: 916–920. [DOI] [PubMed] [Google Scholar]
- Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A et al. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 2010; 141: 315–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Li Q, Mao AP, Hu MM, Shu HB. TRIM4 modulates type I interferon induction and cellular antiviral response by targeting RIG-I for K63-linked ubiquitination. J Mol Cell Biol 2014; 6: 154–163. [DOI] [PubMed] [Google Scholar]
- Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell 2005; 19: 727–740. [DOI] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005; 122: 669–682. [DOI] [PubMed] [Google Scholar]
- Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 2005; 437: 1167–1172. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 2005; 6: 981–988. [DOI] [PubMed] [Google Scholar]
- Wang YY, Liu LJ, Zhong B, Liu TT, Li Y, Yang Y et al. WDR5 is essential for assembly of the VISA-associated signaling complex and virus-triggered IRF3 and NF-kappaB activation. Proc Natl Acad Sci USA 2010; 107: 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Jia X, Xue Q, Dou Z, Ma Y, Zhao Z et al. TRIM14 is a mitochondrial adaptor that facilitates retinoic acid-inducible gene-I-like receptor-mediated innate immune response. Proc Natl Acad Sci USA 2014; 111: E245–E254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LT, Hu MM, Xu ZS, Liu Y, Shu HB. MSX1 Modulates RLR-Mediated Innate Antiviral Signaling by Facilitating Assembly of TBK1-Associated Complexes. J Immunol 2016; 197: 199–207. [DOI] [PubMed] [Google Scholar]
- Lei CQ, Zhong B, Zhang Y, Zhang J, Wang S, Shu HB. Glycogen synthase kinase 3beta regulates IRF3 transcription factor-mediated antiviral response via activation of the kinase TBK1. Immunity 2010; 33: 878–889. [DOI] [PubMed] [Google Scholar]
- Liu S, Chen J, Cai X, Wu J, Chen X, Wu YT et al. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife 2013; 2: e00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 2011; 146: 448–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimoto K, Takahashi H, Hishiki T, Konishi H, Fujita T, Shimotohno K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci USA 2007; 104: 7500–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Han C, Xie B, Hu X, Yu Q, Shi L et al. Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell 2013; 152: 467–478. [DOI] [PubMed] [Google Scholar]
- Hao Q, Jiao S, Shi Z, Li C, Meng X, Zhang Z et al. A non-canonical role of the p97 complex in RIG-I antiviral signaling. EMBO J 2015; 34: 2903–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B, Zhang Y, Tan B, Liu TT, Wang YY, Shu HB. The E3 ubiquitin ligase RNF5 targets virus-induced signaling adaptor for ubiquitination and degradation. J Immunol 2010; 184: 6249–6255. [DOI] [PubMed] [Google Scholar]
- Du J, Zhang D, Zhang W, Ouyang G, Wang J, Liu X et al. pVHL negatively regulates antiviral signaling by targeting MAVS for proteasomal degradation. J Immunol 2015; 195: 1782–1790. [DOI] [PubMed] [Google Scholar]
- Pan Y, Li R, Meng JL, Mao HT, Zhang Y, Zhang J. Smurf2 negatively modulates RIG-I-dependent antiviral response by targeting VISA/MAVS for ubiquitination and degradation. J Immunol 2014; 192: 4758–4764. [DOI] [PubMed] [Google Scholar]
- Zhou X, You F, Chen H, Jiang Z. Poly(C)-binding protein 1 (PCBP1) mediates housekeeping degradation of mitochondrial antiviral signaling (MAVS). Cell Res 2012; 22: 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Z, Fu J, Xiong Y, Tang H. SUMOylation of RIG-I positively regulates the type I interferon signaling. Protein Cell 2010; 1: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Xiong Y, Xu Y, Cheng G, Tang H. MDA5 is SUMOylated by PIAS2beta in the upregulation of type I interferon signaling. Mol Immunol 2011; 48: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming-Ming Hu C-YL, Yang Qing, Xie Xue-Qin, Shu Hong-Bing. Innate immunity to RNA virus is regulated by temporal and reversible sumoylation of RIG-I and MDA5. J Exp Med 2016. in revision. [DOI] [PMC free article] [PubMed]
- Liu X, Lei X, Zhou Z, Sun Z, Xue Q, Wang J et al. Enterovirus 71 induces degradation of TRIM38, a potential E3 ubiquitin ligase. Virol J 2011; 8: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlova A, Erttmann SF, Raffi FA, Schmalz AM, Resch U, Anugula S et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity 2015; 42: 332–343. [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2012; 339: 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 2013; 341: 1390–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 2009; 138: 576–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 2007; 448: 501–505. [DOI] [PubMed] [Google Scholar]
- Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol 2012; 13: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 2010; 11: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen R, Zhou Q, Xu Z, Li C, Wang S et al. LSm14A is a processing body-associated sensor of viral nucleic acids that initiates cellular antiviral response in the early phase of viral infection. Proc Natl Acad Sci USA 2012; 109: 11770–11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ, Mansur DS, Peters NE, Ren H, Smith GL. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. Elife 2012; 1: e00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Pazhoor S, Bao M, Zhang Z, Hanabuchi S, Facchinetti V et al. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc Natl Acad Sci USA 2010; 107: 15181–15186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Kobayashi J, Saitoh T, Maruyama K, Ishii KJ, Barber GN et al. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci USA 2013; 110: 2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao TS, Fitzgerald KA. The cGAS-STING pathway for DNA sensing. Mol Cell 2013; 51: 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzusch PJ, Vance RE. cGAS dimerization entangles DNA recognition. Immunity 2013; 39: 992–994. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008; 455: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 2008; 29: 538–550. [DOI] [PubMed] [Google Scholar]
- Dobbs N, Burnaevskiy N, Chen D, Gonugunta VK, Alto NM, Yan N. STING Activation by Translocation from the ER Is Associated with Infection and Autoinflammatory Disease. Cell Host Microbe 2015; 18: 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Lin H, Wang S, Wang S, Ran Y, Liu Y et al. The ER-associated protein ZDHHC1 is a positive regulator of DNA virus-triggered, MITA/STING-dependent innate immune signaling. Cell Host Microbe 2014; 16: 450–461. [DOI] [PubMed] [Google Scholar]
- Luo WW, Li S, Li C, Lian H, Yang Q, Zhong B et al. iRhom2 is essential for innate immunity to DNA viruses by mediating trafficking and stability of the adaptor STING. Nat Immunol 2016; 17: 1057–1066. [DOI] [PubMed] [Google Scholar]
- Bowie A. The STING in the tail for cytosolic DNA-dependent activation of IRF3. Sci Signal 2012; 5: pe9. [DOI] [PubMed] [Google Scholar]
- Konno H, Konno K, Barber GN. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell 2013; 155: 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol 2015; 15: 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Ye B, Wang S, Zhu X, Du Y, Xiong Z et al. Glutamylation of the DNA sensor cGAS regulates its binding and synthase activity in antiviral immunity. Nat Immunol 2016; 17: 369–378. [DOI] [PubMed] [Google Scholar]
- Wei-Wei Luo H-BS. Delicate Regulations of cGAS-MITA-mediated innate immune response. Cell Mol Immunol 2016. [DOI] [PMC free article] [PubMed]
- Seo GJ, Yang A, Tan B, Kim S, Liang Q, Choi Y et al. Akt kinase-mediated checkpoint of cGAS DNA sensing pathway. Cell Rep 2015; 13: 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Cai X, Wu J, Cong Q, Chen X, Li T et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 2015; 347: aaa2630. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Zou J, Saitoh T, Kumar H, Abe T, Matsuura Y et al. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity 2010; 33: 765–776. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hu MM, Wang YY, Shu HB. TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J Biol Chem 2012; 287: 28646–28655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu X, Cui Y, Tang Y, Chen W, Li S et al. The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 2014; 41: 919–933. [DOI] [PubMed] [Google Scholar]
- Zhong B, Zhang L, Lei C, Li Y, Mao AP, Yang Y et al. The ubiquitin ligase RNF5 regulates antiviral responses by mediating degradation of the adaptor protein MITA. Immunity 2009; 30: 397–407. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhou MT, Hu MM, Hu YH, Zhang J, Guo L et al. RNF26 temporally regulates virus-triggered type I interferon induction by two distinct mechanisms. PLoS Pathog 2014; 10: e1004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MM, Yang Q, Xie XQ, Liao CY, Lin H, Liu TT et al. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity 45: 555–569. [DOI] [PubMed] [Google Scholar]
- Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell Mol Life Sci 2008; 65: 2964–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol 2005; 7: 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal 2010; 3: cm1. [DOI] [PubMed] [Google Scholar]
- Chen R, Li M, Zhang Y, Zhou Q, Shu HB. The E3 ubiquitin ligase MARCH8 negatively regulates IL-1beta-induced NF-kappaB activation by targeting the IL1RAP coreceptor for ubiquitination and degradation. Proc Natl Acad Sci USA 2012; 109: 14128–14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA 2008; 105: 11778–11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Zhang Y, Zhong B, Wang YY, Diao FC, Wang RP et al. RBCK1 negatively regulates tumor necrosis factor- and interleukin-1-triggered NF-kappaB activation by targeting TAB2/3 for degradation. J Biol Chem 2007; 282: 16776–16782. [DOI] [PubMed] [Google Scholar]
- Gong J, Shen XH, Qiu H, Chen C, Yang RG. Rhesus monkey TRIM5alpha represses HIV-1 LTR promoter activity by negatively regulating TAK1/TAB1/TAB2/TAB3-complex-mediated NF-kappaB activation. Arch Virol 2011; 156: 1997–2006. [DOI] [PubMed] [Google Scholar]
- Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS Lett 1999; 442: 147–150. [DOI] [PubMed] [Google Scholar]
- He X, Li Y, Li C, Liu LJ, Zhang XD, Liu Y et al. USP2a negatively regulates IL-1beta- and virus-induced NF-kappaB activation by deubiquitinating TRAF6. J Mol Cell Biol 2013; 5: 39–47. [DOI] [PubMed] [Google Scholar]
- Xiao N, Li H, Luo J, Wang R, Chen H, Chen J et al. Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFalpha-induced cancer cell migration. Biochem J 2012; 441: 979–986. [DOI] [PubMed] [Google Scholar]
- Yasunaga J, Lin FC, Lu X, Jeang KT. Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 tax to negatively regulate NF-kappaB signaling. J Virol 2011; 85: 6212–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 2003; 424: 793–796. [DOI] [PubMed] [Google Scholar]
- Saito K, Kigawa T, Koshiba S, Sato K, Matsuo Y, Sakamoto A et al. The CAP-Gly domain of CYLD associates with the proline-rich sequence in NEMO/IKKgamma. Structure 2004; 12: 1719–1728. [DOI] [PubMed] [Google Scholar]
- Fan YH, Yu Y, Mao RF, Tan XJ, Xu GF, Zhang H et al. USP4 targets TAK1 to downregulate TNFalpha-induced NF-kappaB activation. Cell Death Differ 2011; 18: 1547–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell 2008; 133: 693–703. [DOI] [PubMed] [Google Scholar]
- Zheng H, Li Q, Chen R, Zhang J, Ran Y, He X et al. The dual-specificity phosphatase DUSP14 negatively regulates tumor necrosis factor- and interleukin-1-induced nuclear factor-kappaB activation by dephosphorylating the protein kinase TAK1. J Biol Chem 2013; 288: 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke V, Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J Autoimmun 2012; 39: 77–82. [DOI] [PubMed] [Google Scholar]
- O'Brien BA, Archer NS, Simpson AM, Torpy FR, Nassif NT. Association of SLC11A1 promoter polymorphisms with the incidence of autoimmune and inflammatory diseases: a meta-analysis. J Autoimmun 2008; 31: 42–51. [DOI] [PubMed] [Google Scholar]
- Wolska N, Rybakowska P, Rasmussen A, Brown M, Montgomery C, Klopocki A et al. Brief report: patients with primary Sjogren's syndrome who are positive for autoantibodies to tripartite motif-containing protein 38 show greater disease severity. Arthritis Rheumatol 2016; 68: 724–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamozo S, Akasbi M, Brito-Zeron P, Bosch X, Bove A, Perez-de-Lis M et al. Anti-Ro52 antibody testing influences the classification and clinical characterisation of primary Sjogren's syndrome. Clin Exp Rheumatol 2012; 30: 686–692. [PubMed] [Google Scholar]
- Szczerba BM, Kaplonek P, Wolska N, Podsiadlowska A, Rybakowska PD, Dey P et al. Interaction between innate immunity and Ro52-induced antibody causes Sjogren's syndrome-like disorder in mice. Ann Rheum Dis 2016; 75: 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Nakagawa K, Abe J, Awaya T, Funabiki M, Hijikata A et al. Aicardi-Goutieres syndrome is caused by IFIH1 mutations. Am J Hum Genet 2014; 95: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, del Toro Duany Y, Jenkinson EM, Forte GM, Anderson BH, Ariaudo G et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 2014; 46: 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyck L, De Somer L, Pombal D, Bornschein S, Frans G, Humblet-Baron S et al. Brief report: IFIH1 mutation causes systemic lupus erythematosus with selective IgA deficiency. Arthritis Rheumatol 2015; 67: 1592–1597. [DOI] [PubMed] [Google Scholar]
- Rutsch F, MacDougall M, Lu C, Buers I, Mamaeva O, Nitschke Y et al. A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am J Hum Genet 2015; 96: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MA, Kim EK, Now H, Nguyen NT, Kim WJ, Yoo JY et al. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am J Hum Genet 2015; 96: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]