Abstract
The number of malaria vaccine candidates in preclinical and clinical development is limited. To identify novel blood-stage malaria vaccine candidates, we constructed a library of 1,827P. falciparum proteins prepared using the wheat germ cell-free system (WGCFS). Also, a high-throughput AlphaScreen procedure was developed to measure antibody reactivity to the recombinant products. Purified IgGs from residents in malaria endemic areas have shown functional activity against blood-stage parasites as judged by an in vitro parasite Growth Inhibition Assay (GIA). Therefore, we evaluated the GIA activity of 51 plasma samples prepared from Malian adults living in a malaria endemic area against the WGCFS library. Using the AlphaScreen-based immunoreactivity measurements, antibody reactivity against 3 proteins was positively associated with GIA activity. Since anti-LSA3-C responses showed the strongest correlation with GIA activity, this protein was investigated further. Anti-LSA3-C-specific antibody purified from Malian adult plasmas showed GIA activity, and expression of LSA3 in blood-stage parasites was confirmed by western blotting. Taken together, we identified LSA3 as a novel blood-stage vaccine candidate, and we propose that this system will be useful for future vaccine candidate discovery.
WHO estimated 214 million cases and 438,000 deaths from malaria in 2015, and Plasmodium falciparum was responsible for much of this morbidity and mortality1. The emergence of drug-resistant parasites and insecticide-resistant mosquitoes has greatly hampered malaria control and has accelerated development of new approaches to support malaria eradication and elimination efforts2,3. Moreover, classic studies showing that passive transfer of γ-globulin isolated from adults who lived in a malaria endemic area dramatically reduced parasitemias and alleviated the symptoms in malaria-infected children have pointed to the role of antibodies in protective immune responses4. However, the targets of this naturally acquired immunity are not fully understood.
Malaria vaccine candidates under preclinical and clinical development are currently limited (WHO rainbow table: http://www.who.int/vaccine_research/links/Rainbow/en/index.html), and several blood-stage vaccines, which have reached to clinical trials, failed to show efficacy in field studies or in controlled human malaria infection models5,6. Therefore, more candidates for malaria vaccines for further blood-stage vaccine development are needed. When attempted, the screening for novel candidates has been hampered in part by difficulties in expressing plasmodial proteins in heterologous expression systems. Correct protein conformations are essential to induce protective immunity against malaria7, and it is well acknowledged that recombinant plasmodial proteins cannot always elicit functional antibodies due to the lack of proper conformation8. There are several preceding studies to detect a broader range of antigen-specific immune responses using protein microarrays9,10,11, and an Escherichia coli-based cell-free protein expression system was utilized in those studies to produce the polypeptides. The wheat germ cell-free system (WGCFS) has also been used to express several malaria antigens, and the recombinant proteins produced could elicit in-vitro growth inhibitory antibodies in animals as judged by in vitro functional assays with P. falciparum parasites, such as the growth inhibition assay (GIA)8,12. The results indicate that the recombinant proteins expressed by WGCFS retain (at least in part) critical functional epitopes. Purified IgGs from residents in malaria endemic areas have shown GIA activities13,14. To identify novel blood-stage malaria vaccine candidates, in this study 1,827P. falciparum proteins were expressed using WGCFS, and 51 purified IgGs were prepared from Malian adults who lived in a malaria endemic area. Antigen-specific antibody reactivity of the IgGs against the 1,827 proteins was evaluated using the AlphaScreen procedure. AlphaScreen is a protein-immobilization free procedure which is likely to maintain protein conformation better than the immobilization methods conventionally used in arrays and which allows high-throughput detection of protein-protein interactions15. Using the two data sets, the correlation between antibody responses and GIA activity was evaluated for each protein. We found that the antibody reactivity against three proteins was positively associated with GIA activities. Among the three, anti-C-terminal region of LSA3 (LSA3-C) responses showed the most significant correlation with the GIA activity. Human anti-LSA3-C-specific antibodies purified from Malian adult plasma also showed GIA activity. Previously, LSA3 has been evaluated as a pre-erythrocytic vaccine candidate, and demonstrated a protective effect against P. falciparum sporozoite challenge in a chimpanzee16 and an Aotus monkey model17. Moreover, it has been shown that LSA3 genomic sequence is conserved in the isolates from diverse geographical areas compared to other blood-stage vaccine candidate18. In the present study, we have shown that LSA3 was expressed in blood-stage parasites judged by western blotting, and immunoelectron microscopy showed that LSA3 was localized to the dense granules of merozoites. Taken together, we identified LSA3 as a novel blood-stage vaccine candidate through this study.
Results
Immunoreactivity of the recombinant proteins with Malian adult IgGs
A total of 1,827 recombinant proteins mono-biotinylated at the N-terminus were expressed using WGCFS (Table S1). Antigen-specific IgG responses to these proteins were profiled by an AlphaScreen as described15 and shown schematically in Fig. S1. Immunoreactivity of each test IgG against each antigen was determined, and a mean ASC (log-transformed AlphaScreen count) for each antigen was calculated. A total of 891/1,827 (49%) proteins were considered as immunoreactive to the 51 Malian IgG samples tested (Fig. 1), and the immunoreactive antigens were subjected to subsequent analysis.
Figure 1. Reactivity of Malian adult IgGs to recombinant proteins produced in the WGCFS.
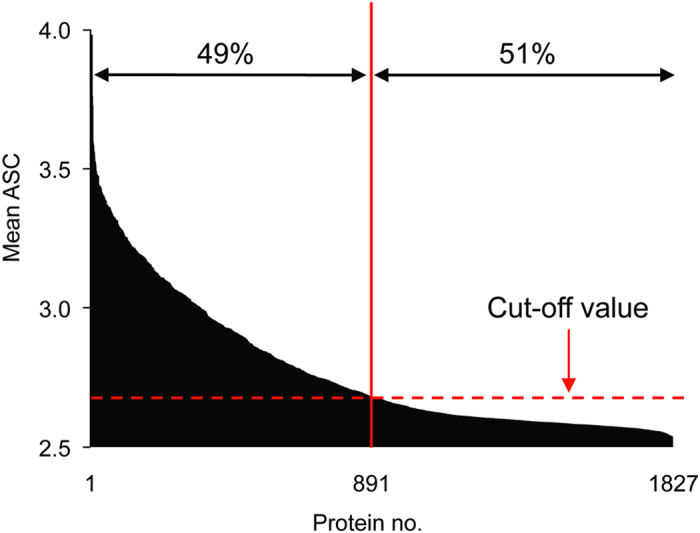
Proteins were sorted by their mean ASC, and the cut-off value was determined as mean plus 3SD of negative control antibodies (dashed line). Approximately 49% of proteins were considered reactive to the Malian IgG samples.
Correlation between GIA activity and antibody response
ASC values for individual antigens were fitted to a Gaussian distribution (data not shown). GIA activity of the 51 IgG samples also presented as a Gaussian distribution (Fig. S2). Therefore, we performed a Pearson correlation test for each individual antigen to determine the correlation between ASC and GIA activity. Because the target molecules of GIA are likely to be exposed to the extracellular environment we selected the proteins harboring a transmembrane domain (TM) and/or signal peptide (SP) from the 891 immunoreactive proteins. As a result, out of the 325 selected proteins, the ASC of 3 proteins showed a significant positive correlation with GIA activity (Table 1, Table S2). These positive proteins were LSA3-C, RAMA, and GLURP. No ASC showed a negative correlation with GIA activity as above. Since ASC of LSA3-C most highly correlated with GIA activity, we further characterized LSA3 in blood stage parasite.
Table 1. The proteins with the positive significant correlations between antibody responses and GIA activity.
| Library ID | Gene ID | Gene name | Expressed region | ASC | r | Unadjusted p-value | Adjusted p-value |
|---|---|---|---|---|---|---|---|
| (aa) | Mean | ||||||
| 1705 | PF3D7_0220000 | Liver stage antigen 3C (LSA3-C) | 750–1433 | 2.84 | 0.571 | 1.18 × 10−5 | 0.004 |
| 1303 | PF3D7_0707300 | Rhoptry-associated membrane antigen (RAMA) | 18–786 | 3.13 | 0.475 | 4.2 × 10−4 | 0.046 |
| 829 | PF3D7_1035300 | Glutamate-rich protein (GLURP) | 34–459 | 3.42 | 0.486 | 2.9 × 10−4 | 0.046 |
GIA activity of human anti-LSA3-C-specific antibody
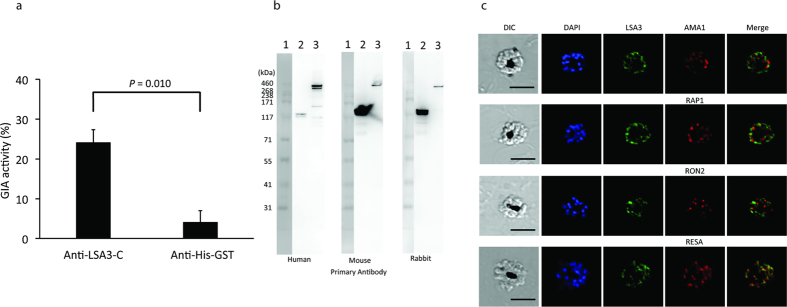
ASC of LSA3-C (V750 to K1433 of LSA3, Library ID:1705, PF3D7_0220000, Fig. 2) highly correlated with GIA data using Malian adult total IgGs (Table 1), suggesting that anti-LSA3-C antibody has functional activity against blood-stage parasites. However, it had earlier been thought that the LSA3 antigen was expressed only in pre-erythrocytic-stage parasites16. Therefore, we next determined the functional activity of human anti-LSA3-C-specific antibodies affinity purified from Malian adult total IgGs. Since the volume of antigen-specific IgG was limited, the human anti-LSA3-C-specific IgG was tested at a single concentration (0.48 mg/ml, which was the highest concentration that could be tested). The antigen-specific IgG exhibited 24% inhibition (Fig. 3a), which was significantly higher than that of the negative control (Student’s t-test; p = 0.010).
Figure 2. Schematic representation of the P. falciparum LSA3 protein.
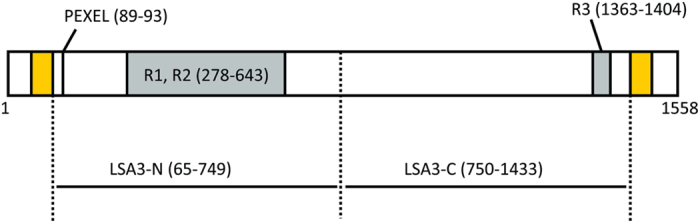
The design of the recombinant proteins expressed; repeat sequence regions (R1, R2, R3), PEXEL motif, and predicted transmembrane domains (in yellow) are shown. The numbers are amino acid positions. The boundaries of the repeat regions were defined by comparison of the primary structure in LSA3 between 3D7 and K1 strain40.
Figure 3. Characterization of LSA3 in blood stage parasites.
(a) Human anti-LSA3-C-specific IgG has significant GIA activity. Human anti-LSA3-C specific IgG was tested at 0.48 mg/ml. Rabbit anti-His-GST IgG was tested at 20 mg/ml as a negative control. Three independent GIA experiments in triplicate were performed, and the mean and SEM are shown. (b) LSA3 was expressed in blood-stage parasites. Western blot was performed with human, mouse or rabbit anti-LSA3-C antibodies. Parasite lysate was obtained from 1 × 104 P. falciparum infected red cells at mixed developmental stages. Molecular weight marker (Lane 1), purified 0.5 ng of C-terminal His-tagged recombinant LSA3-C protein (Lane 2) and the blood-stage parasite lysate (Lane 3) were separated by SDS-PAGE under reducing conditions. (c) IFA of schizont-stage parasites. Human anti-LSA3-C antibody was used for the IFA. Mouse anti-AMA1, -RAP1, -RON2, or -RESA antibodies were used for counter-staining to determine subcellular localization of microneme, rhoptry body, rhoptry neck or dense granule, respectively. Parasite nuclei were stained with DAPI. Scale bars indicate 5 μm.
Characterization of LSA3 in blood stage parasites
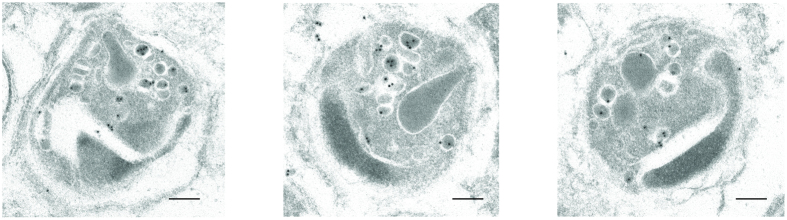
We then characterized LSA3 in the blood-stage parasite. Expression of LSA3 in the blood-stage parasites was first tested by western blotting with trophozoite/schizont-rich 3D7 parasite lysate using the purified human anti-LSA3-C antibody (Fig. 3b). Although the predicted molecular weight of LSA3 is 175 kDa, these antibodies specifically visualized two protein bands of approximately 300 kDa. Consistent with the unexpectedly high molecular weight of LSA3 observed in the western blot, recombinant LSA3-C whose size is predicted to be 80 kDa, migrates at approximately 120 kDa in SDS-PAGE (Fig. 3b, Fig. S3). When western blotting analysis was performed with mouse and rabbit antibodies, which were raised against purified N-terminal GST tagged LSA3-C protein, the same high molecular weight bands were observed (Fig. 3b). Since the sizes of bands determined by the SDS-PAGE were larger than expected, to confirm the specificity of anti-LSA3-C antibody, we performed two experiments. A rabbit was immunized with N-terminal LSA3 protein (LSA3-N, Fig. 2), and western blotting analysis with the anti-LSA3-N antibody specifically visualized protein bands of approximately 300 kDa (Fig. S4) as consistent with the result of anti-LSA3-C antibody. In the second experiment, immunoprecipitation was performed with rabbit anti-LSA3-C antibody using rabbit anti-His-GST antibody as a negative control. The anti-LSA3-C antibody pulled down a protein band which migrated around 300 kDa. Mass spectrometry (MS) analysis of the band detected 7 specific peptides which were only recognized in anti-LSA3-C sample, and 6 of the peptides were matched with LSA3 sequence (Fig. S5). Taken together, the data suggest native LSA3 protein, which is expressed in blood-stage parasites, does not migrate according to the predicted molecular weight.
We further examined the subcellular localization of LSA3 by IFA. As shown in Fig. 3c, LSA3 in schizont-stage parasites co-localized with RESA, suggesting that LSA3 localized to dense granules of merozoites. To confirm this observation, immunoelectron microscopy using rabbit anti-LSA3-C antibody was conducted. As shown in Fig. 4, LSA3 localized to the dense granules of merozoites formed in schizont parasites. IFA results using rabbit anti-LSA3-C antibody were consistent with those using human antibody (Fig. S6).
Figure 4. Immunoelectron microscopy observations of LSA3 in merozoites of schizont-infected erythrocytes.
Rabbit anti-LSA3-C antibody was used for primary antibody. The gold particles were detected specifically at dense granules. Scale bars indicate 200 nm.
Discussion
We generated a large P. falciparum protein library encompassing much of the asexual parasite proteome for the first time using the WGCFS expression system, and this library was screened with antibodies from Malian adults having naturally acquired antibody responses as a result of repeated malaria infections. Antigen-specific antibody responses against 3 proteins, out of 325 immunoreactive ones with TM and/or SP tested, showed significant correlations with the functional activity measured by GIA. One of the key aspects of this study is that we quantified antigen-specific antibody responses using the AlphaScreen that is an immobilization-free method to detect immunoreactions. We have shown for the first time that human antibody against LSA3 (more specifically LSA3-C), which was selected from this study, showed functional activity. The positive GIA result indicates the screening strategy shown in this study is valuable for a future novel blood-stage malaria vaccine candidate discovery.
Although P. falciparum genome information has been available for more than a decade, parasite antigens targeted by anti-malaria immunity in humans remains largely unknown. This bottleneck has partially been attributed to difficulties in expression of the plasmodial proteins. Several studies have aimed to profile host antibodies using protein microarrays containing recombinant P. falciparum proteins synthesized by E. coli-based cell-free protein expression systems10,19. However, a major drawback of these high-throughput protein microarray based approaches may be the conformation of expressed proteins using this system. To the best of our knowledge, none of the malaria recombinant proteins expressed by the E. coli-based cell-free protein expression systems has been shown to induce functional antibodies without codon-optimization and/or a refolding process. In contrast, WGCFS is a eukaryotic plant-based recombinant protein expression system that may offer a better alternative. The system can synthesize recombinant proteins that retain critical functional epitopes, at least in part, as evidenced by the fact that antibodies elicited against the recombinant proteins showed functional activities with live parasites20,21. WGCFS therefore offers a unique and reliable platform for identification of plasmodial proteins that could be important targets of host humoral immune responses. The AlphaScreen-based antibody-antigen reactions take place in homogenous solution rather than in a solid phase and thus this is a sensitive platform for detection of protein-tertiary-structure-dependent antibody immunoreactions22. Here we applied the WGCFS and the AlphaScreen to comprehensively analyze immunoreactions of IgGs obtained from malaria-exposed individuals in Mali. We detected immunoreactivity in approximately half of the proteins (891/1,827), which is higher than the reactivity reported in previous studies (~20%)10,19. While a further head-to-head study is required to compare the two systems directly, the higher immunoreactivity seen in this study might be partially explained by the maintenance of better conformation of proteins in the WGCFS/AlphaScreen system.
To correlate this immunoreactivity with a functional anti-parasite response, we compared these results with the GIA activity data; in this analysis the anti-LSA3-C antibody response showed the most significant correlation with GIA activity (Table 1). In the present study, we synthesized 2 truncated recombinant proteins of LSA3, because a full-length LSA3 protein is too large for WGCFS expression, in terms of the molecular weight (Fig. 2). Although antigen polymorphism is a major obstacle in development of vaccine against blood-stage parasite23, according to the sequences available in PlasmoDB (http://plasmodb.org/), LSA3-C region is conserved compared with other blood-stage vaccine candidates (dNS/dS = 3.3); in contrast the LSA3-N region is relatively polymorphic (dNS/dS = 6.4). A previous study also showed that non-repeat regions of LSA3 gene sequence are highly conserved in the isolates from diverse geographical areas18.
Since GIA activity of anti-LSA3 antibody had not been reported in any species, we affinity purified human anti-LSA3-C-specific antibody and confirmed that the antibody could show GIA activity. The fact that human anti-LSA3-C specific antibody showed GIA activity suggested that the LSA3-C recombinant protein expressed by WGCFS conserved, at least in part, native conformation of the protein in parasites. However, the test concentration in GIA was at least 10-fold higher than seen in human plasmas (data not shown). Therefore, further study is required to determine contribution of each antigen-specific antibody to the GIA activity observed with total IgGs. For unknown reasons, the rabbit anti-LSA3-C antibody did not show any GIA activity (data not shown). Since we could purify functional human antibody with the LSA3-C, the LSA3-C contains at least one functional epitope which is the target of inhibitory human antibody. In the rabbit study, the animal was immunized with truncated LSA3 protein (LSA3-C), which could express different epitopes which were not seen in the (native) full-length LSA3. Thus it could be possible that the immunoreactivity against such artificial epitopes on LSA3-C masked the reactivity against the functional epitope(s) in rabbit when immunized with LSA3-C. Alternatively, it might be also possible that the epitope recognition is not necessarily the same in rabbits and humans for some antigens.
In this study, we have shown that LSA3 is expressed in blood-stage parasites by western blotting analysis, consistent with previous MS/MS analysis that detected LSA3 in ring, trophozoite, schizont, and merozoite stage parasites24,25. The molecular weight estimated from migration in the gel was higher than predicted from amino acid sequence (approximately 175 kDa). In Fig. S3, the recombinant LSA3-C expressed by WGCFS migrated at approximately 120 kDa in SDS-PAGE, which was ~40 kDa heavier than expected (approximately 80 kDa). Because WGCFS is free from glycosylation systems26, the observed slow migration of recombinant LSA3-C protein is likely due to the physiological properties of the LSA3 protein itself. This might explain, at least in part, the reasons why native LSA3 did not migrate at the expected molecular weight. On the other hand, LSA3 might be transcriptionally or post-translationally modified at sporozoite-stage, because the previous western blotting with sporozoite lysate detected a ~175 kDa protein band of LSA316.
Immunoelectron microscopy clearly showed that LSA3 is localized in dense granules (Fig. 4). It has been reported that dense granules secrete their contents into the forming parasitophorous vacuole (PV) during merozoite invasion27. Consistent with other dense granule proteins28, LSA3 is located at the PV of ring-stage parasites (Fig. S7). This is also in line with a previous study showing that LSA3 is localized to the PV of 5- and 6-day-old liver-stage schizonts in the chimpanzee’s liver cells16. Therefore, LSA3 could have a common function during parasite development in both hepatocytes and erythrocytes.
The results reported here are from a limited number of field IgG samples, thus it would be worthwhile to validate the screening results using a larger number of samples and samples from widely different malaria endemic area. A previous cohort study using a P. falciparum protein microarray by other investigators has suggested that higher titers against LSA3 might contribute to the prevention of symptomatic malaria infections during 12 weeks of follow-up after drug treatment29. However, another study using a different P. falciparum protein microarray showed that the levels of anti-LSA3 antibodies did not correlate with subsequent malaria risk during a period of seasonal transmission in 8–10 year old Malian children10. Although GIA has been utilized to identify novel vaccine candidates and prioritize vaccine formulations, there are also reports showing no correlation between GIA activity and clinical protection in several epidemiological studies30. Therefore, further studies are anticipated using the WGCFS/AlphaScreen system established in this study to identify the targets of naturally acquired protective antibody responses. Such studies will include human samples obtained from carefully designed cohort studies and samples assessed by other functional assays. These future studies may identify additional novel malaria vaccine candidates and enhance our understanding of clinical immunity to asexual stages of malaria infections.
Materials and Methods
Production of parasite proteins
We created a large P. falciparum protein library comprised of 1,827 polypeptides derived from 1,565 genes. We first amplified 554 genes by PCR using LATaq PCR kit (Takara Bio, Kusatsu, Japan) and cloned the products into the pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA). Genes encoding an additional 148 proteins were amplified by PCR and cloned into the pEU vector (CellFree Sciences, Matsuyama, Japan). The library also contained an additional 1,125 target proteins derived from full-length cDNA library clones raised from trophozoite-schizont rich 3D7 P. falciparum cultures. The recombinant proteins were expressed without codon optimization using the WGCFS as previously described31. WGCFS synthesis of the P. falciparum protein library was based on the previously described bilayer diffusion system32. For biotinylation of proteins, 500 nM D-biotin (Nacalai Tesque, Kyoto, Japan) was added to both the translation and substrate layers. Crude wheat germ cell-free expressed BirA (1 μl) was added to the translation layer. In vitro transcription and cell-free protein synthesis for the P. falciparum protein library were carried out using the GenDecoder 1000 robotic synthesizer (CellFree Sciences) as previously described33,34. Expression of the proteins was confirmed by western blotting using FITC- or HRP-conjugated streptavidin (ThermoFisher Scientific, Waltham, MA, Code # SA10002 or #21130, respectively).
Detection and quantification of P. falciparum antigen-specific IgG by AlphaScreen
P. falciparum antigen-specific antibodies were quantified by an AlphaScreen as previously reported15 with slight modifications. The protocol was automated by use of the JANUS Automated Workstation (PerkinElmer Life and Analytical Science, Boston, MA). Reactions were carried out in 25 μl of reaction volume per well in 384-well OptiPlate microtiter plates (PerkinElmer). First, 0.1 μl of the translation mixture containing a recombinant P. falciparum biotinylated protein was diluted 50-fold (5 μl), mixed with 10 μl of 10 μg/ml Malian adult IgG in reaction buffer (100 mM Tris-HCL [pH 8.0], 0.01% [v/v] Tween-20 and 0.1% [w/v] bovine serum albumin), and incubated for 30 min at 26 °C to form an antigen-antibody complex. Subsequently, a 10 μl suspension of streptavidin-coated donor-beads and acceptor-beads (PerkinElmer) conjugated with protein G (Thermo Scientific) in the reaction buffer was added to a final concentration of 12 μg/ml of both beads. The mixture was incubated at 26 °C for 1 h in the dark to allow the donor and acceptor-beads to optimally bind to biotin and human IgG, respectively. Upon illumination of this complex, a luminescence signal at 620 nm was detected by the EnVision plate reader (PerkinElmer) and the result was expressed as AlphaScreen counts. The performance of the AlphaScreen was validated with 200 pM of biotinylated-IgG to be Z-factor with more than 0.5. A translation mixture expressing AMA1 incubated with a plasma sample taken from a Thai malaria-immune individual was used as a positive control in each plate. A translation mixture of WGCFS without template mRNA incubated with a plasma sample taken from a Thai malaria naïve individual was used as a negative control in each plate. Reading the plates was conducted in a randomized manner to avoid biases.
Production of mouse and rabbit antisera
As previously described35, we generated rabbit polyclonal antisera against LSA3-C (V750 to K1433 of the 3D7 sequence; PF3D7_0220000) and mouse antisera against AMA1 (Q25 to K546; PF3D7_1133400), RAP1 (M1 to D782; PF3D7_1410400), and RON2 (K84 to Q968; PF3D7_1452000). All antigens were synthesized by WGCFS and purified by GST or hexa-histidine (His) protein-tag fused with the N-terminus of the recombinant proteins. Mouse monoclonal antibody against RESA (PF3D7_0102200) was a kind gift from Robin F. Anders36.
Human plasma collection and IgG preparations
A cohort study was conducted between 2008 and 2011 in three villages of Kenieroba, Fourda and Bozokin in southern Mali. Details of the main cohort study are described elsewhere37, and the study is registered with ClinicalTrials.gov, number NCT00669084. As a part of the study, plasma samples were collected from 51 healthy adults in October, 2008 (middle of the malaria transmission season). Detailed methods for preparation of total IgG and antigen-specific IgG from Malian plasma samples have been described previously38. In brief, total IgG purification was performed using a Protein G column for each plasma sample. For the anti-LSA3-C-specific IgG purification, 4 adult plasma samples with higher anti-LSA3-C titers were pooled first, and the total IgG was applied to a NHS-activated Sepharose 4 Fast Flow column coupled with WGCFS synthesized C-terminal His-tagged LSA3-C protein according to the manufacturer’s instructions.
Western blot analysis
Western blot was conducted with anti-LSA3-C antibodies as previously described8. Purified schizont-rich parasite pellets from P. falciparum 3D7 strain were directly lysed in reducing SDS-PAGE sample buffer. The lysate was boiled at 95 °C for 5 min, centrifuged at 10,000 × g for 10 min at 4 °C, supernatants were collected, and resolved by electrophoresis on a 4–12% Bolt Bis-Tris Plus gel (ThermoFisher Scientific) with MOPS buffer. Following electroblotting, the membrane was incubated with primary antibodies diluted at the following concentrations: human anti LSA3-C antibody, 0.173 μg/ml; mouse anti-LSA3-C antibody, 1:300; rabbit anti-LSA3-C antibody, 1:1,000. After washing, it was incubated with secondary antibodies diluted at the following concentrations: polyclonal rabbit anti-human IgG/HRP (Dako, Glostrup, Denmark, Code # P0214), 1:20,000; ECL peroxidase labeled anti-mouse antibody (GE Healthcare, Chicago, IL, Code # NA931), 1:10000; ECL peroxidase labeled anti-rabbit antibody (GE Healthcare, Code # NA934), 1:10,000.
Indirect immunofluorescence assay (IFA)
IFA was conducted as previously described35. The samples were stained with primary antibodies diluted at the following concentrations in blocking solution at 37 °C for 1 h: human anti-LSA3-C antibody, 0.173 μg/ml; rabbit anti-LSA3-C antibody, 1:500; mouse anti-AMA1 antibody, 1:100; mouse anti-RAP1 antibody, 1:1,000; mouse anti-RON2 antibody, 1:100; mouse anti-RESA monoclonal antibody, 1:100. Secondary antibodies, Alexa Fluor 488-conjugated goat anti-human IgG (Invitrogen, Code # A11013), Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen, Code # A11034) and Alexa Fluor 568-conjugated goat anti-mouse IgG (Invitrogen, Code #A11031), were used at a 1:500 dilution in blocking solution at 37 °C for 30 min. DAPI (4’, 6-diamidino-2-phenylindole) (Invitrogen, Code #D1306) at 2 μg/ml was also added to the secondary-antibody solution to stain the nuclei. Slides were mounted in ProLong Gold Antifade reagent (Invitrogen, Code #P36934) and viewed under a 63 × oil immersion lens. High-resolution image capture and processing were performed with a confocal scanning laser microscope (LSM710; Carl Zeiss MicroImaging, Thornwood, NY). Images were processed in Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
Immunoelectron microscopy
Parasites were fixed and embedded in LR White resin (Polysciences, Warrington, PA)39 and ultrathin sections were immunostained8 as previously described. Rabbit anti-LSA3-C antibody was used at a 1:200 dilution. Samples were examined with a transmission electron microscope (JEM-1230; JEOL, Tokyo, Japan).
P. falciparum growth inhibition assay (GIA)
The GIA against 3D7 strain with 10 mg/ml total human IgGs was performed at the National Institute of Allergy and Infectious Diseases (NIAID) as described previously38. The GIA with human anti-LSA3-C-specific IgG was performed at Ehime University as described elsewhere8. Both GIAs were one-cycle assays, and parasite growth was determined by a biochemical assay at NIAID38, and by counting SYBR Green I positive cells with flow cytometry at Ehime University8.
Ethical approval
The Mali cohort study was reviewed and approved by the Institutional Review Boards (IRB) of the NIAID, National Institutes of Health (NIH) and by the Ethics Committee of the Faculty of Medicine, Pharmacy and Odontostomatology, University of Bamako (Protocol no. 08-I-N120, ClinicalTrials.gov identifier no. NCT00669084). The study to obtain serum samples from Thailand was approved by the Ethics Committee of the Thai Ministry of Public Health and the IRB of the Walter Reed Army Institute of Research (WRAIR 802). Written informed consents were obtained from all adult volunteers. The study was conducted in accordance with approved protocols and regulations. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Ehime University (su-14-6), and the experiments were conducted according to the Ethical Guidelines for Animal Experiments of Ehime University.
Statistical analysis
Raw AlphaScreen counts were converted to logarithmic values, and subsequent analyses were performed with the log-transformed values (ASC). The protein whose mean ASC of the 51 test IgG samples was below the cut-off value (mean plus 3 standard deviations of the negative control) was considered as a non-reactive protein and was excluded from further analysis. For the reactive proteins, a Kolmogorov-Smirnov test was used for testing normality of the distribution, and a Pearson correlation test was used to analyze the correlation between GIA activity and ASC for each antigen. Benjamini-Hochberg’s correction was utilized for multiple comparisons. The R software (version 3.1.2; R Foundation for Statistical Computing) was used for statistical analysis and p < 0.05 was considered significant.
Additional Information
How to cite this article: Morita, M. et al. Immunoscreening of Plasmodium falciparum proteins expressed in a wheat germ cell-free system reveals a novel malaria vaccine candidate. Sci. Rep. 7, 46086; doi: 10.1038/srep46086 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank adult volunteers in Mali and Thailand who participated in the epidemiology studies, and we acknowledge Saibou Doumbia, Drissa Konate, Mory Doumbouya, Abdoul Salam Keita, and Jetsumon Sattabongkot for supporting these studies. We are grateful to Robin F. Anders for providing the anti-PfRESA monoclonal antibody. We also thank the Japanese Red Cross Society for providing human erythrocytes and human plasma. We appreciate Saki Fujii and Masachika Shudo at the Division of Analytical Bio-Medicine, the Advanced Research Support Center (ADRES), Ehime University for their technical assistance. This work was supported in part by MEXT KAKENHI (JP23117008) and JSPS KAKENHI (JP25460517, JP26253026, JP26670202, JP26860279, JP15H05276, JP16K15266) in Japan. This study was also supported in part by the Intramural Research Program of the NIAID, NIH and by the PATH Malaria Vaccine Initiative.
Footnotes
The authors declare no competing financial interests.
Author Contributions E.T., K.M., C.L., T.T. designed research; M.M., D.I., K.M., A.T., A.D., M.T. performed research and analyzed data; R.M.F., M.D. provided human samples; and E.T., M.M., K.M., R.M.F., C.L., T.T. wrote the paper.
References
- WHO. World Malaria Report 2015 (2015).
- Straimer J. et al. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science, doi: 10.1126/science.1260867 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp A. M. et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361, 455–467, doi: 10.1056/NEJMoa0808859 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Mc G. I. & Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature 192, 733–737 (1961). [DOI] [PubMed] [Google Scholar]
- Ogutu B. R. et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS One 4, e4708, doi: 10.1371/journal.pone.0004708 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring M. D. et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS One 4, e5254, doi: 10.1371/journal.pone.0005254 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkholtz L. M. et al. Heterologous expression of plasmodial proteins for structural studies and functional annotation. Malar J 7, 197, doi: 10.1186/1475-2875-7-197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam T. U. et al. Discovery of GAMA, a Plasmodium falciparum merozoite micronemal protein, as a novel blood-stage vaccine candidate antigen. Infect Immun 79, 4523–4532, doi: 10.1128/IAI.05412-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. H. et al. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci USA 102, 547–552, doi: 10.1073/pnas.0408782102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton P. D. et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci USA 107, 6958–6963, doi: 10.1073/pnas.1001323107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu A. et al. Sterile protective immunity to malaria is associated with a panel of novel P. falciparum antigens. Mol Cell Proteomics 10, M111 007948, doi: 10.1074/mcp.M111.007948 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H. et al. Antibodies against a Plasmodium falciparum antigen PfMSPDBL1 inhibit merozoite invasion into human erythrocytes. Vaccine 30, 1972–1980, doi: 10.1016/j.vaccine.2012.01.010 (2012). [DOI] [PubMed] [Google Scholar]
- Bolad A. et al. Antibody-mediated in vitro growth inhibition of field isolates of Plasmodium falciparum from asymptomatic children in Burkina Faso. Am J Trop Med Hyg 68, 728–733 (2003). [PubMed] [Google Scholar]
- Crompton P. D. et al. In vitro growth-inhibitory activity and malaria risk in a cohort study in mali. Infect Immun 78, 737–745, doi: 10.1128/IAI.00960-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Komori H., Nose M., Endo Y. & Sawasaki T. Simple screening method for autoantigen proteins using the N-terminal biotinylated protein library produced by wheat cell-free synthesis. J Proteome Res 9, 4264–4273, doi: 10.1021/pr9010553 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubersies P. et al. Protection against Plasmodium falciparum malaria in chimpanzees by immunization with the conserved pre-erythrocytic liver-stage antigen 3. Nat Med 6, 1258–1263, doi: 10.1038/81366 (2000). [DOI] [PubMed] [Google Scholar]
- Perlaza B. L. et al. Protection against Plasmodium falciparum challenge induced in Aotus monkeys by liver-stage antigen-3-derived long synthetic peptides. Eur J Immunol 38, 2610–2615, doi: 10.1002/eji.200738055 (2008). [DOI] [PubMed] [Google Scholar]
- Prieur E. & Druilhe P. The malaria candidate vaccine liver stage antigen-3 is highly conserved in Plasmodium falciparum isolates from diverse geographical areas. Malaria journal 8, 1 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan D. L. et al. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics 8, 4680–4694, doi: 10.1002/pmic.200800194 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam T. U. et al. Application of wheat germ cell-free protein expression system for novel malaria vaccine candidate discovery. Expert Rev Vaccines 13, 75–85, doi: 10.1586/14760584.2014.861747 (2014). [DOI] [PubMed] [Google Scholar]
- Tsuboi T. et al. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect Immun 76, 1702–1708, doi: 10.1128/IAI.01539-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H. et al. Production of monoclonal antibodies against GPCR using cell-free synthesized GPCR antigen and biotinylated liposome-based interaction assay. Sci Rep 5, 11333, doi: 10.1038/srep11333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan R., Farooq U., Dubey M. & Malla N. Genetic polymorphism in Plasmodium falciparum vaccine candidate antigens. Indian J Pathol Microbiol 48, 429–438 (2005). [PubMed] [Google Scholar]
- Florens L. et al. A proteomic view of the Plasmodium falciparum life cycle. Nature 419, 520–526, doi: 10.1038/nature01107 (2002). [DOI] [PubMed] [Google Scholar]
- Oehring S. C. et al. Organellar proteomics reveals hundreds of novel nuclear proteins in the malaria parasite Plasmodium falciparum. Genome Biol 13, R108, doi: 10.1186/gb-2012-13-11-r108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai K., Sawasaki T. & Endo Y. Practical cell-free protein synthesis system using purified wheat embryos. Nat Protoc 5, 227–238, doi: 10.1038/nprot.2009.207 (2010). [DOI] [PubMed] [Google Scholar]
- Torii M., Adams J. H., Miller L. H. & Aikawa M. Release of merozoite dense granules during erythrocyte invasion by Plasmodium knowlesi. Infect Immun 57, 3230–3233 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen H. E. et al. Biosynthesis, localization, and macromolecular arrangement of the Plasmodium falciparum translocon of exported proteins (PTEX). J Biol Chem 287, 7871–7884, doi: 10.1074/jbc.M111.328591 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent A. E. et al. Plasmodium falciparum Protein Microarray Antibody Profiles Correlate With Protection From Symptomatic Malaria in Kenya. J Infect Dis 212, 1429–1438, doi: 10.1093/infdis/jiv224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K. Progress and prospects for blood-stage malaria vaccines. Expert Rev Vaccines 15, 765–781, doi: 10.1586/14760584.2016.1141680 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawasaki T., Ogasawara T., Morishita R. & Endo Y. A cell-free protein synthesis system for high-throughput proteomics. Proc Natl Acad Sci USA 99, 14652–14657, doi: 10.1073/pnas.232580399 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawasaki T. et al. A bilayer cell-free protein synthesis system for high-throughput screening of gene products. FEBS letters 514, 102–105 (2002). [DOI] [PubMed] [Google Scholar]
- Sawasaki T., Morishita R., Gouda M. D. & Endo Y. Methods for high-throughput materialization of genetic information based on wheat germ cell-free expression system. Methods Mol Biol 375, 95–106, doi: 10.1007/978-1-59745-388-2_5 (2007). [DOI] [PubMed] [Google Scholar]
- Sawasaki T. et al. The wheat germ cell-free expression system: methods for high-throughput materialization of genetic information. Methods Mol Biol 310, 131–144 (2005). [DOI] [PubMed] [Google Scholar]
- Ito D. et al. RALP1 is a rhoptry neck erythrocyte-binding protein of Plasmodium falciparum merozoites and a potential blood-stage vaccine candidate antigen. Infect Immun 81, 4290–4298, doi: 10.1128/IAI.00690-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culvenor J. G., Day K. P. & Anders R. F. Plasmodium falciparum ring-infected erythrocyte surface antigen is released from merozoite dense granules after erythrocyte invasion. Infect Immun 59, 1183–1187 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopera-Mesa T. M. et al. Impact of red blood cell variants on childhood malaria in Mali: a prospective cohort study. Lancet Haematol 2, e140–e149, doi: 10.1016/S2352-3026(15)00043-5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K. et al. Non-apical membrane antigen 1 (AMA1) IgGs from Malian children interfere with functional activity of AMA1 IgGs as judged by growth inhibition assay. PLoS One 6, e20947, doi: 10.1371/journal.pone.0020947 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa M. & Atkinson C. T. Immunoelectron microscopy of parasites. Adv Parasitol 29, 151–214 (1990). [DOI] [PubMed] [Google Scholar]
- Toure-Balde A. et al. Evidence for multiple B-and T-cell epitopes in Plasmodium falciparum liver-stage antigen 3. Infect Immun 77, 1189–1196 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.