Abstract
Background: End-stage renal disease (ESRD) incidence due to Type 2 diabetic nephropathy (DN) is 35–50%, according to the United States Renal Data System.
Methods: A single-center, retrospective cohort study to determine incidence and diagnostic accuracy for Type 2 DN as the primary cause of ESRD (Code 250.40) on the Center for Medicare & Medicaid (CMS) Medical Evidence Report form (CMS2728) submitted at renal replacement therapy initiation. All patients ≥18 years of age with a CMS2728 submitted between 1 March 2006 and 31 March 2015 at a single academic military medical center (ESRD Network 5) were included. Medical records of those with a Code 250.40 diagnosis were reviewed to determine whether they met the Kidney Disease Outcomes Quality Initiative (KDOQI) 2007 criteria for DN.
Results: ESRD incidence secondary to Type 2 DN was 18.7% (56/299 individual CMS2728 submissions over 9.09 years). In all, 12/56 (21.4%) did not meet KDOQI criteria for Type 2 DN. Although all had diabetes, those not meeting criteria had shorter disease duration (P = 0.007), were more likely to have active urine sediment (P = 0.006), and were less likely to have macroalbuminuria (P = 0.037) or retinopathy (P = 0.002) prior to ESRD. On exact logistic regression, retinopathy was significantly associated with KDOQI-predicted DN [odds ratio = 19.16 (confidence interval 2.76–223.7), P = 0.0009].
Conclusions: In this single-center cohort, 21.4% identified as having Type 2 DN as the primary cause of ESRD were incorrectly assigned per KDOQI 2007 clinical criteria. If replicated in larger populations, this could have substantial implications regarding the epidemiology of ESRD in the USA.
Keywords: diabetic nephropathy; end-stage renal disease, ESRD; incidence
Introduction
The incidence of end-stage kidney disease (ESRD) requiring renal replacement therapy (RRT) due to Type 2 diabetic nephropathy (DN) in the Medicare ESRD Program is 35–50%, reported by the United States Renal Data System (USRDS) [1]. At RRT initiation, Type 2 diabetes mellitus (DM) with renal manifestations (Code 250.40) is the most frequently reported primary cause of ESRD on the Centers for Medicare & Medicaid (CMS) ESRD Medical Evidence Report form (CMS2728), accounting for over 40% of all incident ESRD in 2013, with an incidence of approximately 0.2% of the US diabetic population per year [2].
Most receive the diagnosis on clinical presentation alone, based on a well-described and specific disease course [3], and few have a renal biopsy. Although DN has characteristic biopsy findings, there is no specific treatment once established, other than blood pressure and glucose control, and treatment with renin–angiotensin–aldosterone inhibitors. Diagnosis is defined by the 2007 Kidney Disease Outcomes Quality Initiative (KDOQI) practice guidelines [3]. Patients must have either (i) macroalbuminuria (albumin to creatinine ratio >300 mg/g) or (ii) microalbuminuria (30–300 mg protein/g creatinine) and associated diabetic retinopathy, with no evidence of another glomerular or systemic disease associated with ESRD. This includes sudden presentation with nephrotic range proteinuria, active urine sediment or rapid glomerular filtration rate (GFR) decline. It is unknown what percentage of patients identified on the CMS2728 as having Type 2 DN as the primary cause of ESRD actually meet these diagnostic criteria. Because USRDS reports on the causes of ESRD are highly cited and may be used to justify allocation of resources, including research, further scrutiny is warranted. The USRDS Annual Data Report includes the paragraph, ‘Primary Cause of ESRD: a cautionary note’, acknowledging that reliability of clinician-assigned primary causes of ESRD is not well established [4]. Even among patients with glomerular disease diagnoses established by renal biopsy, agreement between the CMS2728 primary cause of ESRD and biopsy results were low, with the cause of ESRD field left blank in 57% of forms [5]. We hypothesized that the CMS2728 would tend to overestimate, rather than underestimate, Type 2 DM as a cause of ESRD.
To investigate this hypothesis, we performed a single center, retrospective cohort study to determine the incidence of Type 2 DM reported as the primary cause of ESRD (Code 250.40) on all adult patients who had a CMS2728 form submitted at RRT initiation over a 9-year period. We further determined the percentage of patients so identified who met KDOQI criteria for Type 2 DN, and compared their demographic and clinic characteristics with those who did not meet KDOQI criteria.
Materials and methods
This was a retrospective, cohort study of all adult military health care beneficiaries (aged 18 years or older) whose CMS2728 form was submitted at initiation of RRT (including kidney transplant) at Walter Reed Army Medical Center/Walter Reed National Military Medical Center, located in ESRD Network 5. The study was conducted between 1 March 2006 and 31 March 2015. The beginning of this time period coincided with implementation of the present CMS2728. On the form, ESRD secondary to Type 2 DM is coded as 250.40. The study was reviewed and approved by the Walter Reed National Military Medical Center Institutional Review Board (Study #413443), as a minimal risk protocol with waiver of informed consent.
Subjects were identified from the Nephrology Service Social Work CMS2728 database, during the time period above. CMS2728 forms were counted to determine incidence denominator. If their RRT modality changed, individual ESRD patients may have had more than one CMS2728 form submitted. In individual patients who had forms dated within 90 days of one another, the earliest dated form was included, and the other(s) excluded. If forms were dated >90 days apart, both submissions were counted toward the incidence denominator.
The database was sorted to identify all those who received a diagnosis code 250.40 (Diabetes with Renal Manifestations Type 2), who were then counted for the incidence numerator, and entered as subjects into the subsequent part of the study. Patients with ESRD secondary to Type 1 DM (Code 250.41) were excluded. Copies of the CMS2728 for each of these were reviewed, as well as the information from the electronic medical record in the years preceding RRT initiation, extending back to 2003. Pertinent demographic and clinical data were collected to include age at ESRD, sex, race, year of RRT initiation, type of RRT (peritoneal dialysis, hemodialysis or transplantation), year of Type 2 DM diagnosis, year of DN diagnosis, diabetes medications, HgbA1C at RRT initiation, body mass index at RRT initiation, history of smoking and history of hypertension/years of hypertension at RRT initiation.
Clinical and historical information were collected to determine whether the subjects met KDOQI 2007 criteria for diagnosis of Type 2 DN: serum creatinine, imaging studies, urine sediment at diagnosis, other renal diagnoses, macroalbuminuria at diagnosis (and amount), microalbuminuria at diagnosis (and amount), retinopathy diagnosis and date of positive or negative ophthalmologic examination, neuropathy diagnosis and date, gastropathy diagnosis and date, CHF diagnosis and date, presence of vascular disease and manifestation. If kidney biopsy was performed, diagnosis was recorded.
Type 2 DN as the cause of ESRD was based on the KDOQI 2007 criteria [3], and was deemed present if: (i) biopsy diagnosis of DN was present, (ii) macroalbuminuria (albumin to creatinine ratio > 300 mg/g) was present or (iii) microalbuminuria (30–300 mg protein/g creatinine) and associated diabetic retinopathy was present.
The diagnosis of Type 2 DN was questioned if the subject presented initially with nephrotic syndrome, active urine sediment, an alternative diagnosis, rapidly declining estimated GFR (eGFR), or was without micro- or macroalbuminuria. Records were reviewed by a primary and secondary reviewer. If there was disagreement, a third reviewer served as the tiebreaker.
Statistical analysis
Overall demographic and clinical features of the subjects with the 250.40 diagnosis are presented using descriptive statistics: mean ± standard deviation or median (range). Patients who did not meet KDOQI clinical or biopsy criteria for DN as primary cause of ESRD were compared with those who did meet criteria using t-test for normally distributed variables, and Fisher’s exact test or the Mann–Whitney U test for variables not normally distributed. We conducted exact logistic regression, due to small sample size, to evaluate whether retinopathy was independently associated with Type 2 DN based on KDOQI criteria, adjusted for age, sex and race. Inter-rater agreement between the primary and secondary reviewer was quantified using the kappa statistic. P < 0.05 was considered statistically significant. Analyses were performed using STATA 13SE (College Station, TX, USA) or GraphPad QuickCalcs (San Diego, CA, USA) online software.
Results
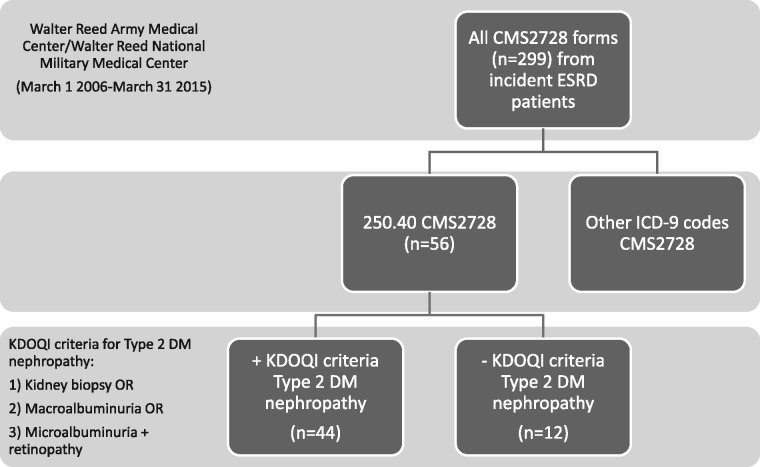
A summary of the conduct of the study is shown in Figure 1. There were 299 CMS2728 forms submitted between March 1 2006 and 31 March 2015 (9.09 years), representing 297 patients, two of whom had two CMS2728 forms each submitted >90 days apart (5 years and 6 years). Of these 299 CMS2728 forms, 56 were coded as representing ESRD due to Type 2 DM [International Classification of Diseases (ICD)-9 Code 250.40]. This represents an incidence of 18.7% over 9.09 years, and was the third most common cause of ESRD coded by our program. For comparison, incidence of ESRD secondary to hypertension (ICD-9 code 40391) was 27.8%, and for primary and secondary glomerulonephritis (GN), 20.1% (Codes 5820, 5821, 5829, 5831, 58321, 58381, 7100, 7101, 7598, 2870, 44620, 44621, 58391).
Fig. 1.
Conduct of the study and identification of study subjects.
The 56 submitted forms represented 56 individual patients (Table 1). The kappa statistic for the two initial reviewers was 0.89. In all, 95% (53/56) were documented as having been seen by a nephrologist prior to ESRD. Of the 56 patients, 12 (21.4%) did not have Type 2 DM as the primary cause of ESRD using KDOQI 2007 criteria upon review of health records (Table 2). Of these 12, two (16.7%) were miscoded. One (Case 1) had nephropathy secondary to Type 1 DM, and should have been coded as ICD-9 Code 250.41. The other (Case 2) had originally had Type 2 DM as the primary cause of ESRD, but lost a subsequent kidney transplant to biopsy-confirmed BK virus-induced nephropathy, and should have been coded as ICD-9 Code 99681.
Table 1.
Demographic and clinical features of patients coded as 250.40 (Type 2 diabetes with renal manifestations) as cause of ESRD on CMS2728 (those meeting KDOQI 2007 guideline criteria for Type 2 DN versus those who did not meet KDOQI criteria are compareda)
| Met KDOQI 2007 criteria for Type 2 DN as cause of ESRD | P-value | ||
|---|---|---|---|
| Yes | No | ||
| (N = 44) | (N = 12) | ||
| Age (years) | 66.8 ± 9.5 | 61.8 ± 10.3 | 0.12 |
| Sex | 0.33 | ||
| Male | 29 (65.9%) | 6 (50.0%) | |
| Female | 15 (34.1%) | 6 (50.0%) | |
| Race | |||
| White | 6 (13.6%) | 0 (0%) | 0.32 |
| African–American | 30 (68.2%) | 8 (66.7%) | 1.00 |
| Asian | 0 (0%) | 2 (16.7%) | 0.04 |
| Other | 8 (18.2%) | 2 (16.7%) | 1.00 |
| RRT type | 43 (98%) HD | 12 (100%) HD | 1.00 |
| Body mass index, kg/m2 | 31.4 ± 7.3 | 29.8 ± 4.2 | 0.48 |
| History of hypertension | 44 (100%) | 12 (100%) | 1.00 |
| History of cardiovascular diseaseb | 30 (68%) | 9 (75%) | 0.73 |
| History of smoking | 13 (30%) (N = 43) | 5 (42%) | 0.50 |
| Years since diagnosis of Type 2 DM | 19 (8–40) (N =33) (N = 11 unknown) | 11 (3–20) (N = 8) (N = 3 unknown, N = 1 Type 1 DM) | 0.007 |
| Active urine sediment | 0 (0%) | 3c (27%) (N = 11) | 0.006 |
| Developed macroalbuminuria prior to ESRD | 44 (100%) | 9 (82%) (N = 11) | 0.037 |
| Microalbuminuria only prior to ESRD | 0 (0%) | 0 (0%) | 1.00 |
| Retinopathy | 38 (86%) | 4 (36%) (N = 11) | 0.002 |
| Biopsy | 1d | 2e (N = 11) | 0.099 |
HD, hemodialysis.
Data are presented as mean ± standard deviation, as median (range) or as number (percentage). Patient records were reviewed by a primary and secondary provider, with a third provider in the event of disagreement, as per Materials and methods.
Defined as history of coronary artery disease, cerebrovascular disease and/or peripheral vascular disease.
All patients with active urine sediment had associated Hepatitis C.
Biopsy results: one patient had BK nephropathy in transplant kidney; one patient had membranoproliferative glomerulonephritis with cryoglobulinemia and background diabetic changes.
Biopsy results: consistent with diabetic nephropathy.
Table 2.
Characteristics of patients coded as having Type 2 DN as the primary cause of ESRD, but who did not meet KDOQI 2007 criteria
| # | Probable primary cause of renal failure (ICD-9-CM Code) | Macro- albuminuria | Micro- albuminuria alone | Retinopathy | Active urine sediment? | Biopsy |
|---|---|---|---|---|---|---|
| 1 | Diabetes with renal manifestations Type 1 (25041) | Yes | No | Yes | No | No |
| 2 | Complications of transplanted kidney (99681) BK nephropathy on transplant biopsy | Yes | No | Yes | No | Yes |
| 3 | Unknown; patient without proteinuria (7999) | No | No | Yes | No | No |
| 4 | Probable Hepatitis C-associated GN with previous AKI (58391) | Yes | No | No | Yes | No |
| 5 | ADPKD; tophaceous gout with obstructive nephrolithiasis (75313) | Yes | No | No | No | No |
| 6 | Possible drug-associated AKI with subsequent CKD (7999) | No | No | No | No | No |
| 7 | ESRD presumed 2° to HTN, presenting with hypertensive urgency (40391) | Yes | No | No | No | No |
| 8 | Probable renal vascular disease; small kidneys, cortical thinning, and renal arterial calcifications (40391) | Yes | No | No | No | No |
| 9 | Probable Hepatitis C-associated GN; history of malignant hypertension (58391) | Yes | No | No | Yes | No (offered) |
| 10 | MPGN with cryoglobulinemia 2° to Hepatitis C on biopsy; diabetic changes also present (58391) | Yes | No | Yes | Yes | Yes |
| 11 | Hypertensive kidney disease; small kidneys bilaterally and renal vascular disease (40391) | Yes | No | No | No | No |
| 12 | Insufficient data to make diagnosis; presented at ESRD post-stroke; presumed 2° to Type 2 DM (7999) | Unknown | Unknown | Unknown | Unknown | Unknown |
AKI, acute kidney injury; HTN, hypertension; ADPKD, autosomal dominant polycystic kidney disease; MPGN, membranoproliferative glomerulonephritis.
Of the remaining 10 patients, although all had Type 2 DM, none appeared to have DN by KDOQI 2007 criteria (Table 2). Three had Hepatitis C-associated GN (one confirmed by biopsy). Three appeared to have hypertensive chronic kidney disease (CKD), two of which had probable renal vascular disease. Two had no history of micro- or macroalbuminuria, and the cause of CKD was unclear. One had polycystic kidney disease and a history of recurrent obstruction due to nephrolithiasis. One, who presented at ESRD after a stroke, had no records available to verify the diagnosis.
Patients who had KDOQI-predicted DN had a Type 2 DM diagnosis more years than those who did not [19 years (8–40) versus 11 years (3–20), P = 0.007]. Those who did not have KDOQI-predicted DN were more likely to have an active urine sediment (P = 0.006), and less likely to have developed macroalbuminuria (P = 0.037) or retinopathy (P = 0.002) prior to ESRD. On exact logistic regression (adjusted for age, sex and race), retinopathy was significantly associated with KDOQI-predicted DN [odds ratio = 19.16 (confidence interval 2.76–223.7), P = 0.0009].
Discussion
Prevalence of Type 2 DM in the USA is 12% for 45–64 year-olds, and 22% among those 65–74 years [6]. In our single-center study, 18.7% of incident ESRD patients were coded on the CMS2728 as having Type 2 DN as the primary cause of ESRD, substantially lower than the national percentage of >40%. However, even in this cohort, over 20% of patients appear to have been incorrectly assigned per KDOQI 2007 clinical criteria. Two were miscoded (one had Type 1 DM and one had biopsy-proven BK nephropathy in a renal allograft). Seven of the 10 who were not miscoded had other, more likely renal diagnoses (including Hepatitis C-associated GN, hypertension/renal vascular disease and polycystic kidney disease), and had ESRD ‘with’ diabetes rather than ESRD ‘from’ diabetes. Type 2 DM is so common that it is likely that some patients starting RRT are indicated as having ESRD secondary to DN merely because they have concurrent Type 2 DM, especially when the patient is not well known to the nephrologist completing the form, as is often the case in patients who begin chronic RRT concurrent with an acute hospitalization. Over-assignment of Type 2 DM as the cause of CKD is suggested by several studies that identify patients with CKD and Type 2 DM who do not have significant proteinuria, one of the clinical diagnostic criteria for DN [7–9]. In a biopsy study of patients with Type 2 DM and CKD (eGFR <60 mL/min/1.73 m2), normoalbuminuric patients were less likely to have typical glomerular changes of DN, but more likely to have arteriosclerosis and tubule-interstitial fibrosis alone [10]. Normoalbuminuric patients without the glomerular changes of DN may have progressive CKD, but this may be due to hypertension, obesity and intrarenal vascular disease rather than DN nephropathy, and the diagnosis can only be clarified by biopsy [11, 12]. Biopsy series of patients with atypical DN presentations (with or without proteinuria) demonstrated that 35–60% had other causes of glomerular disease, and up to 1/3 had nondiabetic renal disease only, including acute tubular necrosis, hypertensive nephrosclerosis and focal segmental glomerulosclerosis [13, 14]. Two out of 34 patients (6%) with Type 2 DM with a typical clinical course and > 500 mg/24 h proteinuria (who would have met the KDOQI 2007 criteria for DN, and had been entered into a clinical trial) did not have DN on biopsy [15]. Thus, it is likely that some patients with ESRD associated with, but not caused by, Type 2 DM, are incorrectly assigned a diagnosis of DN.
The CMS2728 assesses only one component of the KDOQI criteria for DN—diabetic retinopathy. History of macro- or microalbuminuria is not ascertained. According to the USRDS Annual Data Report, in 2013, 14.1% of patients who had Type 2 DM reported as the primary cause of ESRD were also reported as having diabetes with retinopathy. Among patients who had other causes of ESRD, 2.8% were recorded as having diabetes with retinopathy. For purposes of comparison, the other CMS2728 measures of diabetes as a comorbid condition (COMO_DM_INS, COMO_DM_NOMEDS, COMO_DM_ORAL, for diabetes as a comorbid condition with use of insulin, no medications or oral medications, respectively) when assessed together, were present in 90.5% of patients coded as having Type 2 DN as the primary cause of ESRD, and were present in 28.6% of patients with other causes of ESRD (excluding Type 1 or unspecified DM). Comorbid conditions in CMS2728 do not distinguish between Type 1 and Type 2 DM. However, given the small fraction of Type 1 diabetics presenting at ESRD nationally, comorbid diabetes tracks very closely, if not identically, with Type 2 DN as a cause of ESRD.
Study limitations include small sample size, single center series and differences in patient population, suggested by the higher incidence of hypertension and primary/secondary GN in our cohort. Our patient population consists of younger active duty and retired military members and their families, which may account for the greater percentage of incident patients who had ESRD secondary to primary or secondary GN. Nationally, Type 2 DN-associated ESRD incidence is six times that of ESRD secondary to GN, while in our cohort, ESRD incidence secondary to GN is slightly greater than Type 2 DN. Thus, our study may not be able to be extrapolated to the general ESRD population. However, those who received the diagnosis of Type 2 DN were demographically similar to those receiving the diagnosis in ESRD Network 5, where our center is located. In 2014, the mean age among incident Network 5 ESRD patients was 62.7 years and 57.3% were male. In all, 83% of incident ESRD patients in the District of Columbia, and 53% in Maryland, were African–American [16].
Another potential limitation is the diagnostic validity of the KDOQI criteria for DN. The criteria are Grade B ‘moderately strong evidence’ recommendations, especially for patients with Type 1 DM. For Type 2 diabetics, micro- and macroalbuminuria are not as strongly predictive of typical diabetic glomerular changes on biopsy, and are not as strongly associated with retinopathy [8–10, 12, 15, 17]. Although the positive predictive value of retinopathy concurrent with macroalbuminuria is 67–100%, the negative predictive value is quite variable at 20–84% [3].
A study strength was that diagnosis validation was based on manual health record review. A weakness of national registry and health record data is that the KDOQI criteria of albuminuria and diabetic retinopathy may not be recorded in a fashion that facilitates ready retrieval. Thus, similar analyses will depend on manual validation, in the absence of significant advances in natural language analytics.
If the 20% over-assignment of Type 2 DN as the primary cause of ESRD seen in our cohort were replicated in other or larger populations, there could be substantial implications for diagnosis and management of ESRD. Research funding could be affected, as many global and national institutes allocate resources based on the frequency of ESRD causes. A 20% reduction in Type 2 DN as a primary ESRD cause would result in an incidence not much greater than that of hypertensive renal disease, a diagnosis itself in substantial doubt due to recognition of APOL1-mediated renal disease, undiagnosed glomerular disease among all races and other factors. Therapeutic nihilism for DN, due to limited specific treatment options, may unfortunately lead to diagnostic nihilism, especially if finding a condition requiring specific treatment is felt to be unlikely. In our cohort, active urine sediment was a particularly important discriminator and was associated with Hepatitis C-related renal disease, a curable condition with specific therapy.
The original legislation authorizing Health and Human Services’ renal disease registry, of which the USRDS is a primary component, the 1986 Omnibus Budget Reconciliation Act and Section 1881 of the Social Security Act [18], specifically called for ‘research on the causes of end stage renal disease’. Over the years, the USRDS Annual Data Report and independent investigators have contributed substantially to those efforts. One of the implications of over-ascertaining Type 2 DN as a primary cause of ESRD is under-ascertaining other causes of ESRD. These could include treatable diseases, such as Hepatitis C and glomerular disease, as well as new and unsuspected causes due to environmental or other exposures, exemplified by Mesoamerican nephropathy [19]. Non-traditional causes of CKD may go unrecognized in the USA due to the huge penumbra of clinically associated Type 2 DN and hypertension.
Our findings should be replicated in larger and more diverse health settings. The logical next step would be a comparable study in a large health system with queryable health records, including comparison with patients who were not coded as having Type 2 DM as the cause of ESRD. If these findings were replicated, there may be work to do investigating the causes of ESRD and CKD. Although Type 2 DM is an important cause of CKD and ESRD, in many cases it may be an exacerbating factor rather than primary contributor. In particular, these data emphasize the importance of biopsy in patients with atypical features [20], since all of those with active urine sediment likely had a diagnosis other than Type 2 DN. The presence of active urine sediment is not ascertained in current registries and is difficult to abstract from health records.
We conclude that in a single-center setting, reported CMS2728 diagnoses of Type 2 DM as a cause of ESRD were overestimated by approximately 20% when KDOQI 2007 diagnostic criteria for DN were applied. Because of the high prevalence of ESRD reported as secondary to Type 2 DM in the USA, similar overestimation nationally (if validated in larger cohorts) could indicate an opportunity to ‘make space’ for other ESRD diagnoses. Recognition of such conditions, if treatable, could potentially lower the rate of CKD progression and the incidence of ESRD.
Acknowledgements
The views expressed are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense (DoD), or the US Government. The identification of specific products or scientific instrumentation does not constitute endorsement or implied endorsement on the part of the author, the DoD or any component agency. While we generally excise references to products, companies, manufacturers, organizations, etc. in government produced works, the abstracts produced and other similarly situated research presents a special circumstance when such product inclusions become an integral part of the scientific endeavor.
Conflict of interest statement
None declared.
References
- 1. USRDS Annual Report, 2014, Volume 2: End-stage Renal Disease in the United States. Chapter 1.2.F and 1.3.C. http://www.usrds.org/2014/view/v2_01.aspx (8 April 2015, date last accessed)
- 2.Centers for Disease Control and Prevention. National Surveillance: Diabetes Complications: End Stage Renal Disease (2014). http://www.cdc.gov/diabetes/statistics/esrd/fig7.htm (8 April 2015, date last accessed)
- 3. KDOQI Clinical Practice Guideline Diabetes and CKD. Guidelines 1.3 and 1.4. Screening and diagnosis of DKD. Am J Kidney Dis 2007; 49: S1–S180 [Google Scholar]
- 4. United States Renal Data System. 2015. Annual Data Report; Chapter 1: Incidence, prevalence, patient characteristics, and treatment modalities: primary cause of ESRD: a cautionary note. Ann Arbor, MI: USRDS Coordinating Center. http://www.usrds.org/2015/view/v2_01.aspx (12 February 2015, date last accessed)
- 5. Layton JB, Hogan SL, Jennette CE. et al. Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol 2010; 5: 2046–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. National Surveillance: Diagnosed Diabetes: Rate by Age (2014). http://www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm (8 April 2015, date last accessed)
- 7. Kramer HJ, Nguyen QD, Curhan G. et al. Renal insufficiency in the absence of albuminuria and retinopathy among adults with Type 2 diabetes mellitus. JAMA 2003; 289: 3273–3277 [DOI] [PubMed] [Google Scholar]
- 8. Moriya T, Suzuki Y, Inomata S. et al. Renal histological heterogeneity and functional progress in normoalbuminuric and microalbuminuric Japanese patients with type 2 diabetes. BMJ Open Diab Res Care 2014; 2: e000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacIsaac RJ, Tsalamandris C, Panagiotopoulos S. et al. Nonalbuminuria renal insufficiency in type 2 diabetes. Diab Care 2004; 27: 195–200 [DOI] [PubMed] [Google Scholar]
- 10. Ekinci EI, Jerums G, Skene A. et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diab Care 2013; 36: 3620–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marshall SM. Natural history and clinical characteristics of CKD in Type 1 and Type 2 diabetes mellitus. Adv Chronic Kidney Dis 2014; 21: 267–272 [DOI] [PubMed] [Google Scholar]
- 12. Robles NR, Villa J, Gallego RH.. Non-proteinuric diabetic nephropathy. J Clin Med 2015; 4: 1761–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pham TT, Sim JJ, Kujubo DA. et al. Prevalence of non-diabetic renal disease in diabetic patients. Am J Nephrol 2007; 27: 322–328 [DOI] [PubMed] [Google Scholar]
- 14. Sharma SG, Bomback AS, Radhakrishnan J. et al. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol 2013; 8: 1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwartz MM, Lewis EJ, Leonard-Martin T. et al. ; The Collaborative Study Group. Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. Nephrol Dial Transplant 1998; 12: 2547–2552 [DOI] [PubMed] [Google Scholar]
- 16.Mid-Atlantic Renal Coalition 2014 Annual Report. Table 1. ESRD Incidence—One Year Statistics as of 1/1/2014-12/31/2014. http://www.esrdnet5.org/Files/Data-CrownWeb/AR/2014/Table-1.aspx (12 February 2016, date last accessed)
- 17. Tervaert TWC, Mooyaart AL, Amann K. et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010; 21: 556–563 [DOI] [PubMed] [Google Scholar]
- 18.Official Social Security Website. Compilation of the Social Security Laws. Medicare coverage for end stage renal disease patients. https://www.ssa.gov/OP_Home/ssact/title18/1881.htm (3 February 2016, last date accessed)
- 19. Correa-Rotter R, Wessling C, Johnson RJ.. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis 2014; 63: 506–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fiorentino M, Bolignano D, Tesar V. et al. ; ERA-EDTA Immunonephrology Working Group. Renal biopsy in 2015 - from epidemiology to evidence-based indications. Am J Nephrol 2016; 43: 1–19 [DOI] [PubMed] [Google Scholar]