Abstract
Hedgehog (Hh) signalling plays an important role in cancer; however, its mechanism in ovarian cancer migration and invasion remains unclear. In the present study, we aimed to clarify the effect of the Hh signalling pathway on ovarian cancer migration and invasion through the regulation of CD24 expression, both in vitro and in vivo. Patients with ovarian cancer (n = 97) were recruited for this study. Evaluation of the explored the role parameters of patients indicated that CD24 expression was negatively associated with age, histological type and lymph node metastasis (p>0.05), but was positively associated with the clinical stage and pathological grading (p<0.05).The in vitro results indicated that the activator (sonic hedgehog, Shh) and inhibitor (GANT61) of Hh signalling significantly enhanced and reduced CD24 expression, respectively, at both the gene and protein levels (p<0.05).The addition of Shh significantly enhanced cellular migration and invasion of SKOV3 cells in vitro (p<0.05) Down regulation of CD24 using siRNA inhibited the tumour-promoting effects of Shh, and the in vivo results confirmed that GANT61 significantly inhibited CD24 expression and reduced tumour growth (p<0.01). In conclusion, the expression of CD24 can be regulated by Hh signalling, and downregulation of CD24 could play an important role in inhibiting ovarian cancer progression.
Keywords: Hedgehog signalling, CD24, Ovarian cancer, Cellular migration, Cellular invasion.
Introduction
Ovarian cancer is a common cause of morbidity and mortality in women, estimated to affect 7.91 per 100,000 women, with an age-adjusted rate of 5.35 per 100,000 women in China between 1999 and 20101. Several factors play an important role in the development of ovarian cancer, such as increasing age, nulliparity, early menarche or late menopause, and BRCA1 or BRCA2 mutations. Due to the location of tumours in the pelvis, the malignancies are often large and advanced at the time of diagnosis2. Almost75% of women with ovarian cancer are diagnosed at stage III or IV, which have 10-year survival rates of 21% and less than5%, respectively3.
Despite the prevalence of ovarian cancer, its molecular etiology remains mostly unknown. Therefore, it is of great importance to clarify the association of key proteins with ovarian cancer migration and invasion4. In a previous study, we used a cDNA microarray to identify unique downstream targets of the glioma-associated oncogene (GLI) gene, including the adhesion molecule CD24, known to be associated with carcinoma progression 5. Hedgehog (Hh) signalling has been reported to be reactivated in metastases of ovarian, prostatic, gastric, esophageal and pancreatic carcinomas in a ligand-dependent or -independent manner6, 7. Transcription factors of the Gli family (Gli1, Gli2 and Gli3) can regulate Hh target genes by binding to specific elements in their promoter sequences, promoting cellular proliferation, cellular survival, stemness and cell fate determination in a variety of organs 8, 9.
In the present study, we firstly investigated the relationship of CD24 expression with clinic pathological features and prognosis in human ovarian cancer. We then explored the role of CD24 in ovarian cancer cell migration and invasion via upregulating or downregulating its expression, both in vitro and in vivo.
Methods
Patients and tissue samples
Patient samples were obtained after written informed consent had been obtained, in accordance with the ethics committee of the participating institutes and the Declaration of Helsinki. This study was approved by the Institutional Review Board of the Second Affiliated Hospital of Nanchang University.
Tissue samples were collected from 97 patients with ovarian epithelial cell carcinoma who underwent surgical resection at the Second Affiliated Hospital of Nanchang University between 2007 and 2010 (age range 18-55 years). All patients were histopathological diagnosed based on clinical protocols, and none had received pre-surgery chemotherapy or immunotherapy.
All stained sections were independently evaluated and scored by two pathologists with no prior knowledge of the clinic pathological outcome of the patients. The mean percentage of positive cells were scored as 0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%) or 4 (76-100%). The staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). The final histological (h) scores were obtained for each patient by multiplying the score for the percentage of positive cells and the intensity score. Protein expression levels were further analysed by classifying the h values as negative (-) for scores of 0-3, low positive (+) for scores 4-6, medium positive(++)for scores 7-9, or strongly positive (+++) for scores >10 10.
Histopathological analysis
The excised samples were opened along the lesser curvature, and the longitudinal section was fixed in a 10%neutral buffered formalin solution, then embedded in paraffin and processed for histopathological analysis 11. All samples were stained with haematoxylin and eosin (H&E). Histopathological evaluation was performed by a histopathologist with no prior knowledge of the identity of the samples.
Immunofluorescence and immunohistochemistry
The resected tumours were fixed in 10% buffered formalin, embedded in paraffin and mounted on slides. After deparaffinization and rehydration, the tumour sections were incubated in 3% hydrogen peroxide to suppress endogenous peroxidase activity. Antigen retrieval was achieved by microwaving the sections in 10 mM sodium citrate solution (pH 6.0). Sections were then blocked by incubating with 2.5% horse serum, and then antibodies were applied overnight at 4ºC. Antibody detection was achieved using an avidin-biotin complex system (Vector Laboratories, Burlingame, CA). Slides were stained with 3,3′-diaminobenzidine, then washed and counterstained with hematoxylin. Slides were subsequently dehydrated, treated with xylene and mounted.
Cell culture
The human ovarian carcinoma‑derived SKOV3 cell line was cultured in RPMI‑1640 medium, supplemented with foetal bovine serum (FBS; 10%) and penicillin/streptomycin (100 U/ml of each), and cultured in a 5% humidified CO2atmosphere at 37ºC.
Cell migration and invasion assays
Cell migration was measured using a scratch assay. The SKOV3 cells were plated onto 6-well plates to create a confluent monolayer. The cell monolayer was scraped in a straight line using a pipette tipto create a “scratch”. Cells were washed once with the growth medium, then RPMI1640 medium containing 2% FBS was added. The cells were treated with N-Shh conditional medium plus either GANT61 inhibitor or DMSO for 36 hours. Cells were photographed usinga phase-contrast microscope at 0, 12, 24, 36 and 48 hours. The wound area was quantified using Institutes of Health (NIH) Image-Pro Plus software.
The cell invasion assay was performed in Transwell® plates (8-mm pore size, 6.5-mm diameter; Corning Life Sciences, Lowell, MA) which had been pre-coated with Matrigel®basement membrane matrix (1 mg/ml; BD Biosciences, Franklin Lakes, NJ) according to the manufacturer's instructions. Briefly, highly invasive ovarian cancer SKOV3 cells (5 × 104 cells/well) were plated into the upper chamber of the system, and cultured with RPMI1640 supplemented with 2% FBS. The bottom wells in the system were filled with 500 ml N-Shh conditional medium supplemented with 2% FBS. Where applicable, the GLI2 antibody or control IgG (25 mg/ml) was added to the top chamber. After24 hours of incubation, any uninvaded cells in the upper chamber were removed, and cells that had invaded through the Matrigel® matrix membrane were stained with crystal violet for 30 minutes. The number of invading cells was counted under an inverted microscope and photographed.
Reverse-transcription polymerase chain reaction
SKOV3 cells were seeded into 24-well plates (5 × 104 cells per well) for 24 hours, after which N-Shh or GANT61 were added to downregulate or upregulate the Hh signalling pathway, respectively. RNA was isolated using Trizol solution (Life Technologies, Grand Island, NY). After removal of genomic DNA using DNAse I (Ambion, Austin, TX), 2.4 μg of total RNA from SKOV3 cells were reverse-transcribed to cDNA using a commercially available kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed with a 7900HT fast real-time PCR system (ABI, Foster City, CA) using 2×SYBR Green master mix (Bio-Rad, Richmond, Calif). Forty cycles were performed as follows: 95ºC for 30 seconds, 60ºC for 30 seconds, preceded by 1 minute at 95ºC for polymerase activation with the following primers (q-PCR GAPDH sense primer 5'- CAGGGCTGCTTTTAACTCTGGT -3', antisense primer 5'- GATTTTGGAGGGAATCTCGCT -3'; q-PCR CD24 sense primer 5'- GCTAAACGGATTCCAAAGAG-3', antisense primer 5'- CTGGGCGACAAAGTGAGA -3').
Western blotting
Whole-cell lysates were prepared using cell lysis buffer supplemented with protease inhibitor cocktail and 1 mM PMSF12. Protein concentrations were measured and then separated by polyacrylamide gel electrophoresis. After electro transferring to polyvinylidene difluoride membranes, non-specific binding sites were blocked with 5% non-fat milk in TBST for 1 hour at room temperature. Membranes were incubated with primary antibodies overnight at 4°C, washed with TBST, and then incubated with the appropriate HRP-conjugated secondary antibody for 1 hour at room temperature. Immune complexes were visualised using enhanced chemiluminescence. Consistent loading and transfer were confirmed by probing the same membrane with an anti-GAPDH antibody.
siRNA transfection
CD24-miRNAi expression vectors were generated using the BLOCK-iT™ Pol II miR RNAi Expression Vector Kit (K4936-00; Invitrogen, Carlsbad, CA). The oligonucleotide sequences for the miRNAi constructs are presented in Table S1. SKOV3/DDP cells were plated at a density of 18,000 cells/cm2. Transfection was conducted 24 hours after plating, using the RNAi-Max transfection reagent and different concentrations and scrambled siRNA sequences or negative control.
Xenograft tumour model and antitumor effect of CD24 in vivo
SKOV3 cells (5 × 107) were subcutaneously implanted into the flank of BALB/c nu/nu mice. Tumours were allowed to grow until they reached a median size of 100 mm3, then the mice were randomly assigned to receive a subcutaneous injection of either GANT61 (25 mg/kg) in solvent (corn oil:ethanol, 4:1) or an equal volume of solvent only for 15 days. The subcutaneous injections were initiated 4 days after tumour inoculation, and repeated every other day. At the end of the experiment, tumours were excised, weighed, and then prepared for H&E staining and immunohistochemically (IHC) analyses.
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals established by the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Second Affiliated Hospital of Nanchang University.
Statistical analysis
The statistical significance of the relationships between CD24 expression and the clinic pathological parameters was evaluated using Wilcoxon and Kruskal-Wallis tests and Spearman's rank correlation. The data are presented as mean ± SD, and p<0.05 was considered statistically significant 10, 13, 14.
Results
Correlation between the clinicopathological features and CD24expression
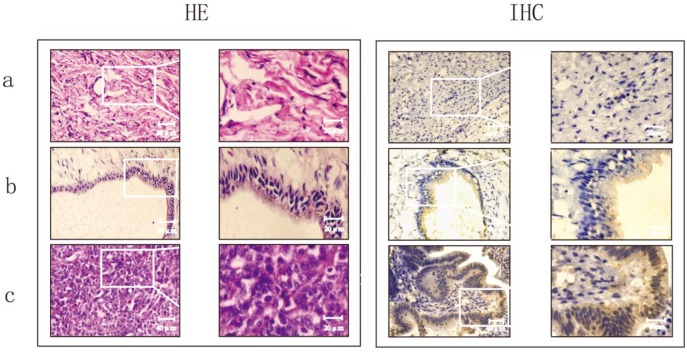
To explore the clinical significance of CD24, 97 patients with ovarian epithelial cell carcinoma were recruited. The histopathological evaluation revealed a significant increase in inflammation in ovarian serous carcinomas compared to normal ovaries and benign ovarian tumours (Fig.1).The proportion of cells that positively expressed CD24 was 55%in borderline tumours and80.95% in malignant tumours, which was significant higher than the21.43% positive cells in benign tumours (p<0.01). There was no significant difference observed between serous cystadeno-carcinoma and mucinous cystadenocarcinoma (Table 1 and Fig. 1).
Figure 1.
Evaluation of the histomorphology and protein expression of CD24 in normal ovaries, ovarian benign tumor and ovarian serious carcinoma using HE staining and immunohistochemistry (IHC).
Table 1.
Expression of CD24 in different ovarian tissues using immunohistochemistry.
| n | CD24 positive | X2 | P | |||
|---|---|---|---|---|---|---|
| n | % | |||||
| Serous cystadenocarcinoma | 50 | |||||
| Benign | 8 | 2 | 25.00 | 10.45 | 0.005<p<0.01 | |
| Borderline | 10 | 5 | 50.00 | |||
| Malignant | 32 | 26 | 81.25 | |||
| Mucinous cystadenocarcinoma | 47 | |||||
| Benign | 6 | 1 | 16.67 | 9.85 | 0.005<p<0.01 | |
| Borderline | 10 | 6 | 60.00 | |||
| Malignant | 31 | 25 | 80.65 | |||
| Total benign | 14 | 3 | 21.43 | |||
| Total borderline | 20 | 11 | 55.00 | 20.00 | <0.005 | |
| Total malignant | 63 | 51 | 80.95 | |||
Moreover, the relationship between CD24 expression and ovarian epithelial cancer indicated that CD24 expression was negatively associated with patient age, histological type and lymph node metastasis (p>0.05), but was positively associated with the clinical stage and pathological grading (p<0.05; Table 2). The proportion of cells positive for CD24 expression in stages III and IV was 94.59%, whereas this was only 61.54% in stages I and II (p <0.005). A high pathological grading (G1) indicated a lower positive rate of CD24 expression (68.97%) than lower pathological grading (G2/G3, 91.18%; 0.025<p<0.05).
Table 2.
Correlation between the protein expression of CD24 and clinicopathological parameters in patients with epithelial ovarian cancer (n=63).
| Characteristics | n | CD24 | |||
|---|---|---|---|---|---|
| positive | X2 | p | |||
| n | % | ||||
| Age | |||||
| ≤50 | 17 | 13 | 76.47 | 0.0358 | 0.75<p<0.90 |
| >50 | 46 | 38 | 82.61 | ||
| Histologic type | |||||
| Serous | 32 | 26 | 81.25 | 0.00374 | p>0.90 |
| Mucinous | 31 | 25 | 80.65 | ||
| Pathological grading | |||||
| G1 | 29 | 20 | 68.97 | 5.01 | 0.025<p<0.05 |
| G2/G3 | 34 | 31 | 91.18 | ||
| Clinical stages | |||||
| I/II | 26 | 16 | 61.54 | 8.78 | p<0.005 |
| III/IV | 37 | 35 | 94.59 | ||
| Lymph node metastasis | |||||
| Yes | 37 | 31 | 83.78 | 0.127 | 0.5<p<0.75 |
| No | 26 | 20 | 76.92 | ||
Effect of CD24 on ovarian cancer cell migration and invasion
To confirm the cDNA microarray results obtained in our previous study, we examined the effect of Shh (activator of Hh signalling) and GANT61 (inhibitor of Hh signalling) on CD24 expression at both gene and protein levels. As shown in Fig. S1, addition of Shh significantly enhanced the CD24 expression both at the gene and protein levels (p<0.05), and GANT61 significantly inhibited CD24 expression (p<0.05).
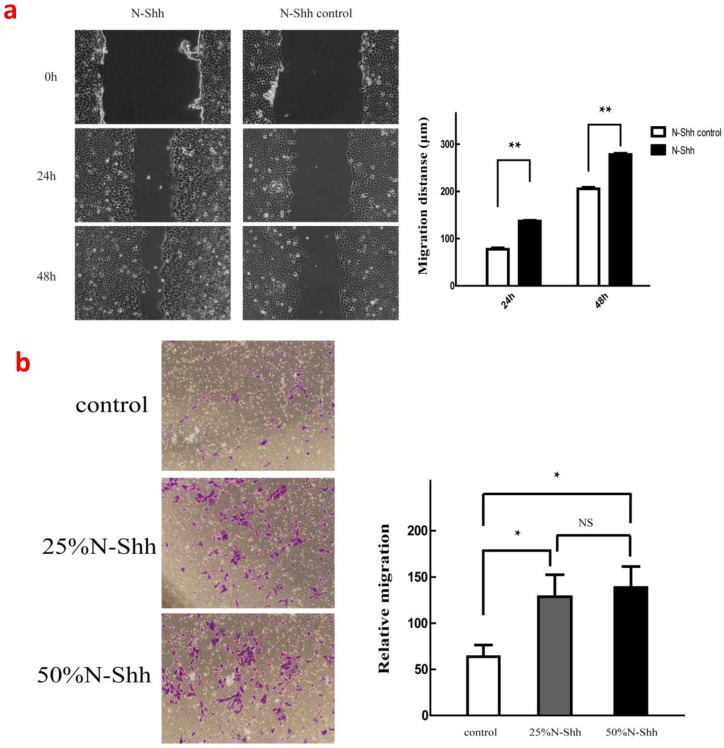
When Shh was added, the activator of Hh signalling significantly enhanced the cellular migration of SKOV3 compared with the control group at both 24 and 48 hours (p<0.01). Furthermore, Shh at 25 and 50% concentrations significantly enhanced the invasion of SKOV3 cells (p<0.05; Fig. 2).
Figure 2.
(a), Effects of 50% N-Shh on cancer cell migration; (b), Effects of 25% and 50% N-Shh on cancer cell invasion.
BlockingCD24 inhibits ovarian cancer cell migration and invasion in vitro
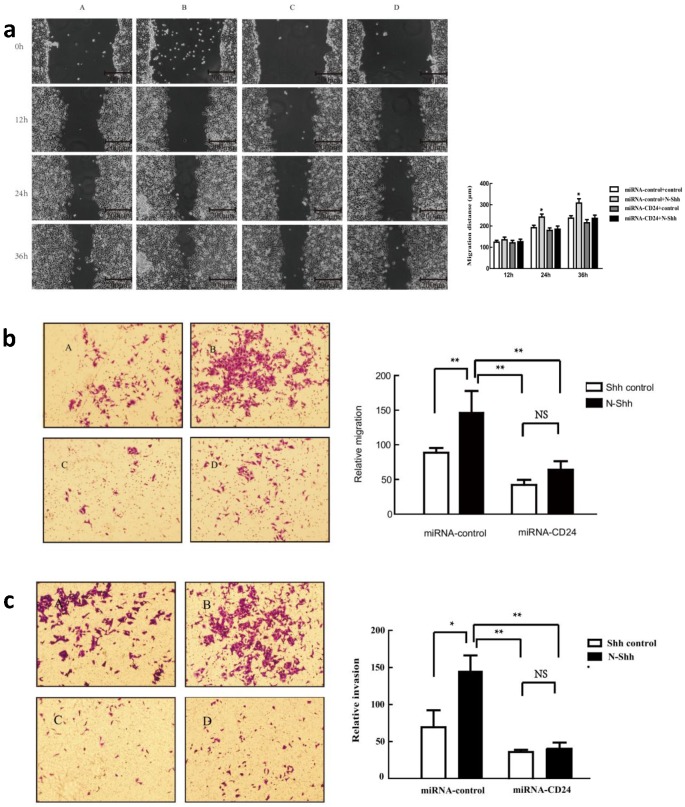
To further determine the effect of CD24 on ovarian cancer cells, a plasmid containing three different fragments that interfere with CD24 was constructed and tested in the SKOV3 cell line. Fragment3 was chosen asit demonstrated the best silencing effect, as determined by Western blot analysis (Table S1 and Fig. S2). We then evaluated the effect of direct silencing of CD24 expression on cell migration and invasion (Fig. 3). Results from the scratch test at 12 hours indicated no obvious changes in cellular migration. However, addition of Shh in the miR-control-N-Shh group resulted in significantly enhanced migration of SKOV3 cells at 24 and 36hours (p<0.05), and silencing CD24 in the miR-CD24-N-Shh group counteracted the effect of Shh (Fig.3a). Similarly, the Transwell results demonstrated that Shh significantly enhanced the cellular migration and invasion of SKOV3 in the control group. The downregulation of CD24 by fragment 3 eliminated its promoting effects, suggesting an important role of CD24as a downstream target of Hh signalling.
Figure 3.
Effects of down-regulation of CD24 on ovarian cancer cell migration (a, b) and invasion (c). (A-D), transfeced with miR-control-control, miR-control-N-Shh, miR-CD24-control and miR-CD24-N-Shh.
BlockingCD24 inhibits ovarian cancer growth in vivo
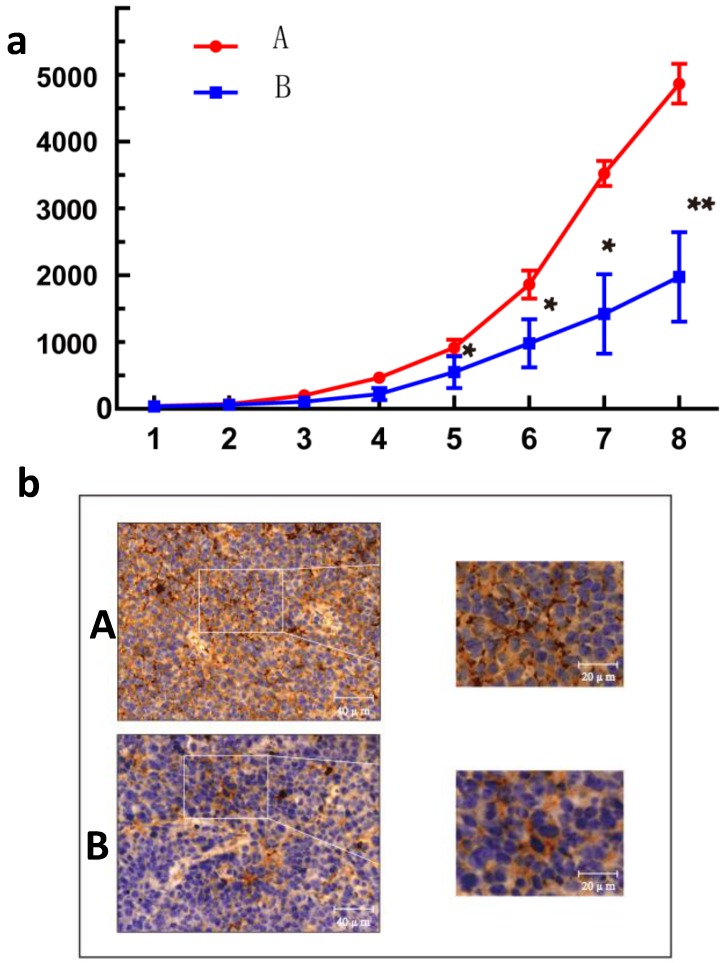
As GANT61 was found to significantly reduce the expression of CD24 (Fig.S1), this inhibitor was used to block the expression of CD24 in vivo. As shown in Fig.4, the IHC results indicated that blocking CD24 by GANT61reduced CD24 expression in the ovarian cancer tumours, and GANT61 successfully reduced the growth of tumours compared with the control on day 8 (p<0.01).
Figure 4.
Effect of CD24 blockade on growth of human ovarian cancer cells in vivo. (a) Treatment with GANT61 led to a significant growth inhibition of xenografted human ovarian cancer tumors. n = 8; *, P<0.05; **, P<0.01. (b) Immunohistochemical image of tumor tissue section. A, control group; B, treatment with GANT61 diminished expression of CD24.
Discussion
Ovarian cancer is a common gynaecological cancer. Many studies have confirmed that approximately 25% of all cancer deaths are related to aberrant activation of the Hh pathway 15. The Hh signalling pathway is a cascade involving Hh, Ptch, Smo and Gli. This signalling pathway can effectively regulate developmental processes in mammals, and plays an important role in regulating the development of embryos, cell differentiation, proliferation, tissue polarity, formation of tumour stem cells and so on16, 17. However, the molecular mechanism of this pathway in ovarian cancer is not well understood.
In our previous studies, we identified that blocking the Hh signalling pathway was associated with significantly downregulated CD24 expression in vitro. CD24 is glycoprotein expressed on the surface of most B lymphocytes and differentiating neuroblasts. In neoplasia, the expression of CD24 has not only been describedin haematological malignancies, but also in a large variety of solid tumours, such as renal cell carcinoma, small cell lung cancer, nasopharyngeal carcinoma, hepatocellular carcinoma, bladder carcinoma, glioma and breast cancer17-19. To date, little is known about the role of CD24 in ovarian cancer via Hh signalling.
In the present study, 97 patients with ovarian epithelial cell carcinoma were recruited. Histopathological evaluation and IHC images revealed a significant increase in inflammation and the number of CD24-positivecellsin ovarian serous carcinoma than in normal ovaries or benign ovarian tumours (Fig.1). CD24 expression was negatively associated with patient age, histological type and lymph node metastasis (p>0.05), but was positively associated with the clinical stage and pathological grading (p<0.05; Table 2), indicating that CD24possesses a strong association with ovarian cancer. Moreover, the in vitro and in vivo results confirmed that the upregulation of CD24 expression significantly enhanced cellular migration and invasion of SKOV3 cells (p<0.05; Fig. 2), and downregulation of CD24 expression reduced the growth of tumours compared with the control group (p<0.01; Fig.4).
Many target genes have been found to be regulated by the Hh pathway, however, only few studies in the literatures have aimed to clarify the connection between CD24 and the Hh signalling pathway. In a previous study, we discovered that the adhesion molecule CD24 may be the downstream target gene of Hh signalling using a bioinformatics approach. In the current study, we have confirmed the key role of the Hh signalling pathway on the expression of CD24. In summary, our findings highlight the potential role of CD24 expression in Shh-stimulated cellular migration and invasion, both in vitro and in vivo. We also present a novel molecular mechanism responsible for Hh signalling-mediated ovarian cancer cell migration and invasion via CD24 expression, which may be useful for the treatment of ovarian cancer.
Supplementary Material
Supplementary figures and tables.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81460392, 81503364, 31560264) and the Jiangxi Government (20161ACB20014, 20151BAB205045, 20153BCB23041, 20151BAB205001).
References
- 1.Wang B, Liu SZ, Zheng RS, Zhang F, Chen WQ, Sun XB. Time trends of ovarian cancer incidence in China. Asian Pac J Cancer P. 2014;15(1):191–3. doi: 10.7314/apjcp.2014.15.1.191. [DOI] [PubMed] [Google Scholar]
- 2.Salehi F, Dunfield L, Phillips KP, Krewski D, Vanderhyden BC. Risk factors for ovarian cancer: an overview with emphasis on hormonal factors. J Toxicol Env Heal B. 2008;11(3-4):301–21. doi: 10.1080/10937400701876095. [DOI] [PubMed] [Google Scholar]
- 3.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. Ca-Cancer J Clin. 2011;61(3):183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Yun R, Yu X, Hu H, Huang G, Tan B, Chen T. Overexpression of Notch3 and pS6 Is Associated with Poor Prognosis in Human Ovarian Epithelial Cancer. Mediat Inflamm. 2016; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Xu R, Zeng C, Lu Q, Huang D, Shi C, Zhang W, Deng L, Yan R, Rao H. Down-regulation of Gli transcription factor leads to the inhibition of migration and invasion of ovarian cancer cells via integrin β4-mediated FAK signaling. Plos One. 2014;9(2):5148–52. doi: 10.1371/journal.pone.0088386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi C, Gao G, Luo S. Hedgehog signaling pathway and ovarian cancer. Chinese J Cancer Res. 2013;25(3):346–53. doi: 10.3978/j.issn.1000-9604.2013.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao X, Siu MKY, Au CWH, Wong ESY, Chan HY, Ip PPC, Ngan HYS, Cheung ANY. Aberrant activation of hedgehog signaling pathway in ovarian cancers: Effect on prognosis, cell invasion and differentiation. Carcinogenesis. 2009;30(1):131–40. doi: 10.1093/carcin/bgn230. (10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiyagarajan S, Bhatia N, Reaganshaw S, Cozma D, Thomastikhonenko A, Ahmad N, Spiegelman VS. Role of GLI2 Transcription Factor in Growth and Tumorigenicity of Prostate Cells. Cancer Res. 2007;67(22):10642–6. doi: 10.1158/0008-5472.CAN-07-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingham PW, Mcmahon AP. Hedgehog signaling in animal development: paradigms and principles. Gene Dev. 2001;15(23):3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 10.Ye YZ, Zhang ZH, Fan XY, Xu XL, Chen ML, Chang BW, Zhang YB. Notch3 overexpression associates with poor prognosis in human non-small-cell lung cancer. Med Oncol; 2013. p. 30. (2) [DOI] [PubMed] [Google Scholar]
- 11.Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, Kalantzopoulos G, Tsakalidou E, Mentis A. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microb. 2004;70(1):518. doi: 10.1128/AEM.70.1.518-526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson SM, Wang X, Evers BM. Triptolide inhibits proliferation and migration of colon cancer cells by inhibition of cell cycle regulators and cytokine receptors. J Surg Res. 2011;168(2):197–205. doi: 10.1016/j.jss.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirone G, Perna S, Shukla A, Marfe G. Involvement of Notch-1 in Resistance to Regorafenib in Colon Cancer Cells. J Cell Physiol. 2016;231(5):1097–105. doi: 10.1002/jcp.25206. [DOI] [PubMed] [Google Scholar]
- 14.Yuan X, Wu H, Xu HX, Xiong HH, Chu Q, Yu SY, Wu GS, Wu KM. Notch signaling: An emerging therapeutic target for cancer treatment. Cancer Lett. 2015;369(1):20–7. doi: 10.1016/j.canlet.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 15.Lum L, Beachy PA. The Hedgehog Response Network: Sensors, Switches, and Routers. Science. 2004;304(5678):1755–9. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 16.Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ, Choudhury SA, Maruyama R. The JAK2/STAT3 signaling pathway is required for growth of CD44+ CD24-stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121(7):2723–35. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips TM, McBride WH, Pajonk F. The response of CD24-/low/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer I. 2006;98(24):1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 18.Kristiansen G, Denkert C, Schlüns K, Dahl E, Pilarsky C, Hauptmann S. CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. Am J Pathol. 2002;161(4):1215–21. doi: 10.1016/S0002-9440(10)64398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristiansen G, Winzer K-J, Mayordomo E, Bellach J, Schlüns K, Denkert C, Dahl E, Pilarsky C, Altevogt P, Guski H. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res. 2003;9(13):4906–13. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables.