Abstract
Purpose: To evaluate the prognostic value of magnetic resonance imaging (MRI)-detected tumor residue after intensity-modulated radiation therapy (IMRT) and its association with post-treatment plasma Epstein-Barr virus deoxyribonucleic acid (EBV DNA) in nasopharyngeal carcinoma (NPC).
Methods and materials: A prospective database of patients with histologically-proven NPC was used to retrospectively analyze 664 cases. Pre- and post-treatment MRI scans were independently reviewed by two senior radiologists who were blinded to clinical findings. Factors significantly associated with MRI-detected tumor residue were identified and included in the following multivariate logistic regression model. Residual risk model were established. Receiver operating characteristic (ROC) identify the optimal cut-off risk score for tumor residue.
Results: MRI-detected residual tumor at three months after IMRT was associated with poor prognosis. The 5-year survival rates for the non-residual and residual groups were: OS (93.8% vs. 76.6%, P<0.001), PFS (84.7% vs. 67.9%, P=0.006), LRFS (93.4% vs. 80.4%, P=0.002), and DMFS (90.3% vs. 87.9%, P=0.305), respectively. Three-month post-treatment EBV DNA was significantly associated with tumor residue (P<0.001). A residual risk score model was established, consisting of T and N categories and post-treatment EBV DNA. ROC identified 22.74 as the optimal cut-off risk score for tumor residue. High-risk score was independently associated with poor treatment outcomes.
Conclusions: MRI-detected tumor residue was an independent adverse prognostic factor in NPC; and significantly associated with three-month post-treatment EBV DNA. As limited resources in some endemic areas prevent patients from undergoing routine post-treatment imaging, our study identifies a selection risk-model, providing a cost-effective reference for the selection of follow-up strategies and clinical decision-making.
Keywords: Nasopharyngeal carcinoma, Intensity-modulated radiation therapy, Tumor residue, Magnetic resonance imaging, Plasma EBV DNA
Introduction
Radiotherapy (RT) is the mainstay treatment for nasopharyngeal carcinoma (NPC) 1, a unique head and neck cancer especially prevalent in southern China 2. Compared to conventional techniques, intensity-modulated radiation therapy (IMRT) has improved treatment outcomes and reduced toxicities in NPC due to superior tumor coverage and organs-at-risk sparing 3.
Assessment of tumor response to RT is of great prognostic significance, as early detection of tumor residue enables effective delivery of salvage treatments (e.g. surgery, boosting irradiation) 4, 5. Additionally, residual tumors respond better to salvage than recurrent disease 6. Therefore, it is necessary to evaluate residual tumor status after RT for NPC; questions remain how and when should we evaluate residual tumors in the IMRT era.
Though biopsy is well recognized as the gold standard for the diagnosis of residual tumors, its routine use is not welcome because of its invasive property. Moreover, 69.4% of recurrent lesions occur outside the nasopharynx, and are difficult to be obtained by biopsy 7. In addition, even for the residual lesions in nasopharynx, the chance of single biopsy missing residual tumors is 26.4% 8. Magnetic resonance imaging (MRI) has recently emerged as the preferred modality, and has a high overall accuracy (92.1%) for detecting residual and/or recurrent NPC at the primary tumor site 9.
When to evaluate residual diseases remains inconclusive. He et al. reported that despite the superiority of IMRT, MRI-detected tumor residue at the end of IMRT was prognostic for poor outcomes in locoregionally-advanced NPC 10. However, initiation of additional treatment too early may lead to over-treatment of patients whose residual tumors may undergo spontaneous histologic remission slowly but firmly after a period of time. It is reported that most spontaneous remissions occur within 12 weeks after treatment; late tumor regression (within 12 weeks) did not jeopardize overall local control 8. On this basis, three months after RT is widely regarded as an appropriate time-point for comprehensive clinical evaluation.
To comprehensively explore the influences of MRI-detected tumor residue on NPC patients in the era of IMRT, we conducted a cohort study to explore the prognostic value of MRI-detected tumor residue at three months after IMRT in NPC. Additionally, as Epstein-Barr virus deoxyribonucleic acid (EBV DNA) is clinically assessed to assist risk stratification, prognostication and relapse supervision 11, 12, we also investigated whether plasma EBV DNA at three months after RT was associated with tumor remission.
Materials and Methods
Study population
A nasopharyngeal carcinoma database that was prospectively maintained by the Sun Yat-Sen University Cancer Center (SYSUCC) between November 2009 and February 2012 was retrospectively analyzed. The database recorded information regarding sociodemographic data, pretreatment evaluation, disease extension, treatment administration, and follow-up status (Supplementary Materials). The patients included in this study fulfilled the following criteria: (1) histologically confirmed, non-metastatic NPC without previous malignant disease or second primary tumor, (2) receipt of radical radiotherapy based on IMRT for the whole course, (3) regular follow-up with complete post-treatment examination, including nasopharyngoscopics, plasma EBV DNA, MRI et al., (4) no evidence of distant metastasis during the first three months after treatment, (5) no previous anticancer treatment or adjuvant chemotherapy. A total of 664 patients were eligible in this analysis (Table 1). All patients were restaged according to the 7th edition of the UICC/AJCC staging system. The institutional review board at SYSUCC approved the analysis of the anonymous data.
Table 1.
Clinical characteristics of the 664 patients with nasopharyngeal carcinoma
| Characteristic | Total (n=664) No. (%) |
Residual status | P-value | |
|---|---|---|---|---|
| Residual (n=135) No. (%) |
Non-residual (n=529) No. (%) |
|||
| Age (years) | 0.038 | |||
| ≤45 | 377 (56.8) | 66 (48.9) | 311 (58.8) | |
| >45 | 287 (43.2) | 69 (51.1) | 218 (41.2) | |
| Gender | 0.385 | |||
| Male | 487 (73.3) | 103 (76.3) | 384 (72.6) | |
| Female | 177 (26.7) | 32 (23.7) | 145 (27.4) | |
| Histological type | 0.514 | |||
| I | 5 (0.8) | 2 (1.5) | 3 (0.6) | |
| II | 39 (5.4) | 7 (5.2) | 32 (6.0) | |
| III | 620 (93.4) | 126 (93.3) | 494 (93.4) | |
| T categorya | <0.001 | |||
| T1 | 130 (19.6) | 10 (7.4) | 120 (22.7) | |
| T2 | 110 (16.6) | 15 (11.1) | 95 (18.0) | |
| T3 | 302 (45.4) | 42 (31.1) | 260 (49.1) | |
| T4 | 122 (18.4) | 68 (50.4) | 54 (10.2) | |
| N categorya | <0.001 | |||
| N0 | 113 (17.0) | 11 (8.1) | 102 (19.3) | |
| N1 | 392 (59.0) | 76 (56.3) | 316 (59.7) | |
| N2 | 105 (15.8) | 34 (25.2) | 71 (13.4) | |
| N3 | 54 (8.1) | 14 (10.4) | 40 (7.6) | |
| Stage a | <0.001 | |||
| I | 41 (6.2) | 0 (0.0) | 41 (7.8) | |
| II | 147 (22.1) | 13 (9.6) | 134 (25.3) | |
| III | 310 (46.7) | 43 (31.9) | 267 (50.5) | |
| IV | 166 (25.0) | 79 (58.5) | 87 (16.4) | |
| Chemotherapy | 0.092 | |||
| Yes | 574 (86.4) | 129 (95.6) | 445 (84.1) | |
| No | 90 (13.6) | 6 (4.4) | 84 (15.9) | |
| Post-EBV DNA | <0.001 | |||
| Undetectable | 621 (93.5) | 116 (85.9) | 505 (95.5) | |
| Detectable | 43 (6.5) | 19 (14.1) | 24 (4.5) | |
Abbreviations: post-EBV DNA = three-month post-treatment plasma Epstein-Barr virus deoxyribonucleic acid.
a According to the 7th edition of the American Joint Committee on Cancer.
Imaging protocol
MRI imaging was performed with a 1.5-T system (Signa CV/i; General Electric Healthcare, Chalfont St. Giles, UK). The region from the suprasellar cistern to the inferior margin of the sternal end of the clavicle was examined with a head-and-neck combined coil. The detailed information was presented in Supplementary Material.
Evaluation of tumor residue
Diagnostic criteria of tumor residue was: (1) tumor residue in nasopharynx, other soft tissues or intracranial spaces, represented as hypo-intense signal on T1-weighted imaging, hyper-intense signal on T2-weighted imaging and enhancement following administration of Gd-DTPA; (2) skull base lesions were considered tumor residue if the bone of the skull base was destructed with soft tissues and the degree, and/or scope of bone strengthening had not decrease or increased compared to pre-treatment images13, 14; (3) regional lymph nodes were diagnosed as residual if they had a short-axis diameter > 10 mm for cervical lymph nodes and > 5 mm for retropharyngeal nodes. Pre- and three-month post-treatment MRI scans for each patient were independently reviewed by two senior radiologists specializing in head and neck cancer who were blinded to clinical findings. Any disagreements were resolved by consensus.
Plasma EBV DNA
Plasma EBV DNA was measured by quantitative polymerase chain reaction (PCR) 15, 16. The cut-off point for three-month post-treatment EBV DNA was 0 copies/ml.
Follow-up and study endpoint
Median follow-up was 50.3 (range: 8.9-69.5) months. The primary endpoint was overall survival (OS), defined as the date from start of treatment to the date of death from any cause. The secondary endpoint was progression-free survival (PFS), defined as the date from start of treatment to the date of treatment failure or death from any cause whichever was first. Locoregional failure-free survival (LRFS) defined as the date from start of treatment to the date of first locoregional failure or death from any cause whichever was first. Distant metastasis failure-free survival (DMFS), defined as the date from start of treatment to the date of first remote failure or death from any cause whichever was first.
Statistical analysis
Survival rates were calculated using the Kaplan-Meier method, and compared by log-rank test 17. Multivariate analyses were used to determine hazard ratios and assess independent significance using the adjusted Cox proportional hazards model with backward elimination 18. Host factors (age, gender), tumor factors (T and N categories), and treatment profiles (chemotherapy) were included as covariates.
To establish the risk score model, firstly univariate analyses (i.e. logistic, Chi squared) were initially performed 19. Next, multivariate logistic regression model with backward elimination was used to establish a model for the binary categorical outcome (i.e. residual tumor or not) and explanatory variables selected in step one. Finally, a model for estimation of residual risk was generated as Z = β0+β1X1+β2X2+...βnXn. (The β0 is the constant of regression coefficients, X1...Xn are independent variables in logistic equation, β1...βn the corresponding coefficients. Individual residual risk scores were calculated using: e Z / (e Z + 1) × 100, where e is the base of natural logarithm and Z calculated according to the logistic regression equation 20. Calibration of the model, to evaluate agreement between expected and actual observed outcomes, was performed using the Hosmer-Lemeshow goodness-of-fit test 21. SPSS version 19.0 software (IBM, Armonk, NY, USA) was used for all analysis; two-tailed P < 0.05 was considered statistically significant.
Results
Characteristics of study population
In total, 49/664 (7.4%) patients died and 108/664 (16.3%) experienced treatment failure during the follow-up, including locoregional recurrence in 53/664 (8.0%) and distant metastasis in 61/664 (9.2%) patients. For the entire cohort, the 5-year survival rates were: OS (90.6%), PFS (81.6%), LRFS (91.0%), and DMFS (89.7%).
135/664 (20.3%) had MRI-detected tumor residue at three months after IMRT and 100/135 (74.1%) had more than one residual lesions. 90/135 (66.7%) residual lesions were located in the nasopharynx; 45/135 (33.3%) residual lesions were located out of the nasopharynx. The locations and distributions of residual tumors were presented in Table 2.
Table 2.
Location and distribution of the tumor residues in the 135 patients with MRI-derived residual tumors
| Regions of the residual tumors a | No. (%) |
|---|---|
| A only | 6 (4.4) |
| B only | 8 (6.0) |
| C only | 0 (0) |
| D only | 26 (19.3) |
| A&B | 22 (16.3) |
| A&C | 1 (0.7) |
| A&D | 23 (17.0) |
| B&C | 9(6.7) |
| B&D | 0 (0) |
| C&D | 2 (1.5) |
| A&B&C | 20 (14.8) |
| A&B&D | 6 (4.4) |
| A&C&D | 1 (0.7) |
| B&C&D | 0 (0) |
| A&B&C&D | 11 (8.1) |
a Regions A: nasopharynx, parapharyngeal space and other surrounding soft tissues; B: skull base and infratemporal fossa; C: intracranial region; and D: retropharyngeal lymph nodes and cervical lymph nodes.
Prognostic value of MRI-detected tumor residue
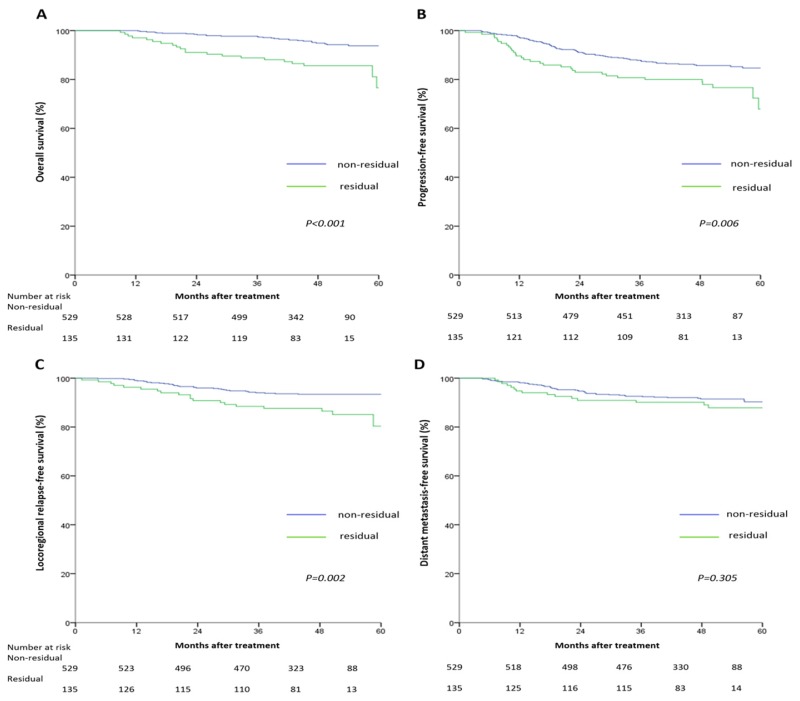
The 5-year survival rates for the non-residual and residual groups were: OS (93.8% vs. 76.6%, P<0.001), PFS (84.7% vs. 67.9%, P=0.006), LRFS (93.4% vs. 80.4%, P=0.002), and DMFS (90.3% vs. 87.9%, P=0.305), respectively. Pairwise comparisons showed OS, PFS and LRFS, but not DFMS, were significantly poorer in the residual tumor group (Figure 1).
Figure 1.
Kaplan-Meier overall survival (A), progression-free survival (B), locoregional relapse-free survival (C), and distant metastasis-free survival (D) curves for the 664 patients with nasopharyngeal carcinoma divided into the residual tumor group and non-residual tumor group. All categories are based on the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer staging system.
Multivariate analyses indicated tumor residue was an independent unfavorable predictor for OS (HR =2.86, 95% CI: 1.62-5.04), PFS (HR =1.61, 95% CI: 1.06-2.44), and LRFS (HR =2.37, 95% CI: 1.35-4.15), but not DMFS, after adjustment (Table 3).
Table 3.
Cox proportional hazards analyses of the prognostic value of tumor residue in the 664 patients with nasopharyngeal carcinoma undergoing IMRT
| Endpoint | Variable | HR | HR (95% CI) | P-valuea |
|---|---|---|---|---|
| OS | Age (>45 vs. ≤45 years) | 2.92 | 1.59-5.36 | 0.001 |
| Residue (yes vs. no) | 2.86 | 1.62-5.04 | <0.001 | |
| PFS | Age (>45 vs. ≤45 years) | 1.59 | 1.09-2.32 | 0.017 |
| N category (N1-3 vs. N0) | 1.94 | 1.01-3.73 | 0.047 | |
| Residue (yes vs. no) | 1.61 | 1.06-2.44 | 0.026 | |
| LRFS | Residue (yes vs. no) | 2.37 | 1.35-4.15 | 0.003 |
| DMFS | Residue (yes vs. no) | NS | --- | --- |
Abbreviations: CI = confidence interval; DMFS = distant metastasis-free survival; HR = hazard ratio; IMRT = intensity-modulated radiotherapy; LRFS = locoregional relapse-free survival; NS = not significant; OS = overall survival.
a The following parameters were included in the Cox proportional hazards model multivariate analysis with backward elimination: age (> 45 vs. ≤ 45 years), gender (female vs. male), T category (T3-4 vs. T1-2), N category (N1-3 vs. N0) and chemotherapy (yes vs. no).
Associations between post-treatment EBV DNA and tumor residue
Univariate logistic analysis indicated three-month post-treatment EBV DNA was significantly associated with tumor residue, and had the highest odds ratio (OR, 3.446; P<0.001; 95% CI: 1.827-6.502) of any significant factor (i.e. age, chemotherapy, T category, N category) identified in univariate analysis. Multivariate logistic regression indicated three-month post-treatment EBV DNA was also independently associated with tumor residue.
Establishment of risk score model
As three-month post-treatment EBV DNA was significantly associated with tumor residue, we established a risk model to indicate tumor residue. The final model identified three-month post-treatment EBV DNA, T and N categories as independent indicators of tumor residue; detailed parameters of the model were reported in Table 4. The Hosmer and Lemeshow goodness-of-fit test showed the final model adequately fitted the data (P=0.658).
Table 4.
Logistic regression analysis of the independent indicators of tumor residue in the 664 patients with nasopharyngeal cancer undergoing IMRT
| Variable | Coefficient | OR | OR (95%CI) | P-value a |
|---|---|---|---|---|
| Post-EBV DNA | 1.536 | 4.65 | 2.27-9.50 | <0.001 |
| T category | ||||
| T1 | Reference | |||
| T2 | 0.588 | 1.80 | 0.75-4.31 | 0.187 |
| T3 | 0.519 | 1.68 | 0.79-3.55 | 0.174 |
| T4 | 2.758 | 15.77 | 7.32-33.98 | <0.001 |
| N category | ||||
| N0 | Reference | |||
| N1 | 0.753 | 2.12 | 1.01-4.45 | 0.046 |
| N2 | 1.475 | 4.37 | 1.92-9.97 | <0.001 |
| N3 | 0.969 | 2.63 | 1.00-6.94 | 0.05 |
| Constant | -3.387 | --- | --- | <0.001 |
Abbreviations: CI = confidence interval; OR = odds ratio; post-EBV DNA= three-month post-treatment Epstein-Barr virus deoxyribonucleic acid; IMRT = intensity-modulated radiotherapy;
a The following parameters were included in the logistic regression analysis with backward elimination: age (> 45 vs. ≤ 45 years), T category, N category and chemotherapy (yes vs. no).
Scoring system with tumor residue
From the logistic regression model, the coefficient Z was calculated as: Z = -3.387 + 1.536 × EBV + 0.588 × T2 + 0.519 × T3 + 2.758 × T4 + 0.753 × N1 + 1.475 × N2 + 0.969 × N3. The value of each parametric variable was 0 (negative) or 1 (positive). Individual risk scores were calculated by the formula: e Z / (e Z + 1) × 100; mean risk score was 17.4 (range, 3-87).
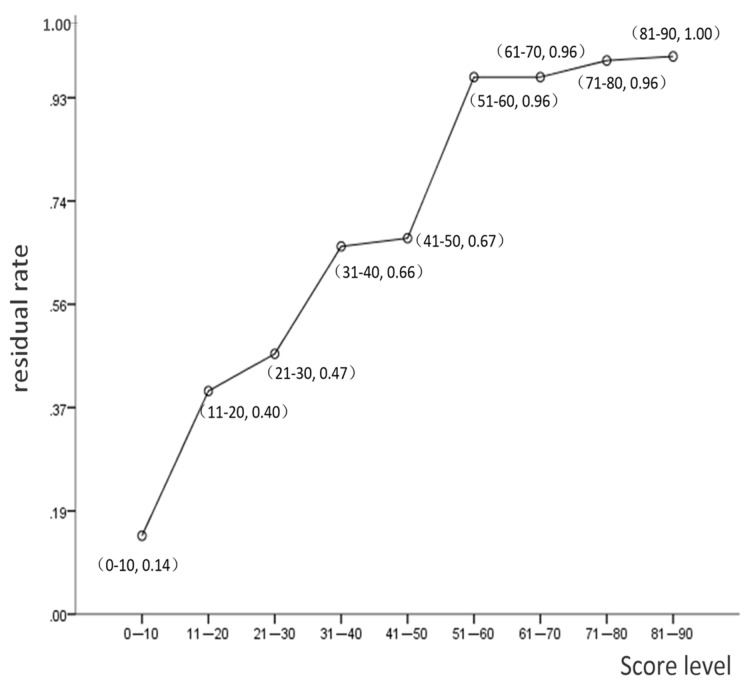
The frequency of tumor residue increased significantly with risk score (Figure 2). Only two patients (of 88; 2.3%) with a risk scores <5 had residual tumors. Receiver operating characteristic (ROC) curve analysis demonstrated 22.74 as the optimal cutoff point with respect to tumor residue (AUC, 0.76; P<0.001; 95% CI: 0.713-0.807). Patients were then separated into two groups, low risk group (risk scores ≤22.74) and high-risk group (risk scores >22.74). In the high-risk group, 60.0% (81/135) patients had tumor residue at three-month after RT compared to only 13.2% (70/529) of the low risk group. The sensitivity, specificity, and accuracy of the risk score model were 60.0%, 86.8%, and 81.3%, respectively.
Figure 2.
Linear distribution of the residual tumor rate according to risk score.
Prognostic value of risk score model
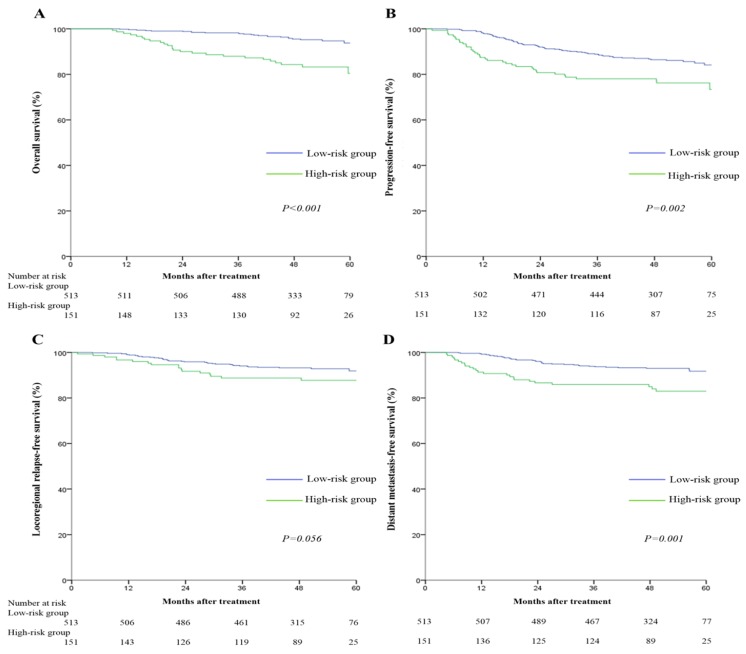
Since tumor residue is a major adverse prognostic factor for clinical outcomes, we analyzed the relationship between risk score model and treatment outcomes of NPC patients. The 5-year survival rates for the low-risk and high-risk groups were: OS (93.8% vs. 80.4%, P<0.001), PFS (84.1% vs. 73.4%, P=0.002), LRFS (91.9% vs. 87.7%, P=0.056), and DMFS (91.7% vs. 83.0%, P=0.001) respectively. Pairwise comparisons showed OS, PFS and DMFS were significantly poorer for the high-risk score group (Figure 3).
Figure 3.
Kaplan-Meier overall survival (A), progression-free survival (B), locoregional relapse-free survival (C), and distant metastasis-free survival (D) curves for the 664 patients with nasopharyngeal carcinoma divided into high-risk and low-risk groups. All categories are based on the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer staging system. The cut-off value for the high-risk group and low-risk group was 22.74.
Multivariate analyses indicated high-risk score remained an independent adverse predictor for OS (HR =3.50, 95% CI: 1.99-6.13), PFS (HR =1.73, 95% CI: 1.16-2.59), and DMFS (HR =2.41, 95% CI: 1.44-4.02; Table 5), but not LRFS, after adjustment.
Table 5.
Cox proportional hazards analyses of the prognostic value of risk score in the 664 patients with nasopharyngeal cancer undergoing IMRT
| Endpoint | Variable | HR | HR (95%CI) | P-value a |
|---|---|---|---|---|
| OS | Age (>45 vs. ≤45 years) | 2.87 | 1.56-5.27 | 0.001 |
| Risk score b (high vs. low) | 3.50 | 1.99-6.13 | <0.001 | |
| PFS | Age (>45 vs. ≤45 years) | 1.58 | 1.08-2.32 | 0.018 |
| N category (N1-3 vs. N0) | 1.93 | 1.06-3.72 | 0.048 | |
| Risk score b (high vs. low) | 1.73 | 1.16-2.59 | 0.008 | |
| LRFS | Risk score b (high vs. low) | NS | --- | --- |
| DMFS | Risk score b (high vs. low) | 2.41 | 1.44-4.02 | 0.001 |
Abbreviations: CI = confidence interval; DMFS = distant metastasis-free survival; HR = hazard ratio; IMRT = intensity-modulated radiotherapy; LRFS = locoregional relapse-free survival; NS = not significant; OS = overall survival.
a The following parameters were included in the Cox proportional hazards model multivariate analysis with backward elimination: age (> 45 vs. ≤ 45 years), gender (female vs. male), T category (T3-4 vs. T1-2), N category (N1-3 vs. N0) and chemotherapy (yes vs. no).
b High-risk score, >22.74; low-risk score, ≤22.74
Discussion
IMRT has proven advantages of better tumor coverage and toxicity profiles in NPC 22. However, the features and prognostic values of tumor response in patients undergoing IMRT remained inconclusive. To the best of our knowledge, this is the first and largest study to investigate MRI-detected tumor residue at three months after IMRT and its association with post-treatment plasma EBV DNA in NPC.
In this study, tumor residue at three months after IMRT was identified as an independently unfavorable prognostic factor in NPC. Moreover, three-month post-treatment EBV DNA was significantly associated with tumor residue. Then we moved one step further to establish a risk score model indicating tumor residue at three months after IMRT, which provide a cost-effective reference for oncologists to evaluate tumor response to IMRT.
Clinically, it is essential for oncologists to evaluate tumor response to RT. Three factors need to be considered: the selection of evaluating approaches, time course of tumor remission, and the cost-effectiveness of evaluations.
Firstly, oncologists should decide the optimal evaluation modality. In this cohort of 664 patients with NPC, only 4.4% of residual lesions were restricted to nasopharynx, parapharyngeal space and/or other surrounding soft tissues, and 19.3% in cervical lymph nodes; the remaining residual lesions were in areas not easily accessible by biopsy (skull base, intracranial areas, retropharyngeal lymph nodes, etc.). Thus, imaging tools (i.e. MRI, PET-CT) are more suitable for comprehensive evaluation of residual tumors. Though PET-CT has a high specificity of 93.4% and is less influenced by RT-induced inflammation 23, there is a trend towards greater overall accuracy for MRI over PET-CT in detection of residual and/or recurrent NPC 9. In addition, due to physiologically high FDG uptake by the brain, PET-CT cannot easily detect intracranial/perineural/pterygopalatine-fossa invasion. Thus, MRI would be the optimal choice to comprehensively evaluate residual tumors in NPC.
The second issue is the optimal evaluation time-point. As the time-course of tumor remission remains controversial, some researchers tend to assess residual status at the end of treatment. He et al. 10 reported MRI-detected tumor residue after completion of IMRT was a significant negative prognostic factor for survival. Other researchers favor evaluations at 3-6 months after treatment. Lin et al. 24 revealed a strong correlation between recurrence and tumor residue at 3-6 months after RT. Clinically, initiation of additional treatment too early may result in over-treatment and unnecessary side effects in patients who may achieve spontaneous histologic remission later. Conversely, delaying salvage treatment too long may carry potential risks, as radioresistant cancer stem cells could repopulate if true persistent disease remained 25. According to Kwong et al. 8 70.4% of patients with positive biopsies at completion of RT achieved complete remission within 12 weeks; the number of patients who achieved spontaneous remission after the twelfth week was very low; in addition, delayed remission before twelve weeks was not prognostic for poor survival. Thus, it was reasonable to set the evaluation time-point at three months after RT. In this study, 20.3% of patients had tumor residue at three months after RT, similarly to studies by Hong et al. 26 (17.2%) and Liu et al. 27 (21.6%); these slight differences may be due to multiple factors including differences in clinical staging, radiation technologies, therapeutic regimens etc.
Last but not least, the cost-effectiveness of evaluation methods should be taken into account, as limited resources in some areas may prevent patients from undergoing routine post treatment imaging (e.g. MRI or PET/CT) in NPC endemic regions. Plasma EBV DNA was found to be a non-invasive and economic approach that offered important information in tumor burden, treatment failures and prognosis in NPC 11. We wondered whether this convenient examination could provide any hints on tumor residue. Interestingly, detectable three-month post-treatment EBV DNA was significantly associated with the presence of tumor residue, and served as the strongest indicator of all clinical variables. The possible reasons for the strong correlation between the post-treatment EBV DNA and tumor residue are that plasma EBV DNA level represents tumor DNA level, and can originate from necrotic or apoptotic cancer cells. The presence of viral DNA in viral-related tumors offers a distinct marker for detection in the blood; thus, patients with detectable EBV DNA levels after treatment are prone to have residue tumors and experience local and distant failure. As three-month post-treatment EBV DNA was highly effective, furthermore we established a risk score model consisting of three-month post-treatment EBV DNA, T and N categories to indicate the presence of tumor residue. The model had an overall accuracy of 81.3%, and thus represents a non-invasive and cost-effective decision-making guide for post-RT evaluations. Based on this model, it is reasonable to recommend low-risk patients with possible RT-induced inflammation undergo less invasive examinations (observation, nasopharyngoscopy and/or MRI). Conversely, MRI, PET-CT, and/or histologic biopsy could be strongly recommended for the high-risk group. In addition, as high-risk score is an unfavorable prognostic factor for survival, it could also be used to guide post-RT therapeutic regimens. Additional chemotherapy, surgery and/or boosting irradiation could possibly be recommended as timely salvage treatments for the high-risk group, whereas the low-risk group may not gain much benefit from further interventions. Clinical trials are warranted to help improve treatment outcomes in NPC.
There are some limitations need to be addressed. MRI observations could be subjective compared to biopsy, and some RT-induced inflammation persisted at three months can be difficult to confirm without pathological verification. However, to minimize potential bias, two experienced radiologists independently assessed all images with disagreements resolved by consensus.
In conclusion, the presence of MRI-detected tumor residue at three months after IMRT was a significant adverse prognostic factor for survival in NPC, and significantly associated with post-treatment EBV DNA. The established risk score model consisting of EBV DNA, T and N categories identifies as a selection criterion for post-treatment imaging. As limited resources in some areas prevent patients from undergoing routine post treatment imaging in NPC endemic regions, our study may provide a valuable reference for accurate post-treatment evaluation and clinical decision-making to improve treatment outcomes in patients with NPC.
Supplementary Material
Supplementary methods.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81372409), the Sun Yat-Sen University Clinical Research 5010 Program (No. 2012011), the Science and Technology project of Guangzhou City, China (No. 132000507), National Natural Science Foundation of China (No. 81402532). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhang LF, Li YH, Xie SH, Ling W, Chen SH, Liu Q. et al. Incidence trend of nasopharyngeal carcinoma from 1987 to 2011 in Sihui County, Guangdong Province, South China: an age-period-cohort analysis. Chinese journal of cancer. 2015;37:15. doi: 10.1186/s40880-015-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee AW, Ma BB, Ng WT, Chan AT. Management of Nasopharyngeal Carcinoma: Current Practice and Future Perspective. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:3356–64. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 3.Wolden SL, Chen WC, Pfister DG, Kraus DH, Berry SL, Zelefsky MJ. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: update of the Memorial Sloan-Kettering experience. International journal of radiation oncology, biology, physics. 2006;64:57–62. doi: 10.1016/j.ijrobp.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 4.Teo P, Leung SF, Choi P, Lee WY, Johnson PJ. Afterloading radiotherapy for local persistence of nasopharyngeal carcinoma. The British journal of radiology. 1994;67:181–5. doi: 10.1259/0007-1285-67-794-181. [DOI] [PubMed] [Google Scholar]
- 5.Wei WI, Ho CM, Yuen PW, Fung CF, Sham JS, Lam KH. Maxillary swing approach for resection of tumors in and around the nasopharynx. Archives of otolaryngology-head & neck surgery. 1995;121:638–42. doi: 10.1001/archotol.1995.01890060036007. [DOI] [PubMed] [Google Scholar]
- 6.Kwong DL, Wei WI, Cheng AC, Choy DT, Lo AT, Wu PM. et al. Long term results of radioactive gold grain implantation for the treatment of persistent and recurrent nasopharyngeal carcinoma. Cancer. 2001;91:1105–13. [PubMed] [Google Scholar]
- 7.Ng SH, Chang JT, Ko SF, Wan YL, Tang LM, Chen WC. MRI in recurrent nasopharyngeal carcinoma. Neuroradiology. 1999;41:855–62. doi: 10.1007/s002340050857. [DOI] [PubMed] [Google Scholar]
- 8.Kwong DL, Nicholls J, Wei WI, Chua DT, Sham JS, Yuen PW. et al. The time course of histologic remission after treatment of patients with nasopharyngeal carcinoma. Cancer. 1999;85:1446–53. doi: 10.1002/(sici)1097-0142(19990401)85:7<1446::aid-cncr4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Comoretto M, Balestreri L, Borsatti E, Cimitan M, Franchin G, Lise M. Detection and restaging of residual and/or recurrent nasopharyngeal carcinoma after chemotherapy and radiation therapy: comparison of MR imaging and FDG PET/CT. Radiology. 2008;249:203–11. doi: 10.1148/radiol.2491071753. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Zhou Q, Shen L, Zhao Y, Lei M, Wei R. et al. A retrospective study of the prognostic value of MRI-derived residual tumors at the end of intensity-modulated radiotherapy in 358 patients with locally-advanced nasopharyngeal carcinoma. Radiation oncology. 2015;10:89. doi: 10.1186/s13014-015-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS. et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. The New England journal of medicine. 2004;350:2461–70. doi: 10.1056/NEJMoa032260. [DOI] [PubMed] [Google Scholar]
- 12.Chan KC. Plasma Epstein-Barr virus DNA as a biomarker for nasopharyngeal carcinoma. Chinese journal of cancer. 2014;33:598–603. doi: 10.5732/cjc.014.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng SH, Chan SC, Yen TC, Liao CT, Chang JT, Ko SF. et al. Comprehensive imaging of residual/ recurrent nasopharyngeal carcinoma using whole-body MRI at 3 T compared with FDG-PET-CT. European radiology. 2010;20:2229–40. doi: 10.1007/s00330-010-1784-9. [DOI] [PubMed] [Google Scholar]
- 14.Liang FY, Sun W, Han P, Lu X, Lian YN, Huang XM. Detecting plasma Epstein-Barr virus DNA to diagnose postradiation nasopharyngeal skull base lesions in nasopharyngeal carcinoma patients: a prospective study. Chinese journal of cancer. 2012;31:142–9. doi: 10.5732/cjc.011.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo YM, Chan LY, Lo KW, Leung SF, Zhang J, Chan AT. et al. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer research. 1999;59:1188–91. [PubMed] [Google Scholar]
- 16.Shao JY, Zhang Y, Li YH, Gao HY, Feng HX, Wu QL. et al. Comparison of Epstein-Barr virus DNA level in plasma, peripheral blood cell and tumor tissue in nasopharyngeal carcinoma. Anticancer research. 2004;24:4059–66. [PubMed] [Google Scholar]
- 17.Hu XJ, Lagakos SW. Nonparametric estimation of the mean function of a stochastic process with missing observations. Lifetime data analysis. 2007;13:51–73. doi: 10.1007/s10985-006-9030-0. [DOI] [PubMed] [Google Scholar]
- 18.Gill RD. Multistate life-tables and regression models. Mathematical population studies. 1992;3:259–76. doi: 10.1080/08898489209525345. [DOI] [PubMed] [Google Scholar]
- 19.Marrelli D, De Stefano A, de Manzoni G, Morgagni P, Di Leo A, Roviello F. Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Annals of surgery. 2005;241:247–55. doi: 10.1097/01.sla.0000152019.14741.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Ye F, Wang D, Sun X, Tong W, Lian G. et al. Protein predictive signatures for lymph node metastasis of gastric cancer. International journal of cancer Journal international du cancer. 2013;132:1851–9. doi: 10.1002/ijc.27864. [DOI] [PubMed] [Google Scholar]
- 21.Fagerland MW, Hosmer DW. A goodness-of-fit test for the proportional odds regression model. Statistics in medicine. 2013;32:2235–49. doi: 10.1002/sim.5645. [DOI] [PubMed] [Google Scholar]
- 22.Lee N, Xia P, Quivey JM, Sultanem K, Poon I, Akazawa C. et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. International journal of radiation oncology, biology, physics. 2002;53:12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 23.Yen RF, Hung RL, Pan MH, Wang YH, Huang KM, Lui LT. et al. 18-fluoro-2-deoxyglucose positron emission tomography in detecting residual/recurrent nasopharyngeal carcinomas and comparison with magnetic resonance imaging. Cancer. 2003;98:283–7. doi: 10.1002/cncr.11519. [DOI] [PubMed] [Google Scholar]
- 24.Lin GW, Wang LX, Ji M, Qian HZ. The use of MR imaging to detect residual versus recurrent nasopharyngeal carcinoma following treatment with radiation therapy. European journal of radiology. 2013;82:2240–6. doi: 10.1016/j.ejrad.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Deng CC, Liang Y, Wu MS, Feng FT, Hu WR, Chen LZ. et al. Nigericin selectively targets cancer stem cells in nasopharyngeal carcinoma. The international journal of biochemistry & cell biology. 2013;45:1997–2006. doi: 10.1016/j.biocel.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Hong J, Yao Y, Zhang Y, Tang T, Zhang H, Bao D. et al. Value of magnetic resonance diffusion-weighted imaging for the prediction of radiosensitivity in nasopharyngeal carcinoma. Otolaryngology-head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2013;149:707–13. doi: 10.1177/0194599813496537. [DOI] [PubMed] [Google Scholar]
- 27.Liu LT, Tang LQ, Chen QY, Zhang L, Guo SS, Guo L. et al. The Prognostic Value of Plasma Epstein-Barr Viral DNA and Tumor Response to Neoadjuvant Chemotherapy in Advanced-Stage Nasopharyngeal Carcinoma. International journal of radiation oncology, biology, physics. 2015;93:862–9. doi: 10.1016/j.ijrobp.2015.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods.