Abstract
Focused Ultrasound (FUS) in combination with microbubbles has been demonstrated to non-invasively open the blood-brain barrier (BBB). When applying this technique for drug delivery purposes, repeated drug administration and BBB opening is likely required. Therefore, it is worth investigating the long-term effects of FUS induced BBB opening. In this study, we focused on the assessment of potential behavior changes in mice that could be attributed to repeated BBB openings up to six months. Animals received either monthly or biweekly unilateral sonications to the striatum throughout the course of monitoring period. Behavioral assessments were conducted using open field and rotarod performance test. Upon completion of each sonication, mice underwent MRI to confirm and assess volume of the BBB opening. No differences in locomotor activity between the BBB-opened and control groups in both the biweekly and monthly-treated mice were evident up to 6 months. Similarly, there was no affinity for a particular turn angle in the sonicated compared to the control animals. However, the positive control exhibited a significant decrease in locomotor activity, as well as rotation ipsilateral to the sonicated hemisphere. Our results based on the assessment using open field and rotarod indicated that repeated openings of the BBB in the striatum using FUS in conjunction with microbubbles over the period of six months and under the parameters used here did not cause motor impairment, behavioral changes, or morphological alterations. This reinforces the safety of repeated and long-term drug delivery using the FUS induced BBB opening.
Keywords: Focused Ultrasound, behavior, neuroscience, cognitive, blood-brain barrier, repeated, longitudinal
Introduction
The onset of Parkinson’s disease (PD) is characterized by the loss of dopaminergic neurons, with this loss confined to the nigro-striatal pathway. Although a comprehensive understanding of the etiology of PD remains under examination, much of the pathophysiology is understood (Katzenschlager and Lees 2002). Treatment of PD has progressed immensely over the decades, namely with the introduction of levodopa in the 1960s (Katzenschlager and Lees 2002). Levodopa alleviates the symptoms of Parkinson’s disease through promotion of dopamine replacement for cells lost in the nigro-striatal pathway. However, effective administration of levodopa must be paired with a peripheral decarboxylase inhibitor (carpidopa); levodopa alone is unable to reach the brain parenchyma in adequate amounts without assistance. In addition, despite the understanding that levodopa metabolizes into dopamine upon entering the brain, using dopamine as a method of therapy is impractical due to its associated impedance of the blood-brain barrier (BBB), and the inability of dopamine to efficiently penetrate the parenchyma. The BBB is a complex homeostatic system which effectively controls and limits influx and efflux of molecules between the brain and the vascular system (Sheikov et al. 2006). Due to factors such as molecular size, hydrophobicity, and atomic charge (Abbott et al. 2006), the BBB severely limits the potential of therapeutic agents to be administered systemically; the system effectively excludes 98% of small molecule drugs and nearly all large molecule drugs (Abbott 2013).
Focused Ultrasound (FUS) in combination with microbubbles displays great potential in the ability to transiently and focally increase BBB permeability (Choi et al. 2010b; Hynynen et al. 2005; McDannold et al. 2006). The role of microbubbles is fundamental to the mechanism of BBB opening with FUS. The acoustic emissions drive the cavitating microbubbles, which in turn present shear stress on the vessel wall (Hosseinkhah et al. 2013). The resulting shear stress is hypothesized to then disrupt the tight junction complex and allow active transport of larger molecules through paracellular or transcellular endocytosis, which may eventually diffuse into the brain parenchyma (Sheikov et al. 2004). Various therapeutic agents have been successfully delivered through the BBB using FUS (Fan et al. 2013; Jordão et al. 2013; Park et al. 2012; Wu et al. 2014). In the case of PD treatment, neurotrophic factors were reported to protect degenerating dopamine neurons as well as promote regeneration of the nigrostriatal dopamine system (Rangasamy et al. 2010). Several studies investigated the feasibility of FUS facilitated delivery of neurotrophic factors to the brain using glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and neuturin (NTN) (Baseri et al. 2012; Wang et al. 2012). The success of this potential therapy would require repeated administration of neurotrophic factors and FUS-induced BBB opening. Therefore, the safety of longitudinal effects of FUS ultrasound on brain therapy is of great interest.
Several previous studies explored the effects of repeated applications of FUS for drug delivery to the brain. Yang et al. (2011) reported that repeated sonications at 20 or 40 min intervals enhanced Evans Blue extravasation in rat brains. Burgess et al. (2014) performed weekly sonications for a total of 3 weeks in Alzheimer Disease modeled mice followed by a week of behavior testing. They reported plaque reduction and increased neuronal plasticity in mice treated with 3-repeated BBB opening alone. Long-term (over 26 weeks) bi-weekly sonications in a single non-human primate were carried out by McDannold et al. (2012), where no significant changes in histological examination were found. In that study, behavioral testing was performed on other animals, which had 5 treatments (over 5–9 weeks). Therefore, it is still worth investigating the long-term effects of repeated BBB opening induced by FUS in a larger number of animals. Though the importance of post-mortem histological assessment must not be diminished, behavioral assessment of FUS is relevant and important for clinical application. In this study, we aimed at investigating the long-term effects of repeated FUS-induced BBB opening in wild type mice.
Methods
Animals
Wild type mice were purchased from Harlan Laboratories (strain C57BL/6) and were 8 weeks of age at the beginning of the experiment. Table 1 summarizes the main experimental groups (n = 5) used in this experiment, where the group name letter stands for either Bi-weekly (B) sonication or Monthly (M) sonication and the number indicates the survival time (in months). The sonication schedules were designed for the delivery of neurotrophic factors, such as GDNF and NTN. It has been shown that the bio-effects induced by GDNF and NTN can last as long as two weeks (Hoane et al. 1999). Therefore, we selected bi-weekly or monthly (a more conservative approach) sonication schemes and behavioral assessments were carried out following each sonication up to 24 weeks (over 30% of their life span). The different survival periods were selected to gain understanding on when potential damage might have occurred. For each group listed in Table 1, an age-matched control group (n = 5) was studied to compare potential behavioral changes. In addition, a sham (S) group (n = 4, no sonication was performed but underwent the procedures) and a positive control (P) group (n = 8) were also included in this study. For each treatment group, an age-matched control group (no treatment) was studied. Mice were housed under a 12 hour light, 12 hour dark cycle with access to food and water ad libitum. All animal procedures were in accordance and were approved by the Institutional Animal Care and Use Committee of Columbia University.
Table 1.
Main experimental groups and survival time (n=5 for each group).
| Sonication Frequency | Group† | Survival Period (months) |
|---|---|---|
| Bi-weekly | B5 | 5 |
| B3 | 3 | |
| B1 | 1 | |
|
| ||
| Monthly | M6 | 6 |
| M4 | 4 | |
| M2 | 2 | |
The group name letter stands for either Bi-weekly (B) sonication or Monthly (M) sonication and the number indicates the survival time (in months).
Experimental setup
A spherical single element FUS 1.5 MHz transducer was driven by a function generator (Agilent technologies) through a power amplifier (E&I, Rochester, NY USA). A 7.5 MHz pulse-echo transducer was positioned through a center hole of the FUS transducer, aligning the foci of the transducers. The pulser-receiver system, which drives the transducer, was connected to a digitizer and was utilized to locate the target of interest (Figure 1).
Figure 1.
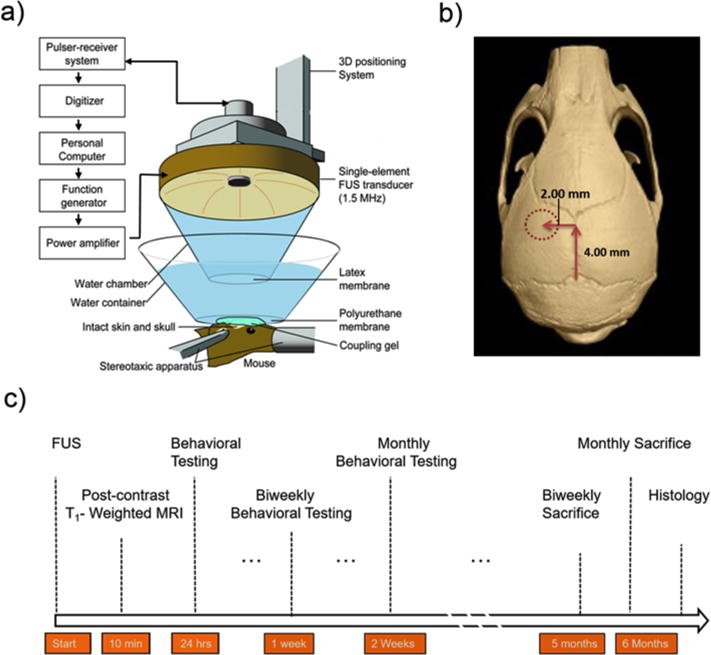
(a) Experimental setup of in vivo focused ultrasound (FUS)-induced blood–brain barrier (BBB) opening experimental setup. (b) The FUS transducer was targeted through the left parietal bone of the mouse skull and into a focal region within the striatum. (c) Experimental timeline for all subjects.
Prior to the procedure, subjects were placed in a chamber filled with 1.25% isoflurane and 0.8 ppm oxygen. Once under anesthesia, the subjects were placed prone onto a stereotaxic apparatus with continuous flow of the anesthetic mixture. The fur on the scalp was removed using an electric clipper and remaining hair was completely removed used by a depilatory cream. Following the application of ultrasound gel to the scalp, a degassed water bath with an acoustically and optically transparent polyurethane membrane base was then placed on the transducer in contact with the gel above the cranium of the mouse.
A grid positioning method was used to identify the striatum of the mouse; this method was similarly used and described in targeting the hippocampus (Choi et al. 2007). Briefly, a metallic grid was placed in alignment with the skull sutures of the mouse, which was visible through the intact scalp after hair removal. The striatum was localized by first identifying the lambda suture and then moving the transducer 2.0 mm lateral of the suture and subsequently 6.0 mm anterior from the top of the skull. The focus of the ultrasound beam was located 3.0 mm below the skull.
Sonication protocol
The commercially available ultrasound contrast agent Definity® (Lantheus Medical Imaging, N Billerica, MA, USA) microbubbles, which is composed of octafluoropropane gas encapsulated in a lipid shell, were used in this study. The mean diameter of the microbubbles range from 1.1–3.3 μm. Due to a shortage of available Definity® supply, for 3 consecutive weeks, in-house manufactured microbubbles were used instead. Similar to Definity®, the in-house manufactured microbubbles were also lipid-shelled polydisperse microspheres (mean diameter 1.4 μm), which were shown to have similar effects on ultrasound-induced BBB opening (Wang et al. 2014). The distribution and concentration of both types of microbubbles were measured with a Coulter Counter Multisizer (Beckman Coulter, Fullerton, CA, USA) and diluted in phosphate buffered saline to a final concentration of 8×108 #/ml before use. A dosage of 1 μl g−1 of body mass was administered via the tail vail immediately prior to the sonication.
Pulsed FUS parameters include an estimated in situ peak-rarefactional pressure (PRP) of 0.45 MPa (assuming 30% attenuation through the skull according our calibration) with a duration of 60s and a pulse repetition frequency of 10 Hz and pulse length of 500 cycles was applied. The control group received no treatment and was housed in the same environment as the treated group. For the positive control, most parameters were identical to the treated group, except the estimated PRP was increased to 1.5 MPa and pulse length was also increased to 10,000 cycles. During each session (bi-weekly or monthly), each subject received a single sonication at the region of interest, while the contralateral side received no treatment. In one cohort, subjects underwent a sham treatment, in which all procedures were performed (including anesthesia and injection of microbubbles) except for the sonication. Further details of experimental timeline can be found in Figure 1.
Magnetic Resonance Imaging
Following each sonication, confirmation of BBB opening was performed using a 9.4 Tesla MRI system (DPX400, Bruker Medical, Boston, MA, USA). The mice were then inserted into the vertical MRI bore and approximately 30 minutes post sonication, 2D FLASH T1-weighted horizontal images of the mouse brain were then acquired (TR/TE= 230/3.3 ms, number of excitations 8, spatial resolution 100 μm × 100 μm, slice thickness 400 μm) after intraperitoneal injection of an MRI contrast agent. The agent was a gadolinium-based, BBB-impermeable compound (Omniscan, amount 0.30 mL, molecular weight 573.7 Da). MRI scans used in this study were acquired 80 minutes post sonication and 50 minutes post gadolinium injection. The volume of BBB opening was quantitatively determined with volumetric measurements of post-contrast T1-weighted MR images (Samiotaki et al. 2012). The BBB-opened region was segmented (intensity higher than 2.5 standard deviations of the background) with a manually positioned elliptic cylinder (major diameter 4.3 mm, minor diameter 3.4 mm, and height 4.5 mm) over the left striatum following the shape of the focal spot in that plane. An additional elliptic cylinder of the same size was placed on the unsonicated (right) side of the brain. Voxels above the segmentation threshold on the unsonicated side were subtracted from the sonicated side to exclude the vessels and ventricles.
Behavioral Testing
Two types of behavior tests were carried out in this study: open field test and rotarod performance test. The open field chamber consisted of a custom-made polycarbonate chamber (dimensions: 27.3 cm × 27.3 cm × 27.3 cm) located in an isolated, soundproof room. Directly above the chamber was a video camera, which interfaced with a computer and tracking software (Noldus, Wageningen, Netherlands). All mice underwent one week of behavioral training prior to the first testing session to establish a baseline. Monthly cohorts were tested two weeks prior to the first sonication and biweekly cohorts were tested one week prior to the first sonication. In addition, mice that received monthly sonications were tested 24 hours and two weeks post each sonication. The cohorts receiving biweekly treatments were tested 24 hours and one week post each sonication.
During open field testing, the subject was placed directly in the center of the field, and video tracking was used to record and measure activity. Mice explored the field ad libitum for a session duration of 10 minutes. Movement patterns were recorded, such as rearing, the frequency of the mouse to stand on their rear legs. Upon completion, the subject was removed, urine and feces were counted and removed, and the chamber was cleaned with ethyl alcohol and disinfectants to remove any trace of the former subject.
Prior to being placed on the rotarod apparatus (Med Associates, St. Albans, VT, USA), mice were allowed to rest for a period of approximately 10 minutes. The rod was 32 mm in diameter and gradually accelerated to a maximum of 40 revolutions per minute. Each subject was tested for three consecutive trails with periods of rest of approximately 20 minutes in between. Due to the length of the study and the possibility of the subjects’ habituation to falling, during initial training, an acoustic aversive stimulus was used to promote active avoidance of falling. Falling off the rotarod, as well as latching on to the rotarod (rotating with the rod) for two consecutive revolutions was considered the end of a trial.
To assess any abnormalities independent of functional motor activity, anxiety was also measured. Thigmotactic activity in the open field, defined as the tendency to stay on the perimeter of the chamber, was examined to assess axiogenic behavior (Simon et al. 1994). Time spent within a six-centimeter square radiating from the center point of the field was measured in relation to thigmotaxis. In addition, weight was measured consistently each week to ensure subjects were gaining weight and maintaining adequate nutrition. All comparisons were made against the corresponding age-matched control group and normalization was also done against the established pre-treatment baseline.
Safety Assessment
For histological assessment, mice were sacrificed one day following the final behavioral assessment and one month following the last sonication. Mice were sacrificed by transcardial perfusion with phosphate buffered saline followed by 4% formaldehyde solution. One week following perfusion, post fixation for paraffin embedding was performed and brain samples were sectioned at 6 μm with 80 μm trimmed between levels, totaling 10 levels. For each level, hematoxylin and eosin (H&E) stain was performed on the first slide of the brain sample. Two mice from each group were also stained with Luxol Fast Blue (H&E-LFB) to stain myelin. Nissl staining was also used to stain for neurons and their nucleus. Morphology was assessed using a bright field microscope (IX-81; Olympus, Melville, NY, USA) with 4×, 10×, and 20× objectives.
Statistics
All statistical analysis was performed using SPSS (IBM, Armonk, NY, USA). Weekly body weights were analyzed using repeated measures analysis of variance with a factor of age in weeks. Open field data gait analysis was performed with repeated analysis of variance with a spatial covariance structure with factors of 5-minute intervals (2 per 10 minute session), and weeks of age. Motor coordination data were first averaged for the three trials and then subjected to a repeated measures model with a covariance structure on the repeated measures of age (in weeks) and experimental schedule (in weeks). Calculations for normalization was performed by dividing post-treatment and pre-treatment and subtracting by one, then converting the value to a percentage. Difference between Definity® and in-house manufactured microbubbles were compared using paired t test. Significance was established at P < 0.05, with high significance at P < 0.01.
Results
Spectra of the received PCD signal showed difference in cavitation dose for 0.45 and 1.5 MPa, with strong broadband noise at 1.5 MPa indicating inertial cavitation (Fig. 2). The opening of the BBB was confirmed with T1 contrast-enhanced MRI scanning. Figure 3 demonstrates the bi-weekly BBB openings in the same mouse over five months. With the ultrasound parameters used here, it was found that the BBB remained open for a maximum of 2 days (with complete closing on day 3, see Supplementary Fig.1). The MRI contrast agent gadolinium diffused efficiently throughout the targeted region of interest and the opening was reproducible in each mouse following sonication throughout the weeks. The average opening volume (VBBB) was determined to be 26.4 ± 0.3 mm3.
Figure 2.
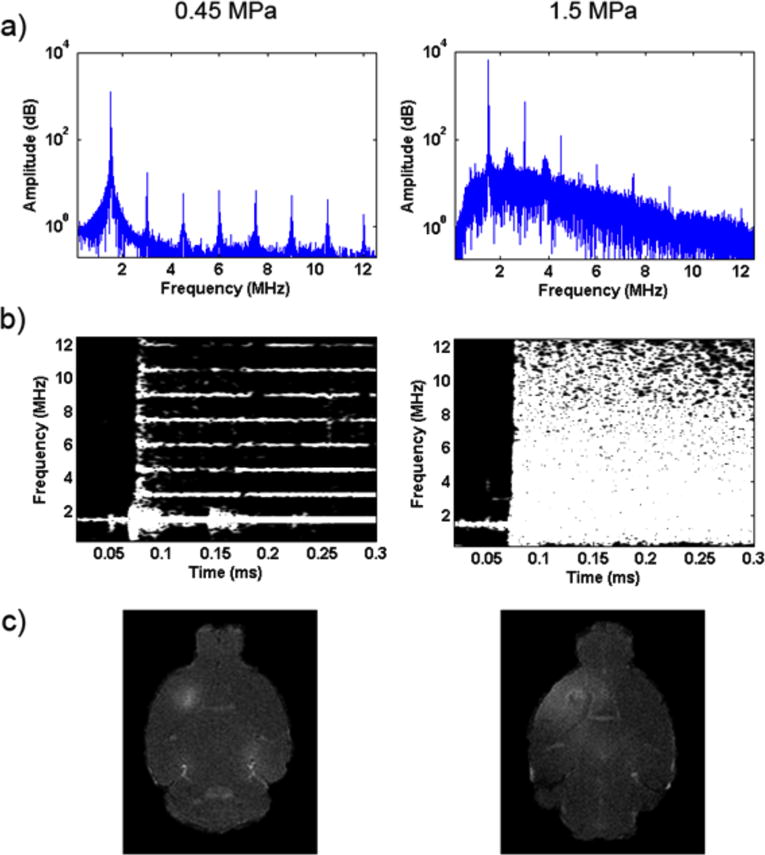
(a–b) PCD spectrogram for 0.45 MPa and 1.5 MPa. 1.5 MPa (positive control) showed strong broadband noise indicating inertial cavitation while 0.45 MPa remained within the stable cavitation threshold. (c) Contrast-enhanced T1 MRI image for the 1.5 MPa (positive control) group showed more dispersed diffusion of gadolinium rather than the localized diffusion of 0.45MPa.
Figure 3.
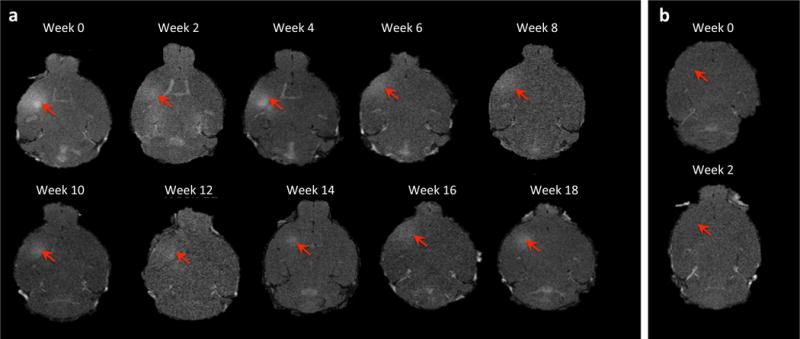
2D horizontal T1-weighted MR images of (a) 5 month, biweekly sonications. MR images were taken following each sonication and show that the BBB was successfully and consistently opened at the target area, the striatum, as well as the peak negative pressure of 0.45 Mpa. Average volume of opening was 26.49mm3. Permeability of monthly sonication cohort had a survival period of 6 months. Average volume of opening was consistent with other test groups and permeability was static throughout the duration of the study. (b) MRI of the sham group where no sonication was applied shows no opening at targeted area.
No difference in overall behavior due to the transient change of microbubbles was observed between weeks 8 and 12 for the biweekly groups, during which Definity was substituted with in-house manufactured polydispersed microbubbles (p = 0.287). Because testing began at different time points within the year for different groups, it was increasingly important for each group not tested on concurrent days to have a respective age matched control group. The distance travelled in the open field for the biweekly groups and sham compared to their control remained consistent throughout the testing duration (Fig. 4-a). The positive control exhibited a significant decrease is distance travelled (p < 0.05) when normalized and compared to their control (Fig. 4-b). For the monthly groups and their respective control, no difference was found in distance travelled (p = 0.429) when tested against time (Fig. 4-c). An increase in overall movement was observed from testing week 0 to week of sacrifice.
Figure 4.
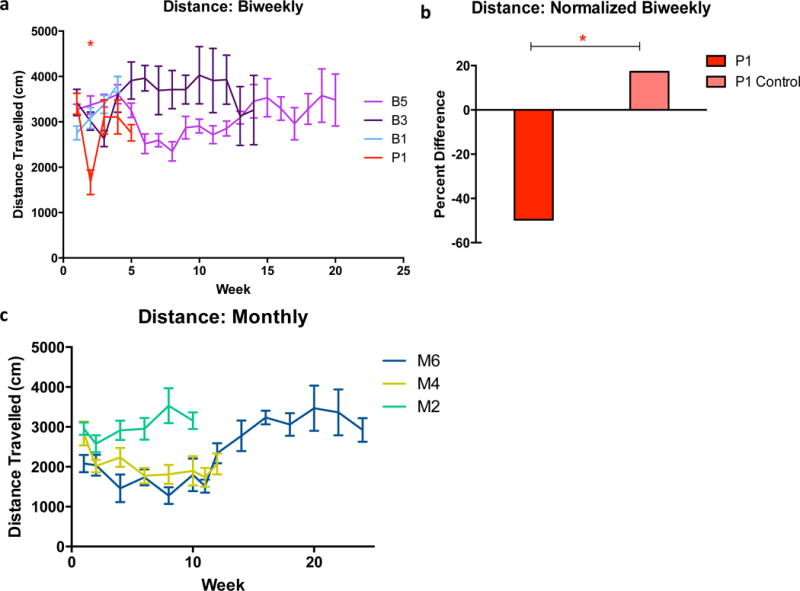
(a) Variances in total distance travelled in the 10-min testing duration for the biweekly cohorts. For all tests, the B control served as a control for B5, B1, and S5 and all groups were tested on the same day. P1 and B3 were tested on different days and therefore had separate controls. * Represents the difference between group P and their control. (b) Distance travelled was normalized from baseline trial to post-treatment treatment and subtracted by one. Normalization of distance travelled after week 1 (24 hours after sonication) shows a significant decrease in distance travelled for the positive control; while the other groups maintained a stable pattern post-sonication. (c) Variances in distance travelled for monthly cohorts. There was no significant time related affect for the monthly treated groups. Patterns of travel remained consistent with their controls throughout the (maximum) 6-month duration. SEM plotted. *(P<0.05).
The total mobility time (time subject spent moving) was insignificant for all biweekly groups except the positive control (p = 0.271. Positive control: p < 0.01) (Fig. 5-a). There was no decrease in mobility over time as shown in the two way repeated ANOVA against time. However, for the positive control, there was a significant decrease in movement after the first sonication (Fig. 5-b), and there was no statistical significance found in subsequent weeks.
Figure 5.
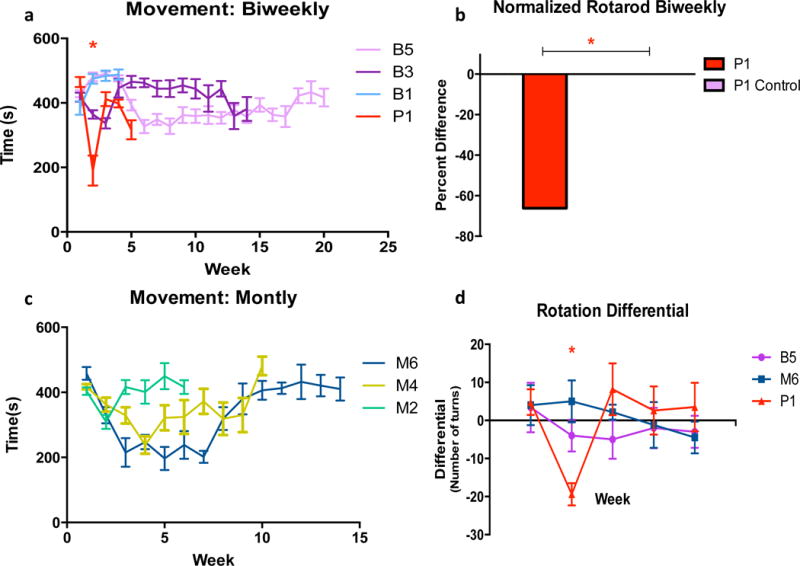
(a) Movement was measured by the amount of time the mouse spent moving during open field trial (600 seconds). Except for the positive control, activity of the mice remained static throughout the duration of the study. (c) However, monthly groups experienced an overall increase in movement over time. (b) Normalized rotarod from initial trial to first treatment. Following learning, mice were able to complete the task, and there was no significant difference between group performance, except for the positive control. P1 control is plotted, however the group achieved proficiency on the task in week 0. (d) Rotation differential was determined by net difference in turn direction. Negative deviation of the rotation differential strongly suggests damage from sonication. Unilateral cerebral damage would cause hemiparesis or hemiparalysis. Positive control displayed a significant affinity to the side corresponding to the sonication, supporting the notion of damage. All other groups did not exhibit a similar pattern. SEM plotted. *(P<0.05).
The rotation differential, determined by the net difference between right and left turns (with the threshold for one turn determined by an angle of 120°), was found to be significant for the positive control only for the subsequent sessions when compared to their control group (p < 0.05) for the week following treatment (Fig. 5-d). However, the following weeks were not significant (p = 0.151) when tested against all biweekly groups.
Minus the positive control, all subjects achieved proficiency in their ability to maintain balance and coordination on the rotarod task prior to exposure to FUS (Fig. 5-b). After treatment, both monthly and biweekly subjects were able to complete the task and remain on the rotarod for the expected duration of 180s over the specified trials (p = 0.477). For the positive control, there was a significant decrease in the latency to fall off the rod (p < 0.05). Latency did, however, increase in the weeks following initial FUS treatment.
Rearing, defined as the animal standing erect on hind legs, was recorded over the duration of the open field task was quantified and measured over time. There was no overall difference in rearing pattern or frequency, with most subjects performing the task along the perimeter of the chamber. The positive control, however, did not perform the rearing pattern following the first sonication. Thigmotaxis, defined by the affinity of mice to remain near the perimeter of the open field, was examined for the second half of the open field test. All treated groups and their controls exhibited similar travel patterns and explored the center of the open field (Fig 6 a–d). The positive control prior to sonication exhibited normal travel patterns associated with low anxiety, however following sonication, the overall decrease in mobility showed abnormal travel patterns (Fig. 6e–f). The sham displayed anxiogenic behavior (Fig. 6g–h), with a decrease in weight, rearing pattern, and decreased overall mobility during week 4 to 5 (Fig. 7). When examining each subject within the group individually, only one did not display the decreased mobility and had an increase in weight. Based on the observation, the non-anxiogenic subject, perceived to be the aggressor, was separated, and the remaining subjects eventually were able to gain and maintain normal weight distribution based on age, as well as decreased anxiogenic behavior (Fig. 6g–h, Fig. 7).
Figure 6.
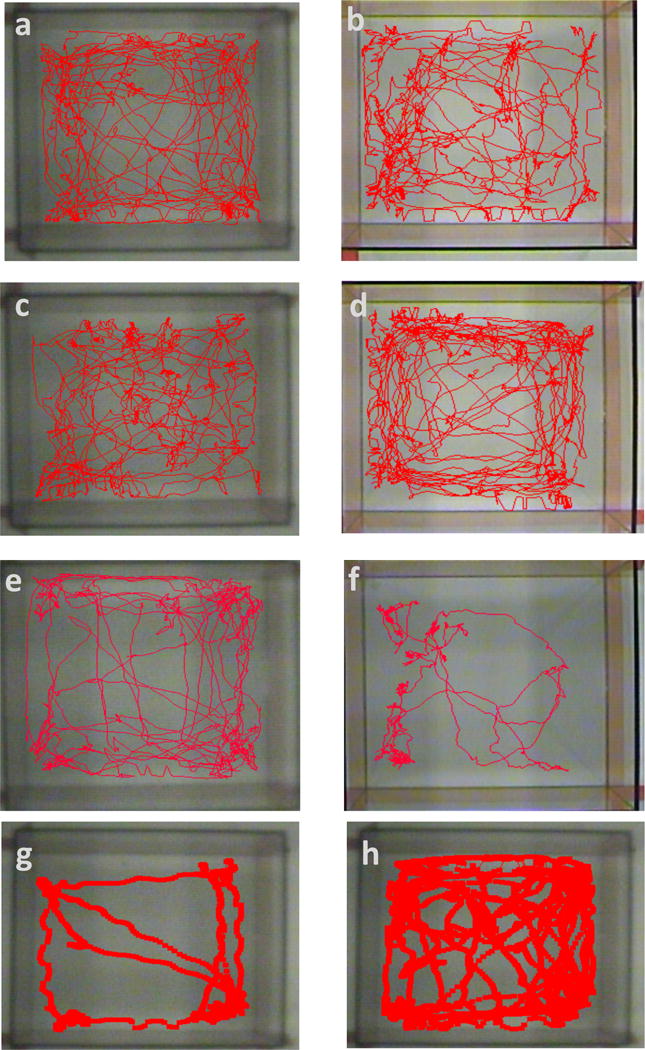
Thigmotactic visualization based on travel path. Comparison of B5 (a–b) and B Control (c–d) from before and after sonication displays similar behavior and no significant difference in time spent in center. In between groups, there was no significant difference in time spent in center. Difference in travel path between positive control before and after sonication (e–f) is also displayed, showing a significant decrease between weeks. (g) A sham mouse exhibited an increased anxiety due to an aggressive mouse in the same cage. The wounded mouse displayed an increase in anxiety. Two weeks after the aggressor was removed, the same mouse exhibited normal behavior (h).
Figure 7.
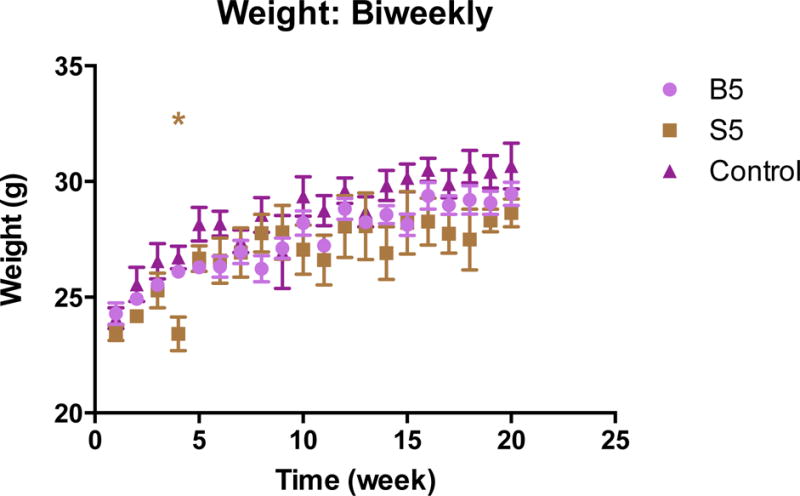
Weight gain of mice throughout weeks of testing. Comparison of 100 c57bl/6 mice on 6% fat diet. Data (provided by Jackson Laboratories) gives normal weight distribution over age, up to 20 weeks. Sham group experienced a decrease in weight due to an aggressive mouse (unrelated to FUS). Upon separation, mice recovered and eventually gained healthy weight. *Represents the difference in Sham and Jackson Laboratories data. SD plotted. *(P<0.05).
Histological analysis was used to detect any permanent damage to the brain, specifically within the region receiving treatment (Fig 8a–f). For all treated cohorts including the positive control with a survival of four weeks following the treatment, there was no evident damage; myelination appeared normal, and no cohort exhibited significant evidence of dark or ischemic neurons. The positive control at 1.5 MPa maintained similar morphology to the 0.45 MPa treated groups (Fig. 8a–c). However the positive control with a survival of 24 hours displayed extravasated erythrocytes (Fig. 8d–f). When staining for Nissl, a loss of neuronal bodies was observed in the positive control at the location of FUS treatment (Fig 9a–c), while the neuronal bodies of the untreated contralateral hemisphere remained did not experience a loss (Fig. 9d–f).
Figure 8.
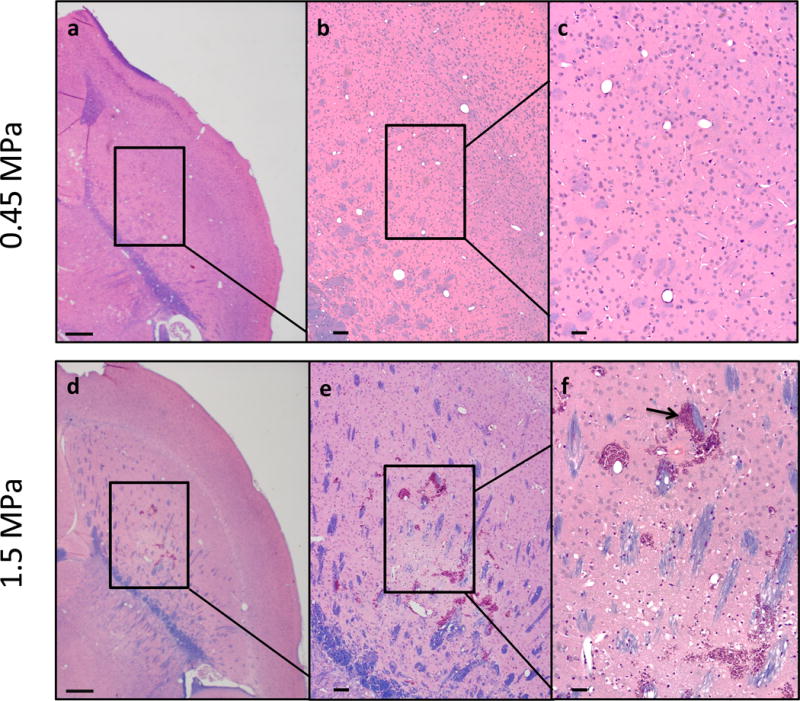
Histological analysis using hemotoxylin and eosin staining followed by luxol fast blue of paraffin embedded sections showed no red blood cell extravasations or dark neurons for all groups, including the positive control at week 4 (a–c). There was also no other abnormal morphology. However, sacrifice at 24 hours following sonication shows hemorrhage (arrow) in the positive control, group P* (d–f). Brains were sectioned horizontally at 6 μm. Scale bars: a, 1mm; b, 200 μm; c, 50 μm.
Figure 9.
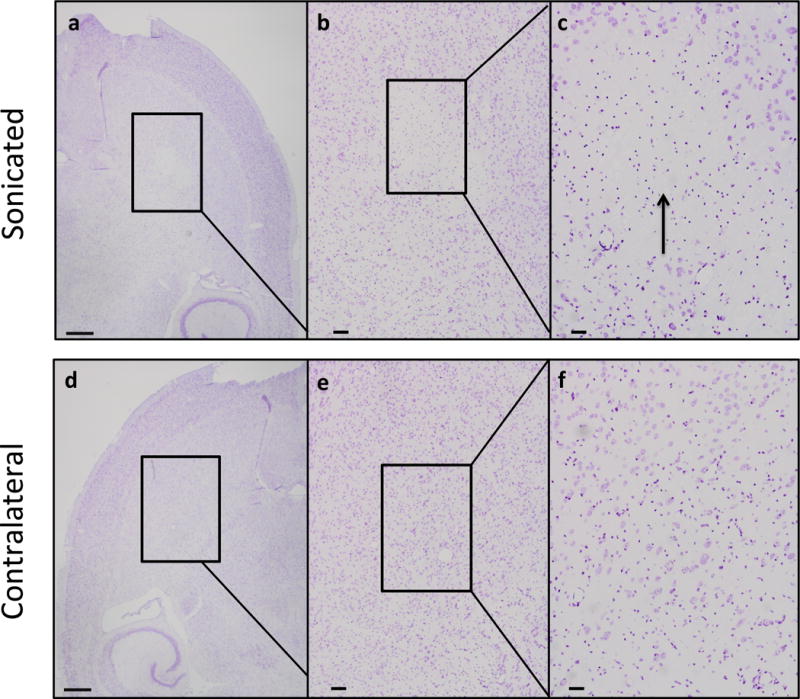
Nissl staining of paraffin embedded sections for the positive control (1.5 MPa) sacrificed 24 hours post sonication showed a loss of neurons (a–c). Other neuronal bodies, however, remained intact. For the contralateral hemisphere of the same subject, there was no pervasive abnormal morphology (d–f). Brains were sectioned horizontally at 6 μm. Scale bars: a, 1mm; b, 200 μm; c, 50 μm.
Discussion
The presented longitudinal analysis of FUS displays a set of parameters in which the treatment of FUS may be applied without causing structural or behavioral deficiencies. Though the use of FUS has many applications, this is the first study to investigate behavioral and motor deficits over durations up to 6 months in murine subjects. Sonication was performed either biweekly, or monthly. And as previously shown, MRI is reliable in monitoring and confirming BBB opening and volume, as well as the parameters.
Using open field and rotarod testing paradigms, as well as histological processing, we were able to quantify and analyze the behavior, as well as examine abnormities that may be attributed to FUS. Open field was used both as a measure of ambulatory activity, as well as anxiogenic activity, while the rotarod was used to monitor motor coordination and, if damage was evident, examine extent of motor instability (Luzzati et al. 2007). Both open field and rotarod are of special relevance in this study due to the required use of the striatum, as it is involved in fine motor control. In our study, injury to the striatum, as suggested in MRI and shown in histology, is consistently manifested behaviorally. Both paradigms have been established and are able to detect cerebral abnormalities (Fujimoto et al. 2004). Overall, all groups that were tested (apart from the positive control) exhibited no abnormal behavior overall.
Despite the fact that some individual subjects experienced an overall decrease in movement, individual characteristics must be examined with a comprehensive approach, whereby certain attributes are considered more indicative evidence than others. Though a vast decrease in distance travelled may initially be attributed to severe cerebral damage (Klein et al. 2012), a lack of evident deficits in any other ambulatory behavior, most emphatically rotation differential, suggests that the decrease may be due to a factor unrelated to FUS treatment, such as environmental factors (Brooks and Dunnett 2009). There was no observed effect relating to age between all groups, with the oldest subject being sacrificed at approximately eight months of age. The only group to achieve an outstanding difference in each variable examined was the positive control. Decrease in motor function and coordination is apparent at a peak negative pressure of 1.5 MPa. However, the cohort was able to recover from the injury, and histological analysis of the cerebral morphology 4 weeks following application of ultrasound found a decrease in neurons, and no other gross abnormalities.
Confirmation of paradigm sensitivity was displayed in the positive control, which exhibited the phenotypic characteristics of severe motor damage. These characteristics include overall decreased locomotor activity and rotation affinity towards the side ipsilateral to damage due to paralysis on the contralateral hemisphere of the subject.
The accelerating rotarod is a paradigm heavily accepted for motor sensitivity and testing (Hamm et al. 1994) and showed no significant difference in latency to fall between groups, further supporting the notion of no damage. The positive control was unable to complete the accelerating rotarod task after the first sonication, but at time of sacrifice was able to maintain coordination and balance for the measured duration (Fig. 5).
Throughout all cohorts, differences in anxiogenic behavior throughout the weeks between treated and control for almost all cohorts were not statistically significant. Though rearing examines motor coordination, it is also indicative of anxiety. There was no significant difference in rearing frequency and pattern between treated cohorts, as well as thigmotaxis (except for the positive control who exhibited poor movement). The sham, S5, exhibited increased anxiety due to exposure of a perceived threatening stimulus. Increased expression of thigmotaxis and a decrease in weight below the average within the age range (Kozak et al. 2010) was displayed by four subjects, which was elicited by one aggressive mouse, while the aggressor exhibited no abnormal anxiogenic behavior and maintained average weight (Fig. 7). Once provided separate cages, the sham group experienced decreased anxiogenic behavior and achieved normal weight gain (Fig. 7). The results show that FUS causes no effect on anxiety behavior in the normal mice. Traumatic brain injury has also been shown to increase anxiogenic behavior in murine subjects (Brooks and Dunnett 2009).
The rotation differential is a heavily utilized method of detecting unilateral damage, and remained a strong indicator in the presented study. Negative deviation constituted damage, and typically, all other motor behaviors followed the inclination of the rotation differential. Negative differential was also observed to be parallel with significantly lower ambulatory movement, as well as poor performance on the rotarod.
The use of in house manufactured bubbles was shown not to cause behavioral alterations and provided an additional paradigm to examine the importance of microbubble composition. Carbon chains forming the lipid shell of the in house made microbubble encapsulated the perflurobutane gas. Definity® microbubbles are made of a proprietary perflutren lipid coated shell which encapsulates octafluropropane gas. Definity® has received approval from the Food and Drug Administration for use as a contrast agent in cardiac imaging. When examining differences from week 8 to week 12 of the 5 month biweekly group (who received the most frequent sonications), it was found that despite variances in composition, both types of bubbles with the parameters tested can induce safe BBB opening and do not alter behavior.
Previous studies from our group indicate that the parameters presented in this study are not the sole parameters that may be applied without causing damage (Tung et al. 2010). Methods of examining the alternate variables in the previous studies were based on histological processing (Baseri et al. 2010). However, the results from our previous studies provided a reference for the upper limit of the pressure variable without inducing damage (Tung et al. 2010). Most pressures at and below 0.45 MPa but above 0.30 MPa using Definity® microbubbles, have been shown to open the BBB with the absence of cerebral damage (Choi et al. 2010a). However, cerebral damage can occur if ultrasound parameters are not carefully chosen. In this study, we deliberately chose 1.5 MPa for the positive control group to demonstrate behavior changes in these subjects. Significant decrease in motor function and coordination is apparent at a peak negative pressure of 1.5 MPa (Fig. 4a,c). Nonetheless, all subjects were able to recover from the injury, and it was found through histological analysis that the cerebral morphology four weeks following application of ultrasound closely related to that of B1 (Fig 8), with a survival period of four weeks and a peak negative pressure within the safety (0.45 MPa) window of parameters, suggesting reversibility of damage (Tung et al. 2010). Histological analysis found no damage between groups over time ranging from one month to six months of survival with repeated FUS application, including the n=5 group P that was sacrificed one month following sonication (Fig 9). The n=3 for group P that was sacrificed 24 hours following sonication exhibited extravasated erythrocytes as well as dark neurons (Fig 8).
Other groups have also shown FUS opening safety and feasibility in other animals. The procedure has been assessed using rats (Kim et al. 2014), rabbits (Hynynen et al. 2005; McDannold et al. 2005), and safety has been further assessed behaviorally in nonhuman primates (McDannold et al. 2012).
Clinical implications
The presented study demonstrates a feasibility examination of the potential of FUS to be used as a form of treatment and essentially eliminate the issue of drug size, hydrophobicity, and other limiting factors for delivery. Using FUS as a potential for therapeutic delivery is not limited to Parkinson’s disease; the use can be applied to many neurodegenerative occurrences, such as Alzheimer’s. For Parkinson’s, however, this offers the possibility of decreasing and potentially eliminating the dependence on carpidopa for levodopa delivery.
Further examining the potential of alternatives offers different varieties of therapeutic agents. Neurotrophic factors are endogenous to the brain and other organs, and we have shown that with FUS, the protein brain-derived neurotrophic factor, with a molecular weight of 27 kDa is able to cross the BBB and retain biological activity (Baseri et al. 2012). Other neurotrophic factors that have been shown to permeate the BBB using FUS include Neurturin, (23.6 kDa), and Glial Cell line derived neurotrophic factor, GDNF (30.1 kDa), all of which have been proposed as potential candidates for Parkinson’s disease therapy.
An area of future study is further investigation and comprehension of neuronal plasticity as it relates to FUS. Though the positive control displayed extensive damage 24 hours following sonication, one month following the high-pressure treatment, there was no evident damage, suggesting brain reparation. Further, others have shown hippocampal neuronal plasticity following FUS application.
The mechanism of repair to the striatum is a worthwhile examination, to reexamine whether neurogenesis occurs in the striatum, which has previously been suggested as a low neurogenesis site (Luzzati et al. 2007).
Another area of particular interest would be monitoring of disruption and damage in vivo. Though behavior has proven an asset and a reliable method of detecting severe damage, discovering a method of examining of in vivo the phenomenon of FUS and the ability to open the BBB, as well as the effect on the peripheral body systems, would be beneficial.
Conclusion
The presented study presented constituted an initial safety and feasibility study to examine the effects of repeated BBB opening using FUS in conjunction with microbubbles, with FUS that may occur over an extended duration of time, as well as behavior changes associated with the treatment. We examined the chronic use of FUS over 6 months, with treatment in the same location, and we have shown the application does not to cause any motor, behavioral, or morphologic deficits or alterations. These results are in support of using FUS as an alternative or complimentary method of noninvasive drug delivery.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institutes of Health (NIH) grants RO1 EB009041, as well as RO1 AG038961, and the Kinetics foundation.
References
- Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–49. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Barrier B, Pathologic I, Burgess A, Yeung S, Aubert I. Alzheimer Disease in a Mouse Model : MR Imaging – guided Focused Ultrasound Targeted to the Hippocampus Opens the Blood. 2014:273. doi: 10.1148/radiol.14140245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseri B, Choi JJ, Deffieux T, Samiotaki G, Tung Y-S, Olumolade O, Small SA, Morrison Barclay III, Konofagou EE. Phys Med Biol. Vol. 57. Department of Biomedical Engineering, Columbia University; New York, NY, USA: 2012. Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood-brain barrier using focused ultrasound and microbubbles; pp. N65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseri B, Choi JJ, Tung Y-S, Konofagou EE. Multi-Modality Safety Assessment of Blood-Brain Barrier Opening Using Focused Ultrasound and Definity Microbubbles: A Short-Term Study. Ultrasound Med Biol. 2010;36:1445–1459. doi: 10.1016/j.ultrasmedbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci Nature Publishing Group. 2009;10:519–529. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Member S, Feshitan JA, Baseri B, Wang S, Tung Y, Borden MA, Konofagou EE. Microbubble-Size Dependence of Focused. 2010a;57:145–154. doi: 10.1109/TBME.2009.2034533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Pernot M, Brown TR, Small SA, Konofagou EE. Spatio-temporal analysis of molecular delivery through the blood–brain barrier using focused ultrasound. Phys Med Biol. 2007;52:5509–5530. doi: 10.1088/0031-9155/52/18/004. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Selert K, Gao Z, Samiotaki G, Baseri B, Konofagou EE. J Cereb Blood Flow & Metab. Vol. 31. Nature Publishing Group; 2010b. Noninvasive and localized blood–brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies; pp. 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C-H, Ting C-Y, Lin H-J, Wang C-H, Liu H-L, Yen T-C, Yeh C-K. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials. 2013;34:3706–3715. doi: 10.1016/j.biomaterials.2013.01.099. [DOI] [PubMed] [Google Scholar]
- Fujimoto ST, Longhi L, Saatman KE, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Gulwadi a G, Morrison S, Hovanesian G, Lindner MD, Tao W. Differential in vivo effects of neurturin and glial cell-line-derived neurotrophic factor. Exp Neurol. 1999;160:235–243. doi: 10.1006/exnr.1999.7175. [DOI] [PubMed] [Google Scholar]
- Hosseinkhah N, Chen H, Matula TJ, Burns PN, Hynynen K. Mechanisms of microbubble-vessel interactions and induced stresses: A numerical study. J Acoust Soc Am. 2013;134:1875–85. doi: 10.1121/1.4817843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Jordão JF, Thévenot E, Markham-Coultes K, Scarcelli T, Weng Y-Q, Xhima K, O’Reilly M, Huang Y, McLaurin J, Hynynen K, Aubert I. Exp Neurol. Elsevier B.V.; 2013. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound; pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenschlager R, Lees AJ. Treatment of Parkinson’s disease: levodopa as the first choice. J Neurol. 2002;249(Suppl):II19–24. doi: 10.1007/s00415-002-1204-4. [DOI] [PubMed] [Google Scholar]
- Kim H, Chiu A, Lee SD, Fischer K, Yoo S-S. Focused ultrasound-mediated non-invasive brain stimulation: examination of sonication parameters. Brain Stimul. 2014;7:748–56. doi: 10.1016/j.brs.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Sacrey L-AR, Whishaw IQ, Dunnett SB. Neurosci Biobehav Rev. Vol. 36. Elsevier Ltd; 2012. The use of rodent skilled reaching as a translational model for investigating brain damage and disease; pp. 1030–1042. [DOI] [PubMed] [Google Scholar]
- Kozak LP, Koza RA, Anunciado-Koza R. Int J Obes (Lond) Suppl 1. Vol. 34. Nature Publishing Group; 2010. Brown fat thermogenesis and body weight regulation in mice: relevance to humans; pp. S23–S27. [DOI] [PubMed] [Google Scholar]
- Luzzati F, De Marchis S, Fasolo A, Peretto P. Adult neurogenesis and local neuronal progenitors in the striatum. Neurodegener Dis. 2007;4:322–327. doi: 10.1159/000101889. [DOI] [PubMed] [Google Scholar]
- McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary Disruption of the Blood-Brain Barrier by Use of Ultrasound and Microbubbles: Safety and Efficacy Evaluation in Rhesus Macaques. Cancer Res. 2012;72:3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51:793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- McDannold NJ, Vykhodtseva NI, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Park E-J, Zhang Y-Z, Vykhodtseva N, McDannold N. J Control Release. Vol. 163. Elsevier B.V.; 2012. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model; pp. 277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy SB, Soderstrom K, Bakay RAE, Kordower JH. Prog Brain Res. Vol. 184. Elsevier B.V.; 2010. Neurotrophic factor therapy for Parkinson’s disease. [DOI] [PubMed] [Google Scholar]
- Samiotaki G, Vlachos F, Tung YS, Feshitan J, Borden M, Konofagou EE. Pressure and microbubble size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo. AIP Conf Proc. 2012;1481:300–306. doi: 10.1002/mrm.23063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Jolesz F, Zhang Y-Z, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med Biol. 2006;32:1399–409. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol Elsevier Ltd. 2004;30:979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Tung Y-S, Vlachos F, Choi JJ, Deffieux T, Selert K, Konofagou EE. In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice. Phys Med Biol. 2010;55:6141–6155. doi: 10.1088/0031-9155/55/20/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Shi Y, Lu L, Liu L, Cai Y, Zheng H, Liu X, Yan F, Zou C, Sun C, Shi J, Lu S, Chen Y. Targeted Delivery of GDNF through the Blood–Brain Barrier by MRI-Guided Focused Ultrasound. In: Smeyne RJ, editor. PLoS One. Vol. 7. 2012. p. e52925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Samiotaki G, Olumolade O, Feshitan JA, Konofagou EE. Microbubble type and distribution dependence of focused ultrasound-induced blood-brain barrier opening. Ultrasound Med Biol. 2014;40:130–7. doi: 10.1016/j.ultrasmedbio.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-K, Yang M-T, Kang K-H, Liou H-C, Lu D-H, Fu W-M, Lin W-L. Targeted delivery of erythropoietin by transcranial focused ultrasound for neuroprotection against ischemia/reperfusion-induced neuronal injury: a long-term and short-term study. PLoS One. 2014;9:e90107. doi: 10.1371/journal.pone.0090107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F-Y, Lin Y-S, Kang K-H, Chao T-K. J Control Release. Vol. 150. Elsevier B.V.; 2011. Reversible blood-brain barrier disruption by repeated transcranial focused ultrasound allows enhanced extravasation; pp. 111–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


