Abstract
Aberrant fucosylation plays a functional role in regulating ontogeny and celluar differentiation and are differentially regulated in cancerous condition, which could provide hallmarks for cancer diagnostics and surveillance. We previously developed a magnetic beads-based lectin ELISA system to measure fucosylated haptoglobin (Hp), which has been reported to be a cancer biomarker through a series of glycoproteomic analysis. In this study, serum fucosylated Hp ratios were measured using our ELISA kit in a separate cohort of 260 patients independently, including 130 healthy controls and 130 patients with hepatocellular carcinoma (HCC). Fucosylated Hp /Hp ratio (levels of fucosylated Hp /levels of protein Hp) and ELISA Index (OD value of fucosylated Hp /OD value of protein Hp) were calculated respectively to reflect Hp fucosylation level on its protein level. Our data showed that fucosylated Hp /Hp ratio (AUC=0.8449) and ELISA Index (AUC=0.8581) had better performance in distinguishing HCC from controls, which indicated that fucosylated Hp ratios could improve the diagnosis and prediction of HCC even with a low level of alpha-fetoprotein (AFP). Additionally, the combination analysis of AFP and fucosylated Hp ratios increased the AUC value for HCC diagnosis.
Keywords: biomarker, fucosylation, haptoglobin, hepatocellular carcinoma.
Introduction
According to the International Agency for Research on Cancer, liver cancer caused 745,500 deaths worldwide during 2012, with half of the total happening in China 1. HCC is the most (70% to 90%) common form of liver cancer with the characteristics of high morbidity and mortality as well as the poor prognosis, which could be induced by multiple etiologies and influenced many risk factors, such as chronic hepatitis B virus (HBV) infection 2, 3. Current surveillance and diagnosis of HCC in high risk population is based on ultrasound and AFP 4. Unfortunately, ultrasound surveillance even performed every three months cannot improve detection of small HCC, on account of limitations in recall procedures 5. As the most commonly used serum biomarker, AFP exists controversies regarding its clinical usefulness for HCC, because of lacking sufficient sensitivity and specificity 4. In order to guide the optimal management and to improve the prognosis as well as the survival rate of HCC patients, it is extremely urgently to discover and validate novel candidate biomarkers for the preclinical prediction and diagnosis of HCC.
Glycosylation is one of post-translation modifications with acting as a key regulatory mechanism controlling several biological processes; it is involved in critical cancer cell processes, such as cell signaling, cellular differentiation and invasion, ligand-receptor interactions and metastasis formation 6. Accumulating evidence suggests that altered glycosylation in serum protein is a frequent event during tumor development and progression, serving as promising biomarkers and providing specific targets for therapeutic intervention. Several typical representatives of glycobiomarkers have been reported to diagnose cancer, such as AFP-L3 (a core fucosylated glycoform) 7, α1-acid glycoprotein (AGP) 8, α1-antitrypsin 9, Hp 10, 11, etc.
In previous study, we demonstrated that various types of fucosylated structure on Hp were increased significantly in serum from HCC patients by lectin blot, high performance liquid chromatography (HPLC), as well as mass spectrometer (MS) 12-14. Recently, we developed an ELISA kit based on Aleuria Aurantia Lectin coated magnetic beads (AAL@Magbeads) to analyze fucosylation changes of Hp from serum samples, which based on the differential binding of Hp to AAL@Magbeads 15. With this method, serum fucosylated Hp could be detected rapidly and sensitively. In this study, both Hp protein level and fucosylated Hp were taken into consideration and we confirmed that fucosylated Hp /Hp ratio and ELISA Index could improve the diagnosis and prediction of HCC even with a low level of AFP. Combination of clinically used biomarker AFP and fucosylated Hp ratios had better performance in distinguishing HCC from controls.
Materials and Methods
Serum Specimens
One hundred thirty HCC patients and 130 healthy volunteers were enrolled in this study. All HCC patients were infected with HBV and were treated at the First Affiliated Hospital of Guangxi Medical University (Nanning, China). The patients who were co-infected with hepatitis C virus (HCV) were excluded from this study. All experiments in this study were performed in accordance with the Declaration of Helsinki. Serum specimens from all patients were collected at the First Affiliated Hospital of Guangxi Medical University. Informed consent was obtained from each patient and this study was approved by the Research Ethics committee of First Affiliated Hospital of Guangxi Medical University, approval number: 2016(KE-Y- 035). The specimens were processed using the standard protocol and stored in aliquots at -80 °C until used.
Detection of Serum Fucosylated Hp
The serum fucosylated Hp levels were determined using our AAL@Magbeads based ELISA system 15. AAL (Vector, Burlingame, CA) coupling to COOH-Magbeads (1 μm diameter particle size, BioCanal Scientific, Jiangsu, China) was performed according to manufacturer's protocol, and the beads were blocked with phosphate buffer saline (PBS) containing 2% bovine serum albumin (BSA) and 2% glycine for four hours. After washing three times with PBST (0.05% Tween20 in PBS, pH 7.2-7.4), AAL@Magbeads were diluted (1 mg/mL) in PBS before use. A 96-well ELISA microplate (Koma biotech, Korea) was blocked with 3% BSA in PBS for one hour. One hundred microliters of AAL@Magbeads was added to the pre-blocked microplate, discarding the supernatant. A concentration gradient (0, 0.5, 1, 1.5, 2, 3, 4, 5 μg) of commercial Hp which was purified from human plasma (Calbiochem, San Diego, CA) was used to establish a standard curve. One hundred microliters of 50-fold diluted serum samples in binding buffer (0.1 M Tris-HCl, 0.15 M NaCl, 1 mM CaCl2, 1 mM MgCl2, pH 7.5) were incubated with AAL@Magbeads for one hour. After washing with PBST, HRP-conjugated antihaptoglobin (R&D system, USA) was applied to each well and incubated for one hour. TMB working solution was added, followed by stop solution. After sufficient color development (15 minutes), the optical density (OD) values were read with a microplate reader at wavelength of 450 nm.
Determination of Total Hp and AFP
The protein abundances of Hp were measured using Human Hp Quantikine ELISA kits (R&D system, USA). A standard curve was generated by Hp standard (3.13, 6.25, 12.5, 25, 50, 100, 200 ng/mL) from this kit. Twenty microliters of 20,000-fold diluted serum from each sample enrolled in the study was examined according to the manufacturer's protocol. The serum AFP level was determined using an electrochemiluminescence immunoassay (ECLIA) system.
Statistical Analysis
All statistical analyses were performed with the SPSS 20.0. Statistical comparisons were determined using analysis of variance (ANOVA) and Wilcoxon rank-sum test. Receive operating characteristic (ROC) curves were generated by sensitivity and 1-specificity. The cutoff was determined as the point in ROC that maximizes the value of sensitivity plus specificity. The value of area under the ROC curve (AUC) was calculated as an indication of the accuracy prediction. Diagnostic models were obtained by binary logistic regression. (Two-tailed) P-value below 0.05 was assumed as statistically significant.
Results
Patients' Characteristics
In this study, 130 patients with HCC and 130 normal controls were used. All the HCC patients were associated with HBV infection and were diagnosed based on histopathology, laboratory and imaging data. Healthy volunteers were characterized by the presence of normal liver biochemistry and no history of liver disease or alcohol abuse. The clinical data of the patients were provided in Table 1.
Table 1.
Characteristics of healthy controls and HCC patients
| Group | N | HCC |
|---|---|---|
| Number | 130 | 130 |
| Gender, female/male | 23/107 | 13/117 |
| Age, year | 46.5 ± 8.1 | 50.0 ± 10.3 |
| HbsAg+, % | 0 | 100 |
| TP, g/L | 74.1 (61.3-88.7) | 66.1 (36.9-85.3) |
| Alb, g/L | 57.5 (45.9-76.8) | 35.7 (16.0-50.1) |
| ALT, U/L | 21.3 (11-40) | 67.7 (13-722) |
| AST, U/L | 19.3 (14-38) | 64.6 (12-924) |
| GGT, U/L | 27.4 (19-46) | 151.4 (16-1280) |
| Tbil, µmol/L | 11.5 (6.7-19.8) | 24.1 (5.7-226.6) |
| AFP, ng/mL | 5.2 (2.0-12.8) | 1700.5 (1.7-36827.0) |
The values supplied in Table 1 were means with SD or range.
AFP was determined using electrochemiluminescence immunoassay (ECLIA) system.
Abbreviation: AFP, α-fetoprotein; Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transpeptidase; Tbil, total bilirubine; TP, total protein.
Measurement of Fucosylated Hp Ratios in Healthy Controls and HCC Patients
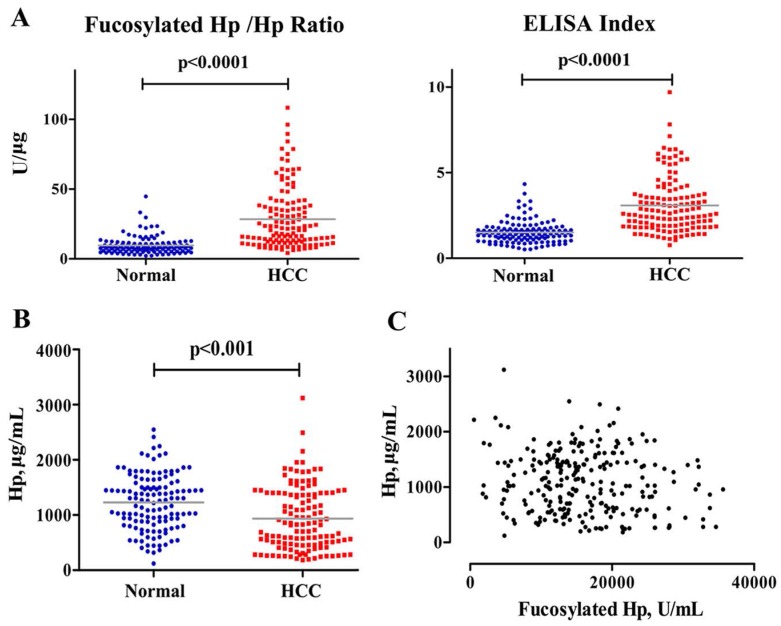
Before serum samples measurement, standard curves were generated for quantifications of Hp and fucosylated Hp with R2 values of 1.000 and 0.998, respectively (data not shown). Furthermore, fucosylated Hp and protein Hp were assayed using the same serum specimens simultaneously. As shown in Fig 1, Hp was decreased in HCC patients compared to levels in normal subjects (p<0.001). Additionally, there was no correlation between Hp and fucosylated Hp levels, suggesting that generation mechanism of them may be different. Fucosylated Hp /Hp ratio was calculated by using the content of individual fucosylated Hp against Hp protein value; ELISA Index was calculated as OD value of fucosylated Hp, divided by OD value of protein Hp, both of which could better reflect Hp fucosylation status on its protein level. Fucosylated Hp /Hp ratio was 28.36±21.79 U/μg in HCC patients and 9.77±7.07 U/μg in normal controls; ELISA index was 3.08±1.60 in patients suffering HCC and 1.51±0.64 in normal controls. Both fucosylated Hp /Hp ratio and ELISA index were significantly increased in HCC patients compared to that in normal controls (p<0.0001).
Fig 1.
Measurements of serum fucosylated Hp ratios and Hp in HCC cases and normal controls. Fucosylated Hp /Hp ratio, ELISA Index (A) and serum Hp levels (B) in HCC patients and normal controls. In each panel, the gray bars indicate mean values. (C) Fucosylated Hp and Hp protein levels were compared in 130 patients with HCC and 130 normal controls.
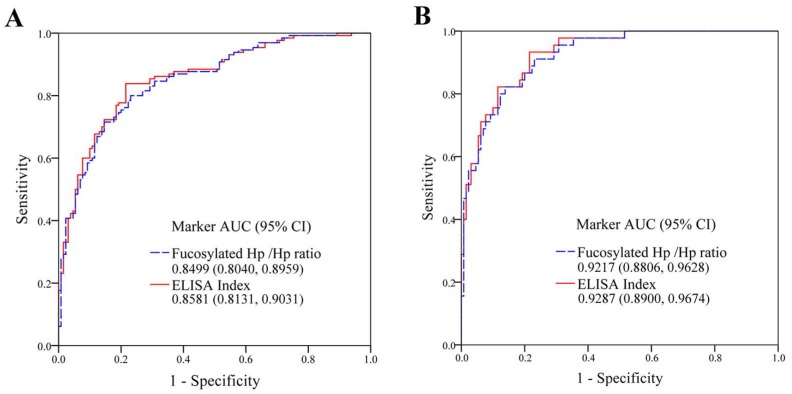
We analyzed serum AFP, fucosylated Hp /Hp ratio (levels of fucosylated Hp /levels of protein Hp) and ELISA Index (OD value of fucosylated Hp /OD value of protein Hp) in the discrimination of HCC from healthy subjects by ROC curves (Fig 2A). For healthy controls and total HCC patients, AUC of fucosylated Hp/Hp ratio was 0.8499 with 81.54% of sensitivity, 80.00% of specificity and 80.76% of accuracy. ELISA Index determined an AUC of 0.8581 with sensitivity of 83.85%, specificity of 80.77% and accuracy of 82.31%. Combination analysis was summarized in Table 2. The predictive models of fucosylated Hp /Hp ratio (1) and ELISA Index (2) for distinguishing HCC from healthy controls were as follow:
Fig 2.
The diagnostic abilities of fucosylated Hp ratios for HCC. (A) ROC curves of fucosylated Hp /Hp ratio and ELISA Index, as the detection of 130 healthy controls and with respect to 130 HCC patients (95% confidence interval, CI). (B) ROC curves of fucosylated Hp /Hp ratio and ELISA Index, as the detection of 130 healthy controls and with respect to 45 AFP-negative HCC patients (95% CI).
Table 2.
Diagnostic values of biomarkers in differentiating HCC from healthy controls
| Biomarkers | AUC | Sensitivity | Specificity | Accuracy | Cut-off |
|---|---|---|---|---|---|
| AFP (ng/mL) | 0.8055 | 66.92% | 89.23% | 79.23% | 13.96 |
| Fucosylated Hp /Hp Ratio (U/μg) | 0.8499 | 81.54% | 80.00% | 80.76% | 10.83 |
| ELISA Index | 0.8581 | 83.85% | 80.77% | 82.73% | 1.78 |
| Fucosylated Hp /Hp Ratio +AFP | 0.9612 | 89.23% | 99.23% | 94.62% | — |
| ELISA Index +AFP | 0.9672 | 90.00% | 99.23% | 94.62% | — |
AUC, area under the receive operating characteristic curve.
| P=exp(-2.327+0.160*Fucosylated Hp /Hp Ratio) /[1+exp(-2.327+0.160*Fucosylated Hp /Hp Ratio)] | (1) |
| P=exp(-3.468+1.709*ELISA Index) /[1+ exp(-3.468+1.709*ELISA Index)] | (2) |
Fucosylated Hp Ratios in HCC Patients with Low AFP Level
Considering different AFP levels, patients with HCC were divided into two groups, which included 85 AFP-positive (AFP+, >20 ng/mL) cases and 45 AFP-negative (AFP-, ≤20 ng/mL) cases. The average age of AFP-HCC patients was 49.6±8.9 years (ranged from 34 to 67 years), with the average level of serum AFP was 5.5±3.7 ng/mL (ranged from 1.7 to 18.8 ng/mL) and CEA was 3.4±5.0 ng/mL (ranged from 0.9 to 33.9 ng/mL), as well as HBV DNA was 4.8*105 copy/mL (ranged from 5.0*103 to 5.9*106 copy/mL). Moreover, average levels of serum AST, ALT, GGT and Alb were 55.0±41.4 U/L, 47.1±43.4 U/L, 126.8±112.5 U/L and 36.3±4.9 g/L, respectively.
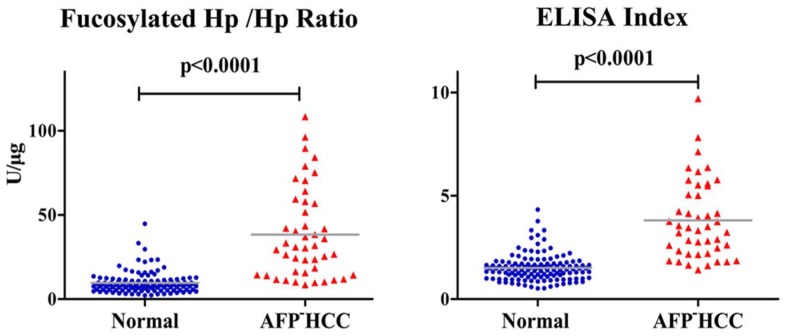
In AFP-HCC patients, average value of fucosylated Hp /Hp ratio and ELISA index was 35.79±24.88 U/μg and 3.81±1.88, respectively, showing an obvious increase compared to normal controls (p<0.0001, Fig 3).
Fig 3.
Fucosylated Hp ratios in normal controls and AFP-negative HCC cases. Serum fucosylated Hp /Hp ratio and ELISA Index in AFP-negative HCC patients and normal controls. In each panel, the gray bars indicate mean values.
ROC analysis was constructed for diagnostic potential evaluation of fucosylated Hp /Hp ratio and ELISA Index for diagnosis AFP-HCC. As shown in Fig 2B, between 45 AFP-HCC cases and 130 normal volunteers, fucosylated Hp /Hp ratio had an AUC of 0.9217 with sensitivity of 91.11% and specificity of 79.23% as well as accuracy of 82.29%; ELISA Index exhibited an AUC of 0.9287 with sensitivity of 93.33% and specificity of 80.77% as well as accuracy of 84.00%, indicating both fucosylated Hp /Hp ratio and ELISA Index could serve as useful glycobiomarkers for AFP-HCC diagnosis. The clinical diagnosis potential of fucosylated Hp /Hp ratio and ELISA Index was shown in Table 3.
Table 3.
Diagnostic values of fucosylated Hp ratios in differentiating AFP-negative HCC patients from healthy controls
| Biomarkers | AUC | Sensitivity | Specificity | Accuracy | Cut-off |
|---|---|---|---|---|---|
| Fucosylated Hp /Hp Ratio (U/μg) | 0.9217 | 91.11% | 79.23% | 82.29% | 10.97 |
| ELISA Index | 0.9287 | 93.33% | 80.77% | 84.00% | 1.79 |
AUC, area under the receive operating characteristic curve.
Discussion
In this study, serum fucosylated Hp levels were significantly increased in HCC patients, indicating its potential utility as a predictive biomarker of HCC. However, fucosylated Hp and total Hp levels were not correlated, suggesting that the production of fucosylated Hp in HCC is not dependent on Hp levels. Considering protein abundance variation, fucosylated Hp /Hp ratio and ELISA Index were established to reflect Hp fucosylation status on its protein level. This study demonstrated that fucosylated Hp /Hp ratio (AUC=0.8449) and ELISA Index (AUC=0.8581) were achieved better performance as predictive hallmarks of HCC when compared with fucosylated Hp alone reported in our previous study 15, which could improve the predictive power and might provide additional information in the distinction of HCC. Also, this potential utility of the fucosylated Hp /Hp ratio and ELISA Index were superior to AFP. Combined with AFP, fucosylated Hp /Hp ratio (AUC=0.9612) and ELISA Index (AUC=0.9672) improve the sensitivity and specificity of HCC diagnosis. In general, AFP level greater than 400 ng/mL has been considered as a serum indicator for HCC; however, only a portion of HCC patients could observe such a high level of AFP and some patients were suffering from HCC even with a low level of AFP (≤20 ng/mL). Furthermore, it was reported that high levels of AFP signified highly malignant tumor and unfavorable prognosis 16, and AFP-HCC patients had a significantly better survival than HCC patients with high AFP levels 17, 18. A novel candidate biomarker for the preclinical prediction and diagnosis of HCC, especially AFP-HCC at early stage, is desperately needed in order to improve the prognosis and increase survival rate. In this study, increased fucosylated Hp level was also observed in AFP-HCC patients, and the detailed analysis of the fucosylation status of Hp was yielding novel candidate indicators for HCC, such as fucosylated Hp /Hp ratio (AUC=0.9217) and ELISA Index (AUC=0.9287). It is suggested that hepatopathic patients should measure serum AFP and fucosylated Hp ratios regularly and quantitatively for supervising and identifying the progression of liver disease as early as possible, which is significant in evaluating the prognosis of the patients. Ultrasound imaging and even biopsy should be applied to hepatopath with low AFP level and high fucosylated Hp ratios for further diagnosis and confirmation.
In the decades, tremendous efforts have been focused on the discovery and verification of novel biomarkers to improve cancer detection. However, most of these hallmarks could be detected in various cancers, instead of in one certain cancer. For instance, the Tn (GalNAc-Ser/Thr) and STn (Sialyl-Tn, NeuAcα2-6GalNAc-Ser/Thr) antigens are aberrant O-glycosylation frequently over-expressed in many cancers 19, 20, which are associated with poor prognosis and tumor metastasis 21, 22. Cumulative studies showed that Tn antigen was highly expressed in cancers of breast 23, pancreas 24, colon 25, 26, lung 27, ovary 28, stomach 29. STn neo- or over-expression has been described in many types of epithelial cancer including pancreas 24, 30, 31, colorectal 26, 32, ovarian 20 and breast cancers 33. Similarly, increased fucosylation of Hp has also been reported in cancers of pancreas 34, prostate 11, lung 35, ovarian 36 and colon 37. Therefore, it is necessary to combine clinically used biomarker AFP with ELISA Index or fucosylated Hp /Hp ratio for HCC diagnosis.
The most-common clinically utilized serological biomarkers for cancer diagnosis, monitoring and recurrence are glycoproteins 38. Currently, these biomarkers have been reported to have aberrant glycosylation in cancer progression, such as AFP for HCC 39, carbohydrate antigen 125 (CA 125) for ovarian cancer 40, prostate-specific antigen (PSA) for prostate cancer 41. As the most typical representative, AFP-L3 is a core-fucosylated glycoform of AFP, which has been approved as a diagnostic index for HCC by the US food and Drug Administration 42.
Basically, fucosylation is regulated by complicated mechanisms and is involved in several factors, fucosyltransferases, GDP-fucose transporter and GDP-fucose synthesis pathway 43. The fucosyltransferase family of enzymes was responsible for the addition of fucose to glycoproteins, which was over expressed in HCC 44. Additionally, the up-regulation of GDP-fucose transporter plays a pivotal role in increased fucosylation in HCC 45. Furthermore, the biosynthesis of GDP-fucose, a sugar nucleotide, is significantly increased in HCC tissues, which is a common donor substrate for all fucosyltransferases, has a similar regulatory influence on the fucosylation 46. These factors might result in the increased levels of fucosylated Hp in patients suffering with HCC. Moreover, it was also reported that pancreatic cancer cells could secrete an inducible factor, IL-6, which stimulated the production of fucosylated Hp in hepatoma cells 46.
Accumulating evidence suggested that fucosylation interfered with key cancer processes as well as the tumor microenvironment, even leading to tumor progression. Fucosylation has been reported to influence many ligand-receptor interactions 47, 48 and play an important role in some signaling pathways, including tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) -mediated apoptosis of tumor cells 49. As Hp could interact with CCR2 and CD163 on tumor-associated macrophages, fucosylation changes of Hp has the potential to modulate macrophage activity and the immune response through influencing the secretion of chemokines, cytokines or proangiogenic factors, creating an microenvironment in favor of tumor growth, angiogenesis, and eventually, tumor metastasis 50.
In summary, our results demonstrated fucosylated Hp /Hp ratio and ELISA Index, could be potential glycobiomarkers for the surveillance and diagnosis of HCC even with a low level of AFP. Moreover, combination of AFP and fucosylated Hp ratios could improve the sensitivity and specificity of HCC diagnosis.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (21505022), National High Tech Program (863 program: 2015AA020108, 2012AA020204), Proteome Information Research Techniques and Analysis (2014DFB30010) and National Basic Research Program of China (973 Program: 2013CB910501).
Author Contributions
S.Z., X.Q., Y.L. and S.S conceived and designed the experiments. W.L. and S.S contributed samples and reagents. S.S. performed the experiments, technical analyses and statistical analyses. S.S and S.Z. wrote the manuscript.
References
- 1.Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73. doi: 10.1053/j.gastro.2011.12.061. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Zhang H, Gu C. et al. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J Natl Cancer Inst. 2009;101:1066–82. doi: 10.1093/jnci/djp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behne T, Copur MS. Biomarkers for hepatocellular carcinoma. Int J Hepatol. 2012;2012:859076. doi: 10.1155/2012/859076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinchet JC, Chaffaut C, Bourcier V. et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987–97. doi: 10.1002/hep.24545. [DOI] [PubMed] [Google Scholar]
- 6.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Imamura H, Matsuyama Y. et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010;45:1272–82. doi: 10.1007/s00535-010-0278-5. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto S, Asao T, Takahashi J. et al. Alpha1-acid glycoprotein fucosylation as a marker of carcinoma progression and prognosis. Cancer. 2004;101:2825–36. doi: 10.1002/cncr.20713. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Ma T, Thakur A. et al. Differentially expressed glycosylated patterns of alpha-1-antitrypsin as serum biomarkers for the diagnosis of lung cancer. Glycobiology. 2015;25:331–40. doi: 10.1093/glycob/cwu115. [DOI] [PubMed] [Google Scholar]
- 10.Nakano M, Nakagawa T, Ito T. et al. Site-specific analysis of N-glycans on haptoglobin in sera of patients with pancreatic cancer: a novel approach for the development of tumor markers. Int J Cancer. 2008;122:2301–9. doi: 10.1002/ijc.23364. [DOI] [PubMed] [Google Scholar]
- 11.Fujita K, Shimomura M, Uemura M. et al. Serum fucosylated haptoglobin as a novel prognostic biomarker predicting high-Gleason prostate cancer. Prostate. 2014;74:1052–8. doi: 10.1002/pros.22824. [DOI] [PubMed] [Google Scholar]
- 12.Shu H, Zhang S, Kang X. et al. Protein expression and fucosylated glycans of the serum haptoglobin-{beta} subunit in hepatitis B virus-based liver diseases. Acta Biochim Biophys Sin (Shanghai) 2011;43:528–34. doi: 10.1093/abbs/gmr038. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Jiang K, Sun C. et al. Quantitative analysis of site-specific N-glycans on sera haptoglobin beta chain in liver diseases. Acta Biochim Biophys Sin (Shanghai) 2013;45:1021–9. doi: 10.1093/abbs/gmt110. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Shu H, Luo K. et al. N-linked glycan changes of serum haptoglobin beta chain in liver disease patients. Mol Biosyst. 2011;7:1621–8. doi: 10.1039/c1mb05020f. [DOI] [PubMed] [Google Scholar]
- 15.Shang S, Sun L, Li W. et al. Rapid diagnosis of hepatocellular carcinoma using a haptoglobin ELISA assay based on AAL-coated magnetic beads. Discov Med. 2016;22:97–104. [PubMed] [Google Scholar]
- 16.Xu J, Liu C, Zhou L. et al. Distinctions between clinicopathological factors and prognosis of alpha-fetoprotein negative and positive hepatocelluar carcinoma patients. Asian Pac J Cancer Prev. 2012;13:559–62. doi: 10.7314/apjcp.2012.13.2.559. [DOI] [PubMed] [Google Scholar]
- 17.Carr BI, Guerra V. Low Alpha-Fetoprotein Levels Are Associated with Improved Survival in Hepatocellular Carcinoma Patients with Portal Vein Thrombosis. Dig Dis Sci. 2016;61:937–47. doi: 10.1007/s10620-015-3922-3. [DOI] [PubMed] [Google Scholar]
- 18.Izumi R, Shimizu K, Kiriyama M. et al. Alpha-fetoprotein production by hepatocellular carcinoma is prognostic of poor patient survival. J Surg Oncol. 1992;49:151–5. doi: 10.1002/jso.2930490305. [DOI] [PubMed] [Google Scholar]
- 19.Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl. 2011;50:1770–91. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julien S, Videira PA, Delannoy P. Sialyl-tn in cancer: (how) did we miss the target? Biomolecules. 2012;2:435–66. doi: 10.3390/biom2040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju T, Wang Y, Aryal RP. et al. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteomics Clin Appl. 2013;7:618–31. doi: 10.1002/prca.201300024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcos NT, Pinho S, Grandela C. et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64:7050–7. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt FC, Figueiredo P, Lacerda M. Simple mucin-type carbohydrate antigens (T, sialosyl-T, Tn and sialosyl-Tn) in breast carcinogenesis. Virchows Arch. 1995;427:251–8. doi: 10.1007/BF00203391. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann BT, Schluter L, Lange P. et al. COSMC knockdown mediated aberrant O-glycosylation promotes oncogenic properties in pancreatic cancer. Mol Cancer. 2015;14:109. doi: 10.1186/s12943-015-0386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konno A, Hoshino Y, Terashima S. et al. Carbohydrate expression profile of colorectal cancer cells is relevant to metastatic pattern and prognosis. Clin Exp Metastasis. 2002;19:61–70. doi: 10.1023/a:1013879702702. [DOI] [PubMed] [Google Scholar]
- 26.Itzkowitz SH, Yuan M, Montgomery CK. et al. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- 27.Laack E, Nikbakht H, Peters A. et al. Lectin histochemistry of resected adenocarcinoma of the lung: helix pomatia agglutinin binding is an independent prognostic factor. Am J Pathol. 2002;160:1001–8. doi: 10.1016/S0002-9440(10)64921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao R, Liu X, Wang Y. et al. Integrated glycomic analysis of ovarian cancer side population cells. Clin Proteomics. 2016;13:32. doi: 10.1186/s12014-016-9131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakeji Y, Tsujitani S, Mori M. et al. Helix pomatia agglutinin binding activity is a predictor of survival time for patients with gastric carcinoma. Cancer. 1991;68:2438–42. doi: 10.1002/1097-0142(19911201)68:11<2438::aid-cncr2820681119>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Schuessler MH, Pintado S, Welt S. et al. Blood group and blood-group-related antigens in normal pancreas and pancreas cancer: enhanced expression of precursor type 1, Tn and sialyl-Tn in pancreas cancer. Int J Cancer. 1991;47:180–7. doi: 10.1002/ijc.2910470204. [DOI] [PubMed] [Google Scholar]
- 31.Nanashima A, Yamaguchi H, Nakagoe T. et al. High serum concentrations of sialyl Tn antigen in carcinomas of the biliary tract and pancreas. J Hepatobiliary Pancreat Surg. 1999;6:391–5. doi: 10.1007/s005340050137. [DOI] [PubMed] [Google Scholar]
- 32.Nakagoe T, Sawai T, Tsuji T. et al. Circulating sialyl Lewis(x), sialyl Lewis(a), and sialyl Tn antigens in colorectal cancer patients: multivariate analysis of predictive factors for serum antigen levels. J Gastroenterol. 2001;36:166–72. doi: 10.1007/s005350170124. [DOI] [PubMed] [Google Scholar]
- 33.Leivonen M, Nordling S, Lundin J. et al. STn and prognosis in breast cancer. Oncology. 2001;61:299–305. doi: 10.1159/000055337. [DOI] [PubMed] [Google Scholar]
- 34.Okuyama N, Ide Y, Nakano M. et al. Fucosylated haptoglobin is a novel marker for pancreatic cancer: a detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int J Cancer. 2006;118:2803–8. doi: 10.1002/ijc.21728. [DOI] [PubMed] [Google Scholar]
- 35.Ferens-Sieczkowska M, Kratz EM, Kossowska B. et al. Comparison of haptoglobin and alpha(1)-acid glycoprotein glycosylation in the sera of small cell and non-small cell lung cancer patients. Postepy Hig Med Dosw (Online) 2013;67:828–36. doi: 10.5604/17322693.1061788. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Zhu J, Yin H. et al. Analysis of glycan variation on glycoproteins from serum by the reverse lectin-based ELISA assay. J Proteome Res. 2014;13:2197–204. doi: 10.1021/pr401061c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SY, Yoon SJ, Jeong YT. et al. N-glycosylation status of beta-haptoglobin in sera of patients with colon cancer, chronic inflammatory diseases and normal subjects. Int J Cancer. 2010;126:142–55. doi: 10.1002/ijc.24685. [DOI] [PubMed] [Google Scholar]
- 38.Reis CA, Osorio H, Silva L. et al. Alterations in glycosylation as biomarkers for cancer detection. J Clin Pathol. 2010;63:322–9. doi: 10.1136/jcp.2009.071035. [DOI] [PubMed] [Google Scholar]
- 39.Taketa K, Endo Y, Sekiya C. et al. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res. 1993;53:5419–23. [PubMed] [Google Scholar]
- 40.Saldova R, Struwe WB, Wynne K. et al. Exploring the glycosylation of serum CA125. Int J Mol Sci. 2013;14:15636–54. doi: 10.3390/ijms140815636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li QK, Chen L, Ao MH. et al. Serum fucosylated prostate-specific antigen (PSA) improves the differentiation of aggressive from non-aggressive prostate cancers. Theranostics. 2015;5:267–76. doi: 10.7150/thno.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oka H, Saito A, Ito K. et al. Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol. 2001;16:1378–83. doi: 10.1046/j.1440-1746.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- 43.Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143:725–9. doi: 10.1093/jb/mvn011. [DOI] [PubMed] [Google Scholar]
- 44.Hutchinson WL, Du MQ, Johnson PJ. et al. Fucosyltransferases: differential plasma and tissue alterations in hepatocellular carcinoma and cirrhosis. Hepatology. 1991;13:683–8. [PubMed] [Google Scholar]
- 45.Moriwaki K, Noda K, Nakagawa T. et al. A high expression of GDP-fucose transporter in hepatocellular carcinoma is a key factor for increases in fucosylation. Glycobiology. 2007;17:1311–20. doi: 10.1093/glycob/cwm094. [DOI] [PubMed] [Google Scholar]
- 46.Noda K, Miyoshi E, Gu J. et al. Relationship between elevated FX expression and increased production of GDP-L-fucose, a common donor substrate for fucosylation in human hepatocellular carcinoma and hepatoma cell lines. Cancer Res. 2003;63:6282–9. [PubMed] [Google Scholar]
- 47.Wang X, Gu J, Ihara H. et al. Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J Biol Chem. 2006;281:2572–7. doi: 10.1074/jbc.M510893200. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto K, Yokote H, Arao T. et al. N-Glycan fucosylation of epidermal growth factor receptor modulates receptor activity and sensitivity to epidermal growth factor receptor tyrosine kinase inhibitor. Cancer Sci. 2008;99:1611–7. doi: 10.1111/j.1349-7006.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moriwaki K, Narisada M, Imai T. et al. The effect of epigenetic regulation of fucosylation on TRAIL-induced apoptosis. Glycoconj J. 2010;27:649–59. doi: 10.1007/s10719-010-9310-5. [DOI] [PubMed] [Google Scholar]
- 50.Dempsey E, Rudd PM. Acute phase glycoproteins: bystanders or participants in carcinogenesis? Ann N Y Acad Sci. 2012;1253:122–32. doi: 10.1111/j.1749-6632.2011.06420.x. [DOI] [PubMed] [Google Scholar]