Abstract
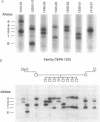
To identify DNA polymorphisms that are abundant in the human genome and are detectable by polymerase chain reaction amplification of genomic DNA, we tested the hypothesis that the polydeoxyadenylate tract of the Alu family of repetitive elements is polymorphic among human chromosomes. We analyzed the 3' ends of three specific Alu sequences and found that two (in the adenosine deaminase gene and the beta-globin pseudogene) were polymorphic. This novel class of polymorphisms, termed AluVpA [Alu variable poly(A)] may represent one of the most useful and informative group of DNA markers in the human genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnou N. P., O'Brien S. J., Shimada T., Nash W. G., Chen M. J., Nienhuis A. W. Chromosomal organization of the human dihydrofolate reductase genes: dispersion, selective amplification, and a novel form of polymorphism. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5170–5174. doi: 10.1073/pnas.81.16.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Chebloune Y., Pagnier J., Trabuchet G., Faure C., Verdier G., Labie D., Nigon V. Structural analysis of the 5' flanking region of the beta-globin gene in African sickle cell anemia patients: further evidence for three origins of the sickle cell mutation in Africa. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4431–4435. doi: 10.1073/pnas.85.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dausset J. Le centre d'étude du polymorphisme humain. Presse Med. 1986 Oct 18;15(36):1801–1802. [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Green P., Helms C., Cartinhour S., Weiffenbach B., Stephens K., Keith T. P., Bowden D. W., Smith D. R., Lander E. S. A genetic linkage map of the human genome. Cell. 1987 Oct 23;51(2):319–337. doi: 10.1016/0092-8674(87)90158-9. [DOI] [PubMed] [Google Scholar]
- Drayna D., White R. The genetic linkage map of the human X chromosome. Science. 1985 Nov 15;230(4727):753–758. doi: 10.1126/science.4059909. [DOI] [PubMed] [Google Scholar]
- Frossard P. M., Coleman R. T., Protter A. A., Seilhamer J. J., Funke H., Assmann G. Deletion polymorphism 5' to the human apolipoprotein AI (apo AI) gene. Nucleic Acids Res. 1986 Nov 11;14(21):8694–8694. doi: 10.1093/nar/14.21.8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M. Efficient computations in multilocus linkage analysis. Am J Hum Genet. 1988 Mar;42(3):498–505. [PMC free article] [PubMed] [Google Scholar]
- Levinson G., Gutman G. A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987 May;4(3):203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M. M., Slightom J. L., Goodman M. Phylogenetic relations of humans and African apes from DNA sequences in the psi eta-globin region. Science. 1987 Oct 16;238(4825):369–373. doi: 10.1126/science.3116671. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Olson M., Hood L., Cantor C., Botstein D. A common language for physical mapping of the human genome. Science. 1989 Sep 29;245(4925):1434–1435. doi: 10.1126/science.2781285. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Ott J. A computer program for linkage analysis of general human pedigrees. Am J Hum Genet. 1976 Sep;28(5):528–529. [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sargentini N. J., Smith K. C. Spontaneous mutagenesis: the roles of DNA repair, replication, and recombination. Mutat Res. 1985 Jul;154(1):1–27. doi: 10.1016/0165-1110(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Semenza G. L., Malladi P., Surrey S., Delgrosso K., Poncz M., Schwartz E. Detection of a novel DNA polymorphism in the beta-globin gene cluster. J Biol Chem. 1984 May 25;259(10):6045–6048. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tautz D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 1989 Aug 25;17(16):6463–6471. doi: 10.1093/nar/17.16.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabl M., Meyer J., Beck-Engeser G., Tenkhoff M., Burrows P. D. Critical test of a sister chromatid exchange model for the immunoglobulin heavy-chain class switch. Nature. 1985 Feb 21;313(6004):687–689. doi: 10.1038/313687a0. [DOI] [PubMed] [Google Scholar]
- Warren A. C., Slaugenhaupt S. A., Lewis J. G., Chakravarti A., Antonarakis S. E. A genetic linkage map of 17 markers on human chromosome 21. Genomics. 1989 May;4(4):579–591. doi: 10.1016/0888-7543(89)90282-6. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Kaplan D. J., States J. C., Akeson A. L., Perme C. M., Bilyk I. J., Vaughn A. J., Lattier D. L., Hutton J. J. Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry. 1986 Dec 16;25(25):8234–8244. doi: 10.1021/bi00373a017. [DOI] [PubMed] [Google Scholar]
- Williams S. M., Strobeck C. Sister chromatid exchange and the evolution of rDNA spacer length. J Theor Biol. 1985 Oct 21;116(4):625–636. doi: 10.1016/s0022-5193(85)80092-8. [DOI] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]
- Woods-Samuels P., Wong C., Mathias S. L., Scott A. F., Kazazian H. H., Jr, Antonarakis S. E. Characterization of a nondeleterious L1 insertion in an intron of the human factor VIII gene and further evidence of open reading frames in functional L1 elements. Genomics. 1989 Apr;4(3):290–296. doi: 10.1016/0888-7543(89)90332-7. [DOI] [PubMed] [Google Scholar]