Highlights
-
•
The complement system plays a central role in age-related macular degeneration (AMD).
-
•
Common and rare genetic variants in complement genes have been identified in AMD.
-
•
Several of the rare variants affect the functioning of the complement system.
-
•
However, a genetic association with AMD cannot always be proven.
-
•
Functional assays can help identify patients for complement inhibiting therapies.
Keywords: Age-related macular degeneration, Complement system, Alternative pathway, Rare genetic variants
Abstract
Age-related macular degeneration (AMD) is a progressive retinal disease and the major cause of irreversible vision loss in the elderly. Numerous studies have found both common and rare genetic variants in the complement pathway to play a role in the pathogenesis of AMD. In this review we provide an overview of rare variants identified in AMD patients, and summarize the functional consequences of rare genetic variation in complement genes on the complement system. Finally, we discuss the relevance of this work in light of ongoing clinical trials that study the effectiveness of complement inhibitors against AMD.
1. Clinical characteristics of age-related macular degeneration (AMD)
Age-related macular degeneration (AMD) is the leading cause of irreversible vision loss among the elderly, accounting for 8.7% of blindness worldwide. AMD is most prevalent in populations of European ancestry with approximately 1–3% of the total population suffering from an advanced form of AMD (Chakravarthy et al., 2010b, Tomany et al., 2004, Wong et al., 2014). Globally, the total number of patients with any type of AMD is expected to increase over the next 25 years to 288 million affected individuals (Wong et al., 2014).
The disease is characterized by a gradual loss of central vision due to photoreceptor cell degeneration in the centre of the retina at the back of the eye, known as the macula. Photoreceptors are in close contact with a layer of cells called the retinal pigment epithelium (RPE). RPE cells support the function of the photoreceptors and play an important role in maintaining retinal homeostasis. In AMD, this natural function of the RPE is disturbed, resulting in the accumulation of retinal waste products called drusen underneath the RPE. Drusen are the tell tale sign of AMD and are easily recognized by ophthalmologists.
AMD is a progressive retinal disease is which the early stage is characterized by relatively few small drusen within the macula. When AMD progresses, drusen size and number increase, eventually leading towards more advanced stages of AMD. Two forms of advanced AMD are distinguished. The first form, neovascular AMD, is characterized by infiltration of abnormal blood vessels into the retina. These newly formed vessels are fragile and when they break, the leakage of blood constituents in the retina leads to sudden vision loss. The second form of advanced AMD, geographic atrophy, is the result of gradual degeneration of the RPE and photoreceptors cells. Although neovascularization occurs in only 15–20% of AMD cases, it is responsible for the vast majority of vision loss caused by AMD. Drugs targeting vascular endothelial growth factor (VEGF), one of the central molecules in neovascularization, have proven to be very successful in neovascular AMD. However, no treatment is available for the remaining majority of early, intermediate or geographic atrophy AMD cases, and furthermore there are no effective means of preventing progression of early to advanced stages (Chakravarthy et al., 2010a, Jager et al., 2008).
2. The complement system plays a central role in the etiology of AMD
2.1. Research on the etiology of AMD: a historical perspective
Today it is known that AMD is the result of a complex interaction of environmental and genetic risk factors. Pooled evidence from numerous studies has demonstrated that environmental factors like aging itself, smoking behavior, and body mass index (BMI) are strong risk factors for AMD. In addition, cataract surgery, cardiovascular disease and family history are also strongly associated (Chakravarthy et al., 2010b). Before any specific gene or biological pathway had been conclusively linked to AMD, studies into the molecular constituents of drusen had already suggested that AMD may have an immunological component. This suggestion arose after proteins involved in inflammation and/or other immune-associated responses, including components of the complement system, were found within drusen (Hageman et al., 2001, Johnson et al., 2001, Mullins et al., 2001).
Evidence for a strong genetic component in AMD arose from twin and family studies. Twin studies observed a high concordance of AMD between monozygotic pairs, even double compared to dizygotic pairs, and estimated that the heritability of AMD may be as high as 45 to 70% (Hammond et al., 2002, Meyers et al., 1995, Seddon et al., 2005). These findings were in line with familial aggregation analyses that observed a higher prevalence of AMD characteristics and an earlier onset of disease symptoms among relatives of patients compared to control families (Klaver et al., 1998, Seddon et al., 1997).
2.2. Genetic evidence for a role of the complement system in AMD
In search for genomic regions implicated in AMD, genetic linkage analyses were done in large family-based studies (Abecasis et al., 2004, Iyengar et al., 2004, Majewski et al., 2003, Seddon et al., 2003, Weeks et al., 2004). Among a few other regions, the findings from these studies strongly and consistently implicated a region on chromosome 1 in the disease. When the first genome-wide association study (GWAS) for AMD was performed in 2005, it identified that same genomic region, which lead to the discovery of a highly associated genetic variant in complement factor H (CFH; Tyr402His) (Klein et al., 2005). These findings were corroborated by three additional studies (Edwards et al., 2005, Hageman et al., 2005, Haines et al., 2005).
Through genetic studies that followed over the next decade, the understanding of the genetic basis of AMD increased dramatically with the identification of disease-associated variants across several biological systems (Fritsche et al., 2013). The genetic link between AMD and the complement system was further expanded when genetic variants in or near complement factor I (CFI), complement component 3 (C3), complement component 2 (C2), complement component 9 (C9), complement factor B (CFB) and vitronectin (VTN) were also found to be associated with the disease (Fagerness et al., 2009, Fritsche et al., 2013, Fritsche et al., 2016, Gold et al., 2006, Maller et al., 2007, Yates et al., 2007) (Table 1). In addition, a common haplotype carrying a deletion of complement factor H related genes CFHR1 and CFHR3 was found to be protective for AMD (Hughes et al., 2006).
Table 1.
Genes in the complement system associated with AMD.
| Gene/Locus | Approach | Referencea |
|---|---|---|
| C2/CFB | Candidate gene | Gold et al. (2006) |
| C3 | Candidate gene/WGS | Maller et al. (2007) and Yates et al., 2007)/(Helgason et al. (2013), Seddon et al. (2013) and Zhan et al. (2013) |
| C9 | Candidate gene | Nishiguchi et al. (2012) and Seddon et al. (2013) |
| CFH | Candidate gene/Linkage/GWAS | Edwards et al. (2005), Hageman et al. (2005), Haines et al. (2005) and Klein et al. (2005)/Raychaudhuri et al. (2011) |
| CFHR1-CFHR3 | Candidate gene | Hughes et al. (2006) |
| CFI | Candidate gene | Fagerness et al. (2009)/van de Ven et al. (2013) |
| VTN | GWAS | Fritsche et al. (2016) |
Reference of first cited association based on common and/or rare genetic variant.
2.3. The role of rare genetic variants in AMD
Common genetic variants (with a minor allele frequency (MAF) of >5% in the population) near the complement genes CFH, C2/CFB, C3 and CFI together explain 40–60% of the heritability of AMD (Fritsche et al., 2014). However, a large fraction of the heritability still remains unknown and is referred to as missing heritability. One hypothesis states that low frequency and rare genetic variants (with a MAF of <1–5% and <1%, respectively) may explain the remaining fraction of the heritability (Manolio et al., 2009). During the past years, genetic studies in AMD have therefore shifted towards the identification of rare genetic variants. However, a practical problem arises when analyzing rare variants. The number of patients and controls needed for the identification of novel variants increases when variants are more rare, since the sample size requirements increase roughly linearly with the inverse of the allele frequency. Therefore, analyses of very large cohorts are required for a comprehensive understanding of the role of rare genetic variants in AMD.
2.4. Genetic approaches to identify rare genetic variants in AMD
In order to discover rare variants investigators resort to other methods of analyses than those methods yielding insight into common variation. An effective approach that can be used to detect rare disease-associated variants is through a GWAS using exome chips. An exome chip is an array containing both common genetic variants as well as rare exonic variants, and is cost-effective in capturing a specific set of variants in large case-control studies. These chips can be customized and enriched for specific variants of interest. The approach is limited in the sense that it cannot discover new genetic variants other than the ones that the chip captures, but after imputation the chip covers over 12 million variants across the genome (Fritsche et al., 2016). A recent large GWAS using exome chips detected 52 (45 common and 7 rare) variants at 34 genomic regions that are independently associated with AMD. More than one third (19/52) of these variants reside in or near a gene of the complement system: C2/CFB, C3, C9, CFH, CFI, and VTN (Table 1). Besides evaluating the association of single genetic variants with the disease, the cumulative number of rare variants detected across an entire gene can be compared between patients and control individuals using burden tests. Interestingly, a significant burden of rare variants in the CFH and CFI genes, in addition to two other genes (TIMP3 and SLC16A1), was observed in AMD (Fritsche et al., 2016).
Another approach that is widely used to detect rare variants is sequence analysis of candidate genes in cases and controls. An advantage of this approach above the use of exome chips is that it can discover new genetic variants, thereby allowing a comprehensive analysis of all genetic variation in a candidate gene or a set of candidate genes. With the development of next-generation sequencing technologies, tens to hundreds of genes can effectively be analyzed in large cohorts consisting of thousands of individuals. The candidate gene approach has been successfully employed in AMD in several studies, which have mainly focused on sequencing of genes of the complement system and other genes previously associated with AMD. These studies lead to the discovery of rare variants in the CFH, CFI, C3 and C9 genes in AMD (Kavanagh et al., 2015, Seddon et al., 2013, Triebwasser et al., 2015, van de Ven et al., 2013, Zhan et al., 2013).
Whereas candidate gene sequencing is a very targeted approach, whole exome sequencing (WES) or whole genome sequencing (WGS) can interrogate genetic variants in all coding regions of the genome (WES) or even the entire genome (WGS). Since WES and WGS are expensive to perform in large cohorts, approaches can be used to enrich for rare variants, for example by analyzing large AMD families. Recent studies in AMD using WES and WGS have successfully identified novel genetic variants in AMD using a case-control cohort (Helgason et al., 2013) or by analyzing multiple affected individuals of large AMD families (Duvvari et al., 2016, Geerlings et al., 2016, Hoffman et al., 2014, Pras et al., 2015, Ratnapriya et al., 2014, Saksens et al., 2016, Yu et al., 2014). New genetic variants were detected in CFH, CFI, C3 and C9, in addition to other genes (FBN2 and HMCN1). Although rare variants segregated with AMD in some of these families (Pras et al., 2015, Ratnapriya et al., 2014, Yu et al., 2014), several variants did not perfectly segregate with the disease, but were enriched in cases compared to control individuals (Geerlings et al., 2016, Hoffman et al., 2014, Saksens et al., 2016). This is in line with the complex etiology of AMD, to which both common and rare genetic variants, and also environmental factors contribute.
3. Rare genetic variants in the complement system
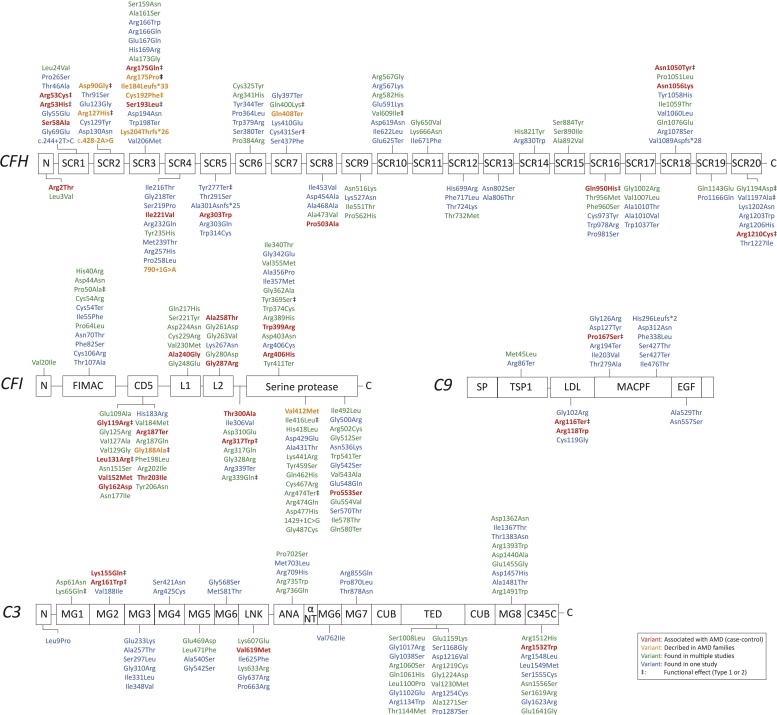
Multiple rare genetic variants in the complement system have been associated with AMD. The following paragraphs summarize these variants, focusing on the ones that were found in more than a single AMD patient. A complete list of rare variants described in literature is presented in Supplementary Table 1 and visualized in Fig. 1.
Fig. 1.
Rare coding variants in CFH, CFI, C3 and C9 found in AMD patients.
Variants are color-coded as follows: significantly (p < 0.05) associated with AMD in one or more AMD case-control cohorts (in red), were found in AMD families (in orange), were found in more than one AMD cohort (in green), or were found in one AMD cohort (in blue). Variants notated with ‡ have a functional effect on the protein or change systemic levels. CFH: Complement Factor H; CFI Complement Factor I; C3 Complement C3; C9 Complement C9.
3.1. Complement factor H
An important rare variant associated with AMD, CFH Arg1210Cys, was discovered after re-sequencing a rare risk haplotype in CFH (Raychaudhuri et al., 2011). The authors demonstrated that the Arg1210Cys variant was highly associated with AMD, independently of the common variant Tyr402His. Moreover, carriers of this variant were significantly younger when the first symptoms of AMD appeared. The Arg1210Cys variant conferred a 47 times higher risk of developing AMD (Fritsche et al., 2016).
Earlier, nonsense variant Gln408Ter and missense variant Arg1078Ser in CFH had already been identified in families that presented with a particular subtype of AMD known as cuticular drusen. Here it was argued that the rare variant, in addition to the common variant CFH Tyr402His, may underlie the phenotype (Boon et al., 2008). In addition, in other families with cuticular drusen, frameshift variants Ile184Leufs*33 and Lys204Thrfs*26 were identified, also independently of CFH Tyr402His (van de Ven et al., 2012).
Splice site variant CFH c.790 + 1G > A and coding variants CFH Arg53Cys, Asp90Gly, Arg127His, Arg175Pro, Arg175Gln, Cys192Phe, and Ser193Leu were identified by WES of AMD families in which known genetic risk factors could not explain the high burden of disease (Geerlings et al., 2016, Wagner et al., 2016, Yu et al., 2014). Variant CFH Pro503Ala was identified using WES in an Amish family after exclusion of the other main risk variants for AMD, and was significantly associated with AMD in an Amish AMD case-control cohort (Hoffman et al., 2014) (Table 2). Next-generation sequencing was performed for CFH in a cohort of 2417 individuals, demonstrating an enrichment of rare variants in functional domains of factor H in AMD. In this study, 65 coding CFH variants were identified of which only 15 rare variants were found in more than one affected individual; the other variants were found only in single cases (Table 3, Supplementary Table 1) (Triebwasser et al., 2015).
Table 2.
Rare variants in complement genes associated with AMD and accompanying OR/LOD scores.
| Gene | Variant | Effect (Odds Ratio) | Significance (P-value and/or LOD score) | Study |
|---|---|---|---|---|
| CFH | Arg2Thr | Risk (14.08) | P = 0.0158** | Fritsche et al. (2016) |
| CFH | Arg53Cys | Risk (22.54) | LOD score 5.07, P = 6.7 × 10−7 P = 0.00118** |
Yu et al. (2014) and Fritsche et al. (2016) |
| CFH | Arg53His | Risk (13.39) | P = 0.01** | Fritsche et al. (2016) |
| CFH | Ser58Ala | Risk (2.82) | P = 0.00702** | Fritsche et al. (2016) |
| CFH | Asp90Gly | Risk | LOD score 1.22, P = 0.009 | Yu et al. (2014) |
| CFH | Arg175Gln | Risk (1.50) | P = 0.04 | Geerlings et al. (2016) |
| CFH | Ser193Leu | Risk | P = 0.01 | Geerlings et al. (2016) |
| CFH | Ile221Val | Risk (11.80) | P = 0.0314** | Fritsche et al. (2016) |
| CFH | Arg303Trp | Risk (12.25) | P = 0.0378** | Fritsche et al. (2016) |
| CFH | Pro503Ala | Risk | P = 9.27 × 10−13 | Hoffman et al. (2014) |
| CFH | Gln950His | Protective (0.72) | P = 0.00258** | Fritsche et al. (2016) |
| CFH | Asn1050Tyr | Protective (0.36) | P = 5.92 × 10−44** | Fritsche et al. (2016) |
| CFH | Asn1056Lys | Protective (0.08) | P = 0.024** | Fritsche et al. (2016) |
| CFH | Arg1210Cys | Risk (31.8) | P = 3.2 × 10−31* | Fritsche et al. (2016) |
| CFI | Gly119Arg | Risk (3.87) | P = 8.6 × 10−11* | Fritsche et al. (2016) |
| CFI | Leu131Arg | Risk | P = 0.02 | Geerlings et al. (2016) |
| CFI | Val152Met | Risk (7.57) | P = 4.65 × 10−4** | Fritsche et al. (2016) |
| CFI | Gly162Asp | Risk (20.29) | P = 0.00231** | Fritsche et al. (2016) |
| CFI | Arg187Ter | Risk (13.63) | P = 0.0175** | Fritsche et al. (2016) |
| CFI | Thr203Ile | Risk (2.46) | P = 0.0344** | Fritsche et al. (2016) |
| CFI | Ala240Gly | Risk (7.43) | P = 0.02 | Kavanagh et al. (2015) |
| CFI | Ala258Thr | Risk (3.88) | P = 6.25 × 10−5** | Fritsche et al. (2016) |
| CFI | Gly287Arg | Risk (4.61) | P = 0.00761** | Fritsche et al. (2016) |
| CFI | Ala300Thr | eRisk (2.67) | P = 0.0144** | Fritsche et al. (2016) |
| CFI | Arg317Trp | Risk (12.20) | P = 1.97 × 10−4** | Fritsche et al. (2016) |
| CFI | Arg339Gln | Risk (11.83) | P = 0.0312** | Fritsche et al. (2016) |
| CFI | Arg406His | Protective (0.10) | P = 0.02 | Kavanagh et al. (2015) |
| CFI | Val412Met | Risk | LOD score 2.51 | Pras et al. (2015) |
| CFI | Pro553Ser | Risk (3.7; 2.69) | P = 0.04; P = 0.03 | Geerlings et al. (2016) and Kavanagh et al. (2015) |
| C3 | Lys155Gln | Risk (3.12) | P = 1.5 × 10−32* | Fritsche et al. (2016) |
| C3 | Arg161Trp | Risk | P = 0.01 | Geerlings et al. (2016) |
| C3 | Val619Met | Risk (2.66) | P = 2.38 × 10−4** | Fritsche et al. (2016) |
| C3 | Arg1532Trp | Risk (12.29) | P = 0.0379** | Fritsche et al. (2016) |
| C9 | Arg116Ter | Protective (0.20) | P = 0.021 | Nishiguchi et al. (2012) |
| C9 | Arg118Trp | Risk (1.12) | OR = 0.04 | Geerlings et al. (2016) |
| C9 | Pro167Ser | Risk (1.79) | P = 1.6 × 10−14* | Fritsche et al. (2016) |
IAMDGC genome-wide significant, Locus-wide conditioned analysis (adjusting for the identified index variant(s) in the locus).
Did not reach genome-wide significance, p < 0.05, Not conditioned for index variant(s) in the locus.OR = Odds-ratio, LOD = logarithm of the odds.
Table 3.
Described functional effects of rare variants in the complement system.
| Gene | Variant | Functional implication | Sources |
|---|---|---|---|
| CFH | Arg53Cys | Reported to possibly affect the local conformation of FH. This variant did not affect levels of FH in serum of 22 individuals, but showed slightly reduced binding affinity to C3b compared to wild type. Marked loss of decay accelerating activity. Trend towards lower cofactor activity for FI. | Yu et al. (2014) |
| Arg53His | Like the 53Cys variant, the 53His variant showed minor decreased affinity to bind C3b. Independent of the C3b affinity, the variant strongly affected co-factor activity of FI. In addition, the variant disrupted decay accelerating activity and was shown to correlate to low C3 levels. | Janssen van Doorn et al. (2013) and Pechtl et al. (2011) | |
| Asp90Gly | No reported effect on FH levels in serum from 22 individuals. No effect on C3b binding affinity and decay accelerating activity. Significantly reduced cofactor activity for FI | Yu et al. (2014) | |
| Arg127His | Reduced FH serum levels in heterozygous and homozygous carriers and no secretion of the protein. | Albuquerque et al. (2012), Falcao et al. (2008) and Wagner et al. (2016) | |
| Arg175Pro | Reduced FH serum levels and no secretion of the recombinant protein. | Wagner et al. (2016) | |
| Arg175Gln | No reported effect on FH levels. Reduced C3b degradation ability. | Geerlings et al. (2016) | |
| Cys192Phe | Lower expression of FH and reduced secretion of the protein. Normal C3 in plasma of one carrier | Triebwasser et al. (2015) and Wagner et al. (2016) | |
| Ser193Leu | No reported effect on FH levels. Reduced C3b degradation ability. | Geerlings et al. (2016) | |
| Tyr277Ter | Lower expression of FH and normal C3 in plasma of one carrier | Triebwasser et al. (2015) | |
| Arg303Gln | Normal plasma levels for FH, FI and C3 | Fakhouri et al. (2008) | |
| Gln400Lys | Lower FH levels, but no effect on plasma concentrations of C3 and FB | Dragon-Durey et al. (2004) | |
| Cys431Ser | Lower expression of FH and normal C3 in plasma of one carrier | Triebwasser et al. (2015) | |
| Val609Asp | Affects FH expression and resulted in decreased alternative pathway activity and C3 level in remission | Szarvas et al. (2016) | |
| Ser890Ile | The variant did not result is differences in FH co-activity with FI. The C3b binding was not affected and FH concentration in plasma were normal. In addition a hemolytic assay showed that the capacity to regulate the alternative pathway on cellular surfaces was normal. | Tortajada et al. (2012) | |
| Gln950His | This variant demonstrated reduced erythrocyte binding and, consequently, increased lysis after serum addition to sheep erythrocytes. Patient plasma levels of FH were not different compared to controls, but transient expression levels of mutant lagged behind that of the wild type. No impaired cofactor binding for FI was observed and normal complementary inhibitory functions were observed. | Mohlin et al. (2015) and Szarvas et al. (2016) | |
| Thr956Met | No effect on C3 or FH levels in plasma. The lysis of erythrocytes was not increased and no effect on protein expression was shown. | Perez-Caballero et al. (2001) and Szarvas et al. (2016) | |
| Val1007Leu | No differences in FH co-activity nor C3b binding; normal hemolytic assay (capacity to regulate the alternative pathway on cellular surfaces); normal FH in plasma | Tortajada et al. (2012) | |
| Asn1050Tyr | Abnormal C3 and normal FH levels in serum | Stahl et al. (2008) | |
| Gln1076Glu | Normal C3 and FH levels in serum | Neumann et al. (2003) | |
| Gly1194Asp | Slightly increased complement regulatory function of mutant FH on cell surfaces (sheep erythrocyte lysis); normal C3 FH and FI levels in serum | Bresin et al. (2013) and Johnson et al. (2010) | |
| Val1197Ala | Normal lysis of sheep erythrocytes, low FH and C3 levels, and shows low binding to surface bound C3b. | Heinen et al. (2006) and Sanchez-Corral et al. (2002) | |
| Arg1203Trp | Hemolytic test showed no lysis | Szarvas et al. (2016) | |
| Arg1210Cys | This variant results in a covalent binding to human serum albumin which hampers all FH functional domains. It also shows reduced binding to heparin and endothelial cells and binding to C3b and C3d is also decreased. No effects on cofactor activity for FI was reported and no effect on erythrocyte lysis was shown. | Ferreira et al. (2009), Jozsi et al. (2006), Manuelian et al. (2003), Recalde et al. (2016) and Sanchez-Corral et al. (2002) | |
| 244 + 2T > C splice site |
Normal expression of FH and high C3 in plasma of one carrier | Triebwasser et al. (2015) | |
| 790 + 1G > A; splice site | Lower expression of FH in three carriers and low C3 in plasma of one carrier | Triebwasser et al. (2015) and Wagner et al. (2016) | |
| CFI | Pro50Ala (Pro32Ala)* |
Elevated FB in plasma; normal C3 and FI in plasma; impaired function towards degradation of the alpha-chains of C4b and C3b in solution when FH was used as cofactor | Bienaime et al. (2010) |
|
Gly119Arg (Gly101Arg)* |
This variant resulted in reduced FI levels in human serum as well as in transient in vitro expression studies. The variant resulted in a lower ability to degrade C3b due to impaired expression and secretion of the mutant protein. | van de Ven et al. (2013), Geerlings et al. (2016) and Kavanagh et al. (2015) | |
|
Leu131Arg (Gly113Arg) |
The variant resulted in a lower ability to degrade C3b which could be due to impaired expression and secretion of the mutant protein. | Geerlings et al. (2016) | |
|
Gly188Ala (Gly170Ala)* |
This variant resulted in lower FI levels in human serum as well as in transient in vitro expression studies. The variant resulted in impaired degradation of C3b. | van de Ven et al. (2013) | |
| Arg202Ile (Arg184Ile)* |
This variant had no effect on FI levels in human serum | Kavanagh et al. (2015) | |
|
Ala240Gly (Ala222Gly)* |
This variant resulted in lower or normal FI levels in human serum/plasma. The degradation of fluid phase C4b and C3b was normal, although the ability to cleave surface-bound C3b was impaired. | Caprioli et al. (2006), Kavanagh et al. (2015) and Nilsson et al. (2010) | |
| Gly261Asp (Gly243Asp)* |
No effect on FI levels in human serum; slightly different migration pattern; normal degradation of C3b and C4b. | Kavanagh et al., 2008, Kavanagh et al., 2015 and Nilsson et al. (2007) | |
| Thr300Ala (Thr282Ala)* |
No effect on FI levels in human serum | Kavanagh et al. (2015) | |
| Arg317Trp (Arg299Trp)* |
Normal FI plasma level and normal functioning on hemolytic assay; only impaired secretion compared to wildtype FI | Caprioli et al. (2006), Kavanagh et al. (2008) and Nilsson et al. (2010) | |
| Arg339Gln (Arg321Gln)* |
Reduced C3, FH, and FB levels, but normal FI levels in serum | Szarvas et al. (2016) | |
| Ile340Thr (Ile323Thr)* |
Normal FI and C3 levels in serum | Bresin et al. (2013) | |
| Tyr369Ser (Ile351Thr)* |
Normal FH and C4 levels; low C3 levels in serum | Chan et al. (2009) | |
|
Arg406His (Arg388His)* |
No effect on FI levels in human serum | Kavanagh et al. (2015) | |
| Ile416Leu (Ile398Leu)* |
Low FI and C3 serum levels; normal FB levels | Bienaime et al. (2010) and Sellier-Leclerc et al. (2007) | |
| His418Leu (His400Leu)* |
Homozygous variation results in FI deficiency (low or undetectable FI and C3 levels) | Vyse et al. (1996) | |
| Lys441Arg (Lys423Arg)* |
This variant had no effect on FI levels in human serum | Cayci et al. (2012) and Kavanagh et al. (2015) | |
| Tyr459Ser (Tyr441Ser)* |
Normal FI and C3 levels in serum | Bienaime et al. (2010) | |
| Arg474Gln (Arg456Gln)* |
Normal FI protein level. | Szarvas et al. (2016) | |
| Arg474Ter (Arg456Ter)* |
Low FI and C3 serum levels; normal FB levels | Bienaime et al. (2010), Fremeaux-Bacchi et al. (2004) and Nilsson et al. (2010) | |
|
Pro553Ser (Pro535Ser)* |
This variant had no effect on FI levels in human serum and slightly lower ability to degrade C3b. | Geerlings et al. (2016) and Kavanagh et al. (2015) | |
| C3 |
Lys65Gln (Lys43Gln)* |
This variants weakened the interaction of C3b and FH and showed reduced MCP binding affinity | Schramm et al. (2015) and Volokhina et al. (2012) |
|
Lys155Gln (Lys131Gln)* |
This variant resulted in significantly reduced cleavage of C3b in fluid phase cofactor assays as well as reduced binding to FH. MCP cofactor activity was not changed. | Fritsche et al. (2016), Helgason et al. (2013), Seddon et al. (2013) and Zhan et al. (2013) | |
|
Arg161Trp (Arg139Trp)* |
Reduced binding activity of C3b to FH in one study and no effect on binding and cleavage of C3 in other studies. MCP binding was reduced, FB binding was increased. This variant is discussed to be a gain-of-function variant of the convertase complex and C3a, C5a, C5b-9 formation was shown to be increased. | Geerlings et al. (2016), Martinez-Barricarte et al. (2015), Roumenina et al. (2012), Schramm et al. (2015) and Volokhina et al. (2012) | |
|
Arg735Trp (Arg713Trp)* |
This variant showed no functional effects on MCP binding, FI cofactor activity, FB binding, CR1 binding and FH binding. | Brackman et al. (2011) and Fremeaux-Bacchi et al. (2008) | |
| Leu1549Met (Leu1527Met)* | No influence on FH, MCP, or CR1 binding | Schramm et al. (2015) | |
| C9 | Arg95Ter | C9 serum concentration was below the level of detection | Fukumori et al. (1989) and Horiuchi et al. (1998) |
| Pro167Ser | Median C9 serum concentration was elevated in carriers compared to non-carriers | Geerlings et al. (2016) | |
Only variants on which functional analysis were done are shown. *described in literature without signaling peptide. Bold: genetic association with AMD through case-control analysis or found in multiple cases of within an AMD family.
Overall, three different splice site variants, ten different nonsense, four different frameshift and 106 different rare missense variants in CFH were detected in AMD case-control and family studies (Supplementary Table 1). Functional variants seem to cluster in SCR 1-4 domains which mediate complement regulation of the protein, and SCR 19-20 which allow attachment of FH to the host cell. In total, 10 of 15 identified functional variants affect amino acid residues in one of these domains. In total, 124 variants were found of which 14 were significantly (p < 0.05) associated with AMD (Table 2). The majority of the coding variants (68/124) were found in only one study cohort and were not significantly associated with AMD.
3.2. Complement factor I
After sequencing the entire CFI gene in a subset of patients, and subsequent replication in a number of large case-control cohorts, the variant Gly119Arg was shown to be strongly associated with AMD (van de Ven et al., 2013). The Gly119Arg variant conferred a 5 times higher risk of developing AMD (Fritsche et al., 2016).
Additional variants in CFI have been identified in AMD families, including Gly188Ala (van de Ven et al., 2013), Leu131Arg (Geerlings et al., 2016), and Val412Met (Pras et al., 2015). It has been demonstrated that the CFI gene is enriched for rare variants four-fold in AMD cases compared to controls, and that these variants largely reside in the catalytic domain (residing in the serine protease domain) of the protein (Seddon et al., 2013). Of the 70 variants identified in this study (Kavanagh et al., 2015), eight coding variants were confirmed in five or more individuals, while the majority of variants were found only once (Supplementary Table 1). None of the variants were individually associated with AMD, although three variants showed a nominal association (CFI Pro553Ser, Arg406His and Ala240Gly) (Table 2).
Overall, one splice site variant, seven different nonsense, and 86 different rare missense variants in CFI were detected in AMD case-control and family studies (Supplementary Table 1). The variants appear to cluster in the serine protease domain, with 42 of 94 identified variants affecting amino acid residues in this domain, which is in accordance with a previous report (Seddon et al., 2013). Of these variants, 14 were significantly (p < 0.05) associated with AMD (Table 2).
3.3. Complement component 3
The Lys155Gln variant in C3 was described to be associated with AMD independently by three studies (Helgason et al., 2013, Seddon et al., 2013, Zhan et al., 2013). The Lys155Gln variant confers a 3 times higher risk of developing AMD (Fritsche et al., 2016).
In a sequencing study of all coding exons of the C3 gene, four other C3 variants were identified: Lys65Gln, Arg161Trp, Arg735Trp and Ser1619Arg (Duvvari et al., 2014). All but Arg161Trp were found to be associated in the index cohort, but none of the rare variant associations were replicated in an independent cohort. The Arg735Trp and Ser1619Arg variants and two additional variants, Val619Met and Lys633Arg, were identified by next-generation sequencing of the C3 gene in 1676 cases and 745 controls, but none of the variants were found to be significantly associated with AMD (Seddon et al., 2013).
Overall, 71 different rare missense variants in C3 were detected in AMD case-control and family studies (Supplementary Table 1). The variants that effect protein function are located at the first and second MG domains. Of these variants, four variants were significantly (p < 0.05) associated with AMD (Table 2). The majority (39/71) of variants were found in only one in one study cohort and were not significantly associated with AMD.
3.4. Complement component 9
Sequence analysis of the C9 gene in 1676 cases and 745 controls demonstrated that the Pro167Ser variant confers an increased risk of developing AMD (Seddon et al., 2013). The Pro167Ser variant was confirmed to confer risk of AMD in other cohorts as well (Saksens et al., 2016), with a 1.7 times increased risk of developing AMD (Fritsche et al., 2016).
Sequence analysis of C9 also identified 2 other variants, Met45Leu and Ile203Val, but these were not found to be significantly associated with AMD (Seddon et al., 2013). In addition, variant Arg118Trp was identified in an AMD family with three affected siblings (Geerlings et al., 2016). The nonsense variant Arg95Ter has been associated with a reduced risk for advanced AMD but is a founder mutation of East Asian origin and extremely rare in European populations (Nishiguchi et al., 2012).
Overall, four nonsense, one frameshift and 15 different rare missense variants in C9 were detected in AMD case-control and family studies (Supplementary Table 1). Of these variants, three were significantly (p < 0.05) associated with AMD (Table 2). The majority (16/20) of variants were found in one study cohort and were not significantly associated with AMD.
4. Functional implication of rare genetic variants
A genetic association provides statistical evidence that a particular variant is implicated in the disease, but if offers no insight into the molecular mechanisms that underlie and lead to the disease. To better understand this, the functional consequences of genetic variants on the complement system need to be investigated. Although the complement system acts locally, complement components or activation products can be detected systemically, for example in serum or plasma. In literature, several studies have described the expression of complement regulators and measurements of both complement components and activation products in AMD patients compared to controls. In addition, for some variants in vitro studies have been performed to examine their effect. In the next paragraphs we summarize the rare variants described in AMD literature, and detail their functional effects (Table 3).
4.1. Complement factor H
Complement factor H (FH) is an inhibitor and plays a key role in the alternative pathway of the complement system. FH protects tissues by inhibiting the formation of excess C3 convertase through competition with factor B (FB) in the binding of C3b, and in addition promoting the decay of surplus C3 convertase.
The Arg1210Cys variant showed reduced binding to C3b, C3d and heparin but normal cofactor activity to factor I (FI) (Manuelian et al., 2003, Sanchez-Corral et al., 2002). Through the introduction of a cysteine residue, Arg1210Cys forms covalent interactions with human serum albumin (Sanchez-Corral et al., 2002). It has been postulated that it is the albumin bound to FH rather than any functional defect of the protein itself that eventually disrupts FH function (Recalde et al., 2016).
Variants Arg53Cys and Asp90Gly are both located within the first four domains of FH which are known to bind C3b, however only Arg53Cys showed minor decreased affinity to bind C3b (Yu et al., 2014). Independent of the C3b affinity, the variants strongly affected co-factor activity of FI. In addition, Arg53Cys disrupted decay accelerating activity and was shown to correlate to low C3 levels (Fakhouri et al., 2010, Servais, 2012, Yu et al., 2014). Later, variants c.790 + 1G > A, Arg127His, Arg175Pro and Cys192Phe were analyzed for levels of serum concentration, and all variants, except Arg127His, had reduced FH serum levels compared to a control group. The coding variants all shown impaired protein secretion (Albuquerque et al., 2012, Wagner et al., 2016).
Variants can be grouped according to effect on the protein function. Type 1 mutations cause lower protein expression levels as a result of misfolding or degradation of the protein, in contrast to type 2 mutations that result in reduced functionality which is not necessarily reflected in protein levels. This distinction is also observed for variants found in CFH. Serum concentration of FH and C3 were measured in plasma samples of carriers of 5 CFH variants (Cys192Phe, Tyr277Ter, Cys431Ser, and two splice-site variants). For these variants, lower FH concentrations were observed in each of the carriers compared to a non-carrier control set (Triebwasser et al., 2015), and can thus be classified as type 1 mutations. For two other variants, Arg175Gln and Ser193Leu, serum levels were normal but these variants exhibited a reduced ability to degrade C3b (Geerlings et al., 2016), suggesting that they are type 2 mutations.
4.2. Complement factor I
Complement factor I (FI) is a serum serine protease that converts C3b and C4b to their inactive form to reduce the formation of the C3 and C5 convertases. Unbound C3b would otherwise result in increased C3 convertase formation and feedback amplification of the alternative pathway.
Overall, many rare variants in CFI result in lower FI levels in serum and consequently lower the regulatory activity of the alternative pathway (Kavanagh et al., 2015, Seddon et al., 2013). Serum measurements found low FI levels for pathogenic variants Ala240Gly and Gly119Arg compared to non-carriers, while serum levels were normal for Pro553Ser and Arg406His (Kavanagh et al., 2015).
In an independent study, CFI Gly119Arg and Gly188Ala resulted in reduced FI levels in plasma, which was supported by in vitro analysis of recombinant FI in human cells, showing that mutant FI is expressed and secreted at lower levels than wild-type FI (van de Ven et al., 2013). Overall plasma samples of carriers of the Gly119Arg variant showed a lower ability to degrade C3b compared to non-carriers, but the ability of recombinant Gly119Arg mutant protein to cleave C3b and C4b was intact. This suggests that this variant is a type 1 mutation and that low expression levels underlie the observed functional effect (van de Ven et al., 2013). Similarly, variant Leu131Arg in CFI showed both impaired FI levels and an inability to properly cleave C3b (Geerlings et al., 2016), supporting that is also a type 1 mutation. A difficult variant to classify is CFI Pro553Ser, which confers risk for AMD in multiple studies (Geerlings et al., 2016, Kavanagh et al., 2015). The variant however does not alter system FI levels, is classified by prediction software as benign, and showed a lower ability to degrade C3b compared to non-carriers controls but not non-carriers cases.
4.3. Complement component 3
Complement factor 3 (C3) is the central player in the activation of the complement system and several rare variants in C3 have been investigated functionally (Table 3).
The C3 Lys155Gln variant is located close to the binding site for FH, and its interaction was analyzed both in silico and in vitro with matching results. The Lys155Gln variant causes inefficient binding of C3 with FH and consequently reduces cofactor mediated cleavage of C3b (Miller, 2012, Seddon et al., 2013). Variant Lys65Gln leads to a decreased binding of FH to C3b and a slightly lowered affinity to the membrane cofactor protein (MCP; also known as CD46). The Arg161Trp variant increases the affinity to bind to FB and thereby creates an overactive C3 convertase accompanied by increased formation of C3a, C5a and C5b-C9. In addition, Arg161Trp also has reduced binding affinities for MCP and FH, which would otherwise both inactivate C3b/C4b through co-factor activity with FI (Roumenina et al., 2012, Schramm et al., 2015, Volokhina et al., 2012). Variant Arg735Trp demonstrated normal MCP, FB, sCR1 and FH binding and proper cleavage by FI (Fremeaux-Bacchi et al., 2008).
4.4. Complement component 9
Complement factor 9 (C9) takes part in the formation of the terminal complement complex (TCC) comprised of several C5b-9 elements. The TCC can be soluble or it can form a scaffold on the surface of the membrane together with multiple (up to 16) C9 proteins to assemble a pore-like structure known as the membrane attack complex (MAC) promoting cell lysis.
Recently it was shown that carriers of the C9 Pro167Ser variant, associated with an increased risk for AMD, have elevated C9 concentrations in serum compared to non-carriers. It was hypothesized that increased C9 levels could result in elevated complement activation which, through lysis of the cells, may contribute to the degenerative process observed in AMD (Geerlings et al., 2016). Asian Founder mutation C9 Arg95Ter is responsible for most Japanese C9 deficiency cases but is simultaneously protective for AMD. It is shown that C9 serum concentrations are low, even below detection level (Fukumori et al., 1989, Horiuchi et al., 1998), suggesting that less MAC can be formed which could otherwise contribute to retinal damage.
5. Complement therapies in AMD
The treatment of neovascular AMD has highly improved with the introduction of anti-neovascularization therapy with VEGF as the principle target. However, VEGF-based treatment is not effective or even not applicable in most AMD patients since only a minority of patients suffer from the neovascular form of AMD. Currently, no available treatment is available for the majority of AMD patients that suffer from early or intermediate AMD or geographic atrophy. Moreover, no effective means other than a modest effect of AREDS supplements to reduce the risk of AMD progression is available (AREDS, 2001, Chew et al., 2015, Chew et al., 2014). Because of the central role of the complement system in AMD, complement inhibition has been considered a potential therapeutic option and several clinical trials have been initiated to investigate this possibility (Table 4) (Rhoades et al., 2015, Volz and Pauly, 2015, Yehoshua et al., 2014).
Table 4.
Clinical trials for AMD targeting the complement system.
| Drug, trade name (company) | Target | Status | Source |
|---|---|---|---|
| Eculizumab, Soliris (Alexion) | Complement component 5 | Phase II has been completed | Yehoshua et al. (2014) |
| Lampalizumab (Genentech, Roche) | Complement factor D | Phase II has been completed; recruitment for phase III clinical trial has started | Rhoades et al. (2015) and Volz and Pauly (2015) |
| Avacincaptad pegol (ARC-1905) Zimura (Ophthotech) | Complement component 5 |
Recruitment for II/III clinical trial has started. | Clinicaltrials.gov |
| Tesidolumab, LFG316 (Novartis) | Complement component 5 |
Recruitment for II/III clinical trial has started | Clinicaltrials.gov |
| CLG561 (Novartis) | Properdin | Recruitment for phase II clinical trial has started | Clinicaltrials.gov |
| POT4, (Potentia Pharmaceuticals and Alcon) | Complement component 3 |
Phase I has been completed. | Clinicaltrials.gov |
Of the studies that published the outcomes, the COMPLETE study was a phase II clinical trial with systemic eculizumab, an humanized IgG antibody that inhibits complement component 5 (C5). The trial results showed that eculizumab was not effective in the treatment of geographic AMD, as the growth of atrophic lesions did not decrease after 6 months of treatment (Yehoshua et al., 2014). The MAHALO study was a phase II clinical trial with lampalizumab, an antibody directed against complement factor D. MAHALO showed promising preliminary results: progression of the geographic atrophy lesion showed a 20% reduction after 18 months of treatment and it was suggested that lampalizumab is most effective in a subpopulation of patients, since an even higher reduction rate was seen in patients carrying a specific CFI genotype (Rhoades et al., 2015, Volz and Pauly, 2015). A phase III trial with lampalizumab is currently ongoing, which will further investigate the role of the CFI genotype on treatment response.
In summary, a number of clinical trials using complement inhibitors in AMD have been performed, or are currently still running. Eculizumab seemed not to be effective, while lampalizumab may have a (limited) beneficial effect in reducing AMD progression.
6. Discussion and conclusion
Just over a decade after the initial discovery of the involvement of CFH in AMD (Klein et al., 2005), basic science has been translated to experimental approaches where complement inhibitors against AMD are now tested in clinical trials. Although the involvement of the complement system in AMD has been firmly established, the limited success of these clinical trials seem to suggest that the drugs currently tested are not entirely effective in the overall study populations (Volz and Pauly, 2015). In part this may be explained by the fact that, besides the complement system, also other biological systems like the extracellular matrix, lipid homeostasis or oxidative stress may contribute substantially to AMD pathogenesis (AREDS, 2001, Fritsche et al., 2016, Shen et al., 2007). At this time, it is not well understood how these pathways interact with each other in the development of the disease. Therefore the complement system is not necessarily the only, or even an appropriate target for any given AMD patient.
It is conceivable that a subset of patients would benefit more from anti-complement therapy than others, in particular those that have a genetic defect in genes of the complement system. It has now been shown that rare genetic variants in complement genes that are genetically associated with AMD often negatively impact the functioning of this system (Geerlings et al., 2016, Kavanagh et al., 2015, Triebwasser et al., 2015, van de Ven et al., 2013, Yu et al., 2014). However, does this mean that all AMD patients carrying a rare variant in the complement system will benefit from complement inhibitors?
Several issues arise when considering the role of rare variants in AMD. To date only few rare variants have been consistently replicated across multiple cohorts (Fritsche et al., 2016, Helgason et al., 2013, Raychaudhuri et al., 2011, Seddon et al., 2013, van de Ven et al., 2013, Zhan et al., 2013). While some rare variants are present relatively abundantly in one population, they are virtually absent in other populations, for example Arg1210Cys in CFH (Duvvari et al., 2015, Miyake et al., 2015, Raychaudhuri et al., 2011, Zhan et al., 2013) and Gly119Arg in CFI (Alexander et al., 2014, Cheng et al., 2015, Kavanagh et al., 2015, van de Ven et al., 2013).
Many other potentially interesting variants have been found, illustrated by this review, but very large cohorts are required to detect a significant genetic association with AMD (Manolio et al., 2009, McCarthy et al., 2008). The question arises whether a rare variant is or is not relevant in the context of the disease if it cannot be genetically associated with AMD. Functional assays could help clarify if a genetic variant has an impact on protein stability or systemic levels. This may provide functional evidence that a variant could be involved in the pathogenesis of the disease in the cases where statistical tests are underpowered to detect any association.
At this time, patients who carry AMD-associated rare variants proven to have a negative impact on function, could be prioritized in clinical trials with complement inhibitors. Across multiple cohorts, numerous of such patients have now been identified. Such focused studies would offer a proof of principle that could later benefit many more patients that have a defective complement system based on functional tests, but carry genetic variants that are too rare to be statistically associated with AMD.
AMD patients should therefore be screened using a functional complement assay in addition to genetic analyses. An exciting future lies ahead in the field of AMD where, for each individual patient, genetic evidence and functional tests come together in a treatment plan that is personalized and tailored to the specific needs and requirements of that patient.
Acknowledgements
The authors would like to thank the International Age-Related Macular Degeneration Genomics Consortium (IAMDGC) for sharing minor allele frequencies and odd-ratio's of rare variants in the CFH, CFI, C3 and C9 genes identified in Fritsche et al 2016, and A. Kwong, X. Zhan, A. Swaroop and G.R. Abecasis for sharing their dataset of rare variants in the CFH, CFI andC3genes identified in Zhan et al. 2013. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement n. 310644 (MACULA).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.molimm.2016.11.016.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- AREDS Age Related Eye Disease Study Research Group, A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. (Chicago, Ill.: 1960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis G.R., Yashar B.M., Zhao Y., Ghiasvand N.M., Zareparsi S., Branham K.E., Reddick A.C., Trager E.H., Yoshida S., Bahling J., Filippova E., Elner S., Johnson M.W., Vine A.K., Sieving P.A., Jacobson S.G., Richards J.E., Swaroop A. Age-related macular degeneration: a high-resolution genome scan for susceptibility loci in a population enriched for late-stage disease. Am. J. Hum. Genet. 2004;74:482–494. doi: 10.1086/382786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque J.A., Lamers M.L., Castiblanco-Valencia M.M., Dos Santos M., Isaac L. Chemical chaperones curcumin and 4-phenylbutyric acid improve secretion of mutant factor H R127H by fibroblasts from a factor H-deficient patient. J. Immunol. 2012;189:3242–3248. doi: 10.4049/jimmunol.1201418. (Baltimore, Md.: 1950) [DOI] [PubMed] [Google Scholar]
- Alexander P., Gibson J., Cree A.J., Ennis S., Lotery A.J. Complement factor I and age-related macular degeneration. Mol. Vis. 2014;20:1253–1257. [PMC free article] [PubMed] [Google Scholar]
- Bienaime F., Dragon-Durey M.A., Regnier C.H., Nilsson S.C., Kwan W.H., Blouin J., Jablonski M., Renault N., Rameix-Welti M.A., Loirat C., Sautes-Fridman C., Villoutreix B.O., Blom A.M., Fremeaux-Bacchi V. Mutations in components of complement influence the outcome of factor I-associated atypical hemolytic uremic syndrome. Kidney Int. 2010;77:339–349. doi: 10.1038/ki.2009.472. [DOI] [PubMed] [Google Scholar]
- Boon C.J., Klevering B.J., Hoyng C.B., Zonneveld-Vrieling M.N., Nabuurs S.B., Blokland E., Cremers F.P., den Hollander A.I. Basal laminar drusen caused by compound heterozygous variants in the CFH gene. Am. J. Hum. Genet. 2008;82:516–523. doi: 10.1016/j.ajhg.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackman D., Sartz L., Leh S., Kristoffersson A.C., Bjerre A., Tati R., Fremeaux-Bacchi V., Karpman D. Thrombotic microangiopathy mimicking membranoproliferative glomerulonephritis. Nephrol. Dial. Transplant. 2011;26:3399–3403. doi: 10.1093/ndt/gfr422. [DOI] [PubMed] [Google Scholar]
- Bresin E., Rurali E., Caprioli J., Sanchez-Corral P., Fremeaux-Bacchi V., Rodriguez de Cordoba S., Pinto S., Goodship T.H., Alberti M., Ribes D., Valoti E., Remuzzi G., Noris M. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J. Am. Soc. Nephrol. JASN. 2013;24:475–486. doi: 10.1681/ASN.2012090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli J., Noris M., Brioschi S., Pianetti G., Castelletti F., Bettinaglio P., Mele C., Bresin E., Cassis L., Gamba S., Porrati F., Bucchioni S., Monteferrante G., Fang C.J., Liszewski M.K., Kavanagh D., Atkinson J.P., Remuzzi G. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayci F.S., Cakar N., Hancer V.S., Uncu N., Acar B., Gur G. Eculizumab therapy in a child with hemolytic uremic syndrome and CFI mutation. Pediatric Nephrol. (Berlin, Germany) 2012;27:2327–2331. doi: 10.1007/s00467-012-2283-9. [DOI] [PubMed] [Google Scholar]
- Chakravarthy U., Evans J., Rosenfeld P.J. Age related macular degeneration. BMJ. 2010;340:c981. doi: 10.1136/bmj.c981. (Clinical research ed.) [DOI] [PubMed] [Google Scholar]
- Chakravarthy U., Wong T.Y., Fletcher A., Piault E., Evans C., Zlateva G., Buggage R., Pleil A., Mitchell P. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. doi: 10.1186/1471-2415-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.R., Thomas C.P., Torrealba J.R., Djamali A., Fernandez L.A., Nishimura C.J., Smith R.J., Samaniego M.D. Recurrent atypical hemolytic uremic syndrome associated with factor I mutation in a living related renal transplant recipient. Am. J. Kidney Dis. 2009;53:321–326. doi: 10.1053/j.ajkd.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.-Y., Yamashiro K., Jia Chen L., Ahn J., Huang L., Huang L., Cheung C.M.G., Miyake M., Cackett P.D., Yeo I.Y., Laude A., Mathur R., Pang J., Sim K.S., Koh A.H., Chen P., Lee S.Y., Wong D., Chan C.M., Loh B.K., Sun Y., Davila S., Nakata I., Nakanishi H., Akagi-Kurashige Y., Gotoh N., Tsujikawa A., Matsuda F., Mori K., Yoneya S., Sakurada Y., Iijima H., Iida T., Honda S., Lai T.Y.Y., Tam P.O.S., Chen H., Tang S., Ding X., Wen F., Lu F., Zhang X., Shi Y., Zhao P., Zhao B., Sang J., Gong B., Dorajoo R., Yuan J.-M., Koh W.-P., van Dam R.M., Friedlander Y., Lin Y., Hibberd M.L., Foo J.N., Wang N., Wong C.H., Tan G.S., Park S.J., Bhargava M., Gopal L., Naing T., Liao J., Guan Ong P., Mitchell P., Zhou P., Xie X., Liang J., Mei J., Jin X., Saw S.-M., Ozaki M., Mizoguchi T., Kurimoto Y., Woo S.J., Chung H., Yu H.-G., Shin J.Y., Park D.H., Kim I.T., Chang W., Sagong M., Lee S.-J., Kim H.W., Lee J.E., Li Y., Liu J., Teo Y.Y., Heng C.K., Lim T.H., Yang S.-K., Song K., Vithana E.N., Aung T., Bei J.X., Zeng Y.X., Tai E.S., Li X.X., Yang Z., Park K.-H. New loci and coding variants confer risk for age-related macular degeneration in East Asians. Nat. Commun. 2015;6 doi: 10.1038/ncomms7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew E.Y., Clemons T.E., Sangiovanni J.P., Danis R.P., Ferris F.L., 3rd, Elman M.J., Antoszyk A.N., Ruby A.J., Orth D., Bressler S.B., Fish G.E., Hubbard G.B., Klein M.L., Chandra S.R., Blodi B.A., Domalpally A., Friberg T., Wong W.T., Rosenfeld P.J., Agron E., Toth C.A., Bernstein P.S., Sperduto R.D. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report no. 3. JAMA Ophthalmol. 2014;132:142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew E.Y., Clemons T.E., Agron E., Launer L.J., Grodstein F., Bernstein P.S. Effect of omega-3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: the AREDS2 randomized clinical trial. JAMA. 2015;314:791–801. doi: 10.1001/jama.2015.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragon-Durey M.A., Fremeaux-Bacchi V., Loirat C., Blouin J., Niaudet P., Deschenes G., Coppo P., Herman Fridman W., Weiss L. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J. Am. Soc. Nephrol. JASN. 2004;15:787–795. doi: 10.1097/01.asn.0000115702.28859.a7. [DOI] [PubMed] [Google Scholar]
- Duvvari M.R., Paun C.C., Buitendijk G.H., Saksens N.T., Volokhina E.B., Ristau T., Schoenmaker-Koller F.E., van de Ven J.P., Groenewoud J.M., van den Heuvel L.P., Hofman A., Fauser S., Uitterlinden A.G., Klaver C.C., Hoyng C.B., de Jong E.K., den Hollander A.I. Analysis of rare variants in the C3 gene in patients with age-related macular degeneration. PLoS One. 2014;9:e94165. doi: 10.1371/journal.pone.0094165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvvari M.R., Saksens N.T., van de Ven J.P., de Jong-Hesse Y., Schick T., Nillesen W.M., Fauser S., Hoefsloot L.H., Hoyng C.B., de Jong E.K., den Hollander A.I. Analysis of rare variants in the CFH gene in patients with the cuticular drusen subtype of age-related macular degeneration. Mol. Vis. 2015;21:285–292. [PMC free article] [PubMed] [Google Scholar]
- Duvvari M.R., van de Ven J.P., Geerlings M.J., Saksens N.T., Bakker B., Henkes A., Neveling K., Rosario M.D., Westra D., van den Heuvel L.P., Schick T., Fauser S., Boon C.J., Hoyng C.B., Jong E.K., Hollander A.I. Whole exome sequencing in patients with the cuticular drusen subtype of age-related macular degeneration. PLoS One. 2016;11:e0152047. doi: 10.1371/journal.pone.0152047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A.O., Ritter R., 3rd, Abel K.J., Manning A., Panhuysen C., Farrer L.A. Complement factor H polymorphism and age-related macular degeneration. Science (New York, N.Y.) 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Fagerness J.A., Maller J.B., Neale B.M., Reynolds R.C., Daly M.J., Seddon J.M. Variation near complement factor I is associated with risk of advanced AMD. Eur. J. Hum. Genetics: EJHG. 2009;17:100–104. doi: 10.1038/ejhg.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri F., Jablonski M., Lepercq J., Blouin J., Benachi A., Hourmant M., Pirson Y., Durrbach A., Grunfeld J.P., Knebelmann B., Fremeaux-Bacchi V. Factor H, membrane cofactor protein, and factor I mutations in patients with hemolysis, elevated liver enzymes, and low platelet count syndrome. Blood. 2008;112:4542–4545. doi: 10.1182/blood-2008-03-144691. [DOI] [PubMed] [Google Scholar]
- Fakhouri F., Roumenina L., Provot F., Sallee M., Caillard S., Couzi L., Essig M., Ribes D., Dragon-Durey M.A., Bridoux F., Rondeau E., Fremeaux-Bacchi V. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J. Am. Soc. Nephrol. JASN. 2010;21:859–867. doi: 10.1681/ASN.2009070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcao D.A., Reis E.S., Paixao-Cavalcante D., Amano M.T., Delcolli M.I., Florido M.P., Albuquerque J.A., Moraes-Vasconcelos D., Duarte A.J., Grumach A.S., Isaac L. Deficiency of the human complement regulatory protein factor H associated with low levels of component C9. Scand. J. Immunol. 2008;68:445–455. doi: 10.1111/j.1365-3083.2008.02152.x. [DOI] [PubMed] [Google Scholar]
- Ferreira V.P., Herbert A.P., Cortes C., McKee K.A., Blaum B.S., Esswein S.T., Uhrin D., Barlow P.N., Pangburn M.K., Kavanagh D. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J. Immunol. 2009;182:7009–7018. doi: 10.4049/jimmunol.0804031. (Baltimore, Md.: 1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeaux-Bacchi V., Dragon-Durey M.A., Blouin J., Vigneau C., Kuypers D., Boudailliez B., Loirat C., Rondeau E., Fridman W.H. Complement factor I: a susceptibility gene for atypical haemolytic uraemic syndrome. J. Med. Genet. 2004;41:e84. doi: 10.1136/jmg.2004.019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeaux-Bacchi V., Miller E.C., Liszewski M.K., Strain L., Blouin J., Brown A.L., Moghal N., Kaplan B.S., Weiss R.A., Lhotta K., Kapur G., Mattoo T., Nivet H., Wong W., Gie S., Hurault de Ligny B., Fischbach M., Gupta R., Hauhart R., Meunier V., Loirat C., Dragon-Durey M.A., Fridman W.H., Janssen B.J., Goodship T.H., Atkinson J.P. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–4952. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche L.G., Chen W., Schu M., Yaspan B.L., Yu Y., Thorleifsson G., Zack D.J., Arakawa S., Cipriani V., Ripke S., Igo R.P., Jr., Buitendijk G.H., Sim X., Weeks D.E., Guymer R.H., Merriam J.E., Francis P.J., Hannum G., Agarwal A., Armbrecht A.M., Audo I., Aung T., Barile G.R., Benchaboune M., Bird A.C., Bishop P.N., Branham K.E., Brooks M., Brucker A.J., Cade W.H., Cain M.S., Campochiaro P.A., Chan C.C., Cheng C.Y., Chew E.Y., Chin K.A., Chowers I., Clayton D.G., Cojocaru R., Conley Y.P., Cornes B.K., Daly M.J., Dhillon B., Edwards A.O., Evangelou E., Fagerness J., Ferreyra H.A., Friedman J.S., Geirsdottir A., George R.J., Gieger C., Gupta N., Hagstrom S.A., Harding S.P., Haritoglou C., Heckenlively J.R., Holz F.G., Hughes G., Ioannidis J.P., Ishibashi T., Joseph P., Jun G., Kamatani Y., Katsanis N., Khan C.N.K., Kim I.K., Kiyohara Y., Klein B.E., Klein R., Kovach J.L., Kozak I., Lee C.J., Lee K.E., Lichtner P., Lotery A.J., Meitinger T., Mitchell P., Mohand-Said S., Moore A.T., Morgan D.J., Morrison M.A., Myers C.E., Naj A.C., Nakamura Y., Okada Y., Orlin A., Ortube M.C., Othman M.I., Pappas C., Park K.H., Pauer G.J., Peachey N.S., Poch O., Priya R.R., Reynolds R., Richardson A.J., Ripp R., Rudolph G., Ryu E. Seven new loci associated with age-related macular degeneration. Nat. Genet. 2013;45:433–439. doi: 10.1038/ng.2578. 439e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche L.G., Fariss R.N., Stambolian D., Abecasis G.R., Curcio C.A., Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annu. Rev. Genomics Hum. Genet. 2014;15:151–171. doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche L.G., Igl W., Bailey J.N., Grassmann F., Sengupta S., Bragg-Gresham J.L., Burdon K.P., Hebbring S.J., Wen C., Gorski M., Kim I.K., Cho D., Zack D., Souied E., Scholl H.P., Bala E., Lee K.E., Hunter D.J., Sardell R.J., Mitchell P., Merriam J.E., Cipriani V., Hoffman J.D., Schick T., Lechanteur Y.T., Guymer R.H., Johnson M.P., Jiang Y., Stanton C.M., Buitendijk G.H., Zhan X., Kwong A.M., Boleda A., Brooks M., Gieser L., Ratnapriya R., Branham K.E., Foerster J.R., Heckenlively J.R., Othman M.I., Vote B.J., Liang H.H., Souzeau E., McAllister I.L., Isaacs T., Hall J., Lake S., Mackey D.A., Constable I.J., Craig J.E., Kitchner T.E., Yang Z., Su Z., Luo H., Chen D., Ouyang H., Flagg K., Lin D., Mao G., Ferreyra H., Stark K., von Strachwitz C.N., Wolf A., Brandl C., Rudolph G., Olden M., Morrison M.A., Morgan D.J., Schu M., Ahn J., Silvestri G., Tsironi E.E., Park K.H., Farrer L.A., Orlin A., Brucker A., Li M., Curcio C.A., Mohand-Said S., Sahel J.A., Audo I., Benchaboune M., Cree A.J., Rennie C.A., Goverdhan S.V., Grunin M., Hagbi-Levi S., Campochiaro P., Katsanis N., Holz F.G., Blond F., Blanche H., Deleuze J.F., Igo R.P., Jr., Truitt B., Peachey N.S., Meuer S.M., Myers C.E., Moore E.L., Klein R. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016;48:134–143. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori Y., Yoshimura K., Ohnoki S., Yamaguchi H., Akagaki Y., Inai S. A high incidence of C9 deficiency among healthy blood donors in Osaka, Japan. Int. Immunol. 1989;1:85–89. doi: 10.1093/intimm/1.1.85. [DOI] [PubMed] [Google Scholar]
- Geerlings M.J., Kremlitzka M., Bakker B., Nilsson S.C., Saksens N.T., Lechanteur Y.T., Pauper M., Corominas J., Fauser S., Hoyng C.B., Blom A.M., de Jong E.K., den Hollander A.I. The functional effect of rare variants in complement genes on C3b degradation in patients with age-related macular degeneration. JAMA Ophthalmol. 2016 doi: 10.1001/jamaophthalmol.2016.4604. Published online December 01, 2016. [DOI] [PubMed] [Google Scholar]
- Gold B., Merriam J.E., Zernant J., Hancox L.S., Taiber A.J., Gehrs K., Cramer K., Neel J., Bergeron J., Barile G.R., Smith R.T., Hageman G.S., Dean M., Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman G.S., Luthert P.J., Victor Chong N.H., Johnson L.V., Anderson D.H., Mullins R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Hageman G.S., Anderson D.H., Johnson L.V., Hancox L.S., Taiber A.J., Hardisty L.I., Hageman J.L., Stockman H.A., Borchardt J.D., Gehrs K.M., Smith R.J., Silvestri G., Russell S.R., Klaver C.C., Barbazetto I., Chang S., Yannuzzi L.A., Barile G.R., Merriam J.C., Smith R.T., Olsh A.K., Bergeron J., Zernant J., Merriam J.E., Gold B., Dean M., Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines J.L., Hauser M.A., Schmidt S., Scott W.K., Olson L.M., Gallins P., Spencer K.L., Kwan S.Y., Noureddine M., Gilbert J.R., Schnetz-Boutaud N., Agarwal A., Postel E.A., Pericak-Vance M.A. Complement factor H variant increases the risk of age-related macular degeneration. Science (New York, N.Y.) 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hammond C.J., Webster A.R., Snieder H., Bird A.C., Gilbert C.E., Spector T.D. Genetic influence on early age-related maculopathy: a twin study. Ophthalmology. 2002;109:730–736. doi: 10.1016/s0161-6420(01)01049-1. [DOI] [PubMed] [Google Scholar]
- Heinen S., Sanchez-Corral P., Jackson M.S., Strain L., Goodship J.A., Kemp E.J., Skerka C., Jokiranta T.S., Meyers K., Wagner E., Robitaille P., Esparza-Gordillo J., Rodriguez de Cordoba S., Zipfel P.F., Goodship T.H. De novo gene conversion in the RCA gene cluster (1q32) causes mutations in complement factor H associated with atypical hemolytic uremic syndrome. Hum. Mutat. 2006;27:292–293. doi: 10.1002/humu.9408. [DOI] [PubMed] [Google Scholar]
- Helgason H., Sulem P., Duvvari M.R., Luo H., Thorleifsson G., Stefansson H., Jonsdottir I., Masson G., Gudbjartsson D.F., Walters G.B., Magnusson O.T., Kong A., Rafnar T., Kiemeney L.A., Schoenmaker-Koller F.E., Zhao L., Boon C.J., Song Y., Fauser S., Pei M., Ristau T., Patel S., Liakopoulos S., van de Ven J.P., Hoyng C.B., Ferreyra H., Duan Y., Bernstein P.S., Geirsdottir A., Helgadottir G., Stefansson E., den Hollander A.I., Zhang K., Jonasson F., Sigurdsson H., Thorsteinsdottir U., Stefansson K. A rare nonsynonymous sequence variant in C3 is associated with high risk of age-related macular degeneration. Nat. Genet. 2013;45:1371–1374. doi: 10.1038/ng.2740. [DOI] [PubMed] [Google Scholar]
- Hoffman J.D., Cooke Bailey J.N., D'Aoust L., Cade W., Ayala-Haedo J., Fuzzell D., Laux R., Adams L.D., Reinhart-Mercer L., Caywood L., Whitehead-Gay P., Agarwal A., Wang G., Scott W.K., Pericak-Vance M.A., Haines J.L. Rare complement factor H variant associated with age-related macular degeneration in the Amish. Invest. Ophthalmol. Visual Sci. 2014;55:4455–4460. doi: 10.1167/iovs.13-13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi T., Nishizaka H., Kojima T., Sawabe T., Niho Y., Schneider P.M., Inaba S., Sakai K., Hayashi K., Hashimura C., Fukumori Y. A non-sense mutation at Arg95 is predominant in complement 9 deficiency in Japanese. J. Immunol. 1998;160:1509–1513. [PubMed] [Google Scholar]
- Hughes A.E., Orr N., Esfandiary H., Diaz-Torres M., Goodship T., Chakravarthy U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat. Genet. 2006;38:1173–1177. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]
- Iyengar S.K., Song D., Klein B.E., Klein R., Schick J.H., Humphrey J., Millard C., Liptak R., Russo K., Jun G., Lee K.E., Fijal B., Elston R.C. Dissection of genomewide-scan data in extended families reveals a major locus and oligogenic susceptibility for age-related macular degeneration. Am. J. Hum. Genet. 2004;74:20–39. doi: 10.1086/380912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager R.D., Mieler W.F., Miller J.W. Age-related macular degeneration. N. Engl. J. Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- Janssen van Doorn K., Dirinck E., Verpooten G.A., Couttenye M.M. Complement factor H mutation associated with membranoproliferative glomerulonephritis with transformation to atypical haemolytic uraemic syndrome. Clin. Kidney J. 2013;6:216–219. doi: 10.1093/ckj/sfs190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.V., Leitner W.P., Staples M.K., Anderson D.H. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp. Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- Johnson S.A., Williams J.M., Hakobyan S., Richards A., Perkins S.J., Marchbank K.J., Goodship T.H.J., Morgan B.P., Taylor C.M., Savage C.O.S. Impact of compound heterozygous complement factor H mutations on development of atypical hemolytic uremic syndrome—a pedigree revisited. Mol. Immunol. 2010;47:1585–1591. doi: 10.1016/j.molimm.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Jozsi M., Heinen S., Hartmann A., Ostrowicz C.W., Halbich S., Richter H., Kunert A., Licht C., Saunders R.E., Perkins S.J., Zipfel P.F., Skerka C. Factor H and atypical hemolytic uremic syndrome: mutations in the C-terminus cause structural changes and defective recognition functions. J. Am. Soc. Nephrol. JASN. 2006;17:170–177. doi: 10.1681/ASN.2005080868. [DOI] [PubMed] [Google Scholar]
- Kavanagh D., Richards A., Noris M., Hauhart R., Liszewski M.K., Karpman D., Goodship J.A., Fremeaux-Bacchi V., Remuzzi G., Goodship T.H., Atkinson J.P. Characterization of mutations in complement factor I (CFI) associated with hemolytic uremic syndrome. Mol. Immunol. 2008;45:95–105. doi: 10.1016/j.molimm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Kavanagh D., Yu Y., Schramm E.C., Triebwasser M., Wagner E.K., Raychaudhuri S., Daly M.J., Atkinson J.P., Seddon J.M. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum. Mol. Genet. 2015;24:3861–3870. doi: 10.1093/hmg/ddv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver C.C., Wolfs R.C., Assink J.J., van Duijn C.M., Hofman A., de Jong P.T. Genetic risk of age-related maculopathy. Population-based familial aggregation study. Arch. Ophthalmol. 1998;116:1646–1651. doi: 10.1001/archopht.116.12.1646. (Chicago, Ill.: 1960) [DOI] [PubMed] [Google Scholar]
- Klein R.J., Zeiss C., Chew E.Y., Tsai J.Y., Sackler R.S., Haynes C., Henning A.K., SanGiovanni J.P., Mane S.M., Mayne S.T., Bracken M.B., Ferris F.L., Ott J., Barnstable C., Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science (New York, N.Y.) 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski J., Schultz D.W., Weleber R.G., Schain M.B., Edwards A.O., Matise T.C., Acott T.S., Ott J., Klein M.L. Age-related macular degeneration–a genome scan in extended families. Am. J. Hum. Genet. 2003;73:540–550. doi: 10.1086/377701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J.B., Fagerness J.A., Reynolds R.C., Neale B.M., Daly M.J., Seddon J.M. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat. Genet. 2007;39:1200–1201. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- Manolio T.A., Collins F.S., Cox N.J., Goldstein D.B., Hindorff L.A., Hunter D.J., McCarthy M.I., Ramos E.M., Cardon L.R., Chakravarti A., Cho J.H., Guttmacher A.E., Kong A., Kruglyak L., Mardis E., Rotimi C.N., Slatkin M., Valle D., Whittemore A.S., Boehnke M., Clark A.G., Eichler E.E., Gibson G., Haines J.L., Mackay T.F.C., McCarroll S.A., Visscher P.M. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelian T., Hellwage J., Meri S., Caprioli J., Noris M., Heinen S., Jozsi M., Neumann H.P., Remuzzi G., Zipfel P.F. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J. Clin. Invest. 2003;111:1181–1190. doi: 10.1172/JCI16651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Barricarte R., Heurich M., Lopez-Perrote A., Tortajada A., Pinto S., Lopez-Trascasa M., Sanchez-Corral P., Morgan B.P., Llorca O., Harris C.L., Rodriguez de Cordoba S. The molecular and structural bases for the association of complement C3 mutations with atypical hemolytic uremic syndrome. Mol. Immunol. 2015;66:263–273. doi: 10.1016/j.molimm.2015.03.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.I., Abecasis G.R., Cardon L.R., Goldstein D.B., Little J., Ioannidis J.P., Hirschhorn J.N. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat. Rev. Genet. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- Meyers S.M., Greene T., Gutman F.A. A twin study of age-related macular degeneration. Am. J. Ophthalmol. 1995;120:757–766. doi: 10.1016/s0002-9394(14)72729-1. [DOI] [PubMed] [Google Scholar]
- Miller, E., 2012. Characterization of Complement C3 Dysregulation Predisposing to Two Human Disease States, Vol. PhD thesis, Washington University, All Theses and Dissertations (ETDs), Paper 719.
- Miyake M., Saito M., Yamashiro K., Sekiryu T., Yoshimura N. Complement factor H R1210C among Japanese patients with age-related macular degeneration. Jpn. J. Ophthalmol. 2015;59:273–278. doi: 10.1007/s10384-015-0394-0. [DOI] [PubMed] [Google Scholar]
- Mohlin F.C., Nilsson S.C., Levart T.K., Golubovic E., Rusai K., Muller-Sacherer T., Arbeiter K., Pallinger E., Szarvas N., Csuka D., Szilagyi A., Villoutreix B.O., Prohaszka Z., Blom A.M. Functional characterization of two novel non-synonymous alterations in CD46 and a Q950H change in factor H found in atypical hemolytic uremic syndrome patients. Mol. Immunol. 2015;65:367–376. doi: 10.1016/j.molimm.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Mullins R.F., Aptsiauri N., Hageman G.S. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye (London, England) 2001;15:390–395. doi: 10.1038/eye.2001.142. [DOI] [PubMed] [Google Scholar]
- Neumann H.P., Salzmann M., Bohnert-Iwan B., Mannuelian T., Skerka C., Lenk D., Bender B.U., Cybulla M., Riegler P., Konigsrainer A., Neyer U., Bock A., Widmer U., Male D.A., Franke G., Zipfel P.F. Haemolytic uraemic syndrome and mutations of the factor H gene: a registry-based study of German speaking countries. J. Med. Genet. 2003;40:676–681. doi: 10.1136/jmg.40.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S.C., Karpman D., Vaziri-Sani F., Kristoffersson A.C., Salomon R., Provot F., Fremeaux-Bacchi V., Trouw L.A., Blom A.M. A mutation in factor I that is associated with atypical hemolytic uremic syndrome does not affect the function of factor I in complement regulation. Mol. Immunol. 2007;44:1835–1844. doi: 10.1016/j.molimm.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Nilsson S.C., Kalchishkova N., Trouw L.A., Fremeaux-Bacchi V., Villoutreix B.O., Blom A.M. Mutations in complement factor I as found in atypical hemolytic uremic syndrome lead to either altered secretion or altered function of factor I. Eur. J. Immunol. 2010;40:172–185. doi: 10.1002/eji.200939280. [DOI] [PubMed] [Google Scholar]
- Nishiguchi K.M., Yasuma T.R., Tomida D., Nakamura M., Ishikawa K., Kikuchi M., Ohmi Y., Niwa T., Hamajima N., Furukawa K., Terasaki H. C9-R95X polymorphism in patients with neovascular age-related macular degeneration. Invest. Ophthalmol. Visual Sci. 2012;53:508–512. doi: 10.1167/iovs.11-8425. [DOI] [PubMed] [Google Scholar]
- Pechtl I.C., Kavanagh D., McIntosh N., Harris C.L., Barlow P.N. Disease-associated N-terminal complement factor H mutations perturb cofactor and decay-accelerating activities. J. Biol. Chem. 2011;286:11082–11090. doi: 10.1074/jbc.M110.211839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero D., Gonzalez-Rubio C., Gallardo M.E., Vera M., Lopez-Trascasa M., Rodriguez de Cordoba S., Sanchez-Corral P. Clustering of missense mutations in the C-terminal region of factor H in atypical hemolytic uremic syndrome. Am. J. Hum. Genet. 2001;68:478–484. doi: 10.1086/318201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras E., Kristal D., Shoshany N., Volodarsky D., Vulih I., Celniker G., Isakov O., Shomron N., Pras E. Rare genetic variants in Tunisian Jewish patients suffering from age-related macular degeneration. J. Med. Genet. 2015;52:484–492. doi: 10.1136/jmedgenet-2015-103130. [DOI] [PubMed] [Google Scholar]
- Ratnapriya R., Zhan X., Fariss R.N., Branham K.E., Zipprer D., Chakarova C.F., Sergeev Y.V., Campos M.M., Othman M., Friedman J.S., Maminishkis A., Waseem N.H., Brooks M., Rajasimha H.K., Edwards A.O., Lotery A., Klein B.E., Truitt B.J., Li B., Schaumberg D.A., Morgan D.J., Morrison M.A., Souied E., Tsironi E.E., Grassmann F., Fishman G.A., Silvestri G., Scholl H.P., Kim I.K., Ramke J., Tuo J., Merriam J.E., Merriam J.C., Park K.H., Olson L.M., Farrer L.A., Johnson M.P., Peachey N.S., Lathrop M., Baron R.V., Igo R.P., Jr., Klein R., Hagstrom S.A., Kamatani Y., Martin T.M., Jiang Y., Conley Y., Sahel J.A., Zack D.J., Chan C.C., Pericak-Vance M.A., Jacobson S.G., Gorin M.B., Klein M.L., Allikmets R., Iyengar S.K., Weber B.H., Haines J.L., Leveillard T., Deangelis M.M., Stambolian D., Weeks D.E., Bhattacharya S.S., Chew E.Y., Heckenlively J.R., Abecasis G.R., Swaroop A. Rare and common variants in extracellular matrix gene Fibrillin 2 (FBN2) are associated with macular degeneration. Hum. Mol. Genet. 2014;23:5827–5837. doi: 10.1093/hmg/ddu276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S., Iartchouk O., Chin K., Tan P.L., Tai A.K., Ripke S., Gowrisankar S., Vemuri S., Montgomery K., Yu Y., Reynolds R., Zack D.J., Campochiaro B., Campochiaro P., Katsanis N., Daly M.J., Seddon J.M. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat. Genet. 2011;43:1232–1236. doi: 10.1038/ng.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalde S., Tortajada A., Subias M., Anter J., Blasco M., Maranta R., Coco R., Pinto S., Noris M., Garcia-Layana A., Rodriguez de Cordoba S. Molecular basis of factor H R1210C association with ocular and renal diseases. J. Am. Soc. Nephrol. JASN. 2016;27:1305–1311. doi: 10.1681/ASN.2015050580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades W., Dickson D., Do D.V. Potential role of lampalizumab for treatment of geographic atrophy. Clin. Ophthalmol. (Auckland, N.Z.) 2015;9:1049–1056. doi: 10.2147/OPTH.S59725. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Roumenina L.T., Frimat M., Miller E.C., Provot F., Dragon-Durey M.A., Bordereau P., Bigot S., Hue C., Satchell S.C., Mathieson P.W., Mousson C., Noel C., Sautes-Fridman C., Halbwachs-Mecarelli L., Atkinson J.P., Lionet A., Fremeaux-Bacchi V. A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood. 2012;119:4182–4191. doi: 10.1182/blood-2011-10-383281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksens N.T., Geerlings M.J., Bakker B., Schick T., Daha M.R., Fauser S., Boon C.J., de Jong E.K., Hoyng C.B., den Hollander A.I. Rare genetic variants associated with development of age-related macular degeneration. JAMA Ophthalmol. 2016;134:287–293. doi: 10.1001/jamaophthalmol.2015.5592. [DOI] [PubMed] [Google Scholar]
- Sanchez-Corral P., Perez-Caballero D., Huarte O., Simckes A.M., Goicoechea E., Lopez-Trascasa M., de Cordoba S.R. Structural and functional characterization of factor H mutations associated with atypical hemolytic uremic syndrome. Am. J. Hum. Genet. 2002;71:1285–1295. doi: 10.1086/344515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm E.C., Roumenina L.T., Rybkine T., Chauvet S., Vieira-Martins P., Hue C., Maga T., Valoti E., Wilson V., Jokiranta S., Smith R.J., Noris M., Goodship T., Atkinson J.P., Fremeaux-Bacchi V. Mapping interactions between complement C3 and regulators using mutations in atypical hemolytic uremic syndrome. Blood. 2015;125:2359–2369. doi: 10.1182/blood-2014-10-609073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J.M., Ajani U.A., Mitchell B.D. Familial aggregation of age-related maculopathy. Am. J. Ophthalmol. 1997;123:199–206. doi: 10.1016/s0002-9394(14)71036-0. [DOI] [PubMed] [Google Scholar]
- Seddon J.M., Santangelo S.L., Book K., Chong S., Cote J. A genomewide scan for age-related macular degeneration provides evidence for linkage to several chromosomal regions. Am. J. Hum. Genet. 2003;73:780–790. doi: 10.1086/378505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J.M., Cote J., Page W.F., Aggen S.H., Neale M.C. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch. Ophthalmol. 2005;123:321–327. doi: 10.1001/archopht.123.3.321. (Chicago, Ill.: 1960) [DOI] [PubMed] [Google Scholar]
- Seddon J.M., Yu Y., Miller E.C., Reynolds R., Tan P.L., Gowrisankar S., Goldstein J.I., Triebwasser M., Anderson H.E., Zerbib J., Kavanagh D., Souied E., Katsanis N., Daly M.J., Atkinson J.P., Raychaudhuri S. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat. Genet. 2013;45:1366–1370. doi: 10.1038/ng.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellier-Leclerc A.L., Fremeaux-Bacchi V., Dragon-Durey M.A., Macher M.A., Niaudet P., Guest G., Boudailliez B., Bouissou F., Deschenes G., Gie S., Tsimaratos M., Fischbach M., Morin D., Nivet H., Alberti C., Loirat C. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. JASN. 2007;18:2392–2400. doi: 10.1681/ASN.2006080811. [DOI] [PubMed] [Google Scholar]
- Servais A.A. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- Shen J.K., Dong A., Hackett S.F., Bell W.R., Green W.R., Campochiaro P.A. Oxidative damage in age-related macular degeneration. Histol. Histopathol. 2007;22:1301–1308. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- Stahl A.L., Vaziri-Sani F., Heinen S., Kristoffersson A.C., Gydell K.H., Raafat R., Gutierrez A., Beringer O., Zipfel P.F., Karpman D. Factor H dysfunction in patients with atypical hemolytic uremic syndrome contributes to complement deposition on platelets and their activation. Blood. 2008;111:5307–5315. doi: 10.1182/blood-2007-08-106153. [DOI] [PubMed] [Google Scholar]
- Szarvas N., Szilagyi A., Csuka D., Takacs B., Rusai K., Muller T., Arbeiter K., Reti M., Haris A., Wagner L., Torok S., Kelen K., Szabo A.J., Reusz G.S., Morgan B.P., Prohaszka Z. Genetic analysis and functional characterization of novel mutations in a series of patients with atypical hemolytic uremic syndrome. Mol. Immunol. 2016;71:10–22. doi: 10.1016/j.molimm.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Tomany S.C., Wang J.J., Van Leeuwen R., Klein R., Mitchell P., Vingerling J.R., Klein B.E., Smith W., De Jong P.T. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Tortajada A., Pinto S., Martinez-Ara J., Lopez-Trascasa M., Sanchez-Corral P., de Cordoba S.R. Complement factor H variants I890 and L1007 while commonly associated with atypical hemolytic uremic syndrome are polymorphisms with no functional significance. Kidney Int. 2012;81:56–63. doi: 10.1038/ki.2011.291. [DOI] [PubMed] [Google Scholar]
- Triebwasser M.P., Roberson E.D., Yu Y., Schramm E.C., Wagner E.K., Raychaudhuri S., Seddon J.M., Atkinson J.P. Rare variants in the functional domains of complement factor H are associated with age-related macular degeneration. Invest. Ophthalmol. Visual Sci. 2015;56:6873–6878. doi: 10.1167/iovs.15-17432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven J.P., Boon C.J., Fauser S., Hoefsloot L.H., Smailhodzic D., Schoenmaker-Koller F., Klevering J., Klaver C.C., den Hollander A.I., Hoyng C.B. Clinical evaluation of 3 families with basal laminar drusen caused by novel mutations in the complement factor H gene. Arch. Ophthalmol. 2012;130:1038–1047. doi: 10.1001/archophthalmol.2012.265. (Chicago, Ill.: 1960) [DOI] [PubMed] [Google Scholar]
- van de Ven J.P., Nilsson S.C., Tan P.L., Buitendijk G.H., Ristau T., Mohlin F.C., Nabuurs S.B., Schoenmaker-Koller F.E., Smailhodzic D., Campochiaro P.A., Zack D.J., Duvvari M.R., Bakker B., Paun C.C., Boon C.J., Uitterlinden A.G., Liakopoulos S., Klevering B.J., Fauser S., Daha M.R., Katsanis N., Klaver C.C., Blom A.M., Hoyng C.B., den Hollander A.I. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat. Genet. 2013;45:813–817. doi: 10.1038/ng.2640. [DOI] [PubMed] [Google Scholar]
- Volokhina E., Westra D., Xue X., Gros P., van de Kar N., van den Heuvel L. Novel C3 mutation p.Lys65Gln in aHUS affects complement factor H binding. Pediatric Nephrol. (Berlin, Germany) 2012;27:1519–1524. doi: 10.1007/s00467-012-2183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz C., Pauly D. Antibody therapies and their challenges in the treatment of age-related macular degeneration. Eur. J. Pharm. Biopharm. 2015;95:158–172. doi: 10.1016/j.ejpb.2015.02.020. [DOI] [PubMed] [Google Scholar]
- Vyse T.J., Morley B.J., Bartok I., Theodoridis E.L., Davies K.A., Webster A.D., Walport M.J. The molecular basis of hereditary complement factor I deficiency. J. Clin. Invest. 1996;97:925–933. doi: 10.1172/JCI118515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E.K., Raychaudhuri S., Villalonga M.B., Java A., Triebwasser M.P., Daly M.J., Atkinson J.P., Seddon J.M. Mapping rare, deleterious mutations in Factor H: association with early onset, drusen burden, and lower antigenic levels in familial AMD. Sci. Rep. 2016;6:31531. doi: 10.1038/srep31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D.E., Conley Y.P., Tsai H.J., Mah T.S., Schmidt S., Postel E.A., Agarwal A., Haines J.L., Pericak-Vance M.A., Rosenfeld P.J., Paul T.O., Eller A.W., Morse L.S., Dailey J.P., Ferrell R.E., Gorin M.B. Age-related maculopathy: a genomewide scan with continued evidence of susceptibility loci within the 1q31, 10q26, and 17q25 regions. Am. J. Hum. Genet. 2004;75:174–189. doi: 10.1086/422476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- Yates J.R., Sepp T., Matharu B.K., Khan J.C., Thurlby D.A., Shahid H., Clayton D.G., Hayward C., Morgan J., Wright A.F., Armbrecht A.M., Dhillon B., Deary I.J., Redmond E., Bird A.C., Moore A.T. Complement C3 variant and the risk of age-related macular degeneration. N. Engl. J. Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- Yehoshua Z., de Amorim Garcia Filho C.A., Nunes R.P., Gregori G., Penha F.M., Moshfeghi A.A., Zhang K., Sadda S., Feuer W., Rosenfeld P.J. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121:693–701. doi: 10.1016/j.ophtha.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Triebwasser M.P., Wong E.K., Schramm E.C., Thomas B., Reynolds R., Mardis E.R., Atkinson J.P., Daly M., Raychaudhuri S., Kavanagh D., Seddon J.M. Whole-exome sequencing identifies rare, functional CFH variants in families with macular degeneration. Hum. Mol. Genet. 2014;23:5283–5293. doi: 10.1093/hmg/ddu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Larson D.E., Wang C., Koboldt D.C., Sergeev Y.V., Fulton R.S., Fulton L.L., Fronick C.C., Branham K.E., Bragg-Gresham J., Jun G., Hu Y., Kang H.M., Liu D., Othman M., Brooks M., Ratnapriya R., Boleda A., Grassmann F., von Strachwitz C., Olson L.M., Buitendijk G.H., Hofman A., van Duijn C.M., Cipriani V., Moore A.T., Shahid H., Jiang Y., Conley Y.P., Morgan D.J., Kim I.K., Johnson M.P., Cantsilieris S., Richardson A.J., Guymer R.H., Luo H., Ouyang H., Licht C., Pluthero F.G., Zhang M.M., Zhang K., Baird P.N., Blangero J., Klein M.L., Farrer L.A., DeAngelis M.M., Weeks D.E., Gorin M.B., Yates J.R., Klaver C.C., Pericak-Vance M.A., Haines J.L., Weber B.H., Wilson R.K., Heckenlively J.R., Chew E.Y., Stambolian D., Mardis E.R., Swaroop A., Abecasis G.R. Identification of a rare coding variant in complement 3 associated with age-related macular degeneration. Nat. Genet. 2013;45:1375–1379. doi: 10.1038/ng.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.