Abstract
Although recombinant glucocerebrosidase (GCase) is the standard therapy for the inherited lysosomal storage disease Gaucher’s disease (GD), enzyme replacement is not effective when the central nervous system is affected. We created a series of recombinant genes/proteins where GCase was linked to different membrane binding peptides including the Tat peptide, the rabies glycoprotein derived peptide (RDP), the binding domain from tetanus toxin (TTC), and a tetanus like peptide (Tet1). The majority of these proteins were well-expressed in a mammalian producer cell line (HEK 293F). Purified recombinant Tat-GCase and RDP-GCase showed similar GCase protein delivery to a neuronal cell line that genetically lacks the functional enzyme, and greater delivery than control GCase, Cerezyme (Genzyme). This initial result was unexpected based on observations of superior protein delivery to neurons with RDP as a vector. A recombinant protein where a fragment of the flexible hinge region from IgA (IgAh) was introduced between RDP and GCase showed substantially enhanced GCase neuronal delivery (2.5 times over Tat-GCase), suggesting that the original construct resulted in interference with the capacity of RDP to bind neuronal membranes. Extended treatment of these knockout neuronal cells with either Tat-GCase or RDP-IgAh-GCase resulted in an >90% reduction in the lipid substrate glucosylsphingosine, approaching normal levels. Further in vivo studies of RDP-IgAh-GCase as well as Tat-GCase are warranted to assess their potential as treatments for neuronopathic forms of GD. These peptide vectors are especially attractive as they have the potential to carry a protein across the blood–brain barrier, avoiding invasive direct brain delivery.
Keywords: Gaucher’s disease (GD), Glucocerebrosidase, Enzyme-replacement therapy, Linker design, Rabies-derived peptide
1. Introduction
Enzyme replacement therapy has greatly impacted the treatment of the visceral manifestations of Gaucher’s disease (GD) for several decades. Gaucher’s disease is an inherited lysosomal storage disease where homozygous and compound heterozygous mutations in the gene for glucocerebrosidase result in deficiency of the enzyme and accumulation of glycosphingolipid substrates glucosylceramide (GlcCer) and glucosylsphingosine (GlcSph). GD is classified into three clinical subtypes: non-neuronopathic type 1, acute neuronopathic type 2, and chronic neuronopathic type 3 (Branco Novo et al., 2012; Cabrera-Salazar et al., 2010; Lee et al., 2005).
The three commercially available recombinant enzymes are targeted to macrophages by mannose-directed endocytosis (Pastores, 2010). In addition to recombinant enzyme therapies, two small molecule glucosylceramide synthase inhibitors have been approved for type 1 GD therapy (Ficicioglu, 2008; Poole, 2014). Although ERT is the standard of care for GD therapy, small molecule therapeutics are valuable treatment options with potential for future growth. Small molecule therapeutics may be at least part of a treatment approach for neuronopathic GD (Cabrera-Salazar et al., 2012). Although three recombinant GCase enzymes and the two glucosylceramide synthase inhibitors are commercially available, all of them treat only the non-neuronopathic type 1 GD. None of these GCase enzymes cross the blood–brain barrier (BBB) due to their size and the lack of mannose receptors on brain endothelia (Grubb et al., 2010), nor do they have any predicted preferential neuronal-targeting properties. Efficiency of uptake of GCase in neuronal cell lines is significantly less than in macrophages and has been shown to be variable based on the cell line used (Schueler et al., 2002). Currently, there is no available therapy for neuronopathic types 2 or 3 GD. As a result, there is a distinct need for an engineered recombinant GCase enzyme for neuronal delivery, enabling the possibility of enzyme replacement therapy for types 2 and 3 GD. A neuron-targeted GCase would need to preferentially bind neurons and ideally, would cross the blood–brain barrier (BBB) increasing the options for therapeutic delivery.
There are a wide variety of peptide delivery vectors capable of facilitating the endocytosis of GCase in neurons. These peptides can be generally separated into two classes, receptor-independent peptides that lack cell type specificity and receptor-dependent peptides that have retained cell-type specificity. These delivery vectors also vary with additional parameters including their ability to cross the BBB and feasibility for use in vivo. The first category of receptor-independent peptides includes cell-penetrating peptides (CPPs) that have the ability to deliver proteins to any cell type (Chauhan et al., 2007; Deshayes et al., 2005; Heitz et al., 2009; Zorko and Langel, 2005). While originally controversial, it is now widely accepted that these peptides are taken into cells through endocytosis. CPPs have been shown to deliver cargo proteins across the BBB (Schwarze et al., 1999), although this has been reported to have very low efficiency (Xia et al., 2001). While a large number of different CPPs exist, Tat, an 11 amino acid peptide originally derived from the transactivator protein of HIV (Tat), was the first to be described (Frankel and Pabo, 1988) and remains one of the more widely used CPPs. Tat is taken up by receptor-independent macropinocytosis and following uptake, most of the Tat-linked cargo protein is sequestered in endosomes (Chauhan et al., 2007; Gillmeister et al., 2011; Gramlich et al., 2013).
Tat has been used, in a limited capacity, to enhance the delivery of lysosomal enzymes in vitro. Treatment of Gaucher fibroblasts with GCase-Tat resulted in approximately 4 and 10 fold more intracellular enzyme activity compared to a control GCase and commercially-available Cerezyme (Genzyme), for types 1 and 2 fibroblasts respectively (Lee et al., 2005). Tat has also been linked to another lysosomal enzyme, galactocerebrosidase (GALC) for delivery to a wider variety of cell types with variable results. Tat improved enzyme delivery to COS-7 cells ~1.5× compared to the control proteins. No improvement in GALC delivery was observed with primary fibroblasts and Tat decreased delivery of GALC to primary mouse cortical neurons (Zhang et al., 2008).
Another class of receptor-dependent cell type-specific peptides derived from neuron-binding virus and toxins may prove to be more promising candidates for neuronal delivery of GCase. Tetanus toxin fragment C (TTC), the non-toxin ~50 kDa neuronal binding domain of tetanus toxin, is one of the best-characterized binding domains from this class. TTC has been used extensively to deliver cargo proteins (Francis et al., 2000, 2004; Li et al., 2009) and has a strong affinity for neurons through its binding of complex gangliosides and protein receptors P15 and SV2A/B (Calvo et al., 2012; Miana-Mena et al., 2002; Yeh et al., 2010). While TTC could dramatically enhance the binding of GCase to neurons, TTC does not cross the blood brain barrier and would raise concerns regarding an in vivo immune response. As a result, the potential of TTC as an in vivo delivery domain is likely also limited. Smaller toxin or virus-derived peptides with lower immunogenic potential would be preferable to TTC. Tet1, a 12 amino acid peptide identified for ganglioside-binding properties similar to TTC, is a small peptide option (Federici et al., 2007). Until recently, the ability of Tet1 to deliver cargo protein was never directly compared to TTC or a CPP such as Tat (Mello et al., unpublished results). As a result, the ability of Tet1 to facilitate neuronal binding of GCase was uncertain. Tet1 has recently been shown to bind brain endothelia, mediating BBB transport (Georgieva et al., 2012).
Rabies-derived peptide (RDP) may have the most potential for the delivery of GCase to the brain. Varieties of RDP have been derived from either of the two regions of the rabies virus glycoprotein (RVG) responsible for neuronal cell binding. Both varieties have been reported to cross the BBB and bind neurons. A 29 amino acid peptide derived from RVG amino acid sequence 175–203 (Lentz, 1990) has been used for delivery with a nine-arginine peptide attached by a sequence of three glycines (Gong et al., 2012; Kumar et al., 2007; Xiang et al., 2011) and by itself (Gao et al., 2014; Liu et al., 2009). Both DNA and protein have been delivered with a 39 amino acid peptide where a 27 amino acid portion derived from RVG sequence 330–356 is connected to a nonarginine by a sequence of 3 glycines (Fu et al., 2012, 2013a,b). RDP has been shown to deliver model protein GFP to neural progenitor cells (NPCs) better than Tat, Tet1, or TTC (Mello et al., unpublished results). A number of proteins have been successfully delivered across the BBB using RDP including luciferase, β-galactosidase, BDNF, GDNF, and GFP (Fu et al., 2012, 2013b)
With the goal of producing a neuronal cell type-targeted recombinant GCase enzyme, 17 modified variants were designed, expressed, and if expressed, successfully purified and assessed for enzyme activity and neuronal binding and internalization. Chronologically, several smaller groups of recombinant GCase enzymes were created, culminating in a final series of experiments where the most promising variant, RDP-IgAh-GCase, was identified. Initial experiments were invaluable in accomplishing this goal, however, the intent of this work was not to perform an exhaustive comparison of RDP-IgAh-GCase and the other 16 variants. Novel enzymes included binding domains Tat, RDP, Tet1, and combinations of Tat and the receptor-dependent peptides. The dual-peptide binding domains were investigated because the combination of Tat and TTC has been shown to work better than either Tat or TTC alone (Gramlich et al., 2013). Design of the candidate genes and enzymes reflected goals of high level expression, enzyme activity, and neuronal binding. GFP was also included in some of the constructs to allow for histologic assessment of binding and internalization. Following initial screening, enzyme internalization of promising GCase variants was assayed quantitatively by lysing cells after protein treatment and quantitating enzyme concentration and activity in the cell lysate. Substrate reduction capabilities for Tat-linked GCase and RDP-IgAh-GCase were also quantitatively tested.
2. Materials and methods
2.1. Construction of glucocerebrosidase expression vectors
All glucocerebrosidase expression vectors were created using the base plasmid pSecTag2a (Life Technologies) containing an IgK leader sequence to promote secretion upstream of the multiple cloning site and both a myc epitope tag and 6× His purification tag downstream of the multiple cloning site. The wild-type human glucocerebrosidase gene (GBA1) sequence was amplified from a vector provided to RAF by Lorne Clark (University of British Columbia, Canada). GBA1 was cloned into the pSecTag2a backbone using EcoRI and EcoRV restriction sites creating pTag GBA. For cloning of additional domains, AgeI and BsiWI restriction sites were introduced in frame at the 5′ and 3′ ends respectively of GBA1. Enhanced green fluorescent protein (EGFP) was amplified by PCR and cloned into the pTag GBA sequence 3′ of the GBA1 gene using the BsiWI and XhoI restriction sites. This plasmid, named, pTag GBA GFP was used for the construction of all EGFP-containing GBA1 expression vectors. The EGFP sequence was derived from Clontech plasmid pEGFP-1.
All cell-penetrating peptides or neuronal-targeting domains were cloned into either pTag GBA or pTag GBA GFP via AscI and AgeI restriction sites. Unless a linker region was specifically designed into the expression vector, only the AgeI restriction site separated the 5′ domain and GBA1. The TTC and Tat-TTC sequences were amplified from the pGEX4T3 TatTTCGFP vector (Gramlich et al., 2013) using PCR. Each of the Tat, Tet1, and Tat-Tet1 sequences were made as duplex oligonucleotides by Integrated DNA Technologies (IDT) and cloned into either pTag GBA or pTag GBA GFP. Tet1 was cloned 3′ of EGFP in one expression vector using the XhoI restriction site. All other sequences were created using the IDT gblock synthetic gene construction service. Sequences constructed as gblocks included Tat 5xTet1, 5xTet1, RDP, RDP GFP, RDP Tat, RDP Flex, RDP IgAh, and RDP Rigid. All gblocks were first cloned into the PCR Blunt (Life Technologies) vector for sequence confirmation before introduction into either pTag GBA or pTag GBA GFP via the AscI and AgeI restriction sites. Tat, RDP, Tet1, and linker region sequences were all codon-optimized for mammalian expression. When synthesized by gblocks (IDT), codon optimization was performed within the manufacturer’s specifications limiting nucleotide repeats.
2.2. Cell culture
HEK 293F suspension cells were maintained at a 40 mL working volume in 125 mL vented non-baffled shake flasks (Corning) and passaged three times per week. For standard passaging, cultures were seeded at 2E5–4E5 cells/mL and not allowed to exceed 2E6 cells/mL to avoid excessive cell aggregation. All suspension cultures were grown at 37 °C and 8% CO2 with shaking at 125 RPM on an innova 2000 shaker (New Brunswick Scientific).
Human neural progenitor cells (NPCs, Lonza) were grown in NPC starting medium (Ebert et al., 2008). Passage 4 vials of neurospheres were thawed into NPC starting medium and allowed to attach over 2–4 days at which time the plated neurospheres were dissociated with Accutase (Sigma) and replated in 50% NPC starting medium and 50% MEM medium containing 5% horse serum. Re-plated NPCs were maintained until dense enough for experimental use and fed 5 mL medium once per week. For protein transduction and imaging, NPCs were plated in poly-L-lysine coated 8-well chambered coverglasses (Nunc) and expanded for 4 days until cells reached a density of ~25,000 cells/cm2.
Glucocerebrosidase-knockout (GCKO) and wild-type (WT) SV40-immortalized mouse neuronal cell lines were used to test recombinant GCase binding. Neuronal cultures were established from the cortices of gba−/− embryos (Tybulewicz et al., 1992) and immortalized with a lentiviral SV40-T construct (Westbroek et al., unpublished results). These cells were maintained in Neurobasal medium supplemented with 1× B27, 1 mM L-glutamine, and 1× penicillin/streptomycin (Life Technologies). Cultures were passaged once a week and medium was changed the day after passaging and every three days afterwards. All culture vessels were coated with poly-L-lysine (Sigma P9155) to facilitate cell attachment. For protein transduction, cells were plated in poly-L-lysine coated 6-well plates and allowed to expanded 4–6 days until 80–90% confluent.
2.3. Generation of iPSC-derived Gaucher neurons
The iPSC-derived Gaucher neurons from a type 2 patient harboring a W184R/D409H genotype (Panicker et al., 2014) and WT neurons were differentiated as described below. The iPSC-derived embryoid bodies were maintained in EB culture medium for 10 days, followed by 4 days in the presence of 5 μM Dorsomorphin and 10 μM SB431542 (Sigma–Aldrich) as previously described (Panicker et al., 2012). EBs were then transferred to Petri dishes coated with Matrigel (BD Biosciences) and maintained in DMEM/F12 media (Invitrogen) plus 1× (v/v) N2 supplement and 20 ng/mL bFGF (Stemgent). After 7–10 days, neural rosettes containing neuronal stem cells (NSCs) started to form and were manually picked and dissociated into single cells using StemPro Accutase (Life Technologies). NSCs were expanded in Neurobasal medium (Life Technologies) containing 1× (v/v) MEM, non-essential amino acids (Life Technologies), 1× (v/v) GlutaMAX-I CTS (Life Technologies), 1× (v/v) B27 supplement (Life Technologies), 1× (v/v) penicillin/streptomycin and 20 ng/mL bFGF (Stemgent). To initiate neuronal differentiation, NSCs were plated on 24 well culture dishes or glass chamber slides coated with 20 mg/mL poly-L-ornithine (Sigma–Aldrich, cat # P3655-50MG) and 10 mg/mL laminin (Life Technologies, cat # 23017-015). NSCs were maintained in neuronal differentiation medium consisting of: Neurobasal medium (Life Technologies) supplemented with 1× (v/v) MEM non-essential amino acids (Life Technologies), 1× (v/v) GlutaMAX-I CTS, 1× (v/v) B27 supplement, BDNF (10 ng/mL) (eBioscience cat # 14–8365), GDNF (10 ng/mL) (eBioscience cat # 14-8506), cAMP (100 nM) (Sigma–Aldrich cat # D-0260), and ascorbic acid (200 μM) (Sigma–Aldrich cat # A-4403). Neuronal cells were maintained in culture for 3–4 weeks with half media changes every 2–3 days.
2.4. Expression of recombinant glucocerebrosidase
All recombinant GCase enzymes were expressed using transient transfection of HEK 293F suspension cells (Life Technologies). HEK 293F cells were transfected at 1E6 cells/mL with 30 mL in 125 mL vented non-baffled shake flasks (Corning) or 120 mL in 500 mL vented non-baffled shake flasks (Corning). Large scale plasmid preps were performed using the Endotoxin-Free Megaprep Kit (Qiagen). Cultures were transfected with 1 μg plasmid DNA for each 1E6 cells in culture. Plasmid DNA was complexed with 2 μL 293fectin reagent (Life Technologies) per μg DNA for 5 min at room-temperature before addition to the culture. Complexes were prepared using OptiMEM serum-free medium containing Gluta-Max (Life Technologies). All HEK 293F culture work was performed using Freestyle 293 medium (Life Technologies).
Transiently transfected batch cultures were maintained at 37 °C and 8% CO2 for 48 h or 96 h. Samples of culture medium and cells were taken during batch culture expression to determine ideal harvest time and whether the extracellular medium, soluble lysate, or insoluble lysate contained the most GCase. All experiments were performed using protein preparations produced via identical expression conditions. Batch cultures were harvested by centrifuging at 2500 × g for 10 min at which time cell-free culture medium was removed and stored at 4 °C until purification. The cell pellets were washed with 1× PBS pH 7.4, centrifuged again, and frozen at −80 °C until ready for cell lysis and protein purification. All 30 mL expression batch cultures were purified directly. In an attempt to improve final yields, medium from test 120 mL cultures was first concentrated ~5–10× using Amicon Ultra 50 kDa molecular weight cutoff centrifugal filters. This medium concentration was investigated to facilitate resin binding with a reasonable volume for the smaller resin bed. Medium was not concentrated before the scaled-up purification performed to assess substrate accumulation and reduction.
2.5. Purification of recombinant glucocerebrosidase
Following protein expression, culture medium or concentrated culture medium containing the expressed GCase was stored at 4 °C until purification. For select GCase that were not secreted, cell pellets were lysed with 1 mL MPER non-denaturing lysis buffer (Pierce) for each 100 mg cell pellet according to the manufacturer’s recommended protocol. Proteins were purified using Ni-NTA Resin (Pierce) and three buffers all with the same 1× PBS pH 7.4 base (20 mM sodium phosphate, 300 mM sodium chloride). Equilibration buffer, Wash Buffer, and Elution Buffer were each pH 7.4 and contained 10 mM, 25 mM, and 250 mM imidazole respectively.
The protein purification method used was a combination of standard batch and column purification methods. An equal volume of cold Equilibration Buffer was added to medium or soluble lysate, up to a 44 mL final volume, and the solution was added to a washed 0.5 mL bed volume of Ni-NTA resin and bound overnight at 4 °C for ~20–21 h. Following overnight binding in batch mode, the resin suspension was equilibrated to room temperature at which time the resin suspension was slowly poured into a 5 mL column to pack the resin bed. The flow-through solution was poured over the resin bed two additional times and allowed to empty by gravity flow at a rate of approximately 1 mL/min. Each resin bed was washed three times with 1 mL, two resin bed volumes, of Wash Buffer. The GCase was eluted off the column with three separate 1 mL volumes of Elution Buffer. Before flowing off the column by gravity flow, each column was capped and the Elution Buffer was allowed to sit on the resin bed for 5 min. All purification samples were run on 4–12% SDS-PAGE gels and stained with SimplyBlue Safestain (Life Technologies). Total protein in GCase-containing elutions was quantitated by Bradford Assay (Thermo Scientific). The specific GCase concentration was determined by densitometry using the ImageLab 3.0 (Biorad) software. All elutions containing reasonable amounts of purified GCase were supplemented with 1 mg/mL BSA and stored at 4 °C until use.
2.6. Glucocerebrosidase activity assay
GCase enzyme activity was determined using a 0.1 M acetate assay buffer pH 5.0 containing 3 mM 4-MUG (4-methylumbelliferyl β-D-glucopyranoside), 0.15% (v/v) Triton X-100, and 0.15% (w/v) taurodeoxycholic acid sodium salt (Calbiochem). Purified GCase or cell lysate was added to the activity assay buffer and incubated at 37 °C for 1 h. The reaction was stopped with the addition of a 2× volume of 0.2 M glycine buffer pH 10.8. Product 4-methylumbelliferone was detected using the Victor 3 fluorimeter (filter set excitation = 355 nm, emission = 460 nm). Background β-glucosidase (GBA2) enzyme activity was subtracted from lysate activity using duplicate controls containing 1 μM conduritol B epoxide (Toronto Research Chemicals Inc.) added to enzyme reactions to inhibit GCase activity. Black 96-well plates were typically used although limited initial work was performed with white 96-well plates. The 4-methylumbelliferone detection is highly linear between 10nM–10 μM in the black 96-well plate and between 10nm–5 μM in the white 96-well plate. Enzyme activity units are defined as nmol 4-methylumbelliferone released per hour.
2.7. Assessing glucocerebrosidase binding and internalization
Due to the large number of recombinant GCase vectors constructed, initial screening of GFP-containing recombinant GCase binding and internalization was accomplished by protein transduction of GCase followed by live-cell imaging of the GFP fluorescence. Proteins used for this study were expressed with 48 h batch cultures. NPCs were treated with 1 μg/mL enzyme in NPC starting medium for 18 h. After the overnight incubation, cells were washed 3× in NPC starting medium without growth factors or heparin and immediately imaged using the Axio Observer Z1 Motorized fluorescent microscope (Carl Zeiss Inc., Oberkochen, Germany). The iPSC-derived Gaucher neurons were treated using an identical method with their appropriate medium. Each protein treatment condition was performed in duplicate, and for each condition, images were collected for a range of exposure times. For each treatment condition, a range of images was taken where signal was completely saturated at the high exposure limit and not visible at the low exposure limit. The 500 ms exposure time was carefully chosen as it was the exposure time where signal was not saturated but was clearly visible for all protein treatment conditions, facilitating an effective qualitative comparison. For this exposure time, three images were collected for each protein treatment condition. Identical exposure times were compared since for direct comparison, the exposure time should not be altered unless there is a compelling reason for doing so (Cromey, 2010).
Quantitative assessment of GCase binding and internalization was performed using GCKO neurons for recombinant GCase enzymes that showed promise in initial screening. All experiments included the appropriate wild-type control. GCKO and WT mouse neurons were plated in poly-L-lysine coated 6-well plates and cells were allowed to expand 4–6 days until 80–90% confluent. Duplicate wells were used for each treatment condition.
Since quantitative experiments required higher treatment concentrations, all proteins used were expressed from 96 h batch cultures. Cells were treated with 12 μg/mL recombinant enzyme overnight for 18 h at which time the cells were extensively washed with 1× PBS to remove excess protein. Recombinant Cerezyme (Genzyme) was obtained from patient infusion bags. Cells were harvested from well plates using TrypLE Express (Life Technologies) and lysed using MPER non-denaturing cell lysis buffer (Pierce). Lysate protein concentrations were determined by Bradford Assay (Thermo Scientific). Enzyme activity in the cell lysates was determined by adding lysate to the 0.1 M acetate assay buffer and incubating at 37 °C for 1 h. Lysate concentration added was always consistent within an experiment; between experiments, concentrations ranged from 10–20 μg/100 μL with a final volume of 200–300 μL per reaction. Standard curves for each recombinant enzyme were generated to quantitate the concentration of enzyme detected in the lysate. Units of enzyme activity in the lysate were calculated using a 4-methylumbelliferone standard curve.
2.8. Assessment of glucosylsphingosine substrate reduction using LC–MS/MS
To assess glucosylsphingosine accumulation and potential reduction with protein treatment, immortalized GCKO and WT mouse neuronal cultures were plated in poly-L-lysine coated 6-well plates and allowed to expand four days until ~80% confluent. To perform the complete experiment, 18 wells of GCKO cells and 6 wells WT cells were plated. Four treatment conditions were carried out: Tat-GCase treatment of GCKO cells, RDP-IgAh-GCase treatment of GCKO cells, an untreated GCKO control, and an untreated WT control. Each treatment condition was performed in duplicate with each of the duplicates consisting of three pooled wells to ensure there was enough material collected to detect glucosylsphingosine accumulation.
Tat-GCase and RDP-IgAh-GCase were expressed and purified using a scaled-up version of the procedure performed for assessing recombinant enzyme binding/internalization (previous section). The transiently transfected flask was scaled up 4×; 120 mL culture was transfected in a 500 mL non-baffled shake flask. Purification scale-up was performed to keep protein concentration similar to the small-scale procedure throughout the purification process. This was done since concentration of protein had been found to reduce enzyme activity (Supplemental Fig. 1). BioRad Econo-Pac polypropylene chromatography columns with a 15 mm internal diameter were chosen so a 2 mL resin bed would have the same bed height compared to when the 5 mL columns were used in the smaller scale procedure.
Aside from the 4× scale-up of production and purification processes, one additional step was performed for the protein purification to investigate substrate accumulation and reduction; final 4 mL elutions were desalted using Zeba 7 kDa MWCO desalting columns with a 10 mL resin bed (Pierce). Buffer was exchanged with 1× PBS pH 7.4 (Life Technologies). Pilot experiments showed that while treatment of the GCKO mouse neuronal cultures with protein elution buffer for <24 h was acceptable, cell loss was high with longer protein treatments. The cell loss with extended treatment was found to result from the components of the elution buffer, likely the 250 mM imidazole. In a mock treatment experiment, dilution of medium with 1× PBS alone did not lead to any appreciable cell detachment and/or death.
After four days of cell growth, duplicate treatment conditions were treated with either 10 μg/mL protein diluted in fresh medium for Tat-GCase or RDP-IgAh-GCase treatment conditions, or fresh medium alone for untreated controls. Every 24 h, for 72 h total, medium was exchanged adding fresh protein or fresh medium only. Following 72 h of treatment, cells were washed 2× with 1× PBS pH 7.4 and harvested by scraping. PBS was removed by centrifugation and cell pellets were frozen at −80 °C. Frozen cell pellets were shipped on dry ice to Cincinnati Children’s Hospital for glucosylsphingosine analysis. Lipid extraction and LC–MS/MS analysis of glucosylsphingosine accumulation was performed as previously described (Sun et al., 2012, 2013). All quantitative glucosylsphingosine data was normalized to total protein detected in the cell lysate.
3. Results
3.1. Protein expression and purification
A total of seventeen GCase expression vectors were created evaluating a number of different parameters including: the presence of a cell-penetrating peptide or neuronal delivery vector, the presence of GFP, the order of protein domains, and the linker regions between domains. All expression vectors used pSecTag2a as the base and contained an IgK leader sequence at the 5′ end and a myc epitope tag and 6×His tag at the 3′ end. Of the 17 recombinant GCase enzymes expressed, only 3 failed to express correctly (Table 1). Tat-TTC-GCase-GFP and RDP-GFP-GCase were expressed as smaller truncated forms at a high level. A low level of full-length Tat-TTC-GCase-GFP was observed via western blot while no full-length RDP-GFP-GCase was observed. Tat-GCase-GFP-5×Tet1 was the only enzyme to not be expressed at all. GFP fluorescence was not even observed in the producer cells and no myc-containing bands were found in the supernatant or soluble lysate of the producer cells.
Table 1.
A compilation of all seventeen recombinant GCase enzymes expressed plus Cerezyme (Genzyme Corp) derived from patient infusion bags. Enzymes are listed in order of specific activity given as units enzyme per mg recombinant protein. One U is defined as the amount of enzyme to release 1 nmol 4-methylumbelliferone per hour. Except for the three enzymes that were not appropriately expressed, expression levels were found to be approximately bimodal where a select group of enzymes were expressed at a low level with final yields of 10 μg/mL or less. Other recombinant GCase enzymes were typically purified at yields several fold higher. Specific activity values represent the average of all preparations of each listed enzyme where proteins were expressed for 48–96 h and not concentrated before purification.
| Summary of recombinant GCase activity and expression level | ||
|---|---|---|
|
| ||
| GCase | Specific activity (U/mg) | Expression level |
| Cerezyme | 1.25E+05 | N/A |
| RDP-GCase | 3.67E +04 | Acceptable |
| Tat-GCase | 3.32E+04 | Acceptable |
| Tat-GCase-GFP-Tet1 | 3.26E+04 | Low |
| Tat-5xTet1-GCase-GFP | 2.79E+04 | Low |
| RDP-Rigid-GCase | 2.53E+04 | Acceptable |
| RDP-Flex-GCase | 2.46E+04 | Acceptable |
| RDP-IgAh-GCase | 2.43E+04 | Acceptable |
| RDP-Tat-GCase | 2.24E+04 | Acceptable |
| GCase (Fishman Lab) | 2.04E+04 | Low |
| Tat-Tet1-GCase-GFP | 1.81E+04 | Acceptable |
| Tat-GCase-GFP | 1.76E+04 | Acceptable |
| RDP-GCase-GFP | 1.53E+04 | Acceptable |
| Tet1-GCase-GFP | 1.23E+04 | Low |
| TTC-GCase-GFP | 8.18E+03 | Acceptable |
| Tat-TTC-GCase-GFP | N/A | Expressed in truncated form |
| RDP-GFP-GCase | N/A | Expressed in truncated form |
| Tat-GCase-GFP-5xTet1 | N/A | Not expressed |
The IgK signal peptide facilitated the secretion of all enzymes (Fig. 1) except for the TTC-containing TTC-GCase-GFP and Tat-TTC-GCase-GFP. Interestingly, the ~100 kDa truncated Tat-TTC-GCase-GFP fragment was secreted while the full length Tat-TTC-GCase-GFP was not. TTC-GCase-GFP was expressed at a high level but found only in the soluble lysate of the producer HEK 293F cells. Secreted GCase was several kilodaltons larger than non-secreted GCase (Fig. 1). This size difference could be due to differences in glycosylation and/or cleavage of the 19 amino acid GCase signal peptide during lysosomal trafficking for the intracellular protein (Berg-Fussman et al., 1993; Grace et al., 1994). The size difference between secreted and intracellular GCase has been observed (Branco Novo et al., 2012). GCase has five N-glycosylation sites, four of which are typically occupied in wild-type GCase (Shaaltiel et al., 2007; Tekoah et al., 2013). Proper glycosylation at only the N19 position is critical for GCase activity (Berg-Fussman et al., 1993).
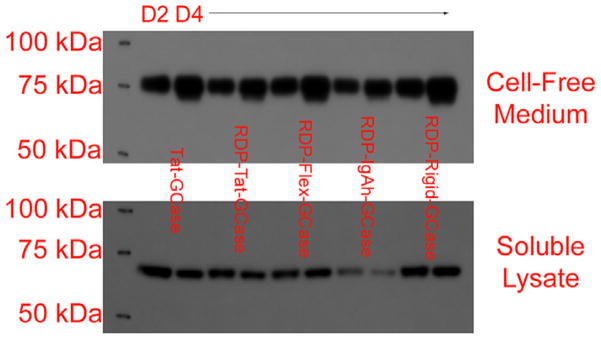
Fig. 1.
The IgK signal peptide facilitated efficient GCase secretion for most engineered enzymes. Predicted molecular weights (polypeptide only): Tat-GCase: 66.1 kDa, RDP-Tat-GCase: 70.7 kDa, RDP-Flex-GCase: 69.7 kDa, RDP-IgAh-GCase: 71.1 kDa, and RDP-Rigid-GCase: 71.2 kDa. Following transient transfection, cell-free medium and HEK 293F soluble lysate were run on 4–12% NuPage Bis-Tris gels and a western blot was performed for the myc epitope tag. For each condition, samples collected both two (D2) and four (D4) days after transfection were run. Three-fold more cell-free supernatant was run on the gels compared to soluble lysate to account for the at least 3× more concentrated soluble lysate. The results shown for the five enzymes were typical for all the secreted glucocerebrosidases. Extending batch duration increased the quantity of secreted protein in the extracellular medium but did not increase the quantity of protein in the soluble lysate. The slightly larger size of the intracellular proteins was predicted to be due to both cleavage of the GCase signal peptide and differences in glycosylation.
All GCase proteins were purified using HisPur Ni-NTA resin (Pierce). While GCase proteins co-purified with a number of other proteins, the GCase was dramatically concentrated (Fig. 2A). Recombinant GCase was not visible in the supernatant via SDS-PAGE and Simply Blue Safestain (Life Technologies) before purification. Along with the assessment of enzyme activity, product quality for all purified proteins was confirmed via SDS-PAGE and Simply Blue Safestain (Fig. 2A) and anti-myc western blot (Fig. 2B). Final yield of purified GCase was calculated by first performing a Bradford assay to determine total protein and then densitometry to account for the impurities in the final elution. Centrifugal filtration was attempted as a strategy to concentrate protein from a larger culture to increase the final purified protein concentration. However, concentrating cell-free supernatant before purification led to a less active final product and as a result, was not further investigated (Supplemental Fig. 1).
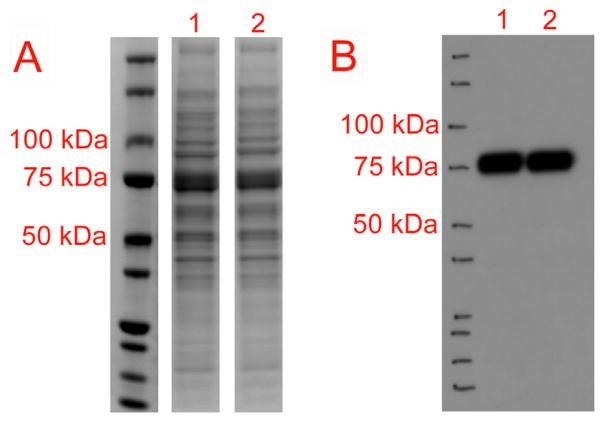
Fig. 2.
The main elutions for Tat-GCase (1) and RDP-IgAh-GCase (2) were run on 4–12% NuPage Bis-Tris SDS-PAGE gels. SimplyBlue Safestain was used to examine total protein in the elution (A) and an anti-myc western blot (B) was utilized to confirm the location of the purified GCase on the gels. Both Tat-GCase and RDP-IgAh-GCase were found to be ~75 kDa, a size consistent with the predicted molecular weights of their glycosylated forms. Comparable SDS-PAGE gels exist for all purified proteins referenced in Table 1.
Although the purpose of this work was to identify a neuron-targeted GCase and not create all possible combinations of GCase and delivery peptide, there was quite a bit of overlap in the design of the recombinant GCase enzymes (Table 1). As a result, several inferences regarding the effect of enzyme design on expression level and activity can be made. First, commercial product Cerezyme (Genzyme Corp.) had a specific enzyme activity that was ~3.5× higher than the most active novel enzyme described here. The higher activity of Cerezyme is due, at least in part, to its increased purity (Van Patten et al., 2007). Second, the presence of CPP Tat or the CPP-containing neuronal delivery domain RDP at the N-terminal end of an expressed glucocerebrosidase appeared to improve the level of expression consistent with previously described attempts at recombinant GCase expression (Lee et al., 2005; Vaags et al., 2005). CPP VP22 has also been shown to enhance GCase expression (Jin et al., 2012). Third, the only full-length GCase to not be secreted, TTC-GCase-GFP, was the least active enzyme by far (Table 1). Fourth, while most enzymes were expressed fairly well, a select few were expressed at lower levels with final elutions containing <10 μg/mL purified protein (Table 1). However, there does not seem to be any correlation between level of protein expression and enzyme specific activity. Finally, the presence of GFP decreased both production yields and specific activity. Specific activity was decreased by a greater degree than what would be expected due to the difference in molecular weight alone. Due to the decrease in activity and yield, after the design of the initial set of expression vectors, GFP was typically not included in later constructs.
3.2. Qualitative assessment of recombinant GCase binding/internalization using fluorescent microscopy
Since initial purification yields were low and the number of recombinant GCase candidates was numerous, initial screening of neuronal binding and internalization was performed by treating NPCs with 1 μg/mL purified protein and using fluorescent microscopy to compare GFP fluorescence (Fig. 3). Qualitatively, NPCs bound similar amounts of Tat-GCase-GFP and TTC-GCase-GFP. Tet1 alone was insufficient for NPC binding nor did Tet1 provide any enhancement in combination with Tat. In fact, combinations of Tat and Tet1 were bound/internalized by NPCs less than Tat-GCase-GFP itself.
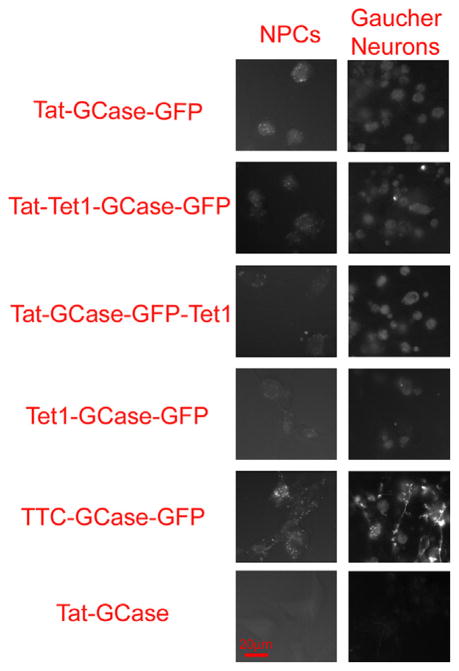
Fig. 3.
Qualitative comparison of recombinant glucocerebrosidase binding and internalization in both neural progenitor cells (NPCs) and iPSC-derived Gaucher neurons. Cells were treated for 18 h with 1 μg/mL purified protein, thoroughly washed, and imaged live using the Zeiss Axio Observer Z1 fluorescent microscope. 400× fluorescent images were taken with a Zeiss AxioCam MRm monochrome camera (1.3 megapixel, 12 bits/pixel) using the C-Apochromat 40×/1.2 W Korr objective. The exposure time for all images was 500 ms.
Protein transduction and imaging GFP fluorescence was repeated using type 2 GD iPSC-derived neurons. While imaging individual neurons was challenging due to the cell clustering, a qualitative comparison was possible. Proportionally to Tat-GCase-GFP, Tet1-GCase-GFP was bound by GD iPSC-neurons better than it had been by NPCs. However, Tet1 alone was not better than Tat nor was any combination of Tat and Tet1. GFP fluorescence in the TTC-GCase-GFP treated Gaucher neurons was qualitatively the brightest. However, TTC-GCase-GFP was not secreted and had much lower enzyme activity than the other enzymes produced (Table 1). Additionally, TTC would be highly immunogenic in vivo; as a result further work with TTC-GCase-GFP was not pursued.
A Tet1-containing construct, Tat-5×Tet1-GCase-GFP, was used to test the hypothesis that multiple repeats of the 12 amino acid Tet1 peptide might lead to enhanced neuronal cell binding. NPCs were treated with Tat-Tet1-GCase-GFP and Tat-5×Tet1-GCase-GFP in parallel, and GFP fluorescence was qualitatively compared. In combination with Tat, additional Tet1 repeats did not appear to offer any binding advantage (data not shown). Furthermore, additional Tet1 repeats led to several-fold lower levels of protein expression.
3.3. Quantitative assessment of RDP-containing GCase binding and internalization in a GCase-knockout mouse neuronal cell line
While initial screening using the qualitative comparison of GFP fluorescence following the protein transduction of GFP-containing GCase enzymes ruled out Tet1 as a GCase delivery vector, a more quantitative method was required for choosing the ideal neuron-targeted GCase. The advantage of the imaging studies was the low concentration of protein needed; 1 μg/mL treatment concentrations led to visible GFP fluorescence after 18 h. To quantify intracellular GCase activity, both higher purified protein yields and a cell line with low wild-type GCase activity were needed.
Quantitatively, recombinant GCase enzymes without GFP and with either Tat or RDP at the N-terminus were evaluated as these attributes tended to give high protein expression enabling treatment of cells with at least 12 μg/mL purified protein. Initial pilot studies tested whether NPCs were a suitable cell line for quantitative work and they were not due to high background wild-type GCase activity. While iPSC-derived Gaucher neurons had very low wild-type activity and were an interesting scientific model, the number of cells required made this cell option expensive and less ideal. The ideal cell type for this work proved to be a SV40-transformed glucocerebrosidase-knockout (GCKO) mouse neuronal cell line (Westbroek et al., unpublished results). Our pilot studies indicated that this cell line had very low activity and preferentially bound RDP and TTC-linked recombinant GFP proteins (data not shown).
For the first RDP-GCase enzyme created, the 39 amino acid RDP peptide was inserted in place of Tat in the Tat-GCase construct. RDP and GCase were separated by only two amino acids, Thr-Gly due to the presence of the AgeI restriction site used in subcloning. The first quantitative experiment performed with the GCKO mouse neurons compared the uptake of Tat-GCase, RDP-GCase, and Cerezyme following an 18 h treatment with 12 μg/mL purified protein. Enzyme uptake was compared two ways: the amount of active GCase harvested in cell lysate and the activity of GCase found in the cell lysate. The amount of enzyme internalized was determined using standard curves of each purified GCase. Standard curves from 50 to 400 ng/mL were prepared fresh for each experiment. While the standard curves were linear above 150 ng/mL purified enzyme, polynomial equations were found to best fit the entire region of interest. Specifically, a second order polynomial fit was used for this initial experiment while, with more data points, a third-order polynomial fit was used for all subsequent experiments. Due to the higher activity of Cerezyme, comparing concentration of active internalized enzyme is the fairest comparison of enzyme delivery.
Unexpectedly, RDP-GCase was not delivered preferentially compared to Tat-GCase and by concentration; both RDP-GCase and Tat-GCase were internalized only marginally better than Cerezyme in the GCKO mouse neuronal line (Table 2). This result was inconsistent with our observation that, in neuronal cell types, RDP improves recombinant protein delivery considerably compared to Tat (Mello et al., unpublished results). This led to the hypothesis that in this first generation RDP-GCase recombinant enzyme, RDP folding or binding was sterically hindered.
Table 2.
Comparison of GCase detected in GCKO mouse neuronal cell lysate following protein treatment with 12 μg/mL recombinant GCase. RDP was not found to improve GCase binding and internalization. Neither Tat nor RDP facilitated significantly better protein transduction efficiency compared to commercially available Cerezyme (Genzyme Corp). Detected GCase concentrations represent the average of duplicate treatment conditions with error indicating one standard deviation. The GCase concentrations for the independent repeats can be found in Supplemental Table 1.
| Comparison of delivered GCase activity in GCKO mouse neuronal cell lysate | ||
|---|---|---|
|
| ||
| Protein | GCase detected (ng/mL) | Normalized to Tat-GCase |
| Tat-GCase | 133.17 ± 18.36 | 1.00 |
| RDP-GCase | 117.10 ± 3.96 | 0.88 |
| Cerezyme | 100.82 ± 5.37 | 0.76 |
3.4. Linker region design is critical to facilitate RDP-mediated neuronal delivery of glucocerebrosidase
Since we had thoroughly established that RDP-GFP preferentially binds neuronal cell types compared to Tat-GFP without a linker region, it was clear that while a linker region between RDP and the recombinant protein of interest is not required in all circumstances, it might be imperative for GCase. The first attempt at relieving this hypothesized steric hindrance was to create RDP-GFP-GCase where GFP would be utilized as the linker region. While the eventual goal would have been to engineer a small peptide linker region, effective RDP-GFP delivery predicted that GFP would be an effective linker region to test the steric hindrance hypothesis. However, since RDP-GFP-GCase was expressed only in a truncated form (Table 1) the small peptide linker region design was attempted.
The four linker regions inserted between the Thr-Gly two amino acid sequence and RDP were Tat, a flexible linker region (Flex), a linker region derived from the flexible hinge region of IgA (IgAh), and a highly alpha-helical linker region (Rigid). A RDP-Tat-GCase enzyme was created since Tat has the length of a typical linker region, 11 amino acids, and most importantly, there was a high degree of confidence that Tat would not interfere with protein expression. The flexible linker region selected was GGGSGGGS. Although shorter than most flexible linkers, this 8-amino acid linker was used successfully in the construction of RDP-linked recombinant proteins (Fu et al., 2012). The IgAh linker region is a typical example of proline-rich semi-rigid linker regions that are commonly used (Bhandari et al., 1986; Evans et al., 1986; Li et al., 2009; Low et al., 1976; Turner et al., 1993). Finally, the rigid A(EAAK)4A 22 amino acid linker was selected for its highly alpha-helical structure, ensuring that the most space possible would be between RDP and GCase (Arai et al., 2001).
Along with Tat-GCase as the control, each of RDP-Tat-GCase, RDP-Flex-GCase, RDP-IgAh-GCase, and RDP-Rigid-GCase was expressed and purified. With four days of expression, all expressed well with final yields from ~40 to 70 μg/mL. All new RDP-linked enzymes had comparable specific activities ranging from 2.24E4 to 2.53E4 U/mg (Table 1).
Duplicate wells containing GCKO mouse neuronal cells were treated with 12 μg/mL of purified Tat-GCase or one of the RDP-Linker-GCase enzymes for 18 h at which time the cells were harvested, lysed, and protein content of the lysates was quantitated. Identical quantities of lysate were added to MUG containing assay buffer and 4-methylumbelliferone fluorescence was compared. Fluorescence from GCase-treated samples was normalized using untreated GCKO cell lysate. The percentage of wild-type activity delivered was calculated using untreated wild-type mouse neuronal cell line lysate. The concentration of GCase found in the lysate was calculated by preparing standard curves of purified GCase.
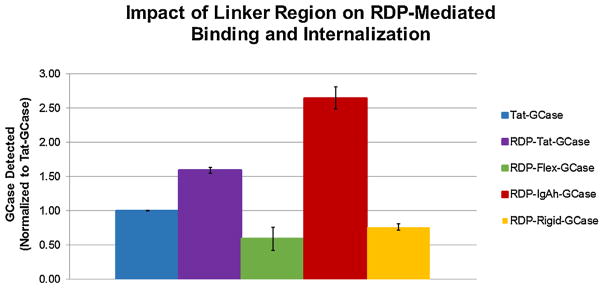
The concentration of GCase detected in the cell lysate was compared for all RDP-Linker-GCase enzymes (Fig. 4). RDP-IgAh-GCase performed the best, as 2.65× more RDP-IgAh-GCase was delivered to the GCKO neurons compared to Tat-GCase. Tat-GCase was bound/internalized better than both RDP-Flex-GCase and RDP-Rigid-GCase. Although RDP-IgAh-GCase is a less active enzyme than Tat-GCase, 2.52× more enzyme activity was delivered to the GCKO cells representing ~10% of wild-type activity. This result was replicated in a second independent experiment (Table 3).
Fig. 4.
The impact of linker region on RDP-Linker-GCase binding and internalization in GCKO mouse neurons. The concentrations of GCase detected in the cell lysates for each treatment condition were quantitated and subsequently normalized to Tat-GCase to facilitate comparison. RDP-IgAh-GCase was internalized 2.65× more than Tat-GCase. All bars in the above graph represent the average of duplicate treatment conditions. Error bars indicate one standard deviation.
Table 3.
Data compilation for the two independent experiments quantitating GCase activity in GCKO mouse neuronal cell lysate following an 18 h protein treatment with 12 μg/mL purified protein. All data points in the above tables represent the average of duplicate treatment conditions with error indicating one standard deviation. Duplicate conditions all gave similar results. Percent of WT activity refers to a comparison with WT cells; all protein treatment was performed with GCKO cells. The GCase concentrations for the independent repeats can be found in Supplemental Table 1.
| Comparison of delivered GCase activity in GCase-Knockout Mouse Neuronal Cell Lysate (Experiment #1) | ||||
|---|---|---|---|---|
|
| ||||
| Protein | GCase detected (ng/mL) | GCase detected (normalized to Tat-GCase) | Percent of WT activity delivered | Percent of WT activity delivered (normalized to Tat-GCase) |
| Tat-GCase | 67.08 ± 0.39 | 1.00 | 3.94 ± 0.03 | 1.00 |
| RDP-Tat-GCase | 106.71 ± 2.64 | 1.59 | 5.89 ± 0.20 | 1.50 |
| RDP-Flex-GCase | 39.77 ± 11.21 | 0.59 | 1.32 ± 0.39 | 0.34 |
| RDP-IgAh-GCase | 177.68 ± 10.58 | 2.65 | 9.92 ± 1.29 | 2.52 |
| RDP-Rigid-GCase | 51.29 ± 3.30 | 0.76 | 1.74 ± 0.12 | 0.44 |
| Comparison of delivered GCase activity in GCase-Knockout Mouse Neuronal Cell Lysate (Experiment #2) | ||||
|
| ||||
| Protein | GCase detected (ng/mL) | GCase detected (normalized to Tat-GCase) | Percent of WT activity delivered | Percent of WT activity delivered (normalized to Tat-GCase) |
|
| ||||
| Tat-GCase | 29.75 ± 2.93 | 1.00 | 1.87 ± 0.23 | 1.00 |
| RDP-IgAh-GCase | 66.18 ± 9.85 | 2.22 | 4.45 ± 0.79 | 2.38 |
3.5. Reduction of glucosylsphingosine accumulation with Tat-GCase and RDP-IgAh-GCase treatment
Production and purification processes were scaled up 4× to produce enough Tat-GCase and RDP-IgAh-GCase to perform the substrate accumulation and reduction assessment. Each scaled up production process was performed in duplicate to produce 8× the final protein typically generated. In these larger preps, final protein concentrations and purity were similar to those from the smaller scale process. Desalting and buffer exchange did not lead to any loss of purified enzyme, however, activity was reduced. Although the reduction in activity was unexpected and unwanted, removal of the 250 mM imidazole was necessary for treatments spanning multiple days. After 72 h of Tat-GCase or RDP-IgAh-GCase treatment, cells appeared normal and looked no different than the GCKO untreated control cells.
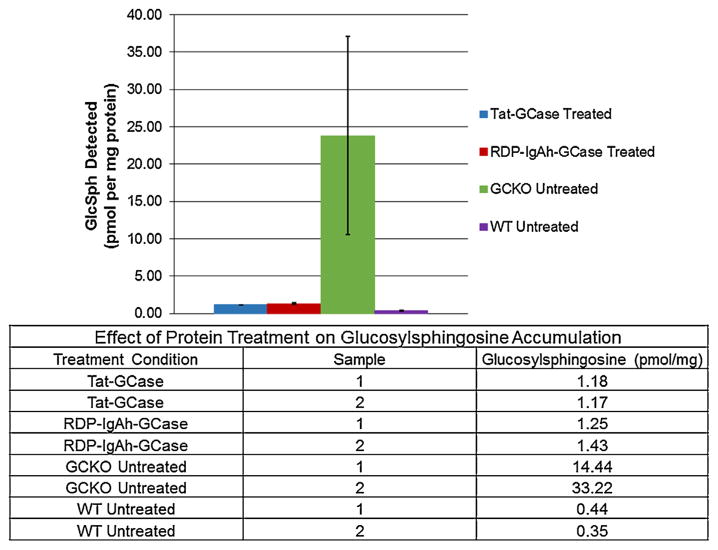
The GCKO mouse neuronal cell line was expected to accumulate glucosylsphingosine but not glucosylceramide (Westbroek et al., unpublished results). This expected result was confirmed; GCKO cells accumulated GlcSph ~60× more than WT cells (Fig. 5). Although there was some variability between the duplicate GCKO negative control samples, even with the lowest value, >30× more glucosylsphingosine was present in the GCKO cells. Treatment with both Tat-GCase and RDP-IgAh-GCase was found to reduce accumulated glucosylsphingosine to essentially wild-type levels (Fig. 5).
Fig. 5.
Treatment of a GCase-Knockout (GCKO) immortalized mouse neuronal cell line with Tat-GCase or RDP-IgAh-GCase dramatically reduced glucosylsphingosine (GlcSph) accumulation. Duplicate sets of three wells were treated with 10 μg/mL purified protein or fresh medium only for 72 h with complete medium changes every 24 h. For each triplicate treatment condition, wells were pooled for analysis. Accumulated GlcSph was quantified using LC–MS/MS analysis and data was normalized to the total protein detected in the cell lysate. Duplicate GlcSph data points, displayed in the table, were averaged for graphical display. Error bars represent one standard deviation from the mean. The GCKO cell line was not found to accumulate glucosylceramide.
4. Discussion
The development of RDP-IgAh-GCase potentially represents a significant advance in recombinant protein therapeutics for neuronopathic Gaucher’s disease therapy. In pursuit of developing an effective neuron-targeted recombinant GCase, seventeen different recombinant enzymes were designed and expressed. Parameters that were varied in recombinant protein design included the type of cell penetrating peptide or neuronal membrane binding vector, the presence of GFP, the order of the different functional domains N-terminus to C-terminus, and the type of linker region used between protein domains. Recombinant proteins were designed in stages where results from each set where employed to optimize the design of subsequent sets of proteins. Although not the original goal of the study, there was enough overlap in the sequence of the recombinant enzymes to make a number of observations regarding recombinant GCase design and expression. All of the recombinant GCase enzymes expressed and successfully purified had specific activities at least 3× lower than commercially available Cerezyme (Genzyme) using the in vitro activity assay with synthetic substrate 4-MUG. Due to this difference in enzyme activity, all protein incubation conditions of cells were normalized by enzyme concentration instead of enzyme activity, a treatment paradigm that has been used with some GCase delivery work (Lee et al., 2005). It is highly likely that increasing the purity of our GCase would improve enzyme specific activity as protein aggregation would be reduced (Van Patten et al., 2007). Improving production yields and purity after protein purification are two important future goals in this work.
The second observation made was that the presence of cell-penetrating peptide Tat or cell-penetrating peptide-containing RDP improved protein expression. The improvement of GCase expression with inclusion of CPPs Tat and VP22 has been previously observed (Jin et al., 2012; Vaags et al., 2005) although the mechanism involved is not currently known. Comparing the production of the GFP-containing recombinant GCase enzymes can provide insight on this observation. Interestingly, while Tat-GCase-GFP, Tat-Tet1-GCase-GFP, and RDP-GCase-GFP all expressed well, Tet1-GCase-GFP did not. Although Tet1 is similar to the size of the Tat peptide, it is not highly cationic. In fact, Tat-Tet1-GCase-GFP was the only Tet1-containing enzyme to be expressed at comparable levels to Tat-GCase-GFP or RDP-GCase-GFP. The only Tat or RDP-containing enzymes to express poorly also contained Tet1 (Table 1). Wild-type GCase did not express nearly as well as any of the Tat-GCase or RDP-GCase enzymes. Overall, at this time, it can only be concluded that a CPP N-terminal to GCase improves production. Although the absence of Tat or RDP at the N-terminus leads to lower production, it does not lead to lower enzyme activity. There does not appear to be any clear relationship between expression level and enzyme activity (Table 1).
Many of the initial GCase expression vectors were designed with a GFP at the C-terminus of the GCase sequence. The motivation for this design was the desire to perform initial screening for cell binding with low concentrations of protein using fluorescent microscopy before using more quantitative methods to assess binding and internalization. While initial screening was performed for the first set of variants (Fig. 3), most subsequently created enzymes were designed without a GFP. This decision was based on two observations: GFP-containing enzymes had lower expression levels compared to their counterparts without GFP and GFP-containing enzymes had lower specific activities than even predicted by their greater molecular weights.
While the initial evaluation of GCase binding and internalization via GFP fluorescence was not a perfect system, it was suitable for eliminating Tet1 from further evaluation for GCase delivery. Qualitatively, Tet1-GCase-GFP was not delivered as well as Tat-GCase-GFP nor did the addition of Tet1 with Tat give any appreciable advantage over Tat alone in either NPCs or iPSC-derived Gaucher neurons (Fig. 3). Furthermore, work in our lab using model protein GFP indicated that Tet1 could not facilitate delivery to NPCs as well as Tat, TTC, or RDP (Mello et al., unpublished results).
While TTC-GCase-GFP did not appear to be bound or internalized better than Tat-GCase-GFP in NPCs qualitatively, it did improve binding with the iPSC-derived Gaucher neurons. While this was an interesting observation regarding the neuronal properties of NPCs, it did not make TTC our preferred delivery vector for GCase. TTC-GCase-GFP is potentially problematic for in vivo delivery due to the presence of highly immunogenic TTC. Furthermore, TTC-GCase-GFP was not secreted during production. While purifying the protein from the soluble lysate is not an issue, we observed that proteins harvested from the soluble lysate were several kDa smaller than their secreted counterparts. TTC-GCase-GFP was by far the least active enzyme purified, possibly due to decreased post-translational modification. N-Glycosylation at the first of the four occupied sites, at amino acid #19, is critical for full catalytic activity (Berg-Fussman et al., 1993).
RDP seemed to be the most promising protein delivery vector for the neuronal targeting of GCase on the basis of its small size, low potential antigenicity, and high level of neuronal binding. Along with the published literature (Fu et al., 2012, 2013b), experience in our lab indicated that model protein RDP-GFP could be delivered to neural progenitor cells ~9× better than Tat-GFP and ~46× better than Tet1-GFP (Mello et al., unpublished results). Along with the promising pilot binding studies, expression of RDP-GCase was high, nearly identical to Tat-GCase. However, our initial recombinant RDP-GCase was unexpectedly not bound or internalized preferentially to Tat-GCase (Table 2). The first expression vector for RDP-GCase was constructed like the expression vector for Tat-GCase, where the RDP sequence replaced the Tat sequence with only a two amino acid linker region separating the delivery peptide from the GCase sequence. We hypothesized that RDP was sterically hindered by GCase and that introduction of a linker region would allow RDP to appropriately bind its receptor(s). Linker region has been shown to be important for recombinant protein function, although it is often ignored (Klement et al., 2015). Additional RDP-GCase enzymes were designed, each with a different linker region. The flexible, semi-rigid IgAh, and rigid linker regions were chosen based upon potential structure while Tat was selected as a linker region to ensure that at least one of the RDP-Linker-GCase enzymes expressed well since we were unsure what affect the addition of space between RDP and GCase would have on expression level. Although expression levels did vary for the RDP-Linker-GCase variants, all expressed fairly. Overall, RDP-IgAh-GCase was found to be bound and internalized preferentially compared to Tat-GCase and all the other RDP-Linker-GCase variants. While there are review articles describing the effect of linker region on recombinant protein design (Chen et al., 2013; George et al., 2003; Yu et al., 2015; Zhang et al., 2009), this work with RDP-GCase is a rare description of the impact of a variety of linker region classes on enzyme functionality and delivery. This work also indicates what while RDP has a lot of potential to mediate the neuronal targeting of cargo proteins, steric hindrance of RDP by the cargo protein is an issue. While the IgAh linker could be useful for all RDP-based delivery, it is possible that linker region will need to be evaluated on a protein-specific basis. Computational tools for predicting the impact of linker regions on protein structure and function are under development and will be very useful for protein-specific evaluations in the future (Yu et al., 2015).
The 19 amino acid IgAh linker, PVPSTPPTPSPSTPPTPSM, was first used by Hallewell and colleagues in 1989 to link superoxide dismutase (SOD) subunits for the purpose of making functional polymers. The IgAh linker was chosen in an attempt to reproduce the partially flexible linkage between Fab and Fc portions of IgA (Hallewell et al., 1989). Our collaborators have used it previously to facilitate the expression of a recombinant TTC-GDNF in a baculovirus expression system (Li et al., 2009). The IgAh linker closely mimics a class of relatively common proline-rich linker regions (Bhandari et al., 1986; Evans et al., 1986; Turner et al., 1993). Proline has been found to be the most preferred amino acid in linkers during linker region analysis. Proline-rich sequences adopt less secondary structure since proline does not hydrogen bond with other amino acids leading to a more extended confirmation (George and Heringa, 2003).
The development of RDP-IgAh-GCase represents the first recombinant glucocerebrosidase capable of effectively binding neurons with the predicted potential to cross the blood brain barrier. There have only been two publications describing the delivery of GCase using methods other than mannose-directed endocytosis. As described, Lee et al. created GCase-Tat and demonstrated that it was delivered preferentially to type 1 and type 2 Gaucher fibroblasts. While it was suggested that GCase-Tat could be used for types 2 and 3 GD therapy, delivery of GCase-Tat to neurons was not demonstrated. This publication is the first to describe the binding and internalization of Tat-GCase in a neuronal cell type. The well-known ability of Tat to move cargo proteins across the BBB makes Tat-GCase more promising for neuronopathic Gaucher disease therapy than mannose-terminal recombinant GCase (Schwarze et al., 1999). However, results presented here indicate that Tat might not provide much of a binding advantage. Besides the creation of GCase-Tat, a receptor-dependent delivery strategy was employed for facilitating the passage of GCase across the BBB. Peptides from apolipoproteins B and E, capable of mediating lysosomal enzyme delivery across the BBB, have been tested and compared to Tat (Böckenhoff et al., 2014; Spencer and Verma, 2007). Specifically, ApoB has been used to deliver GCase across the BBB. ApoB can bind the low-density lipoprotein receptor (LDLR) and mediate transcytosis of cargo protein across the BBB (Spencer et al., 2007). While it can transport proteins across the BBB, it does not target the GCase to neurons once in the CNS.
RDP-IgAh-GCase has the potential to not only target neurons, but to possibly transport GCase across the BBB. The potential to cross the BBB adds a straightforward and non-invasive treatment scheme to be studied for RDP-IgAh-GCase delivery. However, we recognize that the ability of a sufficient quantity of RDP-IgAh-GCase to reach the brain will need to be tested in vivo. Since RDP contains a R9 cell-penetrating peptide, it will retain the ability to bind non-neuronal cells, albeit not with enhanced affinity. As a result, potential delivery of RDP-IgAh-GCase might be performed most efficiently using direct infusion.
In an effort to improve delivery of enzyme to deep brain structures such as the basal ganglia, direct IC injection of GCase using the low pressure infusion parameters of convection enhanced delivery (CED) have been investigated in large animals. This method results in high concentrations in brain regions adjacent to the infusion site as evidenced by enzyme in a vesicular location (Lonser et al., 2005, 2007). CED was employed to attempt to treat an infant with acute neuronopathic GD targeted to the brainstem, the site of severe symptoms. Although the patient tolerated the treatment with some degree of stabilization, distribution of the injectate suggested that multiple intra-cerebral injections would be needed for widespread brain treatment even using CED (Lonser et al., 2007). In a small study, 3 patients with GD underwent intra-cerebroventricular infusion of GCase. As with intra-cerebral injection, failure of this therapy was due to lack of neuronal binding of this unmodified commercial enzyme (Erickson et al., 1997).
The final goal of the presented work was to demonstrate that the active GCase delivered to the GCase-knockout mouse neuronal cell line was functional and capable of reducing accumulated substrate. While enzymatically active RDP-IgAh-GCase and Tat-GCase were detected in cell lysate, we aimed to show that following endocytosis, sufficient enzyme was trafficked to the lysosome to reduce GlcSph accumulation. Cells were treated for 72 h, a treatment time chosen based on both in vitro and in vivo GCase treatment literature (Cabrera-Salazar et al., 2010; Panicker et al., 2012). When the protein-treated and control cells were analyzed by LC–MS/MS to quantitate GlcSph, the GCKO neuronal cell line was found to accumulate ~60× more GlcSph than the WT cells and both RDP-IgAh-GCase and Tat-GCase treatment reduced GlcSph accumulation to essentially wild-type levels. This result fully confirmed that we had delivered active, functional recombinant enzyme. In 2010, Cabrera-Salazar et al. demonstrated that three consecutive daily intra-cerebroventicular injections of reformulated Cerezyme reduced GlcCer and GlcSph levels to wild-type concentrations in the neuronopathic Gaucher’s disease K14 mouse model (Cabrera-Salazar et al., 2010). Neuron-targeted recombinant GCase may prove to be far more effective than enzyme that is internalized via mannose-directed endocytosis.
Recombinant RDP-IgAh-GCase has exciting potential as a therapeutic for neuronopathic Gaucher’s disease due to both its neuron-targeting properties and potential to cross the BBB. RDP-IgAh-GCase was bound/internalized ~2.5 fold over control protein Tat-GCase and both enzymes were found to reduce glucosylsphingosine concentrations to wild-type levels in a GCase-knockout mouse neuronal cell line. In future work, both RDP-IgAh-GCase and Tat-GCase merit in vivo testing to determine their therapeutic potential in Gaucher’s disease animal models. Additionally, while a transient expression system was invaluable for the production of multiple GCase variants in the work presented here, improved production and purification processes will be pursued both to support in vivo testing and facilitate scale-up to meet increased protein needs.
Supplementary Material
Acknowledgments
The authors would like to thank the Department of Veteran’s Administration REAP fellowship for generous funding of this research.
Abbreviation
- GCase
glucocerebrosidase
- GD
Gaucher’s disease
- RDP
rabies-derived peptide
- TTC
tetanus toxin fragment C
- IgAh
peptide from flexible hinge region of IgA
- ERT
enzyme replacement therapy
- GlcCer
glucosylceramide
- GlcSph
glucosylsphingosine
- 4-MUG
4-Methylumbelliferyl β-D-glucopyranoside
- CBE
conduritol B epoxide
- GCKO cell line
glucocerebrosidase-knockout neuronal cell line
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jbiotec.2016.01.015.
Footnotes
Conflicts of interest
The authors declare no competing financial interests.
References
- Arai R, Ueda H, Kitayama A, Kamiya N, Nagamune T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001;14:529–532. doi: 10.1093/protein/14.8.529. [DOI] [PubMed] [Google Scholar]
- Berg-Fussman A, Grace ME, Ioannou Y, Grabowski GA. Human acid beta-glucosidase: N-glycosylation site occupancy and the effect of glycosylation on enzymatic activity. J Biol Chem. 1993;268:14861–14866. [PubMed] [Google Scholar]
- Bhandari DG, Levine BA, Trayer IP, Yeadon ME. 1H NMR study of motility and conformational constraints within the proline-rich N-terminal of the LC1 alkali light chain of skeletal myosin. Eur J Biochem. 1986;160:349–356. doi: 10.1111/j.1432-1033.1986.tb09978.x. [DOI] [PubMed] [Google Scholar]
- Böckenhoff A, Cramer S, Wölte P, Knieling S, Wohlenberg C, Gieselmann V, Galla HJ, Matzner U. Comparison of five peptide delivery vectors for improved brain delivery of the lysosomal enzyme arylsulfatase A. J Neurosci. 2014;34:3122–3129. doi: 10.1523/JNEUROSCI.4785-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco Novo J, Morganit L, Maria Moro A, Paes Leme AF, de Toledo Serrano SM, Raw I, Ho PL. Generation of Chinese Hamster Ovary cell line producing recombinant human glucocerebrosidase. J Biomed Biotechnol. 2012 doi: 10.1155/2012/875383. http://dx.doi.org/10.1155/2012/875383. [DOI] [PMC free article] [PubMed]
- Cabrera-Salazar MA, Bercury SD, Ziegler RJ, Marshall J, Hodges BI, Chuang WL, Pacheco J, Li L, Cheng SH, Scheule RK. Intracerebroventricular delivery of glucocerebrosidase reduces substrates and increases lifespan in a mouse model of neuronopathic Gaucher disease. Exp Neurol. 2010;225:436–444. doi: 10.1016/j.expneurol.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Cabrera-Salazar MA, DeRiso M, Bercury SD, Li L, Lydon JT, Weber W, Pande N, Cromwell MA, Copeland D, Leonard J, Cheng SH, Scheule RK. Systemic delivery of a glucosylceramide synthase inhibitor reduces CNS substrates and increases lifespan in a mouse model of type 2 Gaucher disease. PLoS One. 2012 doi: 10.1371/journal.pone.0043310. http://dx.doi.org/10.1371/journal.pone.0043310. [DOI] [PMC free article] [PubMed]
- Calvo AC, Oliván S, Manzano R, Zaragoza P, Aguilera J, Osta R. Fragment C of tetanus toxin: new insights into its neuronal signaling pathway. Int J Mol Sci. 2012;13:6883–6901. doi: 10.3390/ijms13066883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Tikoo A, Kapur AK, Singh M. The taming of the cell penetrating domain of the HIV Tat: myths and realities. J Control Release. 2007;17:148–162. doi: 10.1016/j.jconrel.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zaro JL, Shen WC. Fusion protein linkers: property, design, and functionality. Adv Drug Deliv Rev. 2013;65:1357–1369. doi: 10.1016/j.addr.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromey DW. Avoiding twisted pixels: ethical guidelines for the appropriate use and manipulation of scientific digital images. Sci Eng Ethics. 2010;16:639–667. doi: 10.1007/s11948-010-9201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62:1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, McMillan EL, Svendsen CN. Isolating, expanding, and infecting human and rodent fetal neural progenitor cells. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc02d02s6. http://dx.doi.org/10.1002/9780470151808.sc02d02s6. [DOI] [PubMed]
- Erickson A, Bembi B, Schiffmann R. Neuronopathic forms of Gaucher’s disease. Baillieres Clin Haematol. 1997;10:711–723. doi: 10.1016/s0950-3536(97)80035-2. [DOI] [PubMed] [Google Scholar]
- Evans JS, Levine BA, Trayer IP, Dorman CJ, Higgins CF. Sequence-imposed structural constraints in the TonB protein of E coli. FEBS Lett. 1986;208:211–216. doi: 10.1016/0014-5793(86)81020-1. [DOI] [PubMed] [Google Scholar]
- Ficicioglu C. Review of miglustat for clinical management in Gaucher disease type 1. Ther Clin Risk Manage. 2008;4:425–431. doi: 10.2147/tcrm.s6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis JW, Brown RH, Jr, Figueiredo D, Remington MP, Castillo O, Schwarzschild MA, Fishman PS, Murphy JR, vanderSpek JC. Enhancement of diphtheria toxin potency by replacement of the receptor binding domain with tetanus toxin C-fragment: a potential vector for delivering heterologous proteins to neurons. J Neurochem. 2000;74:2528–2536. doi: 10.1046/j.1471-4159.2000.0742528.x. [DOI] [PubMed] [Google Scholar]
- Francis JW, Bastia E, Matthews CC, Parks DA, Schwarzschild MA, Brown RH, Jr, Fishman PS. Tetanus toxin fragment C as a vector to enhance delivery of proteins to the CNS. Brain Res. 2004;1011:7–13. doi: 10.1016/j.brainres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Federici T, Liu JK, Teng Q, Yang J, Boulis NM. A means for targeting therapeutics to peripheral nervous system neurons with axonal damage. Neurosurgery. 2007;60:911–918. doi: 10.1227/01.NEU.0000255444.44365.B9. [DOI] [PubMed] [Google Scholar]
- Fu A, Wang Y, Zhan L, Zhou R. Targeted delivery of proteins into the central nervous system mediated by rabies virus glycoprotein-derived peptide. Pharm Res. 2012;29:1562–1569. doi: 10.1007/s11095-012-0667-y. [DOI] [PubMed] [Google Scholar]
- Fu A, Zhang M, Gao F, Xu X, Chen Z. A novel peptide delivers plasmids across blood–brain barrier into neuronal cells as a single-component transfer vector. PLoS One. 2013a doi: 10.1371/journal.pone.0059642. http://dx.doi.org/10.1371/journal.pone. [DOI] [PMC free article] [PubMed]
- Fu A, Zhao Z, Gao F, Zhang M. Cellular uptake mechanism and therapeutic utility of a novel peptide in targeted-delivery of proteins into neuronal cells. Pharm Res. 2013b;30:2108–2117. doi: 10.1007/s11095-013-1068-6. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wang ZY, Zhang J, Zhang Y, Huo H, Wang T, Jiang T, Wang S. RVG-peptide-linked trimethylated chitosan for delivery of siRNA to the brain. Biomacromolecules. 2014;15:1010–1018. doi: 10.1021/bm401906p. [DOI] [PubMed] [Google Scholar]
- George RA, Heringa J. An analysis of protein domain linkers: their classification and role in protein folding. Protein Eng. 2003;15:871–879. doi: 10.1093/protein/15.11.871. [DOI] [PubMed] [Google Scholar]
- Georgieva JV, Brinkhuis RP, Stojanov K, Weijers C, Zuilhof H, Rutjes F, Hoekstra D, van Hest J, Zuhorn IS. Peptide-mediated blood–brain barrier transport of polymersomes. Angew Chem Int Ed. 2012;51:8339–8342. doi: 10.1002/anie.201202001. [DOI] [PubMed] [Google Scholar]
- Gillmeister MP, Betenbaugh MJ, Fishman PS. Cellular trafficking and photochemical internalization of cell penetrating peptide linked cargo proteins: a dual fluorescent labeling study. Bioconjug Chem. 2011;22:556–566. doi: 10.1021/bc900445g. [DOI] [PubMed] [Google Scholar]
- Gong C, Li X, Xu L, Zhang YH. Target delivery of a gene into the brain using the RVG29-oligoarginine peptide. Biomaterials. 2012;33:3456–3463. doi: 10.1016/j.biomaterials.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Grace ME, Newman KM, Scheinker V, Berg-Fussman A, Grabowski GA. Analysis of human acid β-glucosidase by site-directed mutagenesis and heterologous expression. J Biol Chem. 1994;269:2283–2291. [PubMed] [Google Scholar]
- Gramlich PA, Remington MP, Amin J, Betenbaugh MJ, Fishman PS. Tat-tetanus toxin fragment C: a novel protein delivery vector and its use with photochemical internalization. J Drug Target. 2013;21:662–674. doi: 10.3109/1061186X.2013.796954. [DOI] [PubMed] [Google Scholar]
- Grubb JH, Volger C, Sly WS. New strategies for enzyme replacement therapy for lysosomal storage diseases. Rejuvenation Res. 2010;13:229–236. doi: 10.1089/rej.2009.0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallewell RA, Laria I, Tabrizi A, Gunnar C, Getzoff ED, Tainer JA, Cousena LS, Mullenbach GT. Genetically engineered polymers of human CuZn superoxide dismutase. J Biol Chem. 1989;264:5260–5268. [PubMed] [Google Scholar]
- Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Zhu G, Zhao Z, Liu F. VP22 enhances the expression of glucocerebrosidase in human gaucher II fibroblast cells mediated by lentiviral vectors. Clin Exp Med. 2012;12:135–143. doi: 10.1007/s10238-011-0152-7. [DOI] [PubMed] [Google Scholar]
- Klement M, Li C, Loo BLW, Choo AB, Ow DS, Lee D. Effect of linker flexibility and length on the functionality of a cytotoxic engineered antibody fragment. J Biotechnol. 2015;199:90–97. doi: 10.1016/j.jbiotec.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–45. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- Lee KO, Luu N, Kaneski CR, Schiffmann R, Brady RO, Murray GJ. Improved intracellular delivery of glucocerebrosidase mediated by the HIV-1 Tat protein transduction domain. Biochem Biophys Res Commun. 2005;337:701–707. doi: 10.1016/j.bbrc.2005.05.207. [DOI] [PubMed] [Google Scholar]
- Lentz TL. Rabies virus binding to an acetylcholine receptor α-subunit peptide. J Mol Recognit. 1990;3:82–88. doi: 10.1002/jmr.300030205. [DOI] [PubMed] [Google Scholar]
- Li J, Chian RJ, Ay I, Celia SA, Kashi BB, Tamrazian E, Matthews JC, Remington MP, Pepinsky RB, Fishman PS, Brown RH, Jr, Francis JW. Recombinant GDNF: tetanus toxin fragment C fusion protein produced from insect cells. Biochem Biophys Res Commun. 2009;385:380–384. doi: 10.1016/j.bbrc.2009.05.079. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, Lou J, Jiang C. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30:4195–4202. doi: 10.1016/j.biomaterials.2009.02.051. [DOI] [PubMed] [Google Scholar]
- Lonser RR, Walbridge S, Murray GJ, Aizenberg MR, Vortmeyer AO, Aerts JMFG, Brady RO, Oldfield EO. Convention perfusion of glucocerebrosidase for neuronopathic Gaucher’s disease. Ann Neurol. 2005;57:542–548. doi: 10.1002/ana.20444. [DOI] [PubMed] [Google Scholar]
- Lonser RR, Schiffman R, Robison RA, Butman JA, Quezado Z, Walker ML, Morrison PF, Walbridge S, Murray GJ, Park DM, Brady RO, Oldfield EO. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68:254–261. doi: 10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- Low T, Liu YSV, Putnam FW. Structure, function, and evolutionary relationships of Fc domains of human immunoglobulins A, G, M, and E. Science. 1976;191:390–392. doi: 10.1126/science.1246619. [DOI] [PubMed] [Google Scholar]
- Mello N, Gramlich PA, Remington MP, Fishman PS. Rabies glycoprotein derived peptide shows enhanced neuronal internalization of GFP compared to tetanus toxin fragment C, Tat, and Tet-1. 2015 Unpublished results, (in preparation) [Google Scholar]
- Miana-Mena FJ, Roux S, Benichou J, Osta R, Brulet P. Neuronal activity-dependent membrane traffic at the neuromuscular junction. Proc Natl Acad Sci USA. 2002;99:3234–3239. doi: 10.1073/pnas.052023599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker LM, Miller D, Park TS, Patel B, Azevedo JL, Awad O, Masood MA, Veenstra TD, Goldin E, Stubblefield BK, Tayebi N, Polumuri SK, Vogel SN, Sidransky E, Zambidis ET, Feldman RA. Induced pluripotent stem cell model recapitulates pathologic hallmarks of Gaucher disease. Proc Natl Acad Sci USA. 2012;109:18054–18059. doi: 10.1073/pnas.1207889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker LM, Miller D, Awad O, Bose V, Lun Y, Park TS, Zambidis ET, Sgambato JA, Feldman RA. Gaucher iPSC-derived macrophages produce elevated levels of inflammatory mediators and serve as a new platform for therapeutic development. Stem Cells. 2014;32:2338–2349. doi: 10.1002/stem.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastores GM. Recombinant glucocerebrosidase (imiglucerase) as a therapy for Gaucher disease. BioDrugs. 2010;24:41–47. doi: 10.2165/11318540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Poole RM. Eliglustat: first global approval. Drugs. 2014;74:1829–1836. doi: 10.1007/s40265-014-0296-3. [DOI] [PubMed] [Google Scholar]
- Schueler U, Kaneski C, Murray G, Sandhoff K, Brady RO. Uptake of mannose-terminal glucocerebrosidase in cultured human cholinergic and dopaminergic neuron cell lines. Neurochem Res. 2002;27:325–330. doi: 10.1023/a:1014915430398. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Shaaltiel Y, Bartfield D, Hashmueli S, Baum G, Brill-Almon E, Galili G, Dym O, Boldin-Adamsky SA, Silman I, Sussman JL, Futerman AH, Aviezer D. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol J. 2007;5:579–590. doi: 10.1111/j.1467-7652.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- Spencer BJ, Verma IM. Targeted delivery of proteins across the blood–brain barrier. Proc Natl Acad Sci USA. 2007;104:7594–7599. doi: 10.1073/pnas.0702170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liou B, Xu YH, Quinn B, Zhang W, Hamler R, Setchell KDR, Grabowski GA. Ex vivo and in vivo effects of isofagomine on acid β-glucosidase variants and substrate levels in Gaucher disease. J Biol Chem. 2012;287:4275–4287. doi: 10.1074/jbc.M111.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang W, Xu YH, Quinn B, Dasgupta N, Liou B, Setchell KDR, Grabowski GA. Substrate compositional variation with tissue/region and Gba1 mutations in mouse models-implications for Gaucher Disease. PLoS One. 2013 Mar 8; doi: 10.1371/journal.pone.0057560. http://dx.doi.org/10.1371/journal.pone.0057560. [DOI] [PMC free article] [PubMed]
- Tekoah Y, Tzaban S, Kizhner T, Hainrichson M, Gantman A, Golembo M, Avioezer D, Shaaltiel Y. Glycosylation and functionality of recombinant β-glucocerebrosidase from various production systems. Biosci Rep. 2013 doi: 10.1042/BSR20130081. http://dx.doi.org/10.1042/BSR20130081. [DOI] [PMC free article] [PubMed]
- Turner SL, Russell GC, Williamson MP, Guest GR. Restructuring an interdomain linker in the dihydrolipoamide acetyltransferase component of the pyruvate dehydrogenase complex of Escherichia coli. Protein Eng. 1993;6:101–108. doi: 10.1093/protein/6.1.101. [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL, Tremblay ML, LaMarca ME, Willemsen R, Stubblefield BK, Winfield S, Zablocka B, Sidransky E, Martin BM, Huang SP. Animal model of Gaucher’s disease from targeted disruption of the mouse glucocerebrosidase gene. Nature. 1992;357:407–410. doi: 10.1038/357407a0. [DOI] [PubMed] [Google Scholar]
- Vaags AK, Campbell TN, Choy FYM. HIV Tat variants differentially influence the production of glucocerebrosidase in Sf9 cells. Genet Mol Res. 2005;4:491–495. [PubMed] [Google Scholar]
- Van Patten SM, Hughes H, Huff MR, Piepenhagen PA, Waire J, Qiu H, Chandrashekar G, Reczek D, Ward PV, Kutzko JP, Edmunds T. Effect of mannose chain length on targeting of glucocerebrosidase for enzyme replacement therapy of Gaucher disease. Glycobiology. 2007;17:467–478. doi: 10.1093/glycob/cwm008. [DOI] [PubMed] [Google Scholar]
- Westbroek W, Nguyen M, Burnett R, Siebert M, Aflaki E, Behre B, Costa W, Rodriguez-Gil J, Fujiwara H, Sidhu R, Tayebi N, Renvoise B, Cramer C, Pavan W, Ory D, Sidransky E. A novel neuronal cell model for Gaucher type II. 2015 Unpublished results, (in preparation) [Google Scholar]
- Xia H, Mao Q, Davidson BL. The HIV Tat protein transduction domain improves the biodistribution of β-glucuronidase expressed from recombinant viral vectors. Nat Biotechnol. 2001;19:640–644. doi: 10.1038/90242. [DOI] [PubMed] [Google Scholar]
- Xiang L, Zhou R, Fu A, Xu X, Huang Y, Hu C. Targeted delivery of large fusion protein into hippocampal neurons by systemic administration. J Drug Target. 2011;19:632–636. doi: 10.3109/1061186X.2010.523788. [DOI] [PubMed] [Google Scholar]
- Yeh FL, Dong M, Yao J, Tepp WH, Lin G, Johnson EA, Chapman ER. SV2 mediates entry of tetanus neurotoxin into central neurons. PLoS Pathog. 2010 doi: 10.1371/journal.ppat.1001207. http://dx.doi.org/10.1371/journal.ppat.1001207. [DOI] [PMC free article] [PubMed]
- Yu K, Liu C, Kim BG, Lee DY. Synthetic fusion protein design and applications. Biotechnol Adv. 2015;33:155–164. doi: 10.1016/j.biotechadv.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yun J, Shang Z, Zhang X, Pan B. Design and optimization of a linker for fusion protein construction. Prog Nat Sci. 2009;19:1197–1200. [Google Scholar]
- Zhang XY, Dinh A, Cronin J, Li SC, Reiser J. Cellular uptake and lysosomal delivery of galactocerebrosidase tagged with the HIV Tat protein transduction domain. J Neurochem. 2008;104:1055–1064. doi: 10.1111/j.1471-4159.2007.05030.x. [DOI] [PubMed] [Google Scholar]
- Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.