Abstract
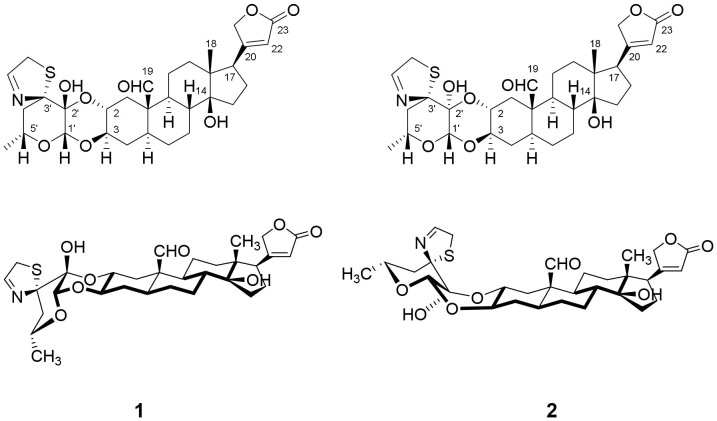
Two stereoisomeric cardenolides, uscharin (1) and a new compound, 2′-epi-uscharin (2), were isolated from the latex of Calotropis gigantea (Asclepiadaceae). Their structures were fully elucidated based on their spectroscopic data, X-ray crystallographic data and chemical evidences. Both epimers (1 and 2) exhibited strong inhibitory effects on HIF-1 activity with different magnitudes. Compound 1 showed much more potent activity than 2 and digoxin, a well-known HIF-1 inhibitor. Discrepancy in potencies between 1 and 2 revealed the contribution of a β-configuration of 2′ hydroxyl moiety for HIF-1 inhibitory activity. This is a first report of the activity of HIF-1 inhibition of thiazoline ring-containing cardenolides.
Cardenolides are classified as a type of steroid containing 23 or 24 carbon atoms with a five or six-membered lactone ring at C-17. The successful isolation of various cardenolides from natural origins together with illustration of their anticancer activities and mechanisms have been reported by many research groups, suggesting that cardenolides give a promise to be potent anticancer agents1,2,3.
Calotropis gigantea, a common plant in Africa, Eastern Asia and Southeast Asia, belongs to the family of Asclepiadaceae which is well known as a rich source of cardenolides2,4 and numerous bioactive chemical constituents such as pregnanones5, triterpenes6, non-protein amino acids1, etc. The latex of this plant has been used for medicinal and insecticidal purposes7. Various pharmacological activities of secondary metabolites isolated from different parts of plants in this genus have been reported, i.e., analgesic, anti-asthmatic, sedative, anti-inflammatory, anti-diarrheal, hepatoprotective, and anticancer activities1,2,3. The phytochemical studies of the latex of C. gigantea revealed the high content of cardiac poisons in its alcoholic extract4,6. In this paper, we report the structural elucidation of uscharin (1) and 2′-epi-uscharin (2) isolated from C. gigantea on the basis of their spectroscopic data and chemical evidences, along with their inhibitory effects on HIF-1 transcription.
Results and Discussion
Structural Elucidation of Compounds 1 and 2
The molecular formula of compound 1 (Fig. 1) was deduced to be C31H41NO8S by the negative HRESIMS (m/z 632.2529, calcd for [M+HCOO]− 632.2535). Its1H and 13C NMR spectra exhibited the presence of an aldehyde moiety (δH 10.00 s, H-19; δC 207.0, C-19) and a butanolactone ring [δH 5.87 s, H-22; δH 4.94 dd (J = 18.0, 1.2 Hz), H-21a and δH 4.79 dd (J = 18.0, 1.8 Hz), H-21b; δC 174.0, C-23; δC 174.3, C-20; δC 117.9, C-22 and δC 73.3, C-21]. The existence of a thiazoline ring was deduced from the characterized signals at δH 7.52 s, H-2″, δH 3.93 dd (J = 16.8, 1.2 Hz), H-1″a and δH 3.86 d (J = 16.8 Hz), H-1″b; δC 160.0, C-2″ and δC 42.7, C-1″. All these typical NMR resonances superimposed to those of uscharin, a known cardenolide8,9,10.
Figure 1. Chemical structures of uscharin (1) and 2′-epi-uscharin (2).
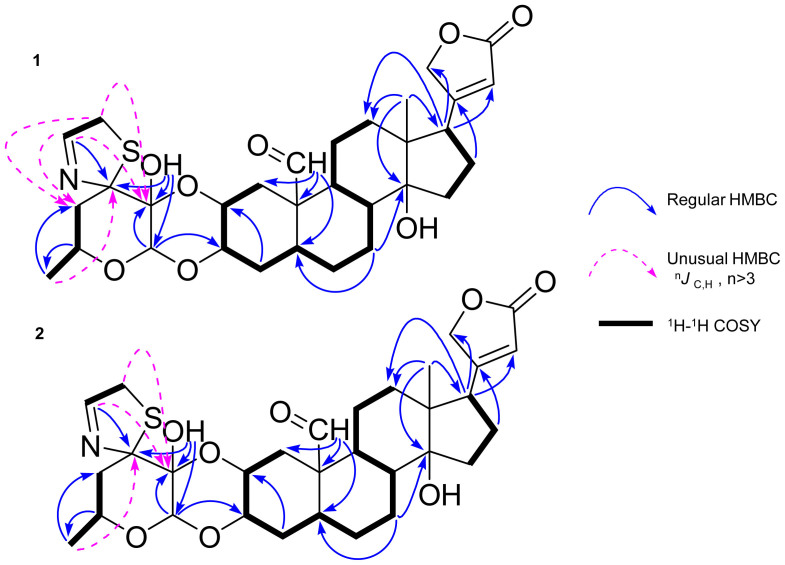
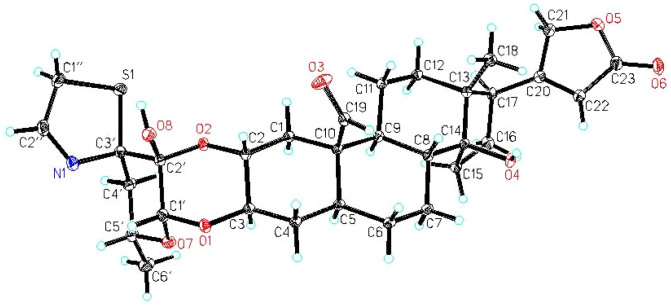
In order to obtain full assignment of 1H NMR data of 1 (Table 1), the protonated carbons were assigned through HSQC, HMBC, 1H-1H COSY and NOSEY correlations. Interestingly, several unusual long-range HMBC correlations (4JC, H) from H-1″ to C-2′ and C-4′, from H-2″ to C-2′ and C-4′, and from H-6′ to C-3′ (Fig. 2) were observed. Since the stereochemistry of 1 in sugar part and thiazoline moiety could not be deduced by NOESY spectroscopic data, we carried out a single crystal X-ray diffraction experiment of compound 1. Bearing a sulfur atom and eight oxygen atoms in the molecule, the final refinement resulted in a small Flack parameter 0.02 (7), which together with the known configuration (C-10 and C-17) of the cardiac steroids allows the assignment of the absolute configuration as shown in Fig. 3. This is the first report of the X-ray crystallographic structure of uscharin along with its fully assigned 1H NMR data in CDCl3.
Table 1. NMR Spectroscopic Data (600 MHz) for Uscharin (1) and 2′-Epi-uscharin (2).
| 1a | 2a | 2b | 2c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Position | type | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) |
| Aglycone moiety | |||||||||
| 1 | CH2 | 36.1 | 2.47, dd (12.6,4.2) | 36.2 | 2.60, dd (12.6,4.2) | 36.9 | 2.45, dd (13.2,4.2) | 37.0 | 2.58, dd (12.6,4.8) |
| 1.14, t (12.0) | 1.23, t (12.0) | 1.16, t (12.6) | 1.23, m | ||||||
| 2 | CH | 69.7 | 3.96, m | 70.6 | 3.84, m | 71.3 | 3.80, m | 70.5 | 4.36, m |
| 3 | CH | 71.4 | 4.08, td (11.4, 4.2) | 71.5 | 4.09, td (10.2, 4.2) | 73.0 | 4.02, m | 72.3 | 4.44, td (10.8, 4.2) |
| 4 | CH2 | 33.0 | 1.78, m | 33.0 | 1.76, m | 34.6 | 1.65, m | 34.2 | 1.72, m |
| 1.44, q (12.2) | 1.47, m | 1.37, m | 1.55, m | ||||||
| 5 | CH | 43.4 | 1.53, m | 43.4 | 1.47, m | 44.6 | 1.55, m | 43.8 | 1.34, m |
| 6 | CH2 | 27.5 | 2.17, m | 27.5 | 1.98, m | 28.9 | 2.02, m | 28.2 | 1.90, m |
| 1.69, m | 1.64, m | 1.65, m | 1.25, m | ||||||
| 7 | CH2 | 27.3 | 1.98, m | 27.3 | 2.14, m | 28.7 | 2.22, m | 28.2 | 2.44, m |
| 1.25, m | 1.24, m | 1.30, m | 1.44, m | ||||||
| 8 | CH | 42.5 | 1.53, m | 42.4 | 1.50, m | 43.6 | 1.57, m | 42.9 | 1.74, m |
| 9 | CH | 48.6 | 1.33, m | 48.6 | 1.32, m | 49.7 | 1.44, m | 49.0 | 1.27, m |
| 10 | C | 52.6 | 52.6 | 54.2 | 53.2 | ||||
| 11 | CH2 | 21.8 | 1.75, m | 21.8 | 1.71, m | 23.1 | 1.72, m | 22.5 | 1.60, m |
| 1.31, m | 1.33, m | 1.28, m | 1.27, m | ||||||
| 12 | CH2 | 39.3 | 1.53, m | 39.4 | 1.53, m | 40.4 | 1.49, m | 39.5 | 1.32, m |
| 1.32, m | 1.32, m | 1.45, m | 1.26, m | ||||||
| 13 | C | 49.3 | 49.3 | 50.9 | 50.1 | ||||
| 14 | C | 85.0 | 85.0 | 85.9 | 84.4 | ||||
| 15 | CH2 | 32.3 | 2.00, m | 32.4 | 2.01, m | 32.9 | 2.10, m | 32.9 | 2.06, m |
| 1.67, m | 1.67, m | 1.68, m | 1.87, m | ||||||
| 16 | CH2 | 26.7 | 2.15, m | 26.7 | 2.14, m | 28.0 | 2.17, m | 27.5 | 2.08, m |
| 1.87, m | 1.86, m | 1.88, m | 1.96, m | ||||||
| 17 | CH | 50.5 | 2.76, dd (9.6,5.4) | 50.5 | 2.75, dd (9.6,5.4) | 52.0 | 2.83, dd (9.0,6.0) | 51.5 | 2.74, m |
| 18 | CH3 | 15.6 | 0.82, s | 15.5 | 0.81, s | 16.2 | 0.82, s | 16.2 | 0.88, s |
| 19 | CH | 207.0 | 10.00, s | 206.8 | 9.94, s | 209.4 | 10.00, s | 208.2 | 9.89, s |
| 20 | C | 174.3 | 174.3 | 178.3 | 176.0 | ||||
| 21 | CH2 | 73.3 | 4.94, dd (18.0, 1.2) | 73.3 | 4.93, dd (18.0, 1.8) | 75.5 | 5.02, dd (18.6, 1.2) | 74.0 | 5.28, dd (18.0, 1.2) |
| 4.79, dd (18.0, 1.8) | 4.78, dd (18.0, 1.2) | 4.91d | 5.03e | ||||||
| 22 | CH | 117.9 | 5.87, s | 117.9 | 5.87, s | 118.1 | 5.90, s | 118.2 | 6.13, s |
| 23 | C | 174.0 | 173.9 | 177.4 | 174.8 | ||||
| Sugar moiety | |||||||||
| 1′ | CH | 94.9 | 5.06, s | 95.7 | 4.67, s | 96.7 | 4.69, s | 96.8 | 5.38, s |
| 2′ | C | 91.5 | 93.8 | 95.6 | 95.8 | ||||
| 3′ | C | 99.5 | 99.8 | 99.4 | 101.0 | ||||
| 4′ | CH2 | 47.0 | 2.23, dd (13.2, 11.6) | 44.6 | 2.71, dd (13.2, 11.4) | 45.9 | 2.66, t (12.0) | 45.8 | 3.30, t (12.6) |
| 1.72, m | 1.73, m | 1.76, m | 2.10, m | ||||||
| 5′ | CH | 68.2 | 4.27, m | 69.4 | 3.86, m | 70.7 | 3.77, m | 70.0 | 4.08, m |
| 6′ | CH | 20.9 | 1.22, d (6.0) | 21.0 | 1.33, d (6.0) | 21.5 | 1.27, d (6.0) | 21.9 | 1.44, d (6.0) |
| 2′-Hydroxyl | OH | 2.86,brs | 3.09, brs | ||||||
| Thiazoline moiety | |||||||||
| 1″ | CH2 | 42.7 | 3.93, dd (16.8,1.2) | 44.6 | 4.01, dd (16.8, 1.2) | 45.4 | 4.00, dd (16.8,1.2) | 45.4 | 4.09, d (15.0) |
| 3.86, d (16.8) | 3.98, dd (16.8, 1.2) | 3.90, d (16.2) | 3.98, dd (16.2, 1.2) | ||||||
| 2″ | CH | 160.0 | 7.52,s | 161.9 | 7.68, s | 165.9 | 7.70, s | 162.7 | 7.75, s |
ain CDCl3, bin CD3OD, cin pyridine-d5, dOverlapped signal with CD3OD, eOverlapped signal with pyridine-d5.
Figure 2. Key HMBC (H to C), 1H-1H COSY correlations of uscharin (1) and 2′-epi-uscharin (2).
Figure 3. X-ray crystallographic structure of uscharin (1) showing the atom labeling scheme.
Compound 2 (Fig. 1) was obtained as a white powder. The negative HRESIMS of m/z 632.2528 (calcd for [M+HCOO]− 632.2535) disclosed its molecular formula to be C31H41NO8S which is the same of 1. Its IR spectrum indicated the existence of hydroxyl groups (3466 cm−1), carbonyl group (1737 cm−1) and olefinic group (1631 cm−1) in the molecule.
The 1H and 13C NMR data of 2 displayed the typical signals of an aldehyde group (δH 9.94 s, H-19; δC 206.8, C-19), a butanolactone ring [δH 5.87 s, H-22; δH 4.93 dd (J = 18.0, 1.8 Hz), H-21a and δH 4.78 dd (J = 18.0, 1.2 Hz), H-21b; δC 173.9, C-23; δC 174.3, C-20; δC 117.9, C-22 and δC 73.3, C-21] and a thiazoline ring [δH 7.68 s, H-2″, δH 4.01 dd (J = 16.8, 1.2 Hz), H-1″a and δH 3.98 dd (J = 16.8, 1.2 Hz), H-1″b; δC 161.9, C-2″ and δC 44.6, C-1″], suggesting compound 2 to be a cardenolide with similar structure of 1. The significant difference between 1 and 2 was found in the signals attributable to glycosidic portion and thiazoline ring (Table 1). The 13C NMR resonances of 2 in sugar and thiazoline moieties shifted downfield at C-2′, C-5′, C-1″ and C-2″ with the differences in the range of 1.2–2.3 ppm, while C-4′ signal shifted upfield with the difference of 2.4 ppm compared with those of 1. The 1H NMR at H-4′a, H-6′, H-1″b and H-2″ shifted downfield, while the H-1′ and H-5′ proton signals shifted upfield with the differences in range of 0.11–0.48 ppm and 0.39–0.41 ppm, respectively.
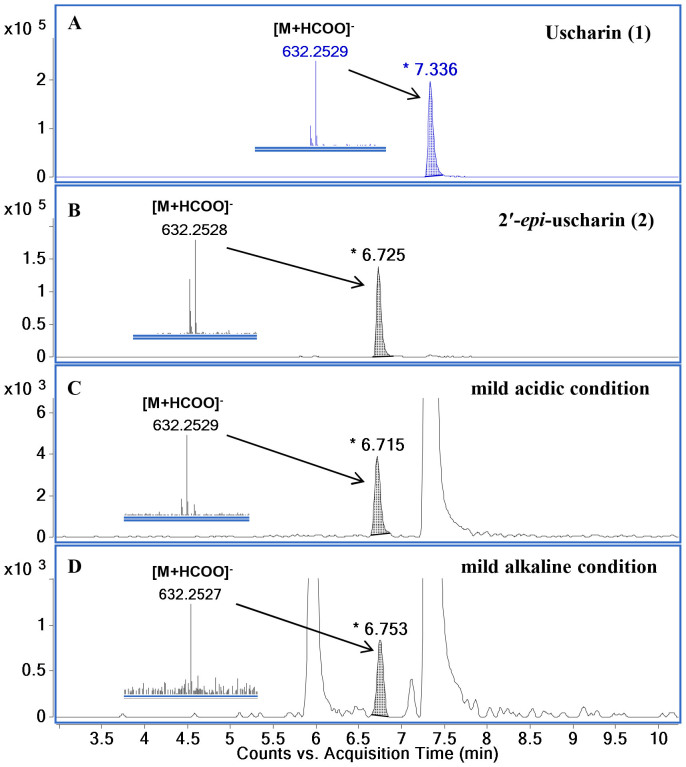
All these spectroscopic data together with similar pattern of their 1H-1H COSY and HMBC correlations (Fig. 2) revealed that the plain chemical structures of 1 and 2 are the same, indicating that 2 is a stereoisomer of 111. Unfortunately, the stereochemistry of 2 in sugar moiety could not be assigned by the correlations obtained from its NOESY spectrum. To prove our expectation, the chemical transformations of 1 to 2 were performed at room temperature under mild acidic and mild alkaline conditions. UHPLC−ESI−TOF−MS analysis was used to monitor the reactions. A product peak of 2 was detected after three and seven days' treatment of 1 in both mild acidic and mild alkaline conditions (Fig. 4). Moreover, 2 was successfully synthesized by using 1 as a starting substrate in a mixture solution of chloroform and methanol containing 2% formic acid with a yield of 3.9%. All these chemical evidences supported that intramolecular hemiketal transformation at C-2′ took place resulting in the conversion of the 2′ hydroxyl group from a β-configuration in 1 to an α-configuration in 2 (Fig. 5)10,11,12. Consequently, a chair conformation of the dioxane ring in 1 converts to a boat conformation in 2 which is a thermodynamically less stable product12. Thus, compound 2 is determined to be a new stereoisomer of 1 and named 2′-epi-uscharin. The complete assignment of 1H and 13C NMR data of 2 is shown in Table 1 in three NMR solvents, i.e., CDCl3, CD3OD and pyridine-d5.
Figure 4.
UHPLC−ESI−TOF−MS chromatograms and HRESIMS data of [M+HCOO]− ion of uscharin (A), 2′-epi-uscharin (B), the reaction mixture in mild acidic condition of 1% formic acid on day 3 (C), and the reaction mixture in mild alkaline condition of 1% ammonium hydroxide on day 7 (D).
Figure 5.
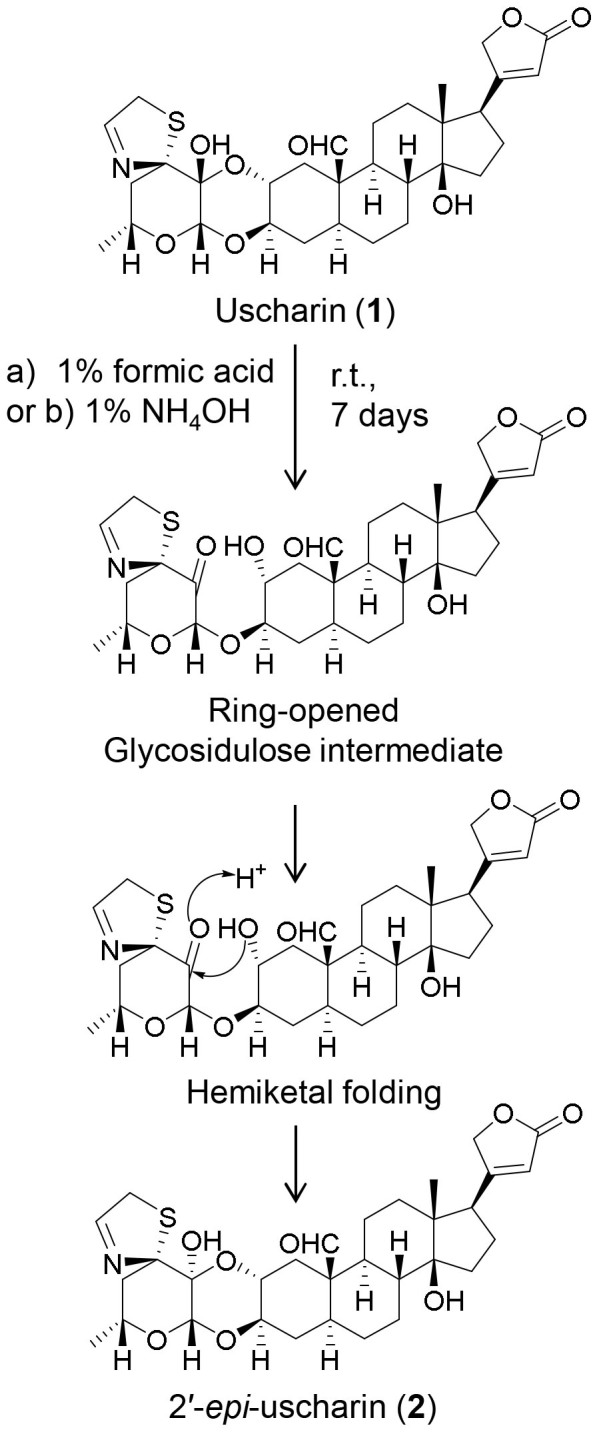
Interconverting between uscharin (1) and 2′-epi-uscharin (2) by intramolecular hemiketal folding of glycosidulose intermediate under a) mild acidic condition, 1% formic acid or b) mild alkaline condition, 1% ammonium hydroxide.
HIF-1 Inhibitory Activity of Compounds 1 and 2
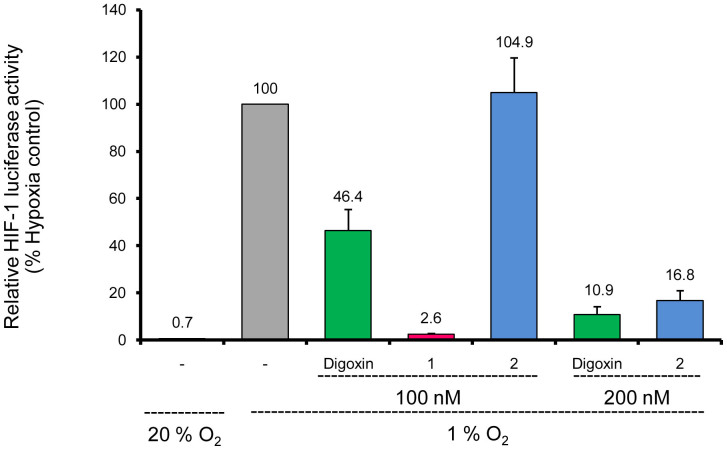
HIF-1 is a transcription factor principally by which cancer cells adapt to the hypoxic microenvironment caused by rapid proliferations. It regulates the expression of hundreds of genes in response to hypoxia, including VEGF, GLUT1, HK1 and HK2, etc. HIF-1 also controls the expression of genes involved in cancer cell immortalization, cancer invasion and metastasis, etc13,14,15,16,17,18,19,20,21. Therefore, compounds that inhibit HIF-1 transcriptional activity show great potential in anticancer therapy. The inhibitory effects of uscharin (1) and 2′-epi-uscharin (2) on HIF-1 transcriptional activity were evaluated by using a T47D cell-based co-transfected firefly and Renilla luciferase reporter assay13. Briefly, human ductal breast epithelial tumor T47D cells were transiently co-transfected with the hypoxia response element (HRE) luciferase and Renilla plasmid which contains Renilla luciferase coding sequences. The HRE contains essential binding sites for HIF-1 which mediates increased transcription in cells that are exposed to hypoxia. Compounds that specifically inhibited hypoxia-induced firefly luciferase activity driven by HIF-1 could be screened out by a decreased ratio of firefly/Renilla luciferase activity under normoxic and hypoxic conditions, compared to that of the blank control (in the absence of any drug)13. As shown in Fig. 6, when the transfected T47D cells were cultured under hypoxic conditions (1% O2), the ratio of firefly/Renilla luciferase activity was 142.9-fold (100/0.7) higher than that when the cells were cultured under normoxic conditions (20% O2) for 24 h. The HIF-1 transcriptional activity in cells treated with digoxin, a well-known HIF-1 inhibitor used as a positive control13, under hypoxic conditions (1% O2) decreased to (46.4 ± 9.1)% and (10.9 ± 3.3)% at the concentrations of 100 nM and 200 nM, respectively (Fig. 6). Under the same tested conditions, 1 remarkably reduced the HIF-1 transcriptional activity to (2.6 ± 0.2)% of that in the blank control (100%), which was 18-fold stronger than digoxin at the concentration of 100 nM. Compound 2 decreased HIF-1 transcriptional activity to (16.8 ± 4.2)% at the concentration of 200 nM, which was comparable to the activity of digoxin. The only difference of an α-configuration at 2′ hydroxyl group in 2 from that of a β-configuration in 1 resulted in a big loss in the inhibitory effect of 2 on HIF-1 transcriptional activity, indicating the importance of the β-configuration at 2′ hydroxyl moiety for such bioactivity.
Figure 6. Inhibition of the hypoxia-induced HIF-1 transcriptional activity of uscharin (1), 2′-epi-uscharin (2) and digoxin by using a T47D cell-based HRE-luciferase reporter assay.
In the present study, uscharin (1) and its stereoisomeric compound 2′-epi-uscharin (2) were isolated from the latex of C. gigantea. Both cardenolides exhibited strong inhibitory effects on HIF-1 transcriptional activity with different magnitudes. Compound 1 displayed much more potent inhibitory activity than digoxin, a positive control, at the concentration of 100 nM, while 2 showed comparable activity with digoxin at the concentration of 200 nM. The bioactive discrepancy between these two epimers indicated the necessity of the β-configuration of 2′-hydroxy group for HIF-1 transcriptional inhibitory activity. This paper is the first report for uscharin and 2′-epi-uscharin as potential HIF-1 inhibitors, and may provide an insight into their anticancer mechanism and guidance for the rational design of effective HIF-1 inhibitors for anticancer drug research and development17,18,19,20,21,22,23,24,25,26. Moreover, this is a first report of the complete assignment of 1H NMR data of uscharin along with its X-ray crystallographic structure.
Methods
General Experimental Procedures
Optical rotations were measured on a Rudolph Research Analytical Autopol I automatic polarimeter. UV spectrum was obtained from a Beckman Coulter DU800 spectrophotometer. IR spectrum was performed on a Perkin Elmer Spectrum One Fourier transform infrared spectrometer. The 1H, 13C and 2D NMR experiments were measured on a Bruker Ascend® 600 NMR spectrometer (600 MHz for 1H and 150 MHz for 13C) with the solvent signal as internal reference. High resolution mass spectra were performed on an Agilent 6230 electrospray ionization (ESI) time-of-flight (TOF) mass spectrometer. UHPLC analyses were carried out on an Agilent 1290 Infinity LC system using an ACQUITY UPLC® BEH C18 column (1.7 µm, 100 × 2.1 mm, Waters®).
Chemicals and Reagents
Absolute ethanol, AR grade, was purchased from Merck (Germany). Chloroform and ethyl acetate, AR grade, were bought from RCI Labscan Limited (Bangkok, Thailand). Methanol, acetronitrile, HPLC grade, and ammonium solution, AR grade, were obtained from Tedia (USA). Formic acid, MS grade, was bought from Sigma-Aldrich (USA). NMR solvents (CDCl3, CD3OD, pyridine-d5) were purchased from Cambridge Isotope Laboratories, Inc. (USA). Digoxin was obtained from Aladdin Industrial Corporation (China). The purity of all tested compounds was more than 95% determined by UHPLC analysis.
Plant Material
The latex of C. gigantea was collected during August–October 2011 from uncultivated land in Lampang, Thailand (latitude/longtitude at 17° 36′ 9″ N/99° 12′ 50″ E). The herbarium specimen was from a shrub which was authenticated by Dr. Li-Ping Bai. A voucher specimen (No. MUST-CG201011) was deposited at State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology.
Extraction and Isolation
The latex of C. gigantea (3 L) was added 95% ethanol to produce a filterable precipitate7. The mixture was sonicated at room temperature then centrifuged. The supernatant was evaporated under reduced pressure to afford a light yellowish residue (240.0 g). The residue was partitioned between EtOAc and H2O and the resulting EtOAc extract (20.7 g) was subjected to normal phase column chromatography (silica gel, CHCl3−MeOH−H2O, 10:0:0 to 6:4:1) to obtain 8 fractions (Fr.1 to Fr.8). Fr.3 (4.0 g) was chromatographed on medium pressure liquid chromatography (C18, MeOH−H2O, 1:1 to 95:5) to give 8 fractions (Fr.3-1 to Fr.3-8). Fr.3-6 (1.3 g) was rechromatographed on medium pressure liquid chromatography under the same above conditions to afford Fr.3-6-1 and uscharin (1, 1.2 g). Fr.3-6-1 (7 mg) was further purified by preparative high performance liquid chromatography (C18, ACN−H2O, 3:7 to 8:2) to afford 2′-epi-uscharin (2, 1 mg).
Uscharin Single Crystal Cultivation
The clear solution of uscharin (20 mg) in MeOH (20 mL) was added several drops of water, and then was kept at ambient temperature for slow evaporation to cultivate a single crystal suitable for X-ray crystallographic measurement.
X-ray Crystallographic Analysis of 1
X-ray diffraction data were collected using an Agilent Gemini S Ultra diffractometer equipped with a Sapphire CCD detector and a graphite monochromated MoKα radiation, λ = 0.71079 Å at 150.0(2) K. Crystal data: C31H41O8NS, M = 520.64 587.71, orthorhombic, space group P212121; unit cell dimensions were determined to be a = 12.5169(4) Å, b = 12.7791(3) Å, c = 18.3728(5) Å, V = 2938.82(14) Å3, Z = 4, Dx = 1.328 g/cm3, F (000) = 1256, μ (MoKα) = 0.162 mm−1. 11064 reflections were collected until θmax = 29.43°, in which independent unique 5369 reflections were observed [F2 > 4σ (F2)]. The structure was solved by direct methods using the SHELXS-97 program, and refined by the full-matrix least-squares calculations. In the structure refinements, hydrogen atoms bonded to carbons were placed geometrically calculated positions, and positions for hydrogen atoms bonded to oxygen were determined from difference Fourier syntheses and included in the calculation of structure factors with isotropic temperature factors. The final refinement gave R = 0.0369, RW = 0.0459, and S = 1.030.
Crystal data of compound 1 was deposited at the Cambridge Crystallographic Data Centre (CCDC number: 989060).
Chemical Transformation of 1 to 2
Uscharin (1) solution of 2 mL (1 mg/mL, MeOH) was added either formic acid (20 μL) or ammonium solution (ammonium hydroxide, 29.3% NH3 basis, 68 μL). The reaction mixtures in both conditions were continuously stirred at room temperature and monitored by UHPLC−ESI−TOF−MS. In order to obtain more compound 2, compound 1 (200 mg) was dissolved in a mixture solution (40 mL) of chloroform and methanol containing 2% formic acid. The reaction solution was continuously stirred under room temperature for 7 days before subjecting to a reverse phase column chromatography (C18, MeOH−H2O, 4:6 to 10:0) to afford compound 2 (7.8 mg).
Reporter Assay for Inhibiting HIF-1 Transcriptional Activity
T47D cells (American Type Culture Collection) were cultured in DMEM medium (Invitrogen), supplemented with 10% (v/v) fetal bovine serum (FBS) (Invitrogen), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) in a humidified atmosphere (5% CO2 and 95% air) at 37°C. Cells (5 × 106 cells) were co-transfected with the HRE-luciferase (Addgene) and Renilla plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The transfected cells were seeded in 96-well plates and cultured in DMEM with 10% FBS for 12 h. In the next day, the cells were treated with tested compounds (100 nM and 200 nM) for 1 h, and then were exposed to hypoxic (1% O2/5% CO2/94% N2) or normoxic (5% CO2/95% air) conditions at 37°C for 24 h. The cells were finally lysed, and luciferase activities of both HRE and Renilla were measured by Dual-Luciferase® reporter assay (Promega) kit according to manufacturer's instructions by using a multimode reader (Infinite 200 PRO, Tecan). HIF-1 transcriptional activity was shown by the ratio of firefly/Renilla luciferase activity. The data were repeated by three independent experiments.
2′-Epi-uscharin (2)
white, amorphous powder; [α]25D −9.62 (c 0.100, MeOH); UV (MeOH) λmax (log ε) = 218 (4.01) nm; IR (KBr) νmax 3466, 2929, 1737, 1631, 1064 cm−1, 1H NMR (600 MHz, CDCl3, CD3OD, pyridine-d5) and 13C NMR (150 MHz, CDCl3, CD3OD, pyridine-d5), see Table 1; HRESIMS m/z 632.2528 [M+HCOO]− (calcd for [C31H41NO8S+HCOO]−, 632.2535).
Supplementary Material
2′-Epi-uscharin from the Latex of Calotropis gigantea with HIF-1 Inhibitory Activity
Acknowledgments
This work was financially supported by Macao Science and Technology Development Fund, MSAR (056/2013/A2 to L.P.B.). The content is solely the responsibility of the authors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions L.P.B., Z.H.J. and L.L. designed experiments. S.P., G.Y.Z. and R.W.J. analyzed data. S.P., L.P.B. and Z.H.J. wrote the paper. S.P., G.Y.Z. and L.P.B. conducted experiments.
References
- Pari K., Rao P. J., Devakumar C. & Rastogi J. N. A novel insect antifeedant nonprotein amino acid from Calotropis gigantea. J. Nat. Prod. 61, 102–104 (1998). [DOI] [PubMed] [Google Scholar]
- Lhinhatrakool T. & Sutthivaiyakit S. 19-Nor- and 18, 20-epoxy-cardenolides from the leaves of Calotropis gigantea. J. Nat. Prod. 69, 1249–1251 (2006). [DOI] [PubMed] [Google Scholar]
- Silva M. C. C. et al. Therapeutic and biological activities of Calotropis procera (Ait.) R. Br. Asian Pac. J. Trop. Med. 3, 332–336 (2010). [Google Scholar]
- Seiber J. N., Nelson C. J. & Lee S. M. Cardenolides in the latex and leaves of seven Asclepias species and Calotropis procera. Phytochemistry 21, 2343–2348 (1982). [Google Scholar]
- Wang Z. N., Wang M. Y., Mei W. L., Han Z. & Dai H. F. A new cytotoxic pregnanone from Calotropis gigantea. Molecules 13, 3033–3039 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S., Das P., Itoh T., Imai K. & Matsumoto T. Latex extractables of Calotropis gigantea. Phytochemistry 23, 2085–2087 (1984). [Google Scholar]
- Bhaskara Rama Murti P. & Seshadri T. R. Chemical composition of Calotropis gigantea. Part I. Wax and resin components of the latex. Proc. Indian Acad. Sci., Section A 18, 145–159 (1943). [Google Scholar]
- Cheung H. T. A., Chiu F. C. K., Watson T. R. & Wells R. J. Cardenolide glycosides of the Asclepiadaceae. New glycosides from Asclepias fruticosa and the stereochemistry of uscharin, voruscharin and calotoxin. J. Chem. Soc., Perkin Trans. 1, 2827–2835 (1983). [Google Scholar]
- Bruschweiler F., Stocklin W., Stockel K. & Reichstein T. Glycosides of Calotropis procera R.Br. Glycosides and aglycones. Helv. Chim. Acta. 52, 2086–2106 (1969). [DOI] [PubMed] [Google Scholar]
- Bruschweiler F., Stockel K. & Reichstein T. Calotropis glycosides, probable partial structure. Glycosides and aglycones. Helv. Chim. Acta. 52, 2276–2303 (1969). [DOI] [PubMed] [Google Scholar]
- Cheung H. T. A., Chiu F. C. K., Watson T. R. & Wells R. J. Reduction of hemiacetal ring-opened forms of Asclepiadaceae cardenolide glycosides. J. Chem. Soc., Perkin Trans. 1, 55–59 (1986). [Google Scholar]
- Lichtenthaler F. W., Cuny E. & Sakanaka O. A concise and general method for doubly attaching 2-ketosugars to aglycon diols: synthesis of the gomphosides and spectinomycin. Angew. Chem. Int. Ed. Engl. 44, 4944–4948 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Digoxin and other cardiac glycosides inhibit HIF-1 α synthesis and block tumor growth. Proc. Natl. Acad. Sci. USA. 105, 19579–19586 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M. Masters of angiogenesis. Nat. Med. 11, 925–927 (2005). [DOI] [PubMed] [Google Scholar]
- Giaccia A., Siim B. G. & Johnson R. S. HIF-1 as a target for drug development. Nat. Rev. Drug Discov. 2, 803–811 (2003). [DOI] [PubMed] [Google Scholar]
- Zhao T. et al. HIF-1-mediated metabolic reprogramming reduces ROS levels and facilitates the metastatic colonization of cancers in lungs. Sci. Rep. 4, 3793; 10.1038/srep03793 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T., Okuyama H., Nishizawa Y., Tomita S. & Inoue M. Hypoxia inducible factor-1α is necessary for invasive phenotype in Vegf-deleted islet cell tumors. Sci. Rep. 2, 494; 10.1038/srep00494 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Li Y., Cornelia R., Swisher S. & Kim H. Regulation of VEGF expression by HIF-1α in the femoral head cartilage following ischemia osteonecrosis. Sci. Rep. 2, 650; 10.1038/srep00650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienes-Martínez R. et al. Autocrine stimulation of clear-cell renal carcinoma cell migration in hypoxia via HIF-independent suppression of thrombospondin-1. Sci. Rep. 2, 788; 10.1038/srep00788 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M. et al. Prostatic ischemia induces ventral prostatic hyperplasia in the SHR; possible mechanism of development of BPH. Sci. Rep. 4, 3822; 10.1038/srep03822 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D. S. et al. Structure-based discovery of natural-product-like TNF-α inhibitors. Angew. Chem. Int. Ed. Engl. 49, 2860–2864 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C. H. et al. A natural product-like inhibitor of NEDD8-activating enzyme. Chem. Commun. 47, 2511–2513 (2011). [DOI] [PubMed] [Google Scholar]
- Leung C. H. et al. A metal-based inhibitor of tumor necrosis factor-α. Angew. Chem. Int. Ed. Engl. 51, 9010–9014 (2012). [DOI] [PubMed] [Google Scholar]
- Welsh S. J. et al. Inhibition of the hypoxia-inducible factor pathway by a G-quadruplex binding small molecule. Sci. Rep. 3, 2799; 10.1038/srep02799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J. et al. Inhibition of endoplasmic reticulum stress alleviates lipopolysaccharide-induced lung inflammation through modulation of NF-kB/HIF-1α signaling pathway. Sci. Rep. 3, 1142; 10.1038/srep01142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2′-Epi-uscharin from the Latex of Calotropis gigantea with HIF-1 Inhibitory Activity