Abstract
Background: The diagnostic role of circulating anti-phospholipase A2 receptor antibodies (anti-PLA2R Abs) is now well recognized in idiopathic membranous nephropathy (iMN). These Abs could also be interesting as predictors of clinical outcome. In this study, we explored the prognostic value of anti-PLA2R Abs measured in a cohort of iMN patients, with a special focus on their ability to detect patients achieving spontaneous remission.
Methods: All adult patients with biopsy-proven iMN diagnosed between 1978 and 2007 were retrospectively screened in our centre. Using a validated enzyme-linked immunosorbent assay, levels of anti-PLA2R Abs were measured from serum samples obtained at the time of renal biopsy and stored at −80°C until processing. Clinical data on disease activity, treatments and outcomes were collected by reviewing patients’ medical records. The association between anti-PLA2R Ab titres and clinical activity/outcome was assessed by Cox proportional hazard and Kaplan–Meier methods.
Results: In this retrospective study, 68 patients were included in the final analysis (median follow-up of 81 months). No significant association was found between anti-PLA2R Ab titres at diagnosis with baseline proteinuria, baseline estimated glomerular filtration rate or chronic kidney disease progression. Spontaneous remission was observed in 22% of patients. Ab titres were significantly and gradually correlated in a dose–response manner with the likelihood of spontaneous remission.
Conclusions: While Ab titres measured at diagnosis were not found to predict the activity of iMN, evaluation of anti-PLA2R Ab titres might prove useful in the early identification of patients likely to achieve spontaneous remission.
Keywords: antibody, disease activity, idiopathic membranous nephropathy, phospholipase A2 receptor, spontaneous remission
Introduction
While immunosuppressive therapeutic regimens are now well validated for the management of patients with idiopathic membranous nephropathy (iMN) [1], such treatments can lead to potentially serious adverse events and must be reserved for patients who could really benefit from the treatment. This point is particularly important, given that approximately one-third of patients with iMN are expected to achieve spontaneous remission with supportive therapy alone [1]. In this context, the recommended strategy is to not introduce immunosuppressive drugs for at least 6 months after diagnosis and to accept implicitly the risk of delaying beneficial treatment for the majority of patients.
M-type phospholipase A2 receptor 1 (PLA2R) has been described as a major antigen in iMN, with anti-PLA2R antibodies (Abs) present in ∼70% of incident patients [2, 3]. The specificity of anti-PLA2R Abs for the diagnosis of iMN, albeit not absolute, is now well established [3–5]. Additionally, several studies have reported a significant correlation between anti-PLA2R Ab titres and factors related to iMN activity, suggesting that these Abs could also be useful in the prediction of disease outcome [4, 6–12].
In this regard, two reports have recently highlighted the potential use of anti-PLA2R Abs to predict spontaneous remission of iMN. Hofstra and colleagues were the first to report that spontaneous remission occurred significantly less frequently in patients with high Ab titres [6]. However, anti-PLA2R Ab measurements were not necessarily made at disease diagnosis and could be delayed by up to 6 months after biopsy. Timmermans and colleagues found that, of 109 patients with iMN, those seronegative for anti-PLA2R Abs had a higher probability for spontaneous remission, compared with seropositive patients. While, in this study, Abs were measured at the time of biopsy, it was, however, not reported whether the Ab titre itself was predictive [5]. Taken together, these results do not provide definitive conclusions on the potential for anti-PLA2R Abs to predict spontaneous remission of iMN.
In this study, we explored the prognostic value of anti-PLA2R Abs measured at the time of diagnostic biopsy in a cohort of iMN patients, with particular focus on using the Abs for the early detection of those who will achieve spontaneous complete remission.
Materials and methods
Patients
In this retrospective study, all adult patients with biopsy-proven iMN diagnosed between January 1978 and December 2007 at the Nephrology Department of Saint-Etienne were included. Potential secondary causes were ruled out using serology, chest X-ray and routine immunological investigations. In our centre, an initial period of supportive treatment (anti-hypertensive and/or anti-proteinuria treatment) was proposed to all patients with suspected iMN for at least 3 months before discussing immunosuppressive treatment.
At the time of diagnostic renal biopsy, each patient agreed to have their Deoxyribonucleic acid (DNA) and serum samples taken and stored at the Nephrology Biological Resources facility. Use of this bio-collection for clinical research was approved by the Commission Nationale Informatique et Liberté and our local institutional board.
Patients on immunosuppressive drug treatment at the time of renal biopsy were not included in the final analysis.
Data collection and outcome definitions
Data on patient characteristics, disease activity, treatment and outcome were obtained by reviewing patients’ medical records.
Complete remission was defined as proteinuria <0.5 g/24 h observed on at least two consecutive consultations. Spontaneous remission was defined as the occurrence of complete remission in the absence of any immunosuppressive drugs. Chronic kidney disease (CKD) stages were defined using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation [end-stage renal disease was defined by either an estimated glomerular filtration (eGFR) rate below 15 mL/min/1.73 m2 (CKD stage 5) or the necessity for dialysis/transplantation].
PLA2R antibody measurements
Serum samples were stored at −80°C and thawed simultaneously for anti-PLA2R Ab measurements by enzyme-linked immunosorbent assay (ELISA). The anti-PLA2R assay was developed by Dr Lambeau’s laboratory (Nice, France) [13]. Briefly, pure recombinant human PLA2R protein, corresponding to the entire extracellular domain, was coated onto ELISA plates in 20 mM Tris pH 8.0 (100 µL/well, 1 µg/mL) and kept at 4°C overnight. Plates were blocked for 2 h with SeramunBlock (Seramun Diagnostica). Patient serum samples were diluted at 1:100 in phosphate-buffered saline/0.1% dry milk and added in duplicate (100 µL/well) to the ELISA plates, which also contained a serial dilution of an iMN standard serum and a quality control calibrator (between plates). After 2 h of incubation at room temperature on a plate shaker, plates were washed four times with phosphate-buffered saline/0.02% Tween 20. Anti-human IgG–horseradish peroxidase conjugate (Southern Biotech #9200-05) diluted 1:7500 in SeramunStab ST plus (Seramun Diagnostica) was added (100 µL/well) and the plates incubated for 1 h at room temperature on a plate shaker. After four washes, tetramethylbenzidine was added, and the reactions were developed for 15 min and then stopped with hydrochloric acid 1.2 N. The plates were read at 450 nm. Sixty-seven serum samples from healthy donors were used to define the normal range, using the mean + 3 standard deviations (SDs). The cut-off value was optimized by receiver operating characteristics curve analysis (cut-off value = 128 RU/mL). A highly positive index patient serum sample was used in each plate to generate a standard curve and a negative control. The PLA2R ELISA test for iMN patients had 100% specificity, 51% sensitivity, 100% positive predictive value, 42.5% negative predictive value and 0.8554 area under the curve [P < 0.001, 95% confidence interval (CI) 0.7905–0.9204].
Distribution of PLA2R Ab titres in tertiles was carried out for the entire study population, including seronegative patients.
Statistical analysis
Data were expressed as mean ± SD if normally distributed, as median (minimum–maximum) if not normally distributed or as frequency. The normality of distribution was assessed by a normal quantile–quantile plot. Patients were grouped in increasing tertiles of anti-PLA2R Ab titres. The relationship between the GFR and proteinuria and tertiles of Ab titres was analysed using the Kruskal–Wallis test. Survival and cumulative incidence curves were drawn using the Kaplan–Meier method. Survival analysis was carried out using univariate and multivariate Cox proportional hazard models. All analyses were performed using R logiciel [14] or GraphPad Prism version 6.00 for Windows.
Results
Patient characteristics
Sixty-eight patients, the majority male, with a mean age of 47 years (± 24) were included in the final analysis. Median follow-up was 81 months. Patient characteristics at baseline for the entire cohort and according to tertiles of anti-PLA2R Ab titres are presented in Table 1. A total of 37 (54%) patients received immunosuppressive treatment during the disease course. Patients were treated with either a combination of alkylating agents and corticosteroids [20 patients (29% of study population)], a combination of a calcineurin inhibitor and corticosteroids [seven patients (10%)], corticosteroids alone [19 patients (28%)] and/or rituximab [five patients (7%)] (Table 2).
Table 1.
Patient characteristics
| Characteristics | Population | First tertile | Second tertile | Third tertile |
|---|---|---|---|---|
| Patients | 68 | 23 | 23 | 22 |
| Anti-PLA2R antibody level (RU/mL) | 345.3 (0–8923) | 1 (0–25.6) | 387.3 (30.3–986.5) | 3589.1 (1034.7–8923) |
| Males | 45 (66) | 13 (57) | 15 (65) | 17 (77) |
| Age (years) | 47 (18–86) | 46 (24–86) | 51 (18–79) | 44 (18–73) |
| Proteinuria (g/24 h) | 3.4 (0.3–52–1) | 3.5 (0.7–12.2) | 4.5 (0.3–52.1) | 2.9 (0.6–13) |
| Serum creatinine (µmol/L) | 88 (46–205) | 81 (46–205) | 88 (46–146) | 94.5 (54–161) |
| Follow-up (months) | 81 (7–415) | 78.4 (22–271) | 91.6 (22–415) | 82 (7–353) |
Data are n (%) or median (minimum–maximum values).
Table 2.
Patient outcomes and immunosuppressive therapies during follow-up
| Outcomes | Population | First tertile | Second tertile | Third tertile | P-value |
|---|---|---|---|---|---|
| Complete remission | 25 (36.7) | 9 | 10 | 6 | 0.51 |
| Spontaneous remission | 15 (22) | 8 | 5 | 2 | 0.11 |
| Stage 3 CKD | 34 (50) | 11 | 11 | 12 | 0.94 |
| Stage 5 CKD | 17 (25) | 5 | 5 | 7 | 0.67 |
| Any IS regimen | 37 (54.4) | 6 | 13 | 18 | |
| Alkylating agent-based regimen | 20 (29.4) | 4 | 5 | 11 | |
| Calcineurin inhibitor-based regimen | 7 (10.3) | 0 | 3 | 4 | |
| Corticosteroids alone | 19 (27.9) | 3 | 7 | 9 | |
| Rituximab | 5 (7.4) | 0 | 2 | 3 | |
| Two or more IS regimens | 13 (19.1) | 1 | 3 | 9 |
IS, immunosuppresive.
Data are n (%). Any IS regimen includes patients treated with steroids alone or with any other IS regimen during follow-up. Ten patients were treated only with steroids (without any other IS therapy during follow-up). Nine patients were treated at one time with steroids alone and later with another IS regimen during follow-up (three patients received an alkylating agent combined with steroids, two patients received rituximab alone and four patients received calcineurin inhibitors later during follow-up). Of 19 patients, 12 (63%) did not achieve remission after treatment (steroid-resistant). Patients treated with steroids alone received 6 months of treatment (with or without an initial intravenous bolus). Twenty patients received a combination of alkylating agents and steroids (alkylating agent-based regimen). Seven patients were treated with a calcineurin inhibitor combined with steroids (calcineurin inhibitor-based regimen) and five patients with rituximab during follow-up.
Overall complete remission was observed in 25 patients (36.7%) and occurred spontaneously in 15 patients (22%).
Association between anti-PLA2R Ab levels and disease activity/outcome
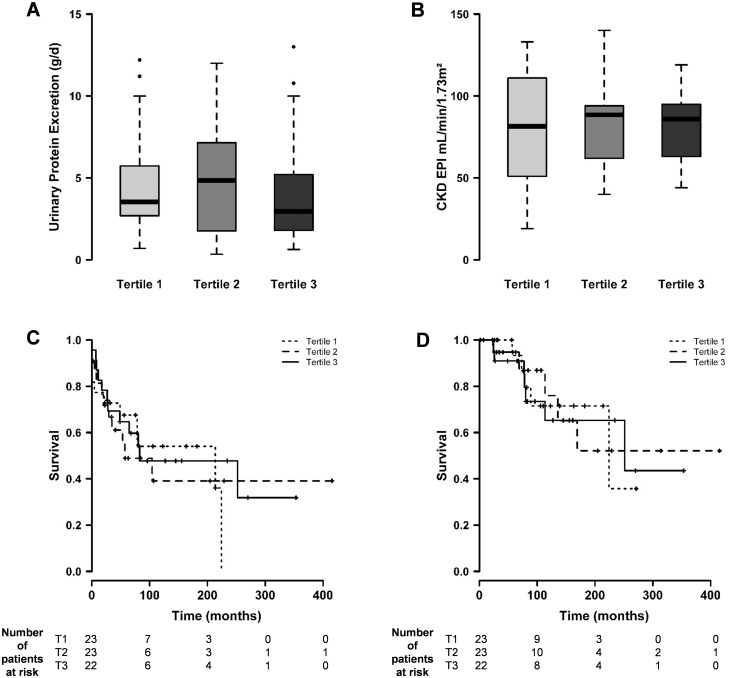
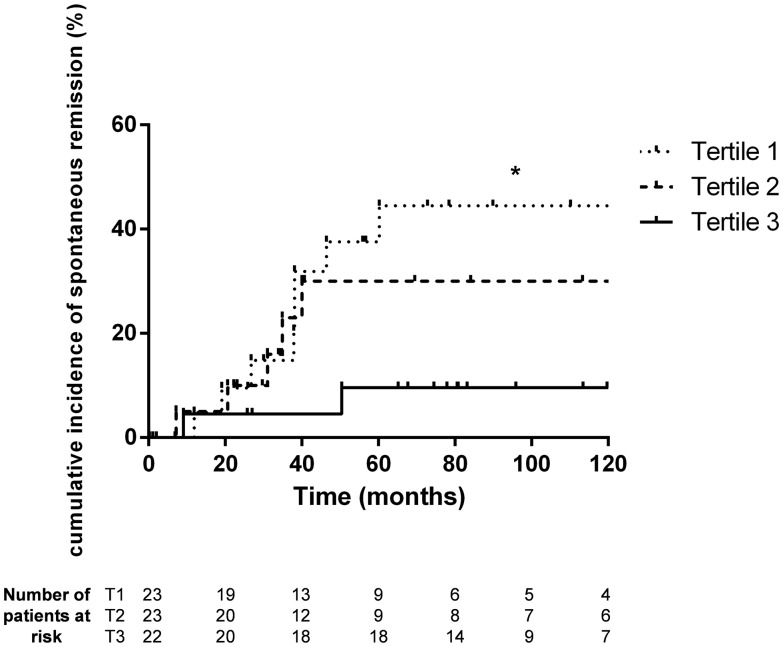
At diagnosis, 41 patients (60%) tested seropositive for anti-PLA2R Abs. No association was observed between anti-PLA2R Ab titres distributed in tertiles and the degree of baseline proteinuria. Similarly, Ab titres were not associated with baseline eGFR values or with the risk of developing stage 3 and 5 CKD (Figure 1 and Table 2). In contrast, we observed a significant and gradual dose–response relationship between anti-PLA2R Ab titres and the likelihood of spontaneous remission (log rank test, P = 0.048) (Figure 2). In Cox multivariate analysis, anti-PLA2R Ab titres were found to be independently associated with spontaneous remission (hazard ratio = 0.378, 95% CI 0.17–0.84, P = 0.017) (Table 3).
Fig. 1.
(A and B) Association between anti-PLA2R antibody levels and disease activity at diagnosis (A = urinary protein excretion, Kruskal–Wallis test, P = 0.64; B = glomerular filtration rate, Kruskal–Wallis test, P = 0.99). (C and D) Association between anti-PLA2R antibody levels and the development of stage 3 (C) and stage 5 (D) CKD (log rank test, P = 0.68 and P = 0.83, respectively). T1, T2 and T3, tertiles 1, 2 and 3.
Fig. 2.
Incidence of spontaneous remission according to the tertile of anti-PLA2R antibody titre. Log rank test, P-value = 0.048 for comparison between all tertiles. Log rank tests for comparison between each tertile: tertile 1 versus tertile 3, P = 0.0167; tertile 1 versus tertile 2, P = 0.38; and tertile 2 versus tertile 3, P = 0.129. *P < 0.05. T1, T2 and T3, tertiles 1, 2 and 3.
Table 3.
Factors associated with spontaneous remission (after Cox multivariate analysis)
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| Male sex | 1.999 (0.61–6.92) | 0.330 |
| Proteinuria at diagnosis | 0.747 (0.55–1.01) | 0.054 |
| eGFR at diagnosis | 0.991 (0.97–1.01) | 0.420 |
| Anti-PLA2R Ab titre | 0.378 (0.17–0.84) | 0.017 |
Discussion
The main result of our study confirmed the association between anti-PLA2R Ab titres measured at the time of renal biopsy in iMN patients and the likelihood of spontaneous complete remission, with a chance of remission three times higher in patients from the lowest tertile of Ab titre. While Hoxha and colleagues [9] did not report similar results in a cohort of 12 iMN patients not treated with immunosuppressive drugs and followed over a period of 15 months, this association has been previously suggested by others [5, 6]. Our data obtained in a large cohort over a long follow-up period give further credence to a relationship between anti-PLA2R Abs and spontaneous remission. Our study supports this relationship by using a real quantitative assay, which was systematically carried out at the time of diagnosis. This method is of importance since it suggests that anti-PLA2R Ab quantification could be included as part of the initial diagnostic investigation of patients with membranous nephropathy. In this respect, our data seem to be in perfect agreement with those reported recently by Timmermans and colleagues [15], with the exception that we considered only complete spontaneous remission whereas the Timmermans group analysed both complete and partial remissions. Interestingly, our data are also directly in line with those from the recent study by Ruggenenti and colleagues [16], showing that, in patients treated with rituximab, complete remission was preceded by complete Ab depletion. Overall, low anti-PLA2R Ab titres, either observed as naturally present at disease diagnosis or induced by immunosuppressive drugs during the course of the disease, are associated with the likelihood of iMN remission.
We detected Abs against PLA2R in 60% of patients with iMN at inclusion, which is comparable to what has been reported [2–9, 17–19]. In our analysis, we purposely included iMN patients with no detectable anti-PLA2R Abs since seronegativity has been previously reported to be associated with a favourable outcome associated with iMN [5]. Additionally, for a given ELISA test, seronegativity is defined according to a pre-defined threshold that shows good diagnostic performance in terms of sensitivity and specificity. This threshold can, however, be lowered without necessarily altering the specificity of the test [5], meaning that, among the so-called seronegative patients, some of them might actually have clinically relevant anti-PLA2R Ab titres, albeit at a quite low level. Conversely, one may speculate that patients may test negative for anti-PLA2R Abs while testing positive for others. In this scenario, it would be questionable to retain anti-PLA2R-seronegative patients in the lower tertile since some of them might nevertheless present strong membranous nephropathy specific autoreactivity. For instance, circulating auto-Abs directed against thrombospondin type-1 domain-containing 7A have recently been described in ∼10% of anti-PLA2R Ab-negative patients [20]. Whether there is a relationship between the titres of anti-thrombospondin type-1 domain-containing 7A and the likelihood of iMN remission has, however, not been explored yet.
Overall, we believe that patients negative for anti-PLA2R Abs at diagnosis can be generally considered as being part of the group of those with the highest probability of progressing towards spontaneous remission, even though a minority of these patients are likely to be positive for other auto-Abs of as yet unexplored clinical significance.
Importantly, when restricting our analysis to the subgroup of seropositive patients, we still observed a similar relationship between low anti-PLA2R Ab titres and the probability of spontaneous remission (even though this association was no longer statistically significant, probably due to a lack of statistical power; n = 41, data not shown).
We did not find an association between anti-PLA2R Ab titres and the severity of iMN as evaluated by proteinuria range and serum creatinine at diagnosis. So far, conflicting data have been reported in the literature regarding the association between anti-PLA2R Ab titres and the clinical activity of iMN. This apparent discrepancy across studies may have various explanations, including: (i) differences in the diagnostic performance of assays used to monitor anti-PLA2R Abs; (ii) failure of proteinuria and serum creatinine to accurately reflect membranous nephropathy clinical activity when these parameters are evaluated at one single time point, as opposed to their respective dynamic change over time; and (iii) absence of concomitant evaluation of Ab deposition in situ, an evaluation that has been suggested to better categorize patients into different groups (PLA2R-related and non-related iMN) with potential prognostic implications [21–24]. Similarly, anti-PLA2R Abs were not found to be associated with the risk of developing CKD stage 3 or 5 in our cohort, an observation that is shared with some previous studies [8], although not with others [7].
The strengths of our study reside in the single-centre study design, which made the management and care of included patients more homogeneous, the relatively long follow-up period, which allowed us to evaluate long-term outcome endpoints, and the setting up and careful preservation of the bio-collection of serum samples, which served to subsequently analyse sera obtained at histological diagnosis. Several limitations to our study must be mentioned. First, its retrospective design did not allow us to control all potential confounders that might have biased the association between Ab titres and outcome. For instance, more patients in the higher tertile of anti-PLA2R Ab titres had received immunosuppressive treatment, an intervention that interferes with the natural history of the disease and could affect the relationship between baseline Ab titres and the risk of progressive CKD. Second, while we observed a gradual dose–response association between anti-PLA2R Abs and spontaneous remission, we were not able to define a threshold of Ab titres to accurately discriminate between patients who did and those who did not progress towards spontaneous remission. This may be due to the relatively low number of patients included in our analysis, so clearly larger studies and/or meta-analyses of existing studies will be necessary to clarify this issue. Third, since we collected sera at the time of diagnosis only, we were not able to evaluate the clinical potential of the longitudinal change in Ab titres, a dynamic parameter that might have better predictive ability [9, 25].
Conclusions
Our data show that anti-PLA2R Ab titres measured at the time of diagnosis in patients with iMN are inversely and independently associated with the likelihood of achieving spontaneous remission. Before implementing the evaluation of anti-PLA2R Ab titres in clinical practice for this specific purpose, further research is needed to confirm this association in larger populations and to define whether a discriminative threshold of Ab titres can be determined.
Conflict of interest statement
We had no involvements that might raise a question of bias in the work reported or in the conclusions, implications or opinions stated.
REFERENCES
- 1. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012; 2: 139–274 [Google Scholar]
- 2. Beck LH, Bonegio RGB, Lambeau G. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Du Y, Li J, He F. et al. The diagnosis accuracy of PLA2R-AB in the diagnosis of idiopathic membranous nephropathy: a meta-analysis. PLoS One 2014; 9: e104936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qin W, Beck LH, Zeng C. et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 2011; 22: 1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Timmermans SA, Damoiseaux JGMC, Heerings-Rewinkel PTJ. et al. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am J Clin Pathol 2014; 142: 29–34 [DOI] [PubMed] [Google Scholar]
- 6. Hofstra JM, Debiec H, Short CD. et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 2012; 23: 1735–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanigicherla D, Gummadova J, McKenzie EA. et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 2013; 83: 940–948 [DOI] [PubMed] [Google Scholar]
- 8. Oh YJ, Yang SH, Kim DK. et al. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS One 2013; 8: e62151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoxha E, Thiele I, Zahner G. et al. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 2014; 25: 1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beck LH, Fervenza FC, Beck DM. et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 2011; 22: 1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoxha E, Harendza S, Pinnschmidt H. et al. M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin J Am Soc Nephrol 2014; 9: 1883–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoxha E, Harendza S, Pinnschmidt H. et al. PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS One 2014; 9: e110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seitz-Polski B, Dolla G, Payré C. et al. Cross-reactivity of anti-PLA2R1 autoantibodies to rabbit and mouse PLA2R1 antigens and development of two novel ELISAs with different diagnostic performances in idiopathic membranous nephropathy. Biochimie 2015; 118: 104–115 [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. 2013
- 15. Timmermans SA, Abdul Hamid MA, Cohen Tervaert JW. et al. Anti-PLA2R antibodies as a prognostic factor in PLA2R-related membranous nephropathy. Am J Nephrol 2015; 42: 70–77 [DOI] [PubMed] [Google Scholar]
- 16. Ruggenenti P, Debiec H, Ruggiero B. et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 2015; 26: 2545–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akiyama S, Akiyama M, Imai E. et al. Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol 2015; 19: 653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hofstra JM, Beck LH, Beck DM, et al. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2011; 6: 1286–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoxha E, Harendza S, Zahner G. et al. An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 2011; 26: 2526–2532 [DOI] [PubMed] [Google Scholar]
- 20. Tomas NM, Beck LH, Meyer-Schwesinger C. et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014; 371: 2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Debiec H, Ronco P.. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 2011; 364: 689–690 [DOI] [PubMed] [Google Scholar]
- 22. Svobodova B, Honsova E, Ronco P. et al. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant 2013; 28: 1839–1844 [DOI] [PubMed] [Google Scholar]
- 23. Larsen CP, Messias NC, Silva FG. et al. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol 2013; 26: 709–715 [DOI] [PubMed] [Google Scholar]
- 24. Qin H-Z, Zhang MC, Le WB. et al. Combined assessment of phospholipase A2 receptor autoantibodies and glomerular deposits in membranous nephropathy. J Am Soc Nephrol 2016; 27: 3195–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bech AP, Hofstra JM, Brenchley PE. et al. Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2014; 9: 1386–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]