Abstract
Aneurysmal subarachnoid hemorrhage (aSAH)-induced cerebral vasospasm and delayed ischemic neurological deficit (DIND) are the major causes of morbidity and mortality in patients with aSAH. The effects of statins-use for patients with aSAH remain controversial. Here,a total of 249 patients from six randomized controlled trials(RCTs) were subjected to meta-analysis. No significant decrease was found in the incidence of vasospasm(RR, 0.80; 95% CI, 0.54–1.17), with substantial heterogeneity (I2 = 49%, P = 0.08), which was verified by the further sensitivity analysis and subgroup meta-analysis. Furthermore, no significant difference was presented in the incidence of poor neurological outcome(RR, 0.94; 95% CI, 0.77–1.16), and potential side effects(RR, 2.49; 95% CI, 0.75–8.33). Nevertheless, significant difference was reported in the occurrence of DIND(RR, 0.58; 95% CI, 0.37–0.92) and mortality(RR, 0.30; 95% CI, 0.14–0.64). At present, although statins-use in the patients with aSAH should not be considered standard care at present, statins-use may have the potential effects in the prevention of mortality in patients with aSAH.
Aneurysmal subarachnoid hemorrhage (aSAH)-induced cerebral vasospasm and delayed ischemic neurological deficit (DIND), especially those associated with arterial vasospasm, remain the major causes of morbidity and mortality in patients with aSAH1. Although treatment with nimodipine confirms the effect of improving outcomes after subarachnoid hemorrhage (SAH), its benefit is modest and the mechanism is uncertain2,3,4. Recently, there has been growing interest in the use of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), which has been demonstrated to raise cerebral endothelial nitric oxide synthase expression, improve endothelial function, increase cerebral blood flow and protect against ischemia5,6. In some clinical studies, statins-use could reduce vasospasm, DIND, and mortality7,8,9,10. Conversely, negative effects of statins-use were presented in other studies11,12,13,14,15,16.
Due to only 5% stroke caused by aSAH17 and a proportion of death in patients with aSAH before admission in hospital, the prospective, multicenter and large sample size randomized controlled trials (RCT) regarding statins-use seems too difficult to complete. Hence, the meta-analysis would be the available methodology to speculate the identified effects of statins-use for patients with aSAH. To our knowledge, four meta-analyses with regards to this aspect had been published previously18,19,20,21. Nevertheless, different viewpoints were raised in the four studies. The effects of statins-use for patients with aSAH still remain controversial and no recommendation was presented in the guideline1,22. In previous meta-analyses, different clinical studies (prospective or retrospective) might enhance the methodological heterogeneity. Besides, a new RCT of simvastatin-use for patients with aSAH has been published16. A further meta-analysis which only included RCTs might be some interesting to explore the effects of statins-use for patients with aSAH. In addition, the potential side effects of statins-use would be investigated in our study.
Results
Study Identification and Selection
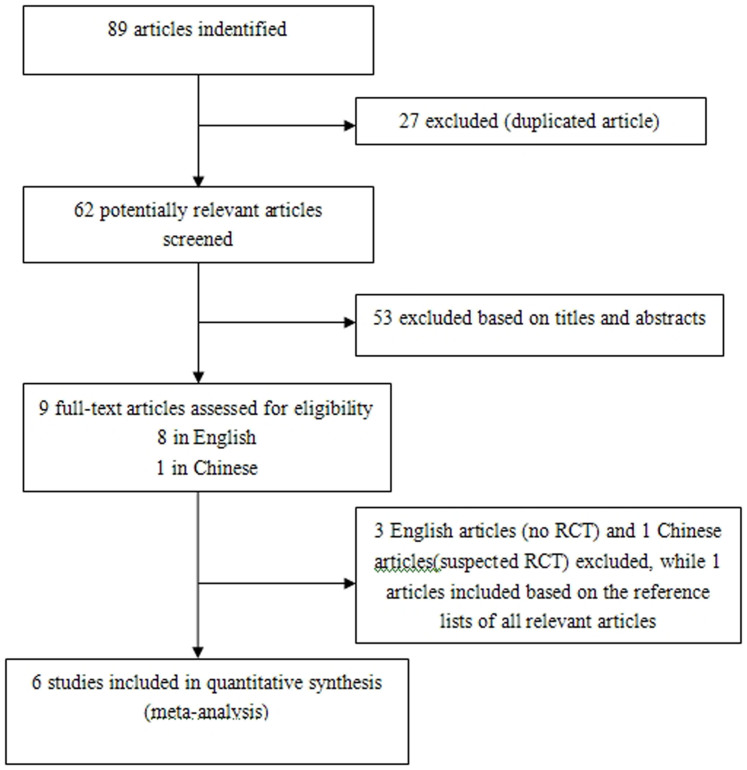
The combined search strategy identified 89 papers. After title, abstract, and full text screening, five RCTs met our inclusion criteria. An additional RCT was identified by hand searching. Thus, eventually six RCTs were included in the present meta-analysis. Unfortunately, we did not receive any missing analytical data for meta-analysis from the corresponding authors of the included studies. The selection process is shown in Figure 1.
Figure 1. Selection process for RCTs.
RCTs: randomized controlled trials.
Study Characteristics
Characteristics of patients with aSAH are presented in Table 1. The six RCTs were all small sample size and single-center studies, enrolling a total of 249 patients7,8,14,15,16,23. Most of patients were female. One RCT has been published as abstract23. One RCT reported the population descent7. Hunt-Hess and WFNS grades were described in four RCTs8,14,15,16. 44 (23%) of 189 patients' initial status was comatose (WFNS or Hunt-Hess grade > IV). Fisher grade was confirmed in three RCTs7,16,23. 10 (10%) of 98 patients were in Fisher grade IV. Management of culprit aneurysms was mentioned in five RCTs7,8,14,15,16. 147(64%) of 228 patients underwent microsurgical clipping. Five studies administered simvastatin at a dose of 80 mg/d within 96 hours for 14–21 days, while one study used pravastatin at a dose of 40 mg/d within 72 hours for 14 days.
Table 1. Characteristics of patients with aSAH in the six RCTs.
| Study ID | Design | Category of statins | Number of patients (S/P) | Patients age (years) | Female | Hunt- Hess or WFNS grade > IV | Fisher scale > IV | Clipping for aSAH | Dose of statins | Initial time and duration of statins treatment | Definiton of vasospasm | Definiton of DIND | Outcome evaluation | Definiton of potential side effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lynch 2005 | RCT | simvastatin | 19/20 | 56 ± 15 | 85%(33/39) | — | 5%(2/39) | 44%(17/39) | 80mg/d | within 48h/14 days | TCD VMCA > 160 cm/sec | clinical manifestation and TCD VMCA > 160 cm/sec or angiography | — | ALT/AST or CK/CPK > 3-fold times the normal (>180 U/L,>1000 U/L respectively) |
| Chou 2008 | RCT | simvastatin | 19/20 | 53 ± 13 | 74%(29/39) | 23%(9/39) | — | 85%(33/39) | 80mg/d | within 96h/21 days | TCD VMCA(peak systolic velocity) > 200 cm/sec and a Lindegaard ratio > 3 or angiography | unaccountable new focal neurological deficit lasting ≥2 hours or GCS↓≥2 points | MRS at discharge | ALT/AST or CPK > 3-fold times the normal (>180 U/L,>1000 U/L respectively) |
| Macedo 2009 | RCT (no blind) | simvastatin | 11/10 | — | — | — | 38%(8/21) | — | 80mg/d | within 72h/21 days | cerebral arteriography examination | Changes in clinical status and CT scan or angiography | GOS at discharge | ALT/AST > 3-fold times the normal (>180 U/L) or creatinine ≥2.5 or total CK ≥1000 U/l |
| Vergouwen 2009 | RCT | simvastatin | 16/16 | 54 ± 11 | 63%(20/32) | 25% (8/32) | — | 22%(7/32) | 80mg/d | within 72h/14 days | TCD VMCA/ACA ≥120 cm/sec | Focal cerebral deficit OR GCS↓≥2 points | GOS at 6 month | ALT/AST > 3-fold times the normal (>180 U/L) |
| Grag 2013 | RCT | simvastatin | 19/19 | 49 ± 9 | 45%(17/38) | 3% (1/38) | 0%(0/38) | 100%(38/38) | 80mg/d | within 96h/14 days | TCD VMCA > 160 cm/sec or angiography | New ischemic neurologic deficits in first two weeks after the ictus(not attributable to be due to hydrocephalus, clip-induced infarct, metabolic derangement, infection or rebleed) | GOS, MRS and MBI at 6 month | ALT/AST or CPK > 3-fold times the normal (>180 U/L,>1000 U/L respectively) |
| Tseng 2005 | RCT | pravastatin | 40/40 | 53 ± 12 | 55%(44/80) | 33% (26/80) | — | 65%(52/80) | 40mg/d | Within 72h/14 days | TCD VMCA > 120 cm/sec with Lindegaard ratio > 3 | focal neurological deficits or GCS↓≥2 points | MRS at discharge | — |
aSAH: aneurysmal subarachnoid hemorrhage; RCTs: randomized controlled trials; TCD: transcranial doppler; MCA: middle cerebral artery; ACA: anterior cerebral artery; DIND: delayed ischemic neurological deficit; GCS: glasgow coma scale; MRS: modified rankin scale; GOS: glasgow outcome scale; MBI: modified barthel index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CK: creatine kinase; CPK: creatine phosphokinase
Three studies used TCD cerebral artery velocity as the detected methodology for vasospasm7,8,15, while the other three required TCD cerebral artery velocity or cerebral arteriographic evidence of vasospasm14,16,23. Definition for DIND also varied. Clinical symptoms, TCD and angiographic results were confirmed as DIND in two RCTs7,23. However, new ischemic neurologic deficits or Glasgow Coma Scale (GCS) were performed as the criteria of DIND in the other RCTs8,14,15,16. GOS and MRS were presented in five RCTs8,14,15,16,23. The timing of outcome assessment varied from as early as hospital discharge8,14,23 to as late as 6 months15,16. Potential side effects were demonstrated in four RCTs7,14,15,16, the definition of that was consistent in the studies.
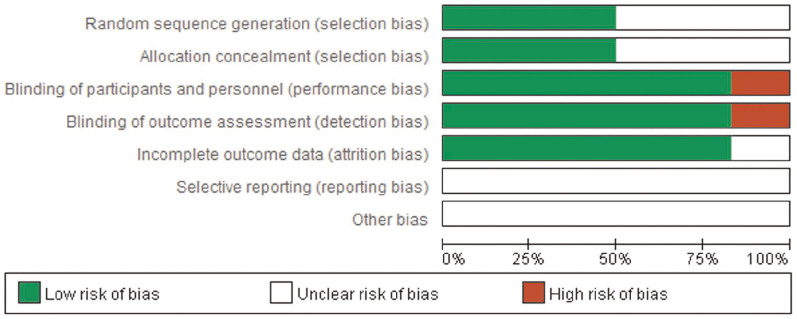
The quality of the included RCTs was assessed by Cochrane risk of bias assessment. If no specific descriptions were found in the trials, we tended to choose the answer of unclear risk. However, the results only reflected our viewpoint (Figure 2).
Figure 2. Quality of the included RCTs assessed by Cochrane risk of bias assessment.
If no specific descriptions were found in the trials, we tended to choose the answer of unclear risk. RCTs: randomized controlled trials.
Meta-analysis Outcomes
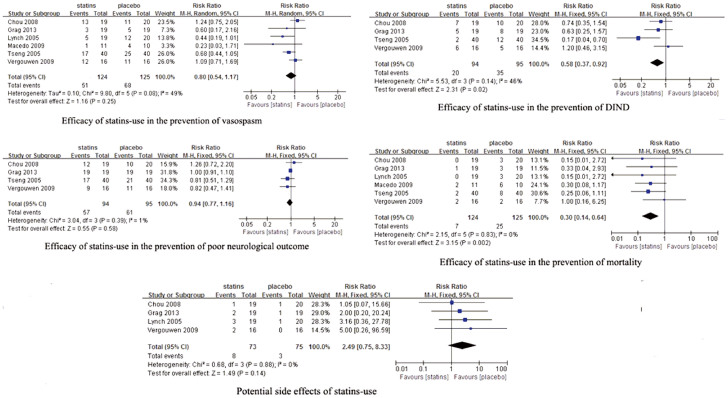
All the pooled results are shown in Figure 3. The incidence of vasospasm was 41% (51/124) and 54% (68/125) in statins-use patients and placebo-use patients respectively. All the six RCTs with 249 patients were combined using a fixed-effects model. statins-use were associated with a slight significant reduction in the incidence of vasospasm (RR, 0.76; 95% CI, 0.59–0.98). However, there was evidence of moderate heterogeneity (I2 = 49%, P = 0.08). A random-effects model was considered more appropriate. The result of meta-analysis using random-effects model showed no statistically significant (RR, 0.80; 95% CI, 0.54–1.17). Further exclusion of any single study did not materially alter the overall combined RR, with a range of from 0.70 (95% CI, 0.45–1.08) to 0.89 (95% CI, 0.61–1.29). The sensitivity analysis were also performed to examine the influence of various criteria on the combined estimates. No significant changes were found using sensitivity analyses, indicating that our results were reliable (Table 2).
Figure 3. Efficacy of statins-use in the prevention of vasospasm, DIND, poor neurological outcome and mortality, and the potential side effects of statins.
DNID: delayed ischemic neurological deficit.
Table 2. Sensitivity analysis based on various criteria for vasospasm.
| outcome | No. patients | No.RCTs | statins | placebo | RR (95%CI) | I2 | P Value for Heterogeneity |
|---|---|---|---|---|---|---|---|
| All trials7,8,14,15,16,23 | 249 | 6 | 51 of 124 | 68 of 125 | 0.80 (0.54–1.17) | 49% | 0.08 |
| Trials with simvastatin-use7,14,15,16,23 | 169 | 5 | 34 of 84 | 43 of 85 | 0.81 (0.49–1.35) | 53% | 0.08 |
| Trials with pravastatin-use8 | 80 | 1 | 17 of 40 | 25 of 40 | 0.68 (0.44–1.05) | — | — |
| Full text trials7,8,14,15,16 | 228 | 5 | 50 of 113 | 64 of 115 | 0.84 (0.58–1.21) | 48% | 0.1 |
| Only TCD vasospasm* 7,14,15 | 110 | 3 | 30 of 54 | 33 of 56 | 0.95 (0.54–1.66) | 64% | 0.06 |
| Quality of trials withouthigh risk7,8,14,15,16 | 228 | 5 | 50 of 113 | 64 of 115 | 0.84 (0.58–1.21) | 48% | 0.1 |
| statins treatment for 14 days7,8,15,16 | 189 | 4 | 37 of 94 | 53 of 95 | 0.74 (0.49–1.12) | 42% | 0.16 |
*in RCT of Chou 2005, 10 patients in statins group and 13 patients in placebo group developed TCD vasospasm.
RCTs: randomized controlled trials; RR: risk ratio; TCD: transcranial doppler.
Although the definition of DIND was mentioned in all the trials, we only acquired the number of patients with DIND in four trials8,14,15,16. We tried to contact the corresponding authors of included RCTs for the missing analytical data, unfortunately, we did not get any reply. Therefore, only four RCTs were included in the meta-analysis of DIND. DIND was observed in 21% (20/94) statins-treated patients and 37% (35/95) placebo-treated patients respectively. Statins-use could significantly reduce the occurrence of DIND (RR, 0.58; 95% CI, 0.37–0.92), with no substantial heterogeneity (I2 = 46%, P = 0.14).
Neurological outcome was evaluated by GOS or MRS in five RCTs8,14,15,16,23. However, the detailed data of neurological outcome was reported in four trials8,14,15,16. For the definition of poor outcome, a GOS of 1 to 4 points was considered equivalent to a MRS of 3 to 6 points21. Poor outcome occurred in 61% (57/94) statins-treated patients, which presented in 64% (61/95) placebo-treated patients. The difference between two groups was not statistically significant (RR, 0.94; 95% CI, 0.77–1.16), with no substantial heterogeneity (I2 = 1%, P = 0.39).
Mortality was observed in all the six RCTs, which occurred in 6% (7/124) statins-treated patients versus 20% (25/125) placebo-treated patients. Statins-use was associated with a significant reduction in the occurrence of mortality (RR, 0.30; 95% CI, 0.14–0.64), with no substantial heterogeneity (I2 = 0%, P = 0.83).
Potential side effects of statins-use were reported in four studies7,14,15,16, which occurred in 11%(8/73) statins-use patients and 4% (3/75) placebo-use patients respectively. No significant difference (RR, 2.49; 95% CI, 0.75–8.33), with no substantial heterogeneity (I2 = 0%, P = 0.88), was found between two groups.
Subgroup meta-analysis Outcomes
According to the solubility in lipids or water, statins are classified as lipophilic statins and hydrophilic statins. Lipophilic statins (simvastatin) cross the blood-brain barrier and penetrate cell membranes more effectively and may be more efficient theoretically in the treatment of intracranial diseases than the hydrophilic statins (pravastatin). In addition, the dose of simvastatin in the included trials was 80 mg/d, while the dose of pravastatin in the included study was 40 mg/d. It may enhance the potential clinical heterogeneity if the trials using simvastatin and the study using pravastatin were pooled together in the meta-analysis. Thus, we further conducted the subgroup meta-analysis. Outcomes from simvastatin or pravastatin are shown in Table 3. According to meta-analysis from simvastatin, the incidence of mortality was significantly reduced in simvastatin-use group, whereas the occurrence of vasospasm, DIND and poor neurological outcome was not materially changed between simvastatin-use group and placebo-use group. Based on the RCT using pravastatin, pravastatin-use could prevent the occurrence of DIND, while no difference was found for vasospasm, mortality and poor neurological outcome between patients with pravastatin-use and patients without pravastatin-use.
Table 3. Subgroup meta-analysis outcomes.
| Outcomes | No. patients | No.RCTs | statins | placebo | RR (95%CI) | I2 | P Value for Heterogeneity |
|---|---|---|---|---|---|---|---|
| Vasospasm | |||||||
| Simvastatin7,14,15,16,23 | 169 | 5 | 34 of 84 | 43 of 85 | 0.81 (0.49–1.35) | 53% | 0.08 |
| Pravastatin8 | 80 | 1 | 17 of 40 | 25 of 40 | 0.68 (0.44–1.05) | — | — |
| DIND | |||||||
| Simvastatin14,15,16 | 109 | 3 | 18 of 54 | 23 of 55 | 0.80 (0.49–1.30) | 0% | 0.6 |
| Pravastatin8 | 80 | 1 | 2 of 40 | 12 of 40 | 0.17 (0.04–0.70) | — | — |
| Poor neurological outcome | |||||||
| Simvastatin14,15,16 | 109 | 3 | 40 of 54 | 40 of 55 | 1.01 (0.82–1.25) | 0% | 0.53 |
| Pravastatin8 | 80 | 1 | 17 of 40 | 21 of 40 | 0.81 (0.51–1.29) | — | — |
| Mortality | |||||||
| Simvastatin7,14,15,16,23 | 169 | 5 | 5 of 84 | 17 of 85 | 0.33 (0.14–0.77) | 0% | 0.74 |
| Pravastatin8 | 80 | 1 | 2 of 40 | 8 of 40 | 0.25 (0.06–1.11) | — | — |
| Potential side effects | |||||||
| Simvastatin7,14,15,16 | 148 | 4 | 8 of 73 | 3 of 75 | 2.49 (0.75–8.33) | 0% | 0.88 |
| Pravastatin8 | — | — | — | — | — | — |
RCTs: randomized controlled trials; DIND: delayed ischemic neurological deficit; RR: risk ratio.
Publication Bias
Publication bias was assessed by funnel plot, but the weak power with only six studies limited the interpretability of the finding.
Discussion
The results from previous meta-analyses remain controversial. Sillberg et al.18 supported all the benefits of statins-use in patients with aSAH from three RCTs. Tseng et al.19 reported that the benefit of statins was the reduced DIND from six RCTs, which was consistent with Kramer et al.20 from four RCTs and two “pseudo” RCTs. Nevertheless, Kramer et al.20 concluded that statins-use was not associated with any reduction in vasospasm, DIND, poor outcome and mortality from four RCTs, two “pseudo” RCTs, five cohort studies and one case–control study, which was also observed by Vergouwen et al.21 from four RCTs. To our knowledge, RCTs are the preferred study design to assess the efficacy of treatment, since the process of randomization ensures that confounders are balanced between different treatment groups. Restricting meta-analysis only to RCTs would be more appropriate to speculate the effects of treatment. At present, there are a new published RCT with respect to statins-use. Given the consideration of the above circumstances and no published large sample size, multicenter RCT of statins-use, it might be a deserved choice for us to further investigate the efficacy and safety of statins-use in patients with aSAH under the current RCTs.
In our study, owing to the inconsistent definitions of vasospasm in the six studies, moderate heterogeneity was presented in the meta-analysis of the incidence of vasospasm. Therefore, we chose random-effects model for this analysis. We found statins-use could not significantly affect the incidence of vasospasm in patients with aSAH. Exclusion of any single study, subgroup meta-analysis and sensitivity analysis based on various conditions did not materially alter the pooled results, which might make our finding reliable. For poor neurological outcome, the results from overall meta-analysis and subgroup meta-analysis consistently indicated that statins-use was not associated with the reduction of poor neurological outcome. Furthermore, Our meta-analysis showed that statins-use significantly decreased the occurrence of DIND. Although no substantial heterogeneity was found in the meta-analysis of the occurrence of DIND, subgroup meta-analysis demonstrated different result that DIND could not be decreased in simvastatin-use patients. Because we only acquired the data of vasospasm-related DIND, which was defined as the DIND associated with severe vasospasm on TCD in the pravastatin-use RCT8, and these was not the accurate data of overall DIND, thus, we should be very cautious to this result. In our opinion, we tended to believe the meta-analysis result from the simvastatin-use RCTs.
Interestingly, our important finding was that statins-use could prevent the occurrence of mortality although it could not significantly ameliorate cerebrovascular events caused by aSAH. To the best of our knowledge, firstly, in a Finnish prospective cohort study24 and in the International Subarachnoid Aneurysm Trial25, cerebrovascular diseases other than recurrent SAH and cardiovascular diseases were important causes of death, indicating that the increased mortality after SAH was caused by other vascular diseases. In addition, patients with SAH may have an increased risk of cardiovascular events because of shared risk factors26. Furthermore, SAH-associated myocardial injury was found by Nguyen et al27 from Aneurysm Surgery Trial. Lastly, in an autopsy study28, SAH accounted for 15.7% cases of the cardiovascular related deaths. Thus, we speculated that the direct cause of death in some SAH patients was not intracranial pathologies (vasospasm, DIND, poor neurological outcome, etc.) but cardiovascular events. However, statins-use could significantly decrease the cardiovascular mortality29,30. Therefore, this might be the reason why statins-use could prevent the occurrence of mortality although it could not significantly ameliorate cerebrovascular events caused by aSAH in patients with aSAH.
There are some potential limitations in our present meta-analysis study. Firstly, so far, published RCTs regarding statins-use for patients with aSAH were all small sample size. Hence, the number of pooled patients in the meta-analysis is only 249. There is a greater chance that the baseline characteristics will be unbalanced between two groups. Second, the RCT with only abstract included in our study presented the high risk in the blinding of participants and blinding of outcome assessment. Finally, substantial differences in the study design among RCTs, including important differences in the treatment and outcome evaluation, could not be actually avoided in the present meta-analysis.
In conclusion, statin-use in the patients with aSAH, which might not be associated with the reduction in the incidence of vasospasm and poor neurological outcome, may reduce the occurrence of DIND and mortality. Furthermore, drug-induced hepatitis or myositis might not be the potential side effects of statins. Owing to the variations in methodology of included RCTs and small sample size, we should be very cautious to treat the results, which should be confirmed in the ongoing multicenter, double-blind RCT(Simvastatin in Aneurysmal Subarachnoid Haemorrhage; http://www.stashtrial.com/home.html). In our opinion, although statins-use in the patients with aSAH should not be considered standard care at present, statins-use may have the potential effects in the prevention of mortality in patients with aSAH. Moreover, cardiovascular risk assessment and subgroup analysis of cardiovascular death might be a deserved choice in the future study regarding statins-use in patients with aSAH.
Methods
Search Strategy and inclusion criteria
PubMed, EMBASE and three Chinese databases (SinoMed, CNKI and VIP) were searched for relevant articles published up to February, 2014. Search terms included “simvastatin”, “pravastatin”, “statin”, “statins” and “aneurysmal subarachnoid hemorrhage”, “aneurysmal subarachnoid haemorrhage” and “random”, “random*”. Furthermore, citations in the retrieved articles were reviewed to search for additional relevant studies. All studies included into this meta-analysis must meet the following criteria: (i) patients with aSAH; (ii) statins compared with placebo agent; (iii) randomized controlled trials. Duplicated studies, review, editorial and comment were excluded.
Data Extraction and Outcome Measures
Two authors(Y.-F.W and W.X) independently extracted the following data: first author, year of publication, number of patients, patients' characteristics, study design, statins group, placebo group, definitions of vasospasm, DIND, poor neurological outcome, mortality, potential side effects, and other outcomes data. Extracted data were checked by another author (J.H). Any disagreements were resolved by discussion and consensus.
Outcomes were the incidence of vasospasm, DIND, poor neurological outcome, mortality and potential side effects. Vasospasm included transcranial doppler(TCD) vasospasm and angiographic vasospasm. DIND was defined as clinical symptoms, TCD or angiographic results and signs of new ischemic neurologic deficits (not attributable to be due to hydrocephalus, clip-induced infarct, metabolic derangement, infection or rebleed). Poor neurological outcome was evaluated by Modified Rankin Scale (MRS) of 3–6 points and Glasgow Outcome Scale (GOS) of 1–4 points at the end of blinded follow-up period21,31,32, which was either at discharge or 6 months after SAH. Serum alanine aminotransferase(ALT)/aspartate aminotransferase(AST) and creatine kinase(CK)/creatine phosphokinase (CPK) > 3-fold times the normal (ALT/AST > 180 U/L, CK/CPK > 1000 U/L) were regarded as potential side effects, which indicated the drug-induced hepatitis or myositis.
Quality Assessment
Cochrane risk of bias assessment33 was used to evaluated the methodologic quality of each trial. This scale consists of seven items including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias, each of which could be divided as low risk, high risk and unclear risk. Each RCT was evaluated independently by two authors(S.-H.S and F. Y). Any disagreements were resolved by consensus between all the authors.
Statistical Analysis
We performed meta-analysis using Cochrane RevMan (version 5.1) software. Differences were expressed as relative risk (RR) with 95% confidence interval(CI) for dichotomous outcomes. Heterogeneity for each pooled summary was estimated using Cochran's Q statistic and the I2 statistic. Substantial heterogeneity will be considered to exist with I2 > 50% or Chi square test P < 0.1. Meta-analysis was performed via fixed-effects model if there was no evidence of statistical heterogeneity. The random-effects model was employed to pool studies when statistical heterogeneity occurred. Meanwhile, we further conducted sensitivity analyses to explore possible explanations for heterogeneity and to examine the influence of various inconsistent criteria on the overall pooled estimate. We also investigated the influence of a single study on the overall pooled estimate by omitting one study in each turn. Publication bias was tested by a funnel plot.
Acknowledgments
This work was partly supported by the National Natural Science Foundation of China (grant No: 81271212 to J. Hai).
Footnotes
The authors declare no competing financial interests.
Author Contributions S.-H.S. and J.H. wrote the main manuscript text, Y.-F.W. and F.Y. prepared table 1,table 2 and table 3. W.X. prepared figure 1, figure 2 and figure 3. S.-H.S., W.X., J.H., Y.-F.W. and F.Y. reviewed the manuscript.
References
- Connolly E. S. Jr et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43, 1711–37 (2012). [DOI] [PubMed] [Google Scholar]
- Bederson J. B. et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council, American Heart Association. Stroke 40, 994–1025 (2009). [DOI] [PubMed] [Google Scholar]
- Dorhout, Mees S. M. et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 3, CD000277 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L. Management of cerebral vasospasm. Neurosurg Rev. 29, 179–93 (2006). [DOI] [PubMed] [Google Scholar]
- Sugawara T., Ayer R. & Zhang J. H. Role of statins in cerebral vasospasm. Acta Neurochir Suppl. 104, 287–90 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirt M. J. et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke 33, 2950–6 (2002). [DOI] [PubMed] [Google Scholar]
- Lynch J. R. et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke 36, 2024–6 (2005). [DOI] [PubMed] [Google Scholar]
- Tseng M. Y., Czosnyka M., Richards H., Pickard J. D. & Kirkpatrick P. J. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation,and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke 36, 1627–32 (2005). [DOI] [PubMed] [Google Scholar]
- Tseng M. Y. et al. Effects of acute pravastatin treatment on intensity of rescue therapy, length of inpatient stay, and 6-month outcome in patients after aneurysmal subarachnoid hemorrhage. Stroke 38, 1545–50 (2007). [DOI] [PubMed] [Google Scholar]
- Parra A. et al. Effect of prior statin use on functional outcome and delayed vasospasm after acute aneurysmal subarachnoid hemorrhage: a matched controlled cohort study. Neurosurgery 56, 476–84 (2005). [DOI] [PubMed] [Google Scholar]
- Kramer A. H. et al. Statin use was not associated with less vasospasm or improved outcome after subarachnoid hemorrhage. Neurosurgery 62, 422–7 (2008). [DOI] [PubMed] [Google Scholar]
- McGirt M. J., Garces Ambrossi G. L., Huang J. & Tamargo R. J. Simvastatin for the prevention of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage: a single-institution prospective cohort study. J. Neurosurg. 110, 968–74 (2009). [DOI] [PubMed] [Google Scholar]
- Kern M., Lam M. M., Knuckey N. W. & Lind C. R. Statins may not protect against vasospasm in subarachnoid haemorrhage. J. Clin Neurosci. 16, 527–30 (2009). [DOI] [PubMed] [Google Scholar]
- Chou S. H. et al. A randomised, double-blind, placebo-controlled pilot study of simvastatin in aneurysmal subarachnoid hemorrhage. Stroke 39, 2891–3 (2008). [DOI] [PubMed] [Google Scholar]
- Vergouwen M. D. et al. Biologic effects of simvastatin in patients with aneurysmal subarachnoid hemorrhage: a double-blind, placebo-controlled randomized trial. J. Cereb Blood Flow Metab. 29, 1444–53 (2009). [DOI] [PubMed] [Google Scholar]
- Garg K. et al. Role of simvastatin in prevention of vasospasm and improving functional outcome after aneurysmal sub-arachnoid hemorrhage: a prospective, randomized, double-blind, placebo-controlled pilot trial. Br J Neurosurg. 27, 181–6 (2013). [DOI] [PubMed] [Google Scholar]
- van Gijn J., Kerr R. S. & Rinkel G. J. Subarachnoid haemorrhage. Lancet 369, 306–18 (2007). [DOI] [PubMed] [Google Scholar]
- Sillberg V. A., Wells G. A. & Perry J. J. Do statins improve outcomes and reduce the incidence of vasospasm after aneurysmal subarachnoid hemorrhage: a meta-analysis. Stroke 39, 2622–6 (2008). [DOI] [PubMed] [Google Scholar]
- Tseng M. Y. Participants in the International Multidisciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Summary of evidence on immediate statins therapy following aneurysmal subarachnoid hemorrhage. Neurocrit Care 15, 298–301 (2011). [DOI] [PubMed] [Google Scholar]
- Kramer A. H. & Fletcher J. J. Statins in the management of patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurocrit Care 12, 285–96 (2010). [DOI] [PubMed] [Google Scholar]
- Vergouwen M. D., de Haan R. J., Vermeulen M. & Roos Y. B. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke 41, e47–52 (2010). [DOI] [PubMed] [Google Scholar]
- Steiner T. et al. European stroke organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 35, 93–112 (2013). [DOI] [PubMed] [Google Scholar]
- Macedo S. et al. Effects of simvastatin in prevention of vasospasm in nontraumatic subarachnoid hemorrhage: preliminary data. Crit Care. 13(Suppl1), P103 (2009). [Google Scholar]
- Ronkainen A. et al. Evidence for excess long-term mortality after treated subarachnoid hemorrhage. Stroke 32, 2850–3 (2001). [DOI] [PubMed] [Google Scholar]
- Molyneux A. J. et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 8, 427–33 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkamp D. J. et al. Excess mortality and cardiovascular events in patients surviving subarachnoid hemorrhage: a nationwide study in Sweden. Stroke 42, 902–7 (2011). [DOI] [PubMed] [Google Scholar]
- Nguyen H. P. et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 113, 327–42 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogeng'o J. A., Gatonga P. & Olabu B. O. Cardiovascular causes of death in an east African country: an autopsy study. Cardiol J. 18, 67–72 (2011). [PubMed] [Google Scholar]
- Barylski M. et al. Lipid and Blood Pressure Meta-Analysis Collaboration Group: Statins decrease all-cause mortality only in CKD patients not requiring dialysis therapy—a meta-analysis of 11 randomized controlled trials involving 21,295 participants. Pharmacol Res. 72, 35–44 (2013). [DOI] [PubMed] [Google Scholar]
- Mills E. J. et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J. Am Coll Cardiol. 52, 1769–81 (2013). [DOI] [PubMed] [Google Scholar]
- Bamford J. M., Sandercock P. A., Warlow C. P. & Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 20, 828 (1989). [DOI] [PubMed] [Google Scholar]
- Jennett B. & Bond M. Assessment of outcome after severe brain damage. Lancet 1, 480–4 (1975). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T. & Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0. (The Nordic Cochrane Centre, Copenhagen, 2011).